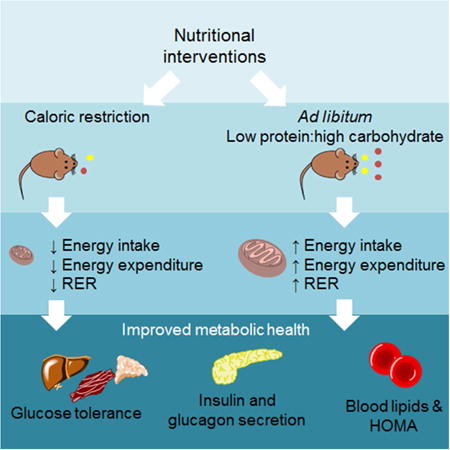
Summary
Both caloric restriction (CR) and low protein, high carbohydrate (LPHC) ad libitum-fed diets increase lifespan and improve metabolic parameters such as insulin, glucose and blood lipids. Severe CR, however, is unsustainable for most people; therefore, it is important to determine whether manipulating macronutrient ratios in ad libitum-fed conditions can generate similar health outcomes. We present the results of a short-term (8 week) dietary manipulation on metabolic outcomes in mice. We compared three diets varying in protein to carbohydrate ratio under both CR and ad libitum conditions. Ad libitum LPHC diets delivered similar benefits to CR in terms of levels of insulin, glucose, lipids and HOMA, despite increased energy intake. CR on LPHC diets did not provide additional benefits relative to ad libitum LPHC. We show that LPHC diets under ad libitum-fed conditions generate the metabolic benefits of CR without a 40% reduction in total caloric intake.
Graphical abstract
Introduction
Caloric restriction (CR) of ∼30-50% increases healthspan, delays the onset of ageing and age-associated diseases, and improves metabolic health in most species (Everitt et al., 2010; Masoro, 2005; Mattison et al., 2012; McCay et al., 1935; Mercken et al., 2012; Weindruch et al., 1986). It is generally thought that CR is mediated directly by the reduction in energy intake impacting on cellular substrates such as NAD+ and AMP, with subsequent downstream effects on nutrient-sensing pathways such as sirtuin (SIRT1), AMP-activated protein kinase (AMPK), mechanistic target of rapamycin (mTOR) and insulin/IGF-1 (Brunet et al., 2004; Fontana et al., 2010; Le Couteur et al., 2012). Although beneficial, CR is unsustainable in the vast majority of humans (Fontana and Partridge, 2015).
More recently, it has been demonstrated in studies using nutritional geometry that the balance of macronutrients has a profound impact on healthspan and lifespan in animals with ad libitum (AL) access to food (Lee et al., 2008; Piper et al., 2011; Solon-Biet et al., 2014). In these studies, CR induced by dietary dilution, did not increase lifespan (Solon-Biet et al., 2014). In AL-fed mice and Drosophila melanogaster, diets low in protein and high in carbohydrates (LPHC) maximized lifespan, while reduction of total energy intake had no positive impact on longevity (Lee et al., 2008; Solon-Biet et al., 2014). Moreover in mice, LPHC diets were associated with improved latelife cardiometabolic health (Solon-Biet et al., 2014) and a younger immune profile (Le Couteur et al., 2014). Low protein intake has also been associated with better health and reduced mortality in observational studies of humans (Levine et al., 2014), while high protein, low carbohydrate diets (HPLC) are associated with higher mortality, cardiovascular disease, and diabetes mellitus (Fontana and Partridge, 2015; Fung et al., 2010; Lagiou et al., 2012; Simpson et al., 2015).
