Abstract
Nonhealing neuropathic foot ulcers remain a significant problem in individuals with diabetes. The gap-junctional protein connexin43 (Cx43) has roles in dermal wound healing and targeting Cx43 signaling accelerates wound reepithelialization. In a prospective, randomized, multi-center clinical trial we evaluated the efficacy and safety of a peptide mimetic of the C-terminus of Cx43, ACT1, in accelerating the healing of chronic diabetic foot ulcers (DFUs) when incorporated into standard of care protocols. Adults with DFUs of at least four weeks duration were randomized to receive standard of care with or without topical application of ACT1. Primary outcome was mean percent ulcer reepithelialization and safety variables included incidence of treatment related adverse events and detection of ACT1 immunogenicity. ACT1 treatment was associated with a significantly greater reduction in mean percent ulcer area from baseline to 12 weeks (72.1% vs. 57.1%; p = 0.03). Analysis of incidence and median time-to-complete-ulcer closure revealed that ACT1 treatment was associated with a greater percentage of participants that reached 100% ulcer reepitheliazation and a reduced median time-to-complete-ulcer closure. No adverse events reported were treatment related, and ACT1 was not immunogenic. Treatment protocols that incorporate ACT1 may present a therapeutic strategy that safely augments the reepithelialization of chronic DFUs.
Keywords: ACT1 peptide, connexin 43, diabetic foot ulcer, wound healing
INTRODUCTION
The global prevalence of diabetes is expected to rise to over 552 million people by 2030 and the management of diabetic foot ulcers (DFUs) remains a therapeutic challenge, afflicting approximately 15% of the world’s diabetic population.1,2 DFUs that remain refractory to conventional treatment protocols may develop soft tissue infection, osteomyelitis, and tissue necrosis; leading to lower-extremity amputation, lengthy hospital stays and costly treatments.3 Standard of care (SOC) protocols involving pressure relief (off-loading), debridement, treatment of infection and ischemia, wound cleansing and saline dressings, provide surprisingly little benefit, where only 25% and 30% of ulcers heal after 12 and 20 weeks, respectively.4,5 The potential of secondary interventions including the use of hyperbaric oxygen, negative pressure devices, and specialized dressings; as well as living skin equivalents, such as Apligraf® (Organogenesis Inc.), Dermagraft® (Shire), and Regranex® (Smith & Nephew), have been evaluated in randomized controlled trials. However, such interventions have been reported as being cumbersome, time-consuming, expensive, largely inefficacious, and may not sufficiently address the underlying mechanisms that contribute to the chronic nature of these ulcers 6.
While the pathophysiology and impaired wound healing response of DFUs is complex and multi-factorial, a growing number of pre-clinical studies have identified the gap junctional protein, connexin43 (Cx43), as a novel therapeutic target in diabetic wound healing.7–9 Abnormal elevation in Cx43 expression and gap junction communication has been reported in human diabetic keratinocytes and fibroblasts, respectively.10,11 ACT1 is a 25 amino acid synthetic peptide containing the carboxy-terminal PDZ binding domain of Cx43. In preclinical studies using a diabetic C57BL/KsJ-m+/+Leptdb(db+/db+) mouse wound model, ACT1 significantly accelerated wound closure, reduced inflammatory neutrophil infiltration and reduced granulation tissue deposition, recapitulating results seen in non-diabetic animal models. 12–14 The molecular mechanism of ACT1 involves modulation of the interaction between Cx43 and its C-terminal binding partners, mediated increases in size and stability of GJ channel aggregates, and a reduction in non-junctional (hemichannel) communication.12,15,16 We performed a multicenter randomized clinical trial to assess the therapeutic potential of ACT1 in augmenting the reepitheliazation of chronic DFUs when incorporated into SOC protocols.
MATERIALS AND METHODS
A randomized, prospective, investigator-blinded, parallel group, multicenter trial targeting patients with cutaneous, full thickness (University of Texas grade 1A), neuropathic diabetic foot ulcers was conducted. The University of Texas classification system is based on ulcer anatomical size and depth as well as presence of infection and ischemia. The study was designed to assess the clinical efficacy and safety of ACT1 in accelerating the closure of refractory DFUs.
