Abstract
Background
Near-infrared spectroscopy (NIRS) measures oxygen metabolism and is increasingly used for monitoring critically-ill neonates. The implications of NIRS-recorded data in this population are poorly understood. We evaluated NIRS monitoring for neonates with seizures.
Methods
In neonates monitored with video-EEG, NIRS-measured cerebral regional oxygen saturation (rSO2) and systemic O2 saturation were recorded every 5 seconds. Mean rSO2 was extracted for 1-hour blocks before, during, and after phenobarbital doses. For each electrographic seizure, mean rSO2 was extracted for a period of 3-times the duration of the seizure before and after the ictal pattern, and during the seizure. Linear mixed models were developed to assess the impact of phenobarbital administration and of seizures on rSO2 and fractional tissue oxygen extraction (FTOE).
Results
For 20 neonates (EGA 39.6±1.5 weeks), 61 phenobarbital doses and 40 seizures were analyzed. Cerebral rSO2 rose (p=0.005), and FTOE declined (p=0.018) with increasing phenobarbital doses. rSO2 declined during seizures, compared with baseline and post-ictal phases (baseline 81.2 vs. ictal 77.7 vs. post-ictal 79.4; p=0.004). FTOE was highest during seizures (p=0.002).
Conclusions
Cerebral oxygen metabolism decreases after phenobarbital administration and increases during seizures. These small, but clear, changes in cerebral oxygen metabolism merit assessment for potential clinical impact.
Introduction
Seizures are common in neonates and often associated with adverse outcomes (1). Whether seizures themselves harm the developing brain or are simply manifestations of abnormal cerebral physiology remains an active debate. Animal data suggest that seizures amplify brain injury (2). Neonates with hypoxic-ischemic encephalopathy (HIE) and high seizure burden show more significant brain injury on MRI and have worse outcomes than those who are seizure-free (3, 4, 5). High seizure burden in critically-ill neonates and older children has also been independently associated with a higher probability and magnitude of neurological decline (6). Long-term outcome studies of neonates with seizures demonstrate that a majority suffer significant neurodevelopmental abnormalities (7).
Phenobarbital is typically used as initial treatment for neonatal seizures, despite incomplete efficacy (7, 8, 9) and concerns that this medication could induce abnormal neuronal apoptosis and cognitive impairment (10). Furthermore the safety of loading dose administration in low birth weight and preterm neonates has been questioned, with currently recommended doses leading to higher than anticipated drug levels (11).
The gold standard for seizure diagnosis in neonates is electroencephalography (EEG) monitoring (12). Recently, additional cerebral monitoring has been advocated. Among clinically-available non-invasive devices, near-infrared spectroscopy (NIRS) has been suggested as a potentially helpful instrument for monitoring brain functional integrity (13, 14). Disturbances in cerebral oxygen metabolism and blood flow, measured by transcranial doppler, have been linked to electrographic seizures (15, 16). NIRS has been shown to reflect cerebral blood flow (17), but its utility as a bedside monitor for infants at risk for neonatal seizures remains uncertain. We hypothesized that this device could provide data on the pathophysiology of neonatal seizures and consequences of their treatment. We aimed to assess the use of NIRS monitoring for neonatal seizures and to determine whether NIRS reveals physiological changes associated with doses of phenobarbital. We hypothesized that cerebral oxygen metabolism would peak during neonatal seizures. We further theorized that phenobarbital administration would be associated with a decline in brain tissue oxygen extraction.
Results
There were 61 doses of phenobarbital administered to 20 patients (mean gestational age 39.55±1.47 weeks), with the majority of neonates receiving more than one dose. There were 40 individual seizures recorded on 11 of the patients. Seizure etiologies included hypoxic ischemic encephalopathy (N=7), neonatal epilepsy syndromes (N=5), arterial ischemic stroke (N=2), sepsis (N=2), benign neonatal seizures (N=1), sinovenous thrombosis (N=1), intracranial hemorrhage (N=1), and uncertain etiology (N=1). Demographic data are presented in Table 1.
Table 1.