Thus, a diet with altered macronutrient composition may be a more feasible intervention than severe CR for managing metabolic health in humans. However, there is a downside: whereas LPHC diets have beneficial effects later in life, they are associated with increased food intake, driven by compensatory feeding for protein (Gosby et al., 2011; Huang et al., 2013; Raubenheimer et al., 2014; Simpson and Raubenheimer, 2005). The clinical consequences of overconsumption are well established, including obesity, metabolic syndrome, type 2 diabetes mellitus, and fatty liver (Dietrich and Hellerbrand, 2014; Nseir et al., 2014; Simpson et al., 2015). Overall, reducing food intake and body weight improves the manifestations of metabolic syndrome and fatty liver (Ajala et al., 2013; Nseir et al., 2014). Effects of macronutrients on these outcomes in humans are less clear, but in general, high carbohydrate diets are thought to contribute to fatty liver and metabolic syndrome, while high protein diets might be protective (Nseir et al., 2014), in part through aiding reduced energy intake (Gosby et al., 2014).
The question arises as to which dietary intervention is more effective at improving metabolic health and whether there is any synergy between these dietary regimens. In this study, we directly compared CR with diets differing in protein to carbohydrate ratio, and evaluated several metabolic and hepatic outcomes. The results indicate that LPHC diets under AL-feeding conditions achieve the metabolic gains seen with CR. As expected, LPHC was associated with increased energy intake, but over a period of 8 weeks this was counterbalanced by increased energy expenditure and did not lead to a significant increase in body adiposity or fatty liver. Additional health improvements were not achieved by combining CR with LPHC diets, although CR prevented the negative metabolic consequences of a HPLC diet in AL-fed mice.
Results
Food and energy intake
Dietary protein to carbohydrate balance (P:C) influenced food and energy intakes in AL-fed animals (Figure 1a-b). AL mice titrated their food intake according to percent dietary protein, with animals on AL LPHC diet consuming the greatest amounts of food and energy. After the 8-week dietary intervention, CR animals had reduced body mass compared with AL-fed animals, while mice on the AL medium P:C (MPMC) diets had the highest body mass (Figure 1c).
Figure. 1. Food and energy intake ± SEM.
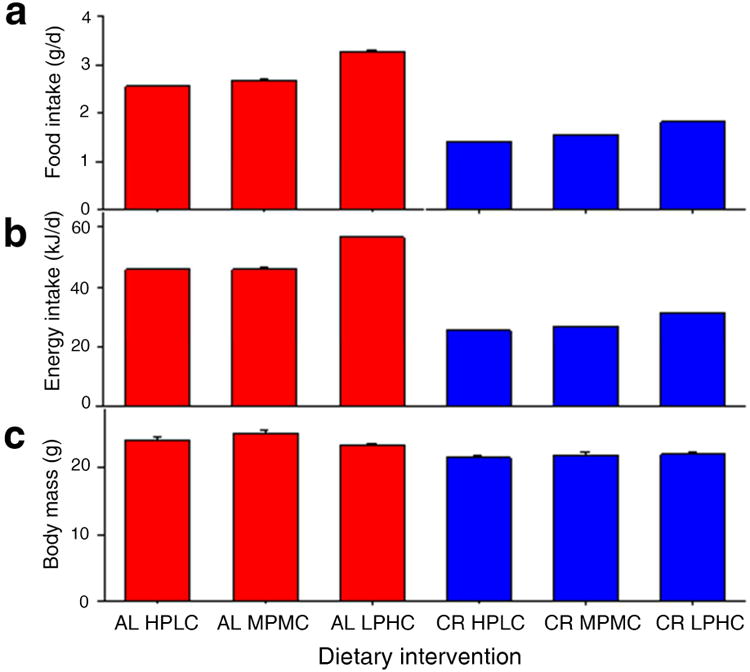
(a) Average food intake (g/day), (b) energy intake (kJ/day) and (c) body weight (g) over 8 weeks of feeding in AL and CR regimes. Note that CR animals were offered exactly 40% of AL counterparts fed the same diet composition (HPLC, MPMC or LPHC). See also Table S2.