Between October 2011 and February 2012 male and female patients, greater than 18 years of age, were recruited and screened by site investigators at eight academic centers located in South Asia. Inclusion criteria (Table S1) included having type 1 or type 2 diabetes with an HbA1c<10% (86 mmol/mol); an ABPI between 0.70 and 1.3 and at least one diagnosed cutaneous, full thickness (University of Texas grade 1A), below ankle surface, viable granulating neuropathic ulcer between 0.5-40 cm2 post-debridement that had remained unresponsive to SOC protocols for at least four weeks prior to screening (i.e. chronic). Exclusion criteria (Table S1) included a change in ulcer size by ≥30% during a 7 day screening period, inability to tolerate offloading, signs of severe clinical infection, an ABPI <0.7 or >1.3 or ankle systolic pressure < 70 mm Hg, , or a heavily exudative (requiring daily dressing changes) ulcer.
Study procedures involved three phases including: screening (day -7 through day 0), treatment (day 0 through week 12), and follow-up (months 4-6). Participants remained in the treatment phase until 100% ulcer reepitheliazation or 12 weeks, whichever occurred first. Baseline assessments (Table 1) were completed on patients who met initial inclusion/exclusion criteria (Table S1). Qualified participants were then registered by the site investigator to receive treatment assignment. A central block randomization (block size 2) list was prepared by an independent statistician using a validated computer program (Statistical Analysis Software (SAS) 9.1.3). Using the Interactive Web Response System (IWRS), participants were randomized 1:1 to either receive 100 μM (0.036%) ACT1 topical formulation plus SOC, or SOC therapy alone. To avoid bias due to baseline ulcer size and duration, ulcers were stratified by size (<10 cm2 and >10 cm2).17 An unblinded coordinator designated by site investigators, received treatment assignments through the IWRS. The trial sponsor, trial monitors, statisticians, investigators, and the observer who performed area closure measurements of ulcer photographs were blinded to treatment assignments.
Table 1.
Baseline characteristics of patients
| ACT1 + Standard of Care (n= 46) | Standard of Care (n=46) | Overall (n=91) | |
|---|---|---|---|
| Clinical Characteristics | |||
|
| |||
| Age (years) | |||
| Mean (±SD) | 50.8 (12.2) | 53.5 (14.9) | 52.2 (13.6) |
|
| |||
| Sex | |||
| Male (%) | 35 (76) | 32 (70) | 67 (73) |
| Female (%) | 11 (24) | 14 (30) | 25 (27) |
|
| |||
| Race | |||
| Indian (%) | 46 (100) | 46 (100) | 91 (100) |
|
| |||
| Weight (kg) | |||
| Mean (±SD) | 66.9 (12.6) | 63.9 (11.2) | 65.4 (12.0) |
|
| |||
| BMI | |||
| Mean (±SD) | 24.7 (4.2) | 24.5 (3.8) | 24.6 (4.0) |
|
| |||
| HbA1c (%) | |||
| Mean (±SD) | 7.4 (1.2) | 7.5 (1.4) | 7.5 (1.3) |
| Range (min : max) | 5.4 : 9.9 | 4.6 : 10.0 | 4.6 : 10.0 |
|
| |||
| Clinical History | |||
|
| |||
| Baseline ulcer area (cm2) | |||
| n=43 | n=43 | ||
| Mean (±SD) | 2.6 (3.0) | 2.6 (3.0) | 2.6 (3.0) |
|
| |||
| Ulcer Duration (weeks) | |||
| Mean (±SD) | 18.7 (20.3) | 23.3 (27.8) | 21.0 (24.3) |
|
| |||
| Ankle Brachial Pressure Index | |||
| Mean (±SD) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1) |
| Range (min : max) | 0.9 : 1.3 | 0.9 : 1.2 | 0.9 : 1.3 |
The study was conducted in compliance with International Conference on Harmonisation Guidelines, principles of the Declaration of Helsinki and with approval by the office of the Drug Controller General of India, and Independent Ethics Committees/Institutional Review Boards (CTRI/2011/09/001984).
Intervention
During the treatment phase, visit protocol for all groups included ulcer cleaning, irrigation, photography, tracing, closure assessment, and dressing, recording of adverse events (AEs) and pain self-assessment. For participants in the treatment group, during SOC protocols a clear gel (1.25% hydroxyethyl cellulose, containing ACT1 (100 μM (0.036%)) was applied topically to the target (largest) ulcer at day 0 (baseline visit) and day 3, and then weekly from weeks 1–12. Preclinical and Phase I dosing studies validate the efficacy and safety of a 100μM ACT1 concentration and the implemented treatment regimen.12–16 In participants receiving SOC alone, as per standard protocols recommended and used by the investigators, Hydroheal Am gel, was incorporated in place of ACT1 on a matched schedule.