Demographics of 20 neonates monitored with simultaneous conventional EEG and NIRS
| Gender | N (%) |
|---|---|
| Male | 10 (50.0) |
| Female | 10 (50.0) |
| Mean (SD) | |
| Birthweight (g) | 3308 (481) |
| Gestational Age (weeks) | 39.6 (1.5) |
| Phenobarbital dose (mg) | 17.4 (13.8; range 6.0–69.0) |
| Dose/birthweight (mg/kg) | 5.2 (4.1; range 2.1–20.3) |
| Seizure duration (sec) | 120.0 (180.4; range 16.0–510.0) |
| Phenobarbital doses per patient | N (%) |
| 1 | 5 (25.0) |
| 2 | 2 (10.0) |
| 3 | 6 (30.0) |
| 4 | 1 (5.0) |
| 5 | 6 (30.0) |
| Seizures per patient | N (%) |
| 0 | 9 (45.0) |
| 1 | 1 (5.0) |
| 2 | 2 (10.0) |
| 4 | 5 (25.0) |
| 5 | 3 (15.0) |
Phenobarbital Models
The phenobarbital doses ranged from 2.1 to 20.3 mg/kg, with 49 maintenance and 12 bolus doses. Absolute changes in rSO2 and FTOE associated with phenobarbital dosing were small (Table 2). The estimated mean left cerebral rSO2 was higher at baseline compared with the hour after phenobarbital administration (75.8±2.84 vs. 74.9±2.80; Bonferroni adjusted p=0.049). In these analyses there was no change in MAP, SaO2, or systemic rSO2 associated with phenobarbital dosing.
Table 2.
Phenobarbital Administration Model Results, Controlling for Dosea
| Outcome: Average | Pre-Dose, Est. mean (95% CI) |
During Dose, Est. mean (95% CI) |
After Dose Est. mean (95% CI) |
p-value |
|---|---|---|---|---|
| rSO2 right cerebral, n=56 | 79.7 (74.4, 85.0) | 79.4 (74.1, 84.7) | 79.0 (73.7, 84.3) | 0.57 |
| rSO2 left cerebral, n=59 | 75.8 (69.1, 82.5) | 75.1 (68.4, 81.7) | 74.9 (68.2, 81.6) | 0.04 |
| rSO2 systemic, n=61 | 77.8 (74.0, 81.6) | 77.6 (73.8, 81.4) | 79.5 (75.7, 83.3) | 0.16 |
| SaO2, n=61 | 96.7 (95.9, 97.6) | 96.7 (95.8, 97.5) | 96.4 (95.6, 97.3) | 0.52 |
| MAP, n=54 | 53.5 (49.8, 57.2) | 54.3 (50.6, 58.0) | 53.1 (49.4, 56.8) | 0.22 |
| FTOE left cerebral, n=59 | 22.0 (15.0, 28.9) | 22.5 (15.6, 29.4) | 22.3 (15.3, 29.2) | 0.42 |
| FTOE right cerebral, n=56 | 17.8 (12.2, 23.3) | 18.0 (12.5, 23.6) | 18.0 (12.4, 23.5) | 0.91 |
| FTOE systemic, n=61 | 19.6 (15.7, 23.5) | 19.7 (15.8, 23.6) | 17.5 (13.6, 21.4) | 0.10 |
Estimated marginal means from linear mixed models with random intercept for patient and dose event with ratio of dose to birthweight (mg/kg) set to the average value of 5.24 (mg/kg). P-value is from an overall Wald Chi-square test for inclusion of time as a predictor in the model. Sample size represents number of dose events included in model.
Models refit to include an interaction between time period (1-hour before, during, and after the dose) and weight-adjusted doses (mg/kg) were significant. For bolus doses, the cerebral rSO2 was significantly higher in the hour after the dose, compared with the pre-dose baseline (83.4±2.4 vs. 79.3±3.5 p=0.005; Figure 1A), while rSO2 was lower in the hour after maintenance doses (81.1±1.69 vs 82.9±1.53, p=0.012). Cerebral rSO2 during the hour of phenobarbital administration did not differ significantly from baseline levels for either maintenance or bolus doses.
Figure 1.