Diet and feeding regime influence metabolic phenotype
After eight weeks, AL HPLC animals demonstrated significantly higher insulin levels and HOMA values and impaired glucose tolerance, relative to other treatment groups (Figure 2a-e, Figure S1 and Table S2). These parameters were more favourable in AL-fed animals with lower dietary P:C. AL LPHC mice showed improved insulin, HOMA, triglyceride and HDLc levels compared with the other AL diets, and their results are comparable to those found in CR-fed animals. These same outcomes were also improved to a similar extent in all CR treatments, regardless of dietary P:C (Table S2). Triglycerides followed a similar pattern, with highest levels in the animals on the AL HPLC and AL MPMC diets, while there were no significant differences between the AL LPHC diets and any of the CR diets. A similar trend was seen for HDLc where the lowest (worse) values were seen for the AL HPLC and AL MPMC diets.
Figure 2. Metabolic phenotype ± SEM.
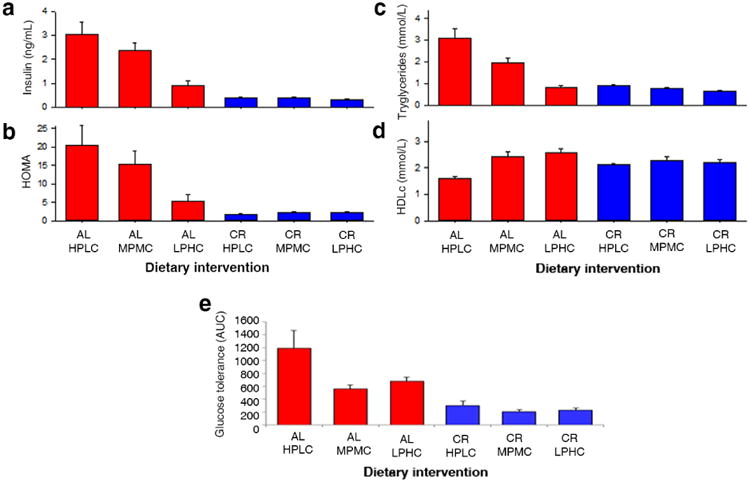
The effect of diets on (a) insulin, (b) HOMA, (c) triglycerides and (d) HDLc and (e) oral glucose tolerance tests. (AL, ad libitum; CR, caloric restricted; HPLC, high ratio of protein to carbohydrate; MPMC, medium protein to carbohydrate ratio; LPHC, low protein to carbohydrate ratio). See also Table S2 and Table S3.
Liver and pancreatic pathology
All diets were associated with normal liver histology regardless of whether mice were fed AL or CR (Figure 3a-b). There were subtle changes in the porosity of the liver sinusoidal endothelium, with lower porosity observed in LPHC compared with MPMC or HPLC mice (1.13%±0.11 vs 1.63%±0.12, P=0.02; Figure 3c-d). There were no obvious changes in pancreatic islet pathology or pancreatic insulin stains (χ2=2.33, df=5, p=0.8; Figure S2) but there was an increase in the intensity of staining for glucagon in AL HPLC mice compared to all other groups (χ2=26.09, df=5, p=<0.0001; Figure 3 e-g). This suggests the AL HPLC diet results in increased glucagon secretion, causing elevated blood glucose levels, and glucose intolerance. The higher insulin and HOMA levels observed (Figure 2a-b) are consistent with this notion.
Figure 3. Hepatic and pancreatic pathology.
