Abstract
Background
Lymphocytic gastritis (LG) is an uncommon entity with varying symptoms and endoscopic appearances. This condition, as well as two forms of H. pylori-negative gastritis (chronic active gastritis [CAG] and chronic inactive gastritis [CIG]), appears to be more common in patients with coeliac disease (CD) based on single-center studies.
Aim
To compare the prevalence of LG, CAG, and CIG among those with normal duodenal histology (or non-specific duodenitis) and those with CD, as defined by villous atrophy (Marsh 3).
Methods
We analyzed all concurrent gastric and duodenal biopsy specimens submitted to a national pathology laboratory during a six-year period. We performed multiple logistic regression to identify independent predictors of each gastritis subtype.
Results
Among patients who underwent concurrent gastric and duodenal biopsy (n=287,503), the mean age was 52 and the majority (67%) was female. Compared to patients with normal duodenal histology, LG was more common inpartial villous atrophy (OR 37.66; 95% CI 30.16–47.03), and subtotal/total villous atrophy (OR 78.57; 95% CI 65.37–94.44). CD was also more common in CAG (OR for partial villous atrophy 1.93; 95%CI 1.49–2.51, OR for subtotal/total villous atrophy 2.42; 95%CI 1.90–3.09) and was similarly associated with CIG (OR for partial villous atrophy 2.04; 95%CI 1.76–2.35, OR for subtotal/total villous atrophy 2.96; 95% CI 2.60–3.38).
Conclusion
LG is strongly associated with CD, with increasing prevalence correlating with more advanced villous atrophy. CAG and CIG are also significantly associated with CD. Future researchshould measure the natural history of these conditions after treatment with a gluten-free diet.
INTRODUCTION
Coeliac disease (CD) is a systemic immune-based disorder triggered by dietary gluten, the protein component of wheat, rye and barley.1 The prevalence of CD has increased in recent decades 2 and the cause for this rise has not been determined. The loss of immune tolerance to gluten can occur at any age, and hypothesized triggers for this event include infections 3 and host factors relating to prenatal exposures4 perinatal events5 and the intestinal microbiome.6 Alteration of the gastric environment has also been proposed as a contributor to the pathophysiology of CD; use of proton pump inhibitors and lack of colonization with Helicobacter pylori have been association with an increased risk of CD.78
Lymphocytic gastritis (LG) is an uncommon histopathologic finding with diverse clinical manifestations and poorly characterized natural history. 9 While it can be idiopathic or drug-induced, LG also appears to be associated with CD, appearing in up to 30% of such patients.10 The clinical significance of LG, in patients with or without CD, is unknown, and there is no recognized treatment strategy for this histologic finding apart from institution of the gluten-free diet in patients with CD. Investigations of LG in patients with CD have been limited to single-center studies involving fewer than 300 patients with CD. Moreover, data regarding other forms of gastritis in patients with CD are lacking. In our recent study that found a lower prevalence of H. pylori colonization in patients with CD, we noted a surprisingly high prevalence of H. pylori-negative gastritis in this population, both chronic active gastritis (CAG) and chronic inactive gastritis (CIG).7 This led us to hypothesize that these gastric abnormalities correlate with the severity of villous atrophy in patients with CD.
We therefore aimed to characterize the relationship between CD and three forms of gastritis: LG, H. pylori-negative CAG, and H. pylori-negative CIG using data from a national pathology database.
METHODS
We performed a cross-sectional analysis of histopathology specimens submitted to Miraca Life Sciences (Irving, TX), a United States pathology laboratory that receives specimens from 43 states, the District of Columbia, and Puerto Rico. Specimens are interpreted at threesites by a group of 35 gastrointestinal pathologists who use standardized reporting language and diagnostic criteria when formulating reports, as previously described.7 We included procedures in which a concurrent gastric and duodenal biopsy specimenwas submitted to during a six-year period (January 2, 2008 through January 2, 2014), excluding patients with a previous diagnosis of upper gastrointestinal surgery or cancer.