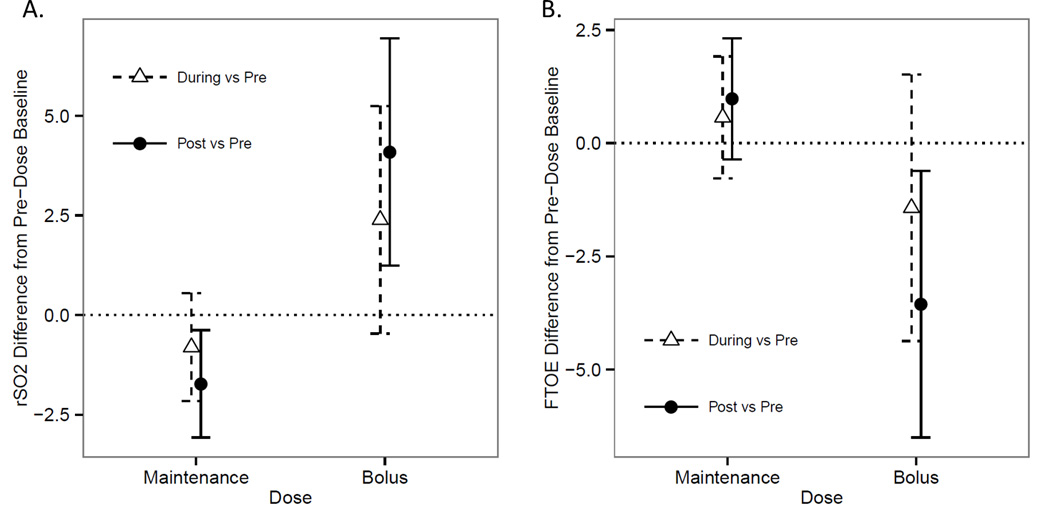
Linear mixed models with interactions were developed to assess the impact of phenobarbital administration on rSO2 (panel A) and FTOE (panel B). Estimated marginal means and standard errors are presented for the difference in rSO2 and FTOE values measured at baseline vs. during phenobarbital dose administration, and at baseline vs. one hour after phenobarbital maintenance (<10mg/kg) and bolus (≥10mg/kg) doses.
SaO2 was slightly higher during the hour of phenobarbital bolus dose administration, compared to baseline (96.9±0.51% vs. 95.4±0.77%, p=0.014), and returned to baseline in the subsequent hour. Maintenance doses were not associated with significant changes in SaO2.
Models of FTOE, as derived from rSO2 and SaO2, predicted reduced cerebral FTOE, by 3.6% on average, after phenobarbital bolus dose administration, compared with baseline (13.5±2.66% vs. 15.81±3.68%, p=0.018; Figure 1B). There was no significant difference in FTOE associated with maintenance doses.
Seizure Models
For data that included all 40 seizures, both right and left cerebral rSO2 declined during seizures, compared with baseline and post-ictal phases (right; 81.2±0.98 vs. 77.7±2.57 vs. 79.4±3.28; p=0.005; left; 77.6±1.35 vs. 74.9±1.96 vs. 75.8±2.79; p=0.004; Table 3). Right and left FTOE were highest during seizures (right: 23.7±1.64 vs. 26.9±2.81 vs. 23.9±2.91; p=0.002; left; 19.9±1.90 vs. 22.6±2.27 vs. 20.0±3.19; p=0.002), compared with the baseline and the post-ictal periods (Table 3). There was no change in SaO2 or systemic NIRS variables (rSO2 or FTOE) associated with seizures. Duration of seizures did not affect the results.
Table 3.
Seizure Model Resultsa
| Pre-Seizure Est. mean (95% CI) |
During Seizure Est. mean (95% CI) |
After Seizure Est. mean (95% CI) |
p-value | |
|---|---|---|---|---|
| rSO2 right cerebral, n=36 | 81.2 (72.0, 90.3) | 77.7 (68.5, 86.8) | 79.4 (70.3, 88.6) | 0.005 |
| rSO2 left cerebral, n=40 | 77.6 (66.6, 88.6) | 74.9 (64.0, 85.9) | 75.8 (64.8, 86.7) | 0.004 |
| rSO2_systemic, n=40 | 70.2 (63.1, 77.4) | 69.6 (62.5, 76.7) | 67.6 (60.4, 74.7) | 0.11 |
| SaO2, n=40 | 96.7 (94.7, 98.6) | 96.1 (94.2, 98.1) | 95.6 (93.6, 97.6) | 0.37 |
| MAP, n=22 | 51.7 (47.5, 56.0) | 50.2 (45.9, 54.5) | 51.7 (47.5, 55.9) | 0.31 |
| FTOE left cerebral, n=40 | 19.9 (8.6, 31.3) | 22.6 (11.2, 34.0) | 20.0 (8.6, 31.4) | 0.002 |
| FTOE right cerebral, n=37 | 23.7 (6.9, 40.5) | 26.9 (10.1, 43.7) | 23.9 (7.1, 40.7) | 0.002 |
| FTOE systemic, n=40 | 27.2 (20.1, 34.3) | 27.9 (20.8, 35.0) | 28.6 (21.6, 35.7) | 0.51 |
Estimated marginal means from linear mixed models with random intercept for patient with time length set to the average value of 178.7 seconds. P-value is from an overall Wald Chi-square test for inclusion of time as a predictor in the model. Sample size represents number of seizure events included in each model.