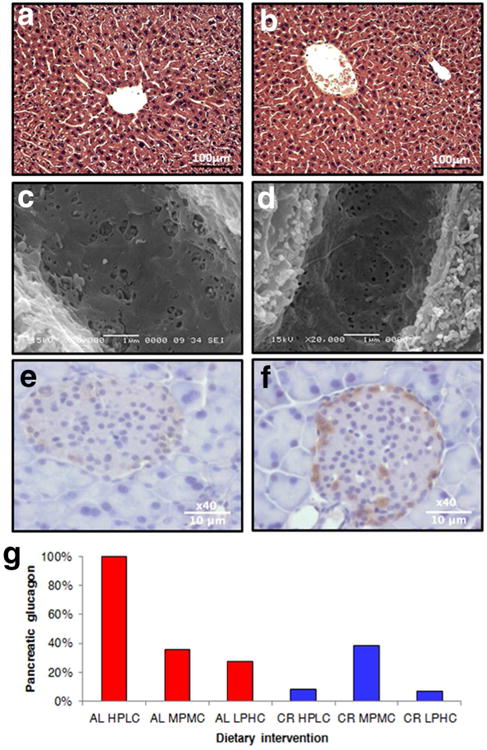
Representative figures are shown of livers stained with hemotoxylin and eosin (a-b), scanning electron microscopy of the liver sinusoidal endothelium (c-d) and glucagon stains of the pancreatic islets (e-f). (g) The effect of diets pancreatic glucagon staining. Percentages indicate the proportion of samples with high intensity staining. (AL ad libitum; CR caloric restricted; HPLC high ratio of protein to carbohydrate; MPMC medium protein to carbohydrate ratio; LPHC low protein to carbohydrate ratio; P-values provided in Table S2). See also Figure S1 and Table S2.
Energy expenditure, RER and body composition
Energy expenditure was significantly higher in the AL LPHC animals compared to all of the CR animals. The respiratory exchange ratio (RER) approached 1 in the AL LPHC animals, indicating carbohydrate was the primary source of energy, compared to the other groups, where the value approached 0.7, indicating utilization of fat (Figure 4a and Table S2). There were discernible effects of dietary interventions on body composition. Body mass was lower in the CR animals (Figure 1c) while, interestingly, % body fat showed opposing patterns, with animals in CR groups tending to have increased adiposity (relative to lean mass) (Figure 4c).
Figure 4. Indirect calorimetry ± SEM.
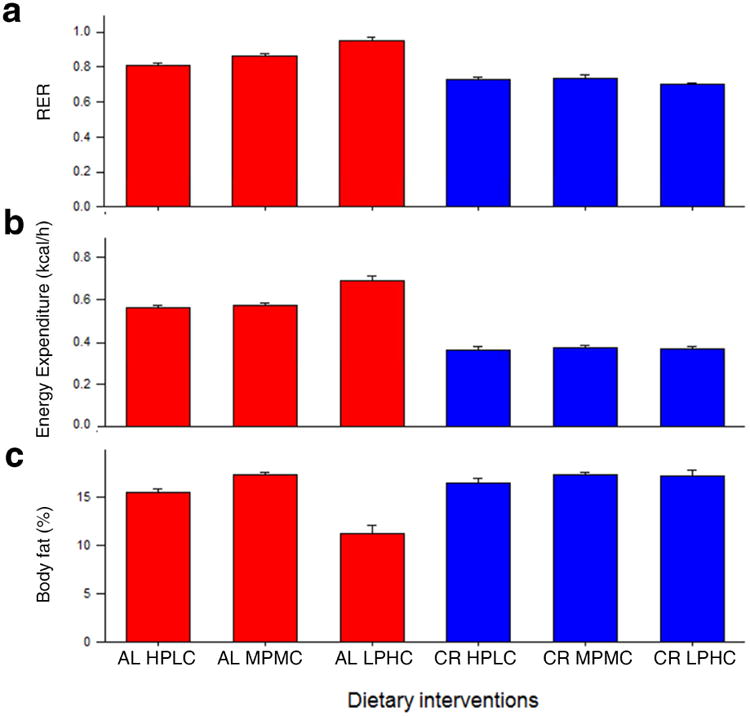
The effect of diets on (a) RER, (b) energy expenditure and (c) body fat. (AL ad libitum; CR caloric restricted; HPLC high ratio of protein to carbohydrate; MPMC medium protein to carbohydrate ratio; LPHC low protein to carbohydrate ratio. See also Table S2.