SOC procedures were maintained in all groups throughout all trial phases and included thorough irrigation with sterile saline, bleeding control, and application of a non-adherent four-layer dressing, which extended 1.27cm beyond the ulcer perimeter and inflamed skin margins, followed by a fine mesh gauze non-occlusive dressing, folded or rolled as a bolster, and enforced off-loading (excluding total contact casting). All treatments were administered by research-trained nurses not acting as investigators.
Outcomes and Follow-up
The primary efficacy endpoint was mean percent ulcer closure (reepithelialization) from baseline to week 12. Secondary efficacy endpoints included mean percent wound closure at 4 weeks, and time to 50% and 100% reepithelialization. Evaluation of ulcer closure was performed by clinically qualified site investigators and independently evaluated by a central evaluator blinded to treatment through computerized planimetry of digital photographs of ulcers using ImageJ (NIH, Bethesda, MD). Incidence of 50% and complete closure at week 12 were incorporated as exploratory endpoints. The safety variable was the incidence of treatment related AEs, determined by vitals, laboratory testing and AE reporting. Testing for anti-ACT1 peptide antibodies was performed at baseline and week 12 using a validated enzyme-linked immunosorbent assay (WuXi Apptec, Philadelphia, PA).
Statistical analyses
Sample size enrolled was calculated with reference to the primary endpoint, assuming a 25% difference in favor of ACT1-treated participants, at a power of 80%, a significance of 95 %(two -sided), a SD of 40%, and adjusted for a 15% dropout rate. Analyses were completed on intent-to-treat (ITT) and per-protocol (PP) populations. ITT analyses included all participants with a baseline visit, thus avoiding bias associated with the non-random participant loss. The PP population excluded participants that died, withdrew consent, or had major protocol violations. Safety analyses were completed on the ITT population. Analyses were performed by an independent statistician using SAS® v9.1.3.
Primary and secondary wound closure endpoints were analyzed using ANCOVA Mixed-Model with Repeated Measure at a 95% CI. As a response variable, the mean percent wound area reduction from baseline to week 12 was adjusted for the strata, wound duration, viable tissue (granulation), exudate level, ankle circumference, and BMI as covariates with treatment group and visit as factors. For data in which the normality assumption was tested and not met, the Wilcoxon Mann-Whitney U test was used. Variables not associated with a defined measurable outcome were assessed on a scale of 1 to 5 (1 = “much worse”; 2 = “worse”; 3 = “same”; 4 = “improved”; 5 = “much improved”) and analyzed by the Wilcoxon Mann-Whitney U test. For time-to-event endpoints (100% and 50% ulcer closure), distribution was estimated by Kaplan-Meier, compared by log-rank test. Median time (i.e. ulcer closure for 50% of participants) was calculated using a 90% CI. Individual and joint effects of covariates on time to closure were evaluated using Cox Proportional Hazard. For time-to-event endpoints (50% and 100% ulcer closure), distribution was estimated by the Kaplan-Meier method, compared by log-rank test. Median time (i.e. ulcer closure for 50% of participants) was calculated using a 90% CI. Individual and joint effects of covariates on time to ulcer closure were evaluated using Cox Proportional Hazard. Closure incidence (50% and 100%) was analyzed overall and by center, by a Fisher’s Exact test (two-tailed), followed by Cochran-Mantel-Haenszel, after adjusting for pooled center. The Breslow-Day test and Cochran-Mantel-Haenszel test was used to determine the significance of a treatment by-pooled-center interaction. Primary analysis was done using Proc Mixed in SAS v9.1.3, where missing values were accounted for using the ANCOVA method with repeated measures and last observation-carried-forward approach was used for performing sensitivity analysis, whereby 100% reduction was carried forward if the ulcer healed and further visits were missed. AEs were evaluated in relation to study drug, seriousness, severity, action taken, and outcome.