Model diagnostics demonstrated one subject to be highly influential in many of the results, based on Cook’s distance values. This infant’s seizures were related to HIE and cerebral rSO2 declined more dramatically during seizures than the other subjects’. Removing this infant’s data changed the statistical significance of the findings (Supplemental Table). The mean left cerebral FTOE remained higher during seizures, compared to baseline (Bonferroni adjusted p=0.033). The previously-significant mean cerebral rSO2 results rose (to p=0.07–0.09), just above the usual significance threshold.
Discussion
This novel use of NIRS monitoring with concurrent conventional EEG to confirm neonatal seizures demonstrates that treatment with loading doses (≥10mg/kg) of phenobarbital is associated with reduced cerebral oxygen metabolism (increased rSO2 and decreased FTOE) that persists for at least an hour after the dose is administered. EEG-confirmed neonatal seizures may, conversely, be associated with increased cerebral oxygen metabolism. The changes in oxygen metabolism were statistically robust, but the absolute differences in rSO2 were small and the rSO2 values always remained within the normal range. Thus, NIRS reveals compelling pathophysiological information, but assessment of potential clinical impact will require further study.
To our knowledge, we are the first to report changes in NIRS-measured cerebral oxygen metabolism associated with phenobarbital dosing for neonatal seizures. We found that brain oxygen metabolism decreased after phenobarbital loading doses. This change was independent of seizure burden, since the seizures analyzed during this study were at separate times from the phenobarbital doses. Notably, the cerebral NIRS changes associated with phenobarbital and with seizures were also independent of systemic NIRS or MAP.
Published reports of NIRS changes associated with neonatal seizures have been confined to case studies. For a 39 week male infant with hypoxic ischemic encephalopathy treated with phenobarbital and phenytoin, prolonged (>7 minute) subclinical seizures were associated with decreased cerebral tissue oxygenation index (analogous to rSO2) (14). This was similar to our finding of lower cerebral rSO2 values during seizures.
Two other case reports of infants monitored by NIRS and EEG demonstrated rSO2 fluctuations during seizures (18, 19). During a 30 minute epoch, the rSO2 of a 2-month-old preterm infant with cortical dysplasia abruptly declined 16 times, 11 of which were coincident with EEG-confirmed seizures (18). The authors noted that the NIRS patterns varied across individual seizures, a finding which is mirrored in our study, in which there was one individual whose seizure data were outliers. In another case study, of a 2.5-month-old with congenital heart disease, a repetitive spike pattern in NIRS-measured cerebral oxygenation was associated with subclinical status epilepticus (19). In all three case studies, and in our results, systemic oxygen saturation remained constant during the seizures.
None of the case studies reported FTOE changes associated with seizures or their treatment, but we showed that FTOE increased during seizures, even after an outlier was excluded. These findings echo previous work that described increased cerebral blood flow during electrographic and electroclinical neonatal seizures (15) and suggest that brain oxygen metabolism increases during neonatal seizures. Our data suggest that seizures place metabolic demands on the newborn brain. However, based solely on our results, we cannot be sure that these metabolic demands cause harm. The rSO2 was always within the expected physiologic range and so the changes associated with seizures would not be expected to induce injury.
Few studies have differentiated between left and right cerebral NIRS measurements. In one study of very preterm neonates, interhemispheric rSO2 and FTOE differences were noted during periods of unstable systemic oxygenation (20). In one of the above case studies, right and left cerebral NIRS measures were recorded for a 30 minute epoch. After administration of pancuronium and subsequent doses of fosphenytoin, a decoupling of right and left NIRS data occurred (18). No physiological explanation was offered, only that the values did not appear to be artifact. Our study results also reflected differences between right and left cerebral NIRS measures, seen for example in the mean rSO2 changes associated with phenobarbital across time (right p=0.57 vs. left p=0.04). Placement of NIRS sensors in clinical practice, on the forehead versus over the parietal regions, may influence the test results and should be taken into consideration when data are interpreted.