Discussion
Our results provide a direct comparison of CR to AL LPHC diets, to determine whether it is possible to generate similar metabolic outcomes achieved with CR using AL diets. Our results show that after 8 weeks, AL-fed LPHC mice had similar metabolic improvements as seen under CR, despite increased energy intake, but without the development of increased body adiposity and fatty liver that is observed in longer term chronic LPHC feeding. Manipulating dietary P:C ratios in animals under CR conditions did not generate any additional benefits in terms of these outcomes, nor did it cause any detrimental effects to the mice.
Mice, like humans and various other species, demonstrate ‘protein leverage’, where protein intake is prioritised over fat and carbohydrates (Gosby et al., 2011; Raubenheimer et al., 2014; Simpson and Raubenheimer, 2005). Such an effect was evident in the present study, with the AL LPHC diet resulting in increased food and energy intake of about 25-30% compared to the AL HPLC diet. Despite this elevated intake, we did not observe increased adiposity, body weight or diet-induced fatty liver in AL LPHC mice. They did, however, show increased energy expenditure, which is consistent with increased diet-induced thermogenesis (DIT) serving to dissipate excess ingested energy and slow development of adiposity (Huang et al., 2013; Stock, 1999).
Exposure to LPHC diets over longer time periods, however, has been associated with increased body weight, adiposity and fatty liver (Huang et al., 2013; Solon-Biet et al., 2014; Sorensen et al., 2008), indicating that mechanisms for compensatory energy expenditure may become progressively less effective with time (Huang et al., 2013). Such mice, albeit more adipose, nonetheless have improved cardiometabolic outcomes, including insulin, GTT, HOMA, lipids and blood pressure (Solon-Biet et al., 2014). It is also important to consider the type of carbohydrate consumed (e.g. starch vs. fructose vs. glucose (Wylie-Rosett et al., 2004)) as this has been shown in rodent studies to have a profound influence on the development of obesity and insulin resistance (Maki and Phillips, 2015; Storlien et al., 1988; Thorburn et al., 1989; Thresher et al., 2000) and thus may have a considerable effect on cardiometabolic health if fructose is a significant component of an LPHC diet.
AL HPLC diets were associated with decreased insulin sensitivity, indicated by elevated circulating insulin, HOMA and pancreatic glucagon. This metabolic dysregulation may be attributed to the upregulation of gluconeogenesis, subsequently increasing glycogen turnover and total hepatic glucose output (Eisenstein et al., 1974; Linn et al., 2000). Whereas HPLC diets do not sustain optimal latelife cardiometabolic health, it is important to note that nutritional requirements change with age, and higher P:C diets are required to support reproduction than sustain maximal lifespan (Simpson et al., 2015; Solon-Biet et al., 2014; Solon-Biet et al., 2015).
Here, we have compared the metabolic effects of short term CR and AL LPHC diets in mice. The results of this study suggest that it may be possible to titrate the balance of macronutrients to gain some of the metabolic benefits of CR, without the challenge of a 40% reduction in caloric intake. A central priority is to further investigate and compare the long-term effects of traditional CR and AL LPHC diets on metabolic health and lifespan in mice and other model organisms, as well as to begin to consider the effects of the type and quality of proteins and carbohydrates.
Experimental Procedures
Animals and dietary interventions
Male C57BL6/J mice (3 weeks old; n=90; Jackson Laboratories, Bar Harbor ME) were housed in groups of 5 at the National Institute of Aging (Baltimore, MD). Animals were kept at 22°C under a 12:12h light-dark cycle (12h dark period starting at 18:00), and were micro-chipped (Biomedic Data Systems, Seaford, DE) for individual identification and temperature quantification. All animal protocols were approved by the Gerontology Research Center Animal Care and Use Committee (352-LEG-2012) of the National Institute on Aging.