RESULTS
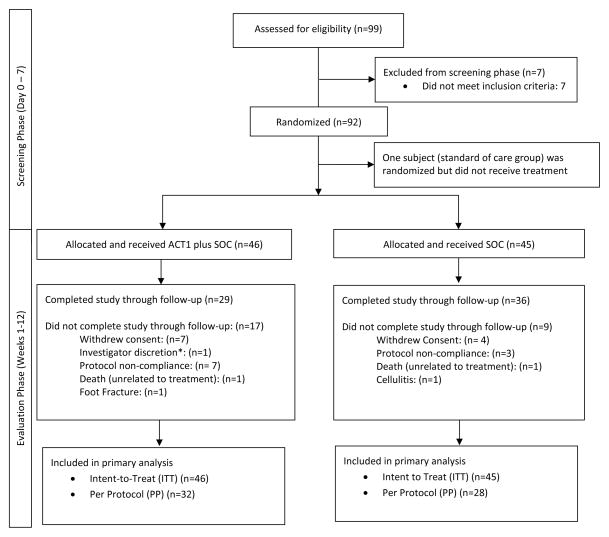
Of the 99 participants screened, 7 participants were excluded, and 92 participants were randomly assigned 1:1 to a treatment protocol involving ACT1 application and SOC protocols or a control treatment involving SOC protocols alone (Figure 1). One participant randomized to the SOC group voluntarily withdrew prior to study treatment. A total of 26 (28.3%) participants discontinued study participation either due to consent withdrawal (n=11), protocol non-compliance (n=10), AEs (n=4), or per investigator discretion (n=1; participant was non- compliant and pursed alternative medicine). Included in the ITT analyses were 14 participants that continued study participation despite experiencing a change in ulcer size by >30% (an exclusion criteria) during the 7 day screening period. Complete wound photograph data were not available for 18 participants. The final analysis sample sizes consisted of an ITT participant population of 91 participants and a PP participant population of 60.
Figure 1.
CONSORT flow diagram of participants
*Withdrawn by Investigator because the subject was non-compliant to the protocol and wanted to pursue alternative medicine.
SOC = standard of care
Randomized groups showed similar baseline patient demographics in terms of mean age, weight, BMI, HbA1c levels and baseline mean ulcer area (Table 1). For 6 participants (3 in each treatment group), baseline ulcer area was incorrectly documented and ulcer area at screening was used in ITT analyses. At study outset, the participant population had an average ulcer size of 2.6 cm2 lasting about 21 weeks.
Primary and Secondary Study Outcomes
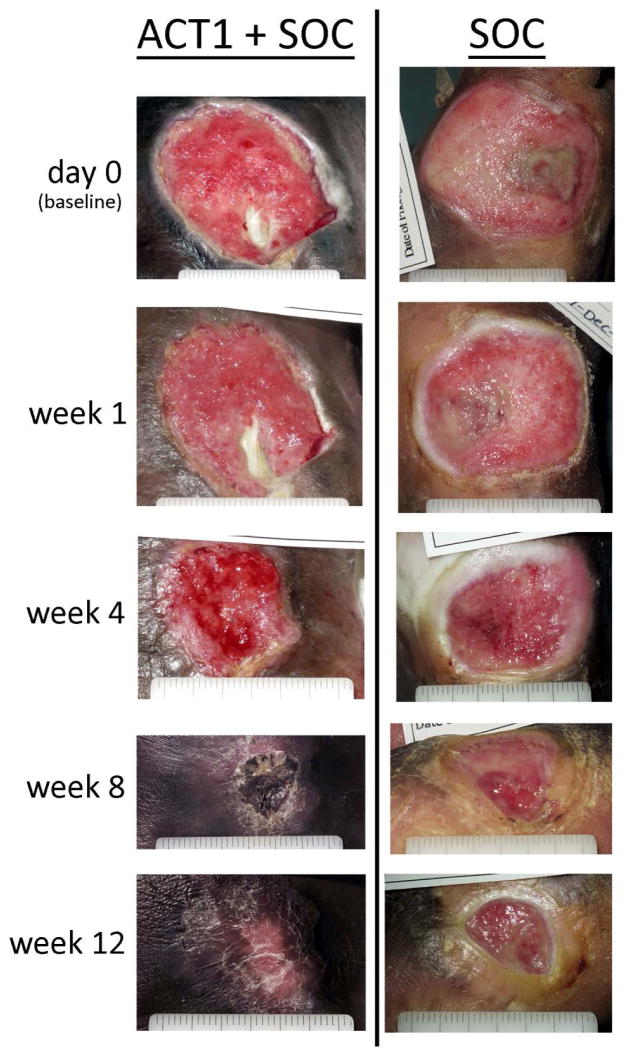
Evaluation of data distribution supported a non-normal distribution (p < 0.001) and the application of non-parametric analyses. In both ITT and PP populations, mean percent ulcer closure from baseline to week 12 was significantly greater in ACT1 treatment group as compared to the control group (Table 2). Representative photographic images are presented in Figure 2. A 4-week percent change in wound area is a predictor of healing outcome at 12 weeks.18 Significant improvement in mean percent closure of the ulcer area from baseline to week 4 was seen with ACT1 in analyses of the PP population (ITT: ~53% for both treatment groups, p > 0.05; PP: 73% (SD 35.52) vs. 47% (43.61), p =0.01).