Our study has some limitations. Although 20 infants were included, not all infants experienced seizures during the simultaneous NIRS-EEG monitoring. We attempted to avert erroneous conclusions related to outliers by limiting the data to a maximum of five seizures or phenobarbital doses per individual subject, and adjusting for subject in the models. There was one outlier subject in our dataset, who had substantial rSO2 changes during seizures. There were no obvious clues in the clinical or EEG data to suggest why that subject’s NIRS patterns differed from the others. The subject was one of seven with HIE and the seizure duration was not different from the others’. This subject’s results dampened our conclusions about cerebral oxygen metabolism during seizures. However, it also suggests that there may be some neonates for whom seizures can produce a clinically meaningful change in cerebral oxygen metabolism. We found no such outliers in the phenobarbital dosing analyses.
While our findings regarding cerebral oxygen metabolism changes associated with phenobarbital loading doses are compelling, we only analyzed data from 1-hour after medication administration. Thus we are unable to comment on how long the documented changes in cerebral rSO2 were sustained. We cannot comment on the effect of phenobarbital blood levels on NIRS results, since most infants did not have clinically-indicated drug levels at the time of monitoring. However, higher doses of phenobarbital were associated with more significant changes in cerebral oxygen metabolism.
Our study subjects had seizures due to a range of etiologies. Due to the small sub-group sample sizes, we did not attempt to determine whether NIRS changes associated with seizures or phenobarbital differed according to the underlying seizure etiology or gestational age. Additionally, we excluded moderate preterm and very preterm infants because their small head size precludes simultaneous monitoring of conventional EEG along with bilateral cerebral NIRS. Therefore, these data may not be directly applicable to one of the populations most commonly monitored with NIRS (16).
Most neonates in this study did not have invasive blood pressure monitoring. Artifact and missing data from non-invasive blood pressure measurements limited potential analysis. We are unable to comment on the possibility that abnormal cerebrovascular autoregulation could have contributed to the NIRS changes observed after phenobarbital administration. However, clinically significant hypotension was not noted for any of these infants.
There were significant strengths to this research. We provide real-world bedside physiologic monitoring data, recorded with readily available clinical NIRS and EEG equipment, on late preterm and term neonates with a variety of seizure etiologies. Our dataset, while limited, is notably larger than previous studies and provides insight for both the physiology of neonatal seizures and their treatment with phenobarbital. All analyzed seizures were identified using the gold standard multichannel conventional EEG. Since neonatal seizures are defined according to their EEG patterns, we did not classify seizures according to the presence or absence of associated clinical signs.
We utilized United States Food and Drug Administration (FDA)-approved neonatal NIRS sensors, which are the sensors most likely to be used in clinical practice in the United States. Others have reported NIRS data gathered with different sensor types. This methodological detail must be accounted for in data interpretation, since neonatal sensor rSO2 results are typically higher than the pediatric sensors’ results (21). We also studied rSO2 recorded over the parietal regions, rather than the typical frontal recording. This provided independent bihemispheric cerebral NIRS data which were recorded from the cerebrovascular watershed regions and co-localize with the most common sites of origin for neonatal seizures (22). We also measured systemic rSO2, pulse oximetry, and, whenever possible, mean arterial blood pressure, in order to exclude changes in systemic physiology as causes for the cerebral oxygen metabolism variation associated with phenobarbital dosing and seizures. Notably, these systemic variables did not fluctuate with seizures or phenobarbital doses.
Conclusions
Typical phenobarbital loading doses are accompanied by prolonged declines in cerebral oxygen extraction and neonatal seizures may be associated with transient increases in oxygen metabolism. Whether these small but statistically significant changes, measured by NIRS, have clinical consequences remains to be determined. Therefore, although NIRS may provide scientifically interesting data, our results alone do not provide rationale for routine clinical NIRS monitoring among neonates with seizures.