Three experimental diets were formulated that differed in protein to carbohydrate ratios based on Solon-Biet et al. (2014) (Solon-Biet et al., 2014). These diets were classified as low protein, high carbohydrate (5% protein; LPHC); medium protein, medium carbohydrate (33% protein; MPMC); and high protein, low carbohydrate (60% protein, HPLC). Fat was fixed at 20% of total energy for all three diets. Experimental diets were isocaloric (4 kcal/g) and contained the same ingredients (Table S1). All diets were manufactured in dry pelleted form by Dyets Inc (Bethlehem, PA). Mice were assigned to one of six different dietary regimes: Ad libitum access to diets where the protein to carbohydrate ratio was either high (AL HPLC), medium (AL MPMC) or low (AL LPHC); and caloric restricted access to diets where the protein to carbohydrate ratio was either high (CR HPLC), medium (CR MPMC) or low (CR LPHC).
At 8 weeks of age, mice underwent a 4-day acclimatisation period of a 50/50 food combination of standard chow and experimental diet, followed by solely experimental diets for the remainder of the study. Mice were randomly assigned to either an AL- or a 40% CR-feeding regime. Bi-weekly food measurements of AL animals were used to calculate daily portions for CR-fed mice, where food was reduced increments of 10% starting at 20% until mice reached 40% CR. AL animals were allowed free access to respective diets for 8 weeks and were fed in the hopper, while CR mice were fed daily at approximately 8am ± 1 hour, with pellets dropped onto the cage of the floor. Body weights and temperature were recorded bi-weekly and food intakes for all groups were quantified at the same time. After 8 weeks of feeding, mice were euthanized and blood and tissues collected for histological and biochemical analyses. On the day of the sacrifice, CR mice were not fed while AL mice were allowed to eat normally.
Body composition
Fat, lean and fluid mass of mice were measured using nuclear magnetic resonance imaging (NMR) with the Minispec LF90 (Bruker Optics, Billerica, MA). Unanaesthetized mice were weighed then scanned.
Glucose and insulin
Oral glucose tolerance tests (OGTT) were performed after 8 weeks of experimental diets. Mice were fasted overnight (16 h) prior to testing then gavaged with a 30% glucose solution (1.5g kg-1 body weight) and blood glucose measurements recorded at 0, 15, 30, 60 and 120 min via tail snip using a handheld glucometer (Bayer, Mishawaka, IN). The incremental area under the curve was calculated using mean values per cage. Insulin was measured in fasting blood samples using an enzyme-linked immunosorbent assay (Crystal Chem, Downers Grove, IL). The homeostatic model assessment (HOMA; http://www.dtu.ox.ac.uk/homa), which reflects insulin resistance, was determined from the product of the fasting glucose and insulin.
Metabolic rate
In order to estimate whole animal metabolic rate, substrate utilization, and physical activity, 8 animals per group were housed individually and assessed by indirect calorimetry in an open-circuit oxymax chamber (Comprehensive Lab Animal Monitory System, CLAMS; Columbus instruments, Columbus, OH). Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured over 48 hours and maintained at 24°C under at 12:12 h light-dark cycle. Mice were acclimatized to metabolic cage conditions for 8 h prior to the start of data recording. The respiratory exchange ratio (RER) was calculated as a ratio of VCO2 produced/VO2 consumed. An RER of 0.7 indicates that fat is the predominant fuel source, while an RER closer to 1.0 indicates that carbohydrate is the primary fuel.
Liver and pancreatic pathology
Paraffin-embedded liver tissue was sectioned and stained with Hemotoxylin and Eosin. Embedded pancreas tissue was sectioned and probed with monoclonal anti-glucagon antibody (Sigma G2654), monoclonal anti-insulin antibody (Sigma I2018) and anti-mouse IgG1 produced in rabbit (Sigma SAB3701171). The extent of fatty liver and glucagon staining intensity was assessed and scored (0, +, ++, +++) by four independent observers blinded to the tissue category. Liver tissue was also needle-perfused with saline, followed by 3% glutaraldehyde/2% paraformaldehyde in 0.1M sodium cacodylate buffer (pH 7.4), 2% (w/v) sucrose and 2mM CaCl2. Following post-fixation with osmium tetroxide, graded dehydration in ethanol and hexamethyldisilazane, 1mm3 blocks of liver were sputter coated with platinum and examined using a JEOL 6380 scanning electron microscope (JEOL, Japan) at 20,000× magnification. Ten random images were taken per sample and fenestration diameter analysed using ImageJ software (Cogger et al., 2015).