Table 2.
Effect of ACT1 on ulcer area and ulcer closure from baseline
| ACT1 + SOC intent-to-treat (ITT) (n=46) |
SOC intent-to-treat (ITT) (n=45)* |
ACT1 + SOC per protocol (PP) (n=32) |
SOC per protocol (PP) (n=28) |
|
|---|---|---|---|---|
| Mean % area reduction + difference at week 12 | ||||
|
| ||||
| Mean percent wound closure† m, (SD) | 72.1 (128.5) | 57.1 (80.9) | 93.6 (17.7) | 52.1 (84.1) |
| % Difference between treatment groups | 15.0% | … | 41.5% | … |
| p value | 0.03 | … | 0.01 | … |
|
| ||||
| Incidence of 100% ulcer closure at week 12 | ||||
|
| ||||
| Number (%) | 28 (60.9%) | 17 (37.8%) | 26 (81.3%) | 14 (50.0%) |
| p value | 0.03 | … | 0.01 | … |
|
| ||||
| Incidence of 50% ulcer closure at week 12 | ||||
|
| ||||
| Number (%) | 31 (67.4%) | 20 (44.4%) | 29 (90.6%) | 17 (60.7%) |
| p value | 0.03 | … | 0.01 | … |
|
| ||||
| Kaplan Meier weeks to 100% ulcer closure | ||||
|
| ||||
| Median weeks (90% confidence interval) | 6.0 (5.0 to 11.0) | 11.0 (9.0 to 18.3) | 6.0 (4.0 to 10.9) | 14.6 (9.0 to 18.3) |
| p value | 0.03 | … | 0.01 | … |
|
| ||||
| Kaplan Meier weeks to 50% ulcer closure | ||||
|
| ||||
| Median weeks (90% confidence interval) | 2.0 (2.0 to 3.1) | 3.3 (3.0 to 4.1) | 2.0 (2.0 to 3.1) | 4.1 (3.0 to 5.0) |
| p value | 0.54 | … | 0.21 | … |
|
| ||||
| Cox hazard ratio (HR) for 100% ulcer closure | ||||
|
| ||||
| HR (95% confidence interval) | 1.6 (0.7 to 3.6) | … | 2.3 (0.9 to 5.7) | … |
| p value | 0.23 | … | 0.07 | … |
92 subjects were enrolled in the study. One subject was randomized to the SOC group but did not receive any study treatment and therefore excluded from ITT.
Number of subjects having non-missing observations: ITT (N=63); PP (N=57)
Abbreviations: SOC, standard of care
Figure 2.