Coeliac Disease
Patients were considered to have CD if their duodenal biopsy demonstrated villous atrophy (corresponding to a Marsh score of 3),11 with accompanying duodenal intraepithelial lymphocytosis(Figure 1). Patients were further subdivided into partial villous atrophy (Marsh 3A) and subtotal or total villous atrophy (Marsh 3B/C). Although duodenal intraepithelial lymphocytosis with normal villous architecture may represent a mild histologic form of CD (Marsh 1), due to its relative lack of specificity we have not included patients with this finding in our definition of CD, in accordance with our previous analyses.7,12,13 Patients without these findings were classified as having a normal duodenal biopsy; those with duodenitis (active mucosal inflammation with or without erosions or foveolar cell metaplasia) were classified as normal for the purposes of this analysis.
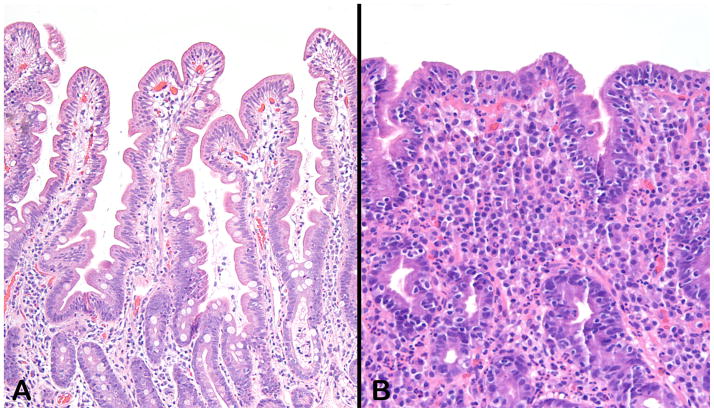
Figure 1.
Sections from normal duodenal mucosa (A) and from a mucosal biopsy with coeliac disease (B). In contrast to the long villi with only minimal numbers of intraepithelial lymphocytes, panel B shows an epithelium studded with lymphocytes and a lamina propria obliterated by a mixed inflammatory infiltrate consisting of lymphocytes, plasma cells, eosinophils, and rare neutrophils. The normal mucin content of the normal goblet cells, evident in panel A, is completely depleted in the mucosa depicted in panel B. Both sections were stained with hematoxylin and eosin and photographed at an original magnification of 10X.
Lymphocytic Gastritis
LG was defined as the presence of at least 25 lymphocytes per 100 epithelial cells on the surface and foveolar epithelium, with chronic inflammation also noted in the lamina propria (Figure 2).
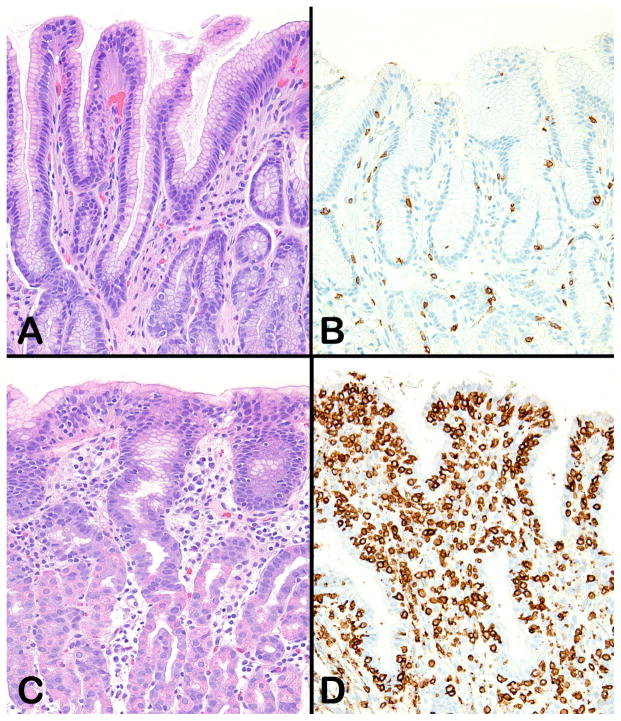
Figure 2.
Normal gastric mucosa stained with hematoxylin and eosin (A) and an anti-CD3 immunohistochemical stain (B). The normal gastric epithelium contains no intraepithelial lymphocytes; the rare CD3-positive cells seen in panel B are in the lamina propria. Panel C shows transitional gastric mucosa from a patient with lymphocytic gastritis. Although intraepithelial lymphocytes can be seen, particularly in the surface epithelium, their large numbers are best appreciated in sections stained with an anti-CD3 immunohistochemical stain (D). Original magnification 200X for all panels.