Methods
The University of Michigan Institutional Review Board approved this study and the parent of each infant provided written informed consent. Each infant’s chart was systematically reviewed for demographic and clinical details. Infants who required video-EEG monitoring due to clinical suspicion for seizures were included. Premature infants (≤35 weeks gestation) and those with multiple congenital anomalies were excluded. Infants were not excluded based on any other seizure etiology.
Near-infrared spectroscopy
Late-preterm and full-term neonates monitored with conventional video-EEG due to suspected seizures were simultaneously monitored with NIRS. Neonatal NIRS sensors (Invos 5100c, Somanetics Corp, Troy, MI) were placed over the bilateral parietal regions (the cerebrovascular watershed areas) and the thigh (systemic control). NIRS-measured cerebral regional oxygen saturation (rSO2) and systemic O2 saturation (SaO2, measured via pulse oximetry) were recorded every 5 seconds and downloaded to a research computer for post-hoc analyses. Fractional tissue oxygen extraction (FTOE) was calculated (FTOE = (SaO2 – rSO2) / SaO2).
Phenobarbital
The infants’ medical records were systematically reviewed for phenobarbital dosing (mg/kg/dose) and administration data. A loading dose (≥ 10mg/kg) of phenobarbital is typically administered over 20 minutes. Mean rSO2 was extracted for 1-hour epochs before (baseline), during, and after each phenobarbital dose. To avoid erroneous conclusions based on over-representation of individual subjects, a maximum of 5 phenobarbital doses were analyzed per study subject and statistical models took subject into account. Time periods for phenobarbital dose data did not overlap with the seizure data (see below). Linear mixed models with interactions were developed to assess the impact of phenobarbital administration on rSO2 and FTOE, adjusted for dose (mg/kg).
Seizures
Electrographic seizures were defined as abnormal EEG events greater than 10 seconds in duration, with clear evolution of frequency, morphology, and/or location, and a minimum 2uV peak-to-peak amplitude (23). For each electrographic seizure, mean and standard deviation of rSO2 values were extracted for a period of 3-times the duration of the seizure before and after the ictal pattern, as well as during the seizure. This duration was selected in order to account for potential effects of variation in seizure duration (for example, a prolonged seizure might result in a more prolonged post-ictal change in cerebral metabolism than a brief seizure). A maximum of 5 seizures were analyzed for each individual subject. Seizure times did not overlap with the above phenobarbital administration times. Linear mixed models and post-hoc pairwise comparisons were employed to evaluate seizure data, adjusted for seizure duration. Bonferroni-adjusted p-values are presented.
Statistical Models
Most neonates had more than one dose of phenobarbital or more than one seizure. Furthermore, each individual administration of medication or seizure produced three measurements, one for each time period (before, during, and after the seizure or phenobarbital dose). To account for this, linear mixed models with random intercepts for patient and phenobarbital dose or seizure event were employed. Models were run separately for phenobarbital and seizure data, including weight-adjusted dosage (mg/kg) in phenobarbital models and length of seizure in seizure models as covariates. Significance of the effect of time period on the NIRS data was determined by a Wald Chi-square test on the inclusion of time period coefficients in the models. Due to the small amount of missingness combined with the relatively small sample size, listwise deletion was used in the presence of missing data. All analyses were performed using Stata version 13.1 (College Station, Texas).
To investigate whether weight-adjusted phenobarbital dose affected NIRS data, a second set of models was developed to include an interaction between time period and weight-adjusted dosage (mg/kg). Dose was categorized into bolus (≥10 mg/kg) or maintenance (<10mg/kg) for a clinically meaningful comparison of time effect differences across dose values. The significance of interaction terms was determined through an overall Wald Chi-square test. Effects of phenobarbital across the three time-points were investigated through plots of estimated marginal means and differences between time periods for the categories of weight-adjusted doses (bolus vs maintenance).
Supplementary Material
Acknowledgements
This work was supported by NICHD (5K23HD068402) and the University of Michigan’s Janette Ferrantino Investigator Award and Charles Woodson Pediatric Biostatistics Fund. Near-infrared spectroscopy machines were donated by Somanetics Corporation (Troy, MI, USA) for research use in our neonatal intensive care unit, but Somanetics had no input into study design, data analysis, writing of the manuscript, or the decision to submit this manuscript.