Statistical analysis
Data are presented as mean ± SEM and differences considered significant when P<0.05. Comparisons between feeding regimes and diets on various responses were analysed using ANOVA and post hoc Fisher LSD tests when indicated. Fisher LSD test was used to test for differences between AL LPHC and CR diets. Two-group comparisons were performed using Students t-tests and Mann-Whitney rank sum tests (Sigmaplot v 11.2.0.5, Systat Software Inc). Intensity of glucagon and insulin staining was compared using a Chi-square test in Microsoft Excel.
Supplementary Material
Highlights.
Ad libitum low protein, high carbohydrate diets (LPHC) improve metabolic health
Caloric restriction combined with LPHC diet does not provide added health benefits
Energy intake and energy expenditure are increased on LPHC diets
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (Project grant 571328, Program grant 571408), and the Ageing and Alzheimers Institute (AAAI) of Concord Hospital. This study was funded in part by the National Institute on Aging Intramural Research Program of the National Institutes of Health. S.J.M. was supported by a National Medical Health and Research Council of Australia CJ Martin Early Career Fellowship (RGMS ID 2010-01671). R.dC is supported by the Intramural Research Program of the National Institute on Aging. DR is an Adjunct Professor in the New Zealand Institute for Advanced Study, Massey University, Auckland. We are grateful to Dawn Nines, Justine Lucas and Dawn Phillips-Boyer for their animal care and assistance. We also thank Dr. Alistair Senior for his helpful comments and advice.
Footnotes
Author Contributions: S.M.S. designed and performed the experiments, analysed the data and wrote the manuscript. S.J.M. performed the experiments. S.C.P.C. wrote the manuscript and contributed to data analysis. V.C.C. and R.G. performed the histology. A.C.M. assisted in preparation of histological samples. V.C.C., D.R. and R.dC. assisted in the preparation of the manuscript. D.G.LC. and S.J.S. supervised the project, contributed to data analysis and the writing of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97:505–516. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Cogger VC, O'Reilly J, Warren A, Le Couteur DG. A standardized method for the analysis of liver sinusoidal endothelial cells and their fenestrations by scanning electron microscopy. J Vis Exp. 2015:e52698. doi: 10.3791/52698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:637–653. doi: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Eisenstein AB, Strack I, Steiner A. Glucagon stimulation of hepatic gluconeogenesis in rats fed a high-protein, carbohydrate-free diet. Metab Clin Exp. 1974;23:15–23. doi: 10.1016/0026-0495(74)90099-7. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Rattan SI, Le Couteur DG, de Cabo R. Calorie Restriction Aging and Longevity. New York: Springer Press; 2010. [Google Scholar]
- Fontana L, Partridge L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153:289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosby AK, Conigrave AD, Lau NS, Iglesias MA, Hall RM, Jebb SA, Brand-Miller J, Caterson ID, Raubenheimer D, Simpson SJ. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS One. 2011;6:e25929. doi: 10.1371/journal.pone.0025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosby AK, Conigrave AD, Raubenheimer D, Simpson SJ. Protein leverage and energy intake. Obes Rev. 2014;15:183–191. doi: 10.1111/obr.12131. [DOI] [PubMed] [Google Scholar]
- Huang X, Hancock DP, Gosby AK, McMahon AC, Solon SM, Le Couteur DG, Conigrave AD, Raubenheimer D, Simpson SJ. Effects of dietary protein to carbohydrate balance on energy intake, fat storage, and heat production in mice. Obesity (Silver Spring) 2013;21:85–92. doi: 10.1002/oby.20007. [DOI] [PubMed] [Google Scholar]
- Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. doi: 10.1136/bmj.e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. J Gerontol A Biol Sci Med Sci. 2012;67:169–174. doi: 10.1093/gerona/glr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Tay SS, Solon-Biet SM, Bertolino P, McMahon AC, Cogger VC, Pichaud N, Horan M, Correa C, Melvin RG, et al. The Influence of Macronutrients on Splanchnic and Hepatic Lymphocytes in Aging Mice. J Gerontol A Biol Sci Med Sci. 2014:1–9. doi: 10.1093/gerona/glu196. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine Morgan E, Suarez Jorge A, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola Mario G, Guevara-Aguirre J, Wan J, et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T, Santosa B, Gronemeyer D, Aygen S, Scholz N, Busch M, Bretzel RG. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia. 2000;43:1257–1265. doi: 10.1007/s001250051521. [DOI] [PubMed] [Google Scholar]
- Maki KC, Phillips AK. Dietary substitutions for refined carbohydrate that show promise for reducing risk of type 2 diabetes in men and women. J Nutr. 2015;145:159S–163S. doi: 10.3945/jn.114.195149. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Age Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay C, Crowell M, Maynard L. The effect of retarded growth upon the length of life and upon ultimate size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nseir W, Hellou E, Assy N. Role of diet and lifestyle changes in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20:9338–9344. doi: 10.3748/wjg.v20.i28.9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raubenheimer D, Machovsky-Capuska GE, Gosby AK, Simpson S. Nutritional ecology of obesity: from humans to companion animals. Br J Nutr. 2014:1–14. doi: 10.1017/S0007114514002323. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the balance back in diet. Cell Metab. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Solon-Biet S, McMahon A, Ballard JWO, Ruohonen K, Wu L, Cogger V, Warren A, Huang X, Pichaud N, Melvin RG, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Walters KA, Simanainen UK, McMahon AC, Ruohonen K, Ballard JWO, Raubenheimer D, Handelsman DJ, Le Couteur DG, Simpson SJ. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc Natl Acad Sci USA. 2015;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen A, Mayntz D, Raubenheimer D, Simpson SJ. Protein-leverage in Mice: The Geometry of Macronutrient Balancing and Consequences for Fat Deposition. Obesity. 2008;16:566–571. doi: 10.1038/oby.2007.58. [DOI] [PubMed] [Google Scholar]
- Stock MJ. Gluttony and thermogenesis revisited. Int J Obes Relat Metab Disord. 1999;23:1105–1117. doi: 10.1038/sj.ijo.0801108. [DOI] [PubMed] [Google Scholar]
- Storlien LH, Kraegen EW, Jenkins AB, Chisholm DJ. Effects of sucrose vs starch diets on in vivo insulin action, thermogenesis, and obesity in rats. Am J Clin Nutr. 1988;47:420–427. doi: 10.1093/ajcn/47.3.420. [DOI] [PubMed] [Google Scholar]
- Thorburn AW, Storlien LH, Jenkins AB, Khouri S, Kraegen EW. Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr. 1989;49:1155–1163. doi: 10.1093/ajcn/49.6.1155. [DOI] [PubMed] [Google Scholar]
- Thresher JS, Podolin DA, Wei Y, Mazzeo RS, Pagliassotti MJ. Comparison of the effects of sucrose and fructose on insulin action and glucose tolerance. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1334–1340. doi: 10.1152/ajpregu.2000.279.4.R1334. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The Retardation of Aging in Mice by Dietary Restriction: Longevity, Cancer, Immunity and Lifetime Energy Intake. J Nutr. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Wylie-Rosett J, Segal-Isaacson CJ, Segal-Isaacson A. Carbohydrates and increases in obesity: does the type of carbohydrate make a difference? Obes Res. 2004;12 Suppl 2:124S–129S. doi: 10.1038/oby.2004.277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