Chronic H. pylori-negative Gastritis
Chronic active gastritis was defined as the presence of polymorphonuclear cells in the lamina propria in the absence of H. pylori organisms.14 Chronic inactive gastritis was defined as the presence of dense populations of lymphocytes and plasma cells within the lamina propria, in the absence of activity or H. pylori organisms.15 Gastric specimens were considered to be H. pylori-negative if H. pylori was not detected on a specific polyclonal immunochemical stain (Cell Marque Corporation, Rocklin, California)that is routinely performed on all gastric specimens.
Statistical Analysis
We calculated the prevalence of LG, CAG, and CIG according to the following a priori categories: age (0–19, 20–39, 40–59, and >60 years), gender, and duodenal histology. The latter category was divided into the following categories: normal, duodenal intraepithelial lymphocytosis (DIL) with normal villi, partial villous atrophy, and subtotal/total villous atrophy. For the analysis of LG (but not CAG or CIG) we also calculated the prevalence stratified by H. pylori colonization. We used the chi square test to compare the prevalence of these gastritis subtypes in each of these categories, and we subsequently performed multivariate logistic regression using models that included all of the above variables to report odds ratios (OR) and corresponding 95% confidence intervals (CI) for the independent association between each variable and the presence of LG, CAG, or CIG.
All p values reported are two-sided. This study was deemed “non-human subjects research” by the Institutional Review Board of Columbia University Medical Center since all data was de-identified prior to being provided to the investigators.
RESULTS
Of 292,336 individuals who underwent concurrent gastric and duodenal biopsy during the specified time period. 4,833 were excluded due to a history of upper gastrointestinal surgery or cancer, leaving 287,503 for this analysis. Demographic and histologic characteristics are listed in Table 1. The median age was 53 years, and the majority were older than 40. Some 67% were female. Overall 64% of gastric biopsies were normal. LG was present in 818 (0.3%) individuals, chronic active H. pylori-negative gastritis (CAG) was present in 4,619 (2%) and chronic inactive H. pylori-negative gastritis (CIG) was present in 16,155 (6%), including 15,882 (5.5%) who had no evidence of H. pylori on immunostain. CD was present in 3,948 individuals (1.4%), including 2062 (0.7%) with partial villous atrophy and 1,886 (0.7%) with subtotal/total villous atrophy. Among the 3,948 patients with CD only 619 (16%) had normal gastric histology.
Table 1.
Characteristics of patients who underwent concurrent gastric and duodenal biopsy during a six year period (n=287,503):
| Characteristic | Number (%) |
|---|---|
|
| |
| Age, years | |
|
| |
| Mean/median (SD) | 51.7/53 (18) |
| 0–19 | 12,415 (4) |
| 20–39 | 60,360 (21) |
| 40–59 | 110,210 (38) |
| ≥60 | 104,518 (36) |
|
| |
| Gender* | |
| Male | 96,722 (34) |
| Female | 190,678 (67) |
|
| |
| Gastrichistology | |
| Normal | 183,325 (64) |
| Active H. pylori gastritis | 27,366 (10) |
| Chronic active gastritis, H. pylori negative | 4,619 (2) |
| Chronic inactive gastritis | 16,155 (6) |
| Lymphocytic gastritis | 818 (0.3) |
| Reactive gastropathy | 46,790 (16) |
| Intestinal metaplasia | 20,223 (7) |
| Atrophic gastritis | 1,647 (0.6) |
|
| |
| Duodenal histology | |
| Normal/duodenitis | 264,739 (92) |
| Duodenal intraepithelial lymphocytosis | 18,816 (7) |
| Partial villous atrophy | 2,062 (0.7) |
| Subtotal/total villous atrophy | 1,886 (0.7) |
Gender data missing for 103 patients (0.04%)
Increasing age was associated with an increased risk of LG (Table 2), while gender and H. pylori status were not. The prevalence of LG was 7.3% in patients with CD and the degree of villous atrophy directly correlated with the probability of concurrent LG; those with partial villous atrophy had an LG prevalence of 5.0%, while those with subtotal/total villous atrophy had an LG prevalence of 9.7%. On multivariate analysis, age remained a significant predictor of LG (OR for age ≥60 years compared to 20–39 years 1.83; 95%CI 1.51–2.21). Among individuals older than 60 years who had CD (n=1,246), 128 (10.3%) had LG. CD was strongly associated with LG on multivariate analysis (OR for partial villous atrophy 37.66; 95% CI 30.16–47.03, OR for subtotal/total villous atrophy 78.57; 95% CI 65.37–94.44).