References
- 1.Ramantani G. Neonatal epilepsy and underlying aetiology: to what extent do seizures and EEG abnormalities influence outcome? Epileptic Disord. 2013;15:365–375. doi: 10.1684/epd.2013.0619. [DOI] [PubMed] [Google Scholar]
- 2.Sampath D, White AM, Raol YH. Characterization of neonatal seizures in an animal model of hypoxic-ischemic encephalopathy. Epilepsia. 2014;55:989–993. doi: 10.1111/epi.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr. 2009;155:318–323. doi: 10.1016/j.jpeds.2009.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr. 2011;159:731–735. doi: 10.1016/j.jpeds.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyatt JS, Gluckman PD, Liu PY, et al. Determinants of outcomes after head cooling for neonatal encephalopathy. Pediatrics. 2007;119:912–921. doi: 10.1542/peds.2006-2839. [DOI] [PubMed] [Google Scholar]
- 6.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–1438. doi: 10.1093/brain/awu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Heide MJ, Roze E, van der Veere CN, Ter Horst HJ, Brouwer OF, Bos AF. Long-term neurological outcome of term-born children treated with two or more anti-epileptic drugs during the neonatal period. Early Hum Dev. 2012;88:33–38. doi: 10.1016/j.earlhumdev.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 9.Blume HK, Garrison MM, Christakis DA. Neonatal Seizures: Treatment and treatment variability in 31 United States Pediatric Hospitals. J Child Neurol. 2009;24:148–154. doi: 10.1177/0883073808321056. [DOI] [PubMed] [Google Scholar]
- 10.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oztekin O, Kalay S, Tezel G, Akcakus M, Oygur N. Can we safely administer the recommended dose of Phenobarbital in very low birth weight infants? Childs Nerv Syst. 2013;29:1353–1357. doi: 10.1007/s00381-013-2094-8. [DOI] [PubMed] [Google Scholar]
- 12.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society's Guideline on Continuous Electroencephalography Monitoring in Neonates. J Clin Neurophysiol. 2011;28:611–617. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 13.Lemmers PMA, Zwanenburg RJ, Benders MJ, et al. Cerebral oxygenation and brain activity after perinatal asphyxia: does hypothermia change their prognostic value? Pediatr Res. 2013;74:180–185. doi: 10.1038/pr.2013.84. [DOI] [PubMed] [Google Scholar]
- 14.Silas R, Sehgal A, Walker AM, Wong FY. Cerebral oxygenation during subclinical seizures in neonatal hypoxic-ischaemic encephalopathy. Eur J Paediatr Neurol. 2012;16:304–307. doi: 10.1016/j.ejpn.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Boylan GB, Panerai RB, Rennie JM, Evans DH, Rabe-Hesketh S, Binnie CD. Cerebral blood flow velocity during neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 1999;80:105–110. doi: 10.1136/fn.80.2.f105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toet MC, Lemmers PMA. Brain monitoring in neonates. Early Hum Dev. 2009;85:77–84. doi: 10.1016/j.earlhumdev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji M, Duplessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res. 1998;44:591–595. doi: 10.1203/00006450-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Diaz GA, Cesaron E, Alfonso I, Dunoyer C, Yaylali I. Near infrared spectroscopy in the management of status epilepticus in a young infant. Eur J Paediatr Neurol. 2006;10:19–21. doi: 10.1016/j.ejpn.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Giorni C, Chiara LD, Cilio MR, et al. The usefulness of near-infrared spectroscopy for detecting and monitoring status epilepticus after pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2009;23:668–671. doi: 10.1053/j.jvca.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Lemmers PMA, Van Bel F. Left-to-right differences of regional cerebral oxygen saturation and oxygen extraction in preterm infants during the first days of life. Pediatr Res. 2009;65:226–230. doi: 10.1203/PDR.0b013e318191fb5d. [DOI] [PubMed] [Google Scholar]
- 21.Dix LM, van Bel F, Baerts W, Lemmers PM. Comparing near-infrared spectroscopy devices and their sensors for monitoring cerebral oxygen saturation in the neonate. Pediatr Res. 2013;74:557–563. doi: 10.1038/pr.2013.133. [DOI] [PubMed] [Google Scholar]
- 22.Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol. 2007;118:2156–2161. doi: 10.1016/j.clinph.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American Clinical Neurophysiology Society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–173. doi: 10.1097/WNP.0b013e3182872b24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