Diabetic foot ulcer healing from baseline through week 12 in a participant treated with ACT1 and standard of care (SOC) as compared to a participant receiving standard of care alone
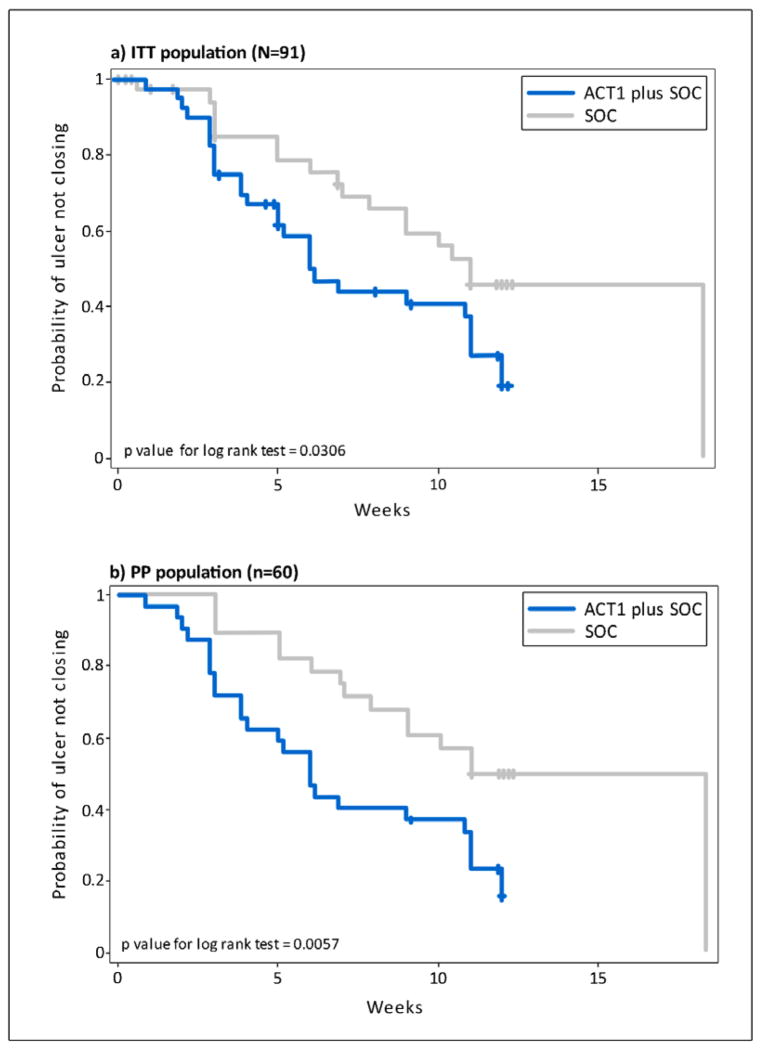
Categorical analysis of incidence and time to ulcer closure, including all study centers, revealed that ACT1 treatment was associated with a significantly greater percentage of participants that reached 100% ulcer reepitheliazation (ITT: p = 0.03 PP: p = 0.01, Chi-square test) as well as a significantly shorter median time to 100% ulcer reepitheliazation during the 12 weeks of efficacy assessments (ITT: p = 0.03; PP: p = 0.01) (Table 2 and Figure 3). These results were associated with an approximately 62% (ITT) to 70% (PP) probability of achieving 100% ulcer closure if ACT1 treatment was received (ITT: 1.6; PP: 2.3; Cox hazard ratio; Table 2).
Figure 3.
Kaplan-Meier plot of time to 100% ulcer closure with ACT1 and standard of care (SOC) compared to standard of care alone; a) Intent to treat (ITT) population, b) Per Protocol (PP) population
While categorical analyses of incidence of 50% ulcer closure was significantly higher in the ACT1 treatment group (ITT: p = 0.03; PP: p = 0.01; Chi-square test) the median time taken to achieve 50% wound closure was not statistically significantly different (ITT: p = 0.54; PP: p = 0.21) (Table 2). Cox proportional hazard regression analysis indicated that participants treated with ACT1 in association with SOC protocols had a 49% (ITT) to 55% (PP) probability of achieving 50% wound closure (ITT: 1.15; PP:1.23). Treatment group, baseline ulcer depth, ulcer size, ulcer duration, and BMI did not show a significant association with time to 100% or 50% ulcer reepitheliazation. There was no significant difference in the number of responders center-wise between treatment groups(ITT :p =.27 ; PP: p = 0.39).
Pain and Adverse Events
Pain intensity was self-assessed and recorded using a visual analog scale of 1 to 10 (1 = “no pain” and 10 = “extreme pain”) during all visits. There was no statistical difference in mean intensity of pain from baseline to week 12 between groups for either ITT (ACT1: 0.5 (SD 1.46); SOC alone: 0.3 (SD 0.93); p = 0.85) or PP (ACT1: 0.4 (SD 1.22); SOC alone: 0.3 (SD 0.81); p = 0.97) populations.