Table 2.
Univariate and multivariate analysis of predictors of lymphocytic gastritis.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
|
|
||||
| Characteristic | Prevalence of Lymphocytic Gastritis | P value | OR (95% CI) | P value |
|
| ||||
| Age, years | <0.0001 | |||
| 0–19 | 26 (0.2) | 0.75 (0.50–1.45) | 0.1860 | |
| 20–39 | 160 (0.3) | 1.0 (ref) | ref | |
| 40–59 | 246 (0.2) | 0.94 (0.77–1.53) | 0.5632 | |
| ≥60 | 386 (0.4) | 1.83 (1.51–2.21) | <0.0001 | |
|
| ||||
| Gender | 0.3463 | |||
| Male | 288 (0.3) | 1.0 (ref) | ref | |
| Female | 530 (0.3) | 0.88 (0.76–1.02) | 0.0925 | |
|
| ||||
| H. pylori status | 0.1249 | |||
| H. pylori | 65 (0.2) | 0.87 (0.67–1.12) | 0.2765 | |
| No H. pylori | 753 (0.3) | 1.0 (ref) | ref | |
|
| ||||
| Duodenal histology | <0.0001 | |||
| Normal/duodenitis | 385 (0.15) | 1.0 (ref) | ref | |
| Duodenal intraepithelial lymphocytosis | 146 (0.8) | 6.15 (5.06–7.47) | <0.0001 | |
| Partial villous atrophy | 104 (5.0) | 37.66 (30.16–47.03) | <0.0001 | |
| Subtotal/total villous atrophy | 183 (9.7) | 78.57 (65.37–94.44) | <0.0001 | |
H. pylori-negative CAG was most common in children, prevalent in 2% of individuals younger than 20 years (Table 3). In contrast, H. pylori-negative CIG was most common in older individuals, affecting 6.1% of subjects older than 60, compared to 5.1% of children (Table 4). These differences in CAG and CIG prevalence according to age remained significant on multivariate analysis. CD was more likely in those with CAG (OR for partial villous atrophy 1.93; 95%CI 1.49–2.51, OR for subtotal/total villous atrophy 2.42; 95%CI 1.90–3.09) and was similarly associated with CIG (OR for partial villous atrophy 2.04; 95%CI 1.76–2.35, OR for subtotal/total villous atrophy 2.96; 95% CI 2.60–3.38).
Table 3.
Univariate and multivariate analysis of predictors of chronic active (H. pylori-negative) gastritis.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
|
|
||||
| Characteristic | Prevalence of Chronic Active Gastritis | P value | OR (95% CI) | P value |
|
| ||||
| Age, years | <0.0001 | |||
| 0–19 | 249 (2.0) | 1.21 (1.05–1.39) | 0.0091 | |
| 20–39 | 1,017 (1.7) | 1.0 (ref) | ref | |
| 40–59 | 1,612 (1.5) | 0.88 (0.82–0.96) | 0.0022 | |
| ≥60 | 1,741 (1.7) | 1.03 (0.95–1.11) | 0.4620 | |
|
| ||||
| Gender | 0.8880 | |||
| Male | 1,550 (1.6) | 1.0 (ref) | ref | |
| Female | 3,069 (1.6) | 0.99 (0.93–1.06) | 0.8405 | |
|
| ||||
| Duodenal histology | <0.0001 | |||
| Normal/duodenitis | 4,027 (1.5) | 1.0 (ref) | ref | |
| Duodenal intraepithelial lymphocytosis | 464 (2.5) | 1.65 (1.50–1.82) | <0.0001 | |
| Partial villous atrophy | 60 (2.9) | 1.93 (1.49–2.51) | <0.0001 | |
| Subtotal/total villous atrophy | 68 (2.6) | 2.42 (1.90–3.09) | <0.0001 | |
Table 4.
Univariate and multivariate analysis of predictors of chronic inactive (H. pylori-negative) gastritis.
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
|
|
||||
| Characteristic | Prevalence of Chronic Inactive Gastritis | P value | OR (95% CI) | P value |
|
| ||||
| Age, years | <0.0001 | |||
| 0–19 | 634 (5.1) | 0.91 (0.84–1.0) | 0.05 | |
| 20–39 | 3,384 (5.6) | 1.0 (ref) | ref | |
| 40–59 | 5,447 (4.9) | 0.89 (0.85–0.93) | <0.0001 | |
| ≥60 | 6,417 (6.1) | 1.14 (1.09–1.19) | <0.0001 | |
|
| ||||
| Gender | 0.0040 | |||
| Male | 5,176(5.4) | 1.0 (ref) | ||
| Female | 10,699(5.6) | 1.05 (1.01–1.08) | 0.0106 | |
|
| ||||
| Duodenal histology | <0.0001 | |||
| Normal/duodenitis | 14,046(5.3) | 1.0 (ref) | ref | |
| Duodenal intraepithelial lymphocytosis | 1,361 (7.2) | 1.42 (1.34–1.51) | <0.0001 | |
| Partial villous atrophy | 210 (10.2) | 2.04 (1.76–2.35) | <0.0001 | |
| Subtotal/total villous atrophy | 265 (14.1) | 2.96 (2.60–3.38) | <0.0001 | |
Duodenal intraepithelial lymphocytosis with normal villous architecture was positively associated with LG (OR 6.15; 95% CI 5.06–7.47), CAG (OR 1.65; 95%CI 1.50–1.82), and CIG (OR 1.42; 95%CI 1.34–1.51). In each category of gastritis, the association with intraepithelial lymphocytosis was weaker than the association with CD.
DISCUSSION
In this analysis of a nationwide pathology database of patients undergoing concurrent gastric and duodenal biopsies, we found that the prevalence of LG was significantly greater in those with CD (7.3%) than those without CD (0.15%), with an increasing prevalence correlating with the degree of villous atrophy. While gender and H. pylori status were not associated with LG, increasing age was, with the consequence that patients with CD older than 60 years had a prevalence of 10.3%. H. pylori-negative CAG and CIG were also more common in patients with CD than those with normal duodenal biopsies, and a similar relationship between degree of villous atrophy and prevalence of gastritis was present. Villous atrophy was an independent risk factor for LG, CAG, and CIG on multivariate analysis.
The association between CD and LG is well established. The common feature of intraepithelial lymphocytosis in these two entities was invoked in an early series of patients with LG16 and subsequent studies confirmed this association, 9,10,17 with one series noting that increased gastric intraepithelial lymphocyte counts decreased on follow-up biopsy in 5 patients.18 In a study of 39 patients with CD and LG, Bhatti and colleagues found that these patients had more severe villous atrophy, higher transglutaminase antibody levels, and lower serum albumin than those patients with CD who did not have LG. 19
More recently LG has been reported as a drug-induced entity; in a case series of 22 patients with severe sprue-like enteropathy attributed to the angiotensin receptor blocker olmesartan, LG was reported in 5 patients.20 Our current study, which includes the largest number of CD patients with LG to date, found a gradient of LG prevalence according to the degree of villous atrophy, suggesting that LG reflects the host immune response to dietary gluten.
We found that there was no association between gastric H. pylori colonization and LG. This is consistent with a recent single-center investigation by Nielsen, et al that found that among 56 cases of LG, 54 had a predominant intraepithelial lymphocytosis and lacked H. pylori, while the 2 H. pylori-positive cases had a prominent neutrophilic infiltrate in the epithelium and lamina propria.21 Given that our previous study found a negative association between H. pylori and CD,7 and that the present analysis found a positive association between LG and CD, it is not surprising that on multivariate analysis (Table 2), H. pylori was negatively associated with LG, though this finding did not meet statistical significance (OR 0.87; 95%CI 0.67–1.12).
To our knowledge, this is the first investigation testing for an association between H. pylori-negative CAG or CIG with CD. This study has a number of strengths, including its large sample size and uniform definitions and reporting terminology. The multicenter setting allowed us to generate prevalence data more reflective of the true prevalence of LG and non-H. pylori CAG and CIG as compared to single-center studies. Our study was limited by the lack of serological details of patients with villous atrophy, leaving open the possibility of misclassification, since not all patients with villous atrophy have CD (though this is the most common cause of villous atrophy, even among patients with negative CD serologies).22 We also lacked details regarding the clinical presentation of patients with these three types of gastritis. As such, the clinical implications of these gastric histologic abnormalities, and how they may affect of the natural history of CD, are not known. As this was a cross-sectional study, we do not know if gastritis preceded CD, occurred synchronously, or appeared subsequent to the development of villous atrophy. It therefore cannot be determined whether LG, CAG, or CIG triggers CD in certain individuals, is a consequence of gluten exposure, or reflects an autoimmune diathesis that is independent of CD activity.