A total of 28 AEs were reported in 22 participants (Table 3). AEs did not segregate in terms of type or number and there were no significant differences between the groups in terms of complications, conditions or disorders (p ≥ 1.0; Table 3). The majority of AEs, were mild (20 events; 71.4%). The remaining eight were classified as moderate (4, (14.3 %)) or (severe (4; 14.3%)). Of the 28 events, 19 (67.9%) recovered, 3 (10.7%) recovered with sequelae, 4 (14.3%) remained at study conclusion, and 2 (9.1%) resulted in participant death. Five(5.4%) (ACT1: n = 2; SOC alone: n = 3) serious AEs were reported, where foot fracture and death due to an unknown cause were reported in ACT1 treated participants, and inadequate control of diabetes, cellulitis, and death by myocardial infarction, were reported in control participants. None of the AEs were related to the study drug or treatment. Anti-ACT1 antibodies were not detected in sera at screening or study conclusion.
Table 3.
Summary of adverse events by MedDRA system organ class and preferred safety population (n=92)
| ACT1 + Standard of Care (n=46); n (%)* | Standard of Care (n=46)†; n (%) | Overall (n=92); n (%) | p- value‡ | ||||
|---|---|---|---|---|---|---|---|
| Subjects with at least one adverse event (AE) | 11 (23.9) | 11 (23.9) | 22 (23.9) | ≥1.00 | |||
| Subjects reporting 1 AE | 9 (19.6) | 7 (15.2) | 16 (17.4) | 0.58 | |||
| Subjects reporting > 1 AE | 2 (4.3) | 4 (8.7) | 6 (6.5) | 0.68 | |||
|
| |||||||
|
Adverse Events
|
n (%) | AEs Reported | n (%) | AEs Reported | n (%) | AEs Reported | |
|
Serious Adverse Events
|
|||||||
| myocardial infarction | 0 (0.0) | 0 | 1 (2.2) | 1 | 1 (1.1) | 1 | … |
| death (unknown cause) | 1 (2.2) | 1 | 0 (0.0) | 0 | 1 (1.1) | 1 | … |
| foot fracture | 1 (2.2) | 1 | 0 (0.0) | 0 | 1 (1.1) | 1 | … |
| diabetes mellitus (inadequate control) | 0 (0.0) | 0 | 1 (2.2) | 1 | 1 (1.1) | 1 | … |
| cellulitis | 0 (0.0) | 0 | 1 (2.2) | 1 | 1 (1.1) | 1 | … |
|
Other Adverse Events
|
… | ||||||
| toothache | 0 (0.0) | 0 | 1 (2.2) | 1 | 1(1.1) | 1 | … |
| impaired healing | 0 (0.0) | 0 | 1 (2.2) | 1 | 1 (1.1) | 1 | … |
| respiratory tract infection | 0 (0.0) | 0 | 1 (2.2) | 1 | 1 (1.1) | 1 | … |
| wound complication§ | 5 (10.9) | 6 | 3 (6.5) | 4 | 8 (8.7) | 10 | 0.71 |
| back pain | 0 (0.0) | 0 | 1 (2.2) | 1 | 1 (1.1) | 1 | … |
| pain in extremity | 0 (0.0) | 0 | 2 (4.3) | 2 | 2 (2.2) | 2 | … |
| headache | 4 (8.7) | 4 | 1 (2.2) | 1 | 5 (5.4) | 5 | 0.36 |
| pneumonitis | 1 (2.2) | 1 | 0 (0.0) | 0 | 1 (1.1) | 1 | … |
| blister | 0 (0.0) | 0 | 1 (2.2) | 1 | 1 (1.1) | 1 | … |
| Total number of AEs in 22 subjects | 13 | 15 | 28 | 0.71 | |||
Percentage was calculated by taking respective column header group count as denominator.
Represents total number of subjects enrolled in standard of care group.
P-value was calculated by comparing two treatment groups using the Chi-square test.
Described by inflammation, ulcer irritation, and minor changes in ulcer size.
DISCUSSION
In this pilot clinical efficacy study, DFU treatment protocols incorporating the topical application of the Cx43 peptide mimetic, ACT1, showed a significantly greater reduction in percent ulcer closure, shorter median time-to-healing, and higher proportion of healing than control protocols. The significant effects of ACT1 treatment on accelerating ulcer reepitheliazation and decreasing median time-to-complete-ulcer closure was statistically demonstrated in the analyses of both the entire randomized population (ITT) and the population subset lacking missing data (PP). Clinical potential is further supported by a safety profile indicating ACT1 was not associated with AE incidence and was not immunogenic.
The incorporation of ACT1 in SOC protocols resulted in a significantly greater mean percent ulcer reepitheliazation from baseline to week 12. However, there was a difference associated with the mean percent ulcer reduction between the ITT (−15%) and PP (−41%) population analyses. At week 4, ITT analyses indicated a mean percent ulcer closure that was comparable between the two treatment groups (53%), while the same analyses of the PP population showed a significantly larger wound area reduction in ACT1-treated ulcers, beyond the 53% threshold that has been identified as predictive of complete healing within 12 weeks19, compared to control treated ulcers, which were on average below 50%. Differences between ITT and PP healing rates likely stem from the ITT inclusion of a number of subjects that exhibited changes in ulcer size ≥30% during the screening period (despite being stipulated as an exclusion criteria), and indicate that participants adhering to protocol achieved the highest incidences of complete closure and the largest absolute reductions in risk of non-closing wounds.
Regulatory authorities have used incidence of 100% wound closure by end-of-study as a primary endpoint for marketing approval (Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds-Developing Products for Treatment, 2006, www.fda.gov). The increase in incidence of 100% complete ulcer closure at week 12 associated with ACT1 incorporation into SOC protocols in the ITT population set (23.1%) and PP population set (31.3%) are robust compared to similar reports and may directly translate to significant pharmacoeconomic benefit.2,18
Current SOC protocols for DFU management dictating debridement, off-loading, cleansing, infection management, and moisture-retaining dressings, usually resolve only 25% of chronic lesions in 12 weeks.4,5 The ulcers analyzed in this study had a baseline average size of 2.59 cm2 and a duration of 21 weeks, ulcers that are larger and older-than-median chronic ulcers described in large population studies.20 Incorporation of ACT1 may thus be useful in the treatment of larger, more challenging ulcers. Studies with larger sample sizes are needed21 and the reported predictions require confirmation in trials involving larger populations.
While the incorporation of ACT1 into SOC protocols significantly accelerated the rate of DFU healing, the healing rates associated with the control group were comparably higher than those reported in similar clinical trials18,22–24. This is likely a result of a protocol design mandating wound care at weekly study center visits as compared to self-administration protocols.18 These results may also reflect an improvement associated with the modernization of SOC protocols over the last decade.
Connexin signaling has critical roles in cell and tissue homeostasis, and acute or systemic toxicity is a reasonable concern25. In alignment with extensive preclinical animal efficacy and toxicology data, ACT1 application was not associated with immunogenicity nor a significantly increased proportion or distribution of AEs.12–14,26 The clean safety profile of ACT1 may be linked to the relatively short in vivo half -life of the peptide.
This pilot study supports the clinical potential of ACT1 in the treatment of chronic DFUs but has several limitations with regards to study sample size, a significant gender-gap, lack of a longer follow-up period to fully evaluate ulcer recurrence, and variations in outcome data depending on population (ITT or PP) analyzed. Underlying cultural bias likely resulted in the male biased gender-gap and further emphasizes the need for large global multi-center clinical studies. Furthermore, DFU recurrence is a significant concern and while recurrence data indicated no significance difference between ACT1- and control-treated DFUs, extended trials to fully investigate recurrence incidence of are needed.
The onset, progression and refractory wound healing phenotype in individuals with diabetes is associated with alteration in connexin expression, phosphorylation, functionality, and degradation. 7,8 Connexin deregulation may be linked to abnormal glucose levels27, in addition to intrinsic factors such as disruption in proinflammatory cytokine or apoptosis-regulating gene expression28. During normal wound healing processes, Cx43 in the wound edge slowly downregulates over ~48 hours, during which time keratinocytes adopt a migratory phenotype.13,29–32 Cx43 downregulation is correlated with increases in TGF-β mRNA and collagen α-1 and decreases in chemokine ligand-2 and TNFα, resulting in the promotion of angiogenesis, fibroblast migration, and keratinocyte proliferation, and a decrease in infiltrating neutrophils and macrophages at the wound site.13,29–32 In injured diabetic skin, wound edge keratinocytes upregulate Cx43 and form a thickened bulb