Gastric biopsies are often taken when a patient undergoes duodenal biopsy. This is advocated partially to explore for the presence of H pylori gastritis that may be a cause of DIL.23 The very frequent finding of abnormal gastric histology in patients with CD is corroborated in our data, with only 16% having normal gastric histology in this group. This finding may be noted by the clinician, but there is typically no further action initiated after the report is noted. This is because we have no information about the clinical significance of non-Helicobactergastritis. Our results support the strong association between CD and gastric pathology, with increasing rates of gastric pathology corresponding to the severity of villous atrophy. These findings open avenues of research regarding the role of the stomach in the pathogenesis of CD. Specifically, questions arise about the influence of gastric pathology on digestion, drug metabolism or the microbiome as well on the long-term effect on general health of an individual. In recent years there has been consideration that long term blocking of gastric acid production by PPIs may have a deleterious effect on health.824 Longitudinal studies of patients with gastric pathology in those with CD are warranted, especially in children.
We acknowledge that in this observational study we restricted our analysis to those individuals who underwent concurrent gastric and duodenal biopsy. Among all patients undergoing duodenal biopsy during the time period of this analysis (January 2, 2008 through January 2, 2014), 65% of those with CD had a gastric biopsy, compared to 82% of those without CD. As such, the prevalence estimates of LG may differ from that of a protocolized study in which every patient undergoing duodenal biopsy undergoes a concurrent gastric biopsy. Nevertheless, our risk estimates would only be biased if those patients with CD and LG were more likely to undergo gastric biopsy compared to non-CD patients with LG. Given that gastric biopsy is determined by a multitude of factors apart from CD (particularly the endoscopic appearance of the stomach) it is unlikely that these large difference in gastritis prevalence is driven by these different rates of gastric biopsy.
In conclusion, we found a strong association between CD and LG, and a weaker (but significant) association between CD and two other forms of H. pylori-negative gastritis, CAG and CIG. For all three of these gastritis subtypes, these outcomes were more common among patients with more severe villous atrophy. Future studies should determine the clinical implications and natural history of gastritis among patients with CD.
Acknowledgments
Grant Support (Funding):
BL: The National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR000040)
Abbreviations used in this article
- CD
coeliac disease
- OR
Odds Ratio
- CI
Confidence interval
Footnotes
Disclosures:
All authors declare that they have no conflicts of interest and nothing to declare.
Details of ethics approval: This project (IRB-AAAF1598) was approved by the Institutional Review Board of Columbia University on April 1, 2010.
Guarantor of the Article: Dr. Lebwohl
Authors Contributions:
Study concept and design: BL, PHRG, RMG
Acquisition of data: BL, RMG
Analysis and interpretation of data: BL, PHRG, RMG
Drafting of the manuscript: BL, RMG
Critical revision of the manuscript for important intellectual content: BL, PHRG, RMG
Statistical analysis: BL
Study supervision: RMG
All authors approve the final manuscript submitted and they approve the authorship list.
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–43. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stene LC, Honeyman MC, Hoffenberg EJ, et al. Rotavirus infection frequency and risk of celiac disease autoimmunity in early childhood: a longitudinal study. Am J Gastroenterol. 2006;101:2333–40. doi: 10.1111/j.1572-0241.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 4.Stordal K, Haugen M, Brantsaeter AL, Lundin KE, Stene LC. Association between maternal iron supplementation during pregnancy and risk of celiac disease in children. Clin Gastroenterol Hepatol. 2014;12:624–31. doi: 10.1016/j.cgh.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marild K, Stephansson O, Montgomery S, Murray JA, Ludvigsson JF. Pregnancy outcome and risk of celiac disease in offspring: a nationwide case-control study. Gastroenterology. 2012;142:39–45. doi: 10.1053/j.gastro.2011.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivares M, Neef A, Castillejo G, et al. The HLA-DQ2 genotype selects for early intestinal microbiota composition in infants at high risk of developing coeliac disease. Gut. 2015;64:406–17. doi: 10.1136/gutjnl-2014-306931. [DOI] [PubMed] [Google Scholar]
- 7.Lebwohl B, Blaser MJ, Ludvigsson JF, et al. Decreased risk of celiac disease in patients with Helicobacter pylori colonization. Am J Epidemiol. 2013;178:1721–30. doi: 10.1093/aje/kwt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebwohl B, Spechler SJ, Wang TC, Green PH, Ludvigsson JF. Use of proton pump inhibitors and subsequent risk of celiac disease. Dig Liver Dis. 2014;46:36–40. doi: 10.1016/j.dld.2013.08.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmack SW, Lash RH, Gulizia JM, Genta RM. Lymphocytic disorders of the gastrointestinal tract: a review for the practicing pathologist. Advances in anatomic pathology. 2009;16:290–306. doi: 10.1097/PAP.0b013e3181b5073a. [DOI] [PubMed] [Google Scholar]
- 10.Brown IS, Smith J, Rosty C. Gastrointestinal pathology in celiac disease: a case series of 150 consecutive newly diagnosed patients. Am J Clin Pathol. 2012;138:42–9. doi: 10.1309/AJCPE89ZPVJTSPWL. [DOI] [PubMed] [Google Scholar]
- 11.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–54. [PubMed] [Google Scholar]
- 12.Carmack SW, Genta RM. The diagnostic value of the duodenal biopsy: a clinico-pathologic analysis of 28,000 patients. Dig Liver Dis. 2010;42:485–9. doi: 10.1016/j.dld.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Lebwohl B, Kapel RC, Neugut AI, Green PH, Genta RM. Adherence to biopsy guidelines increases celiac disease diagnosis. Gastrointest Endosc. 2011;74:103–9. doi: 10.1016/j.gie.2011.03.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genta RM, Sonnenberg A. Helicobacter-negative gastritis: a distinct entity unrelated to Helicobacter pylori infection. Aliment Pharmacol Ther. 2015;41:218–26. doi: 10.1111/apt.13007. [DOI] [PubMed] [Google Scholar]
- 15.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol; International Workshop on the Histopathology of Gastritis; Houston. 1994; 1996. pp. 1161–81. [DOI] [PubMed] [Google Scholar]
- 16.Haot J, Hamichi L, Wallez L, Mainguet P. Lymphocytic gastritis: a newly described entity: a retrospective endoscopic and histological study. Gut. 1988;29:1258–64. doi: 10.1136/gut.29.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feeley KM, Heneghan MA, Stevens FM, McCarthy CF. Lymphocytic gastritis and coeliac disease: evidence of a positive association. J Clin Pathol. 1998;51:207–10. doi: 10.1136/jcp.51.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jevon GP, Dimmick JE, Dohil R, Hassall EG. Spectrum of gastritis in celiac disease in childhood. Pediatr Dev Pathol. 1999;2:221–6. doi: 10.1007/s100249900117. [DOI] [PubMed] [Google Scholar]
- 19.Bhatti TR, Jatla M, Verma R, Bierly P, Russo PA, Ruchelli ED. Lymphocytic gastritis in pediatric celiac disease. Pediatr Dev Pathol. 2011;14:280–3. doi: 10.2350/10-05-0833-OA.1. [DOI] [PubMed] [Google Scholar]
- 20.Rubio-Tapia A, Herman ML, Ludvigsson JF, et al. Severe spruelike enteropathy associated with olmesartan. Mayo Clin Proc. 2012;87:732–8. doi: 10.1016/j.mayocp.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen JA, Roberts CA, Lager DJ, Putcha RV, Jain R, Lewin M. Lymphocytic gastritis is not associated with active Helicobacter pylori infection. Helicobacter. 2014;19:349–55. doi: 10.1111/hel.12139. [DOI] [PubMed] [Google Scholar]
- 22.DeGaetani M, Tennyson CA, Lebwohl B, et al. Villous atrophy and negative celiac serology: a diagnostic and therapeutic dilemma. Am J Gastroenterol. 2013;108:647–53. doi: 10.1038/ajg.2013.45. [DOI] [PubMed] [Google Scholar]
- 23.Bao F, Bhagat G. Histopathology of celiac disease. Gastrointest Endosc Clin N Am. 2012;22:679–94. doi: 10.1016/j.giec.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Freedberg DE, Lebwohl B, Abrams JA. The impact of proton pump inhibitors on the human gastrointestinal microbiome. Clin Lab Med. 2014;34:771–85. doi: 10.1016/j.cll.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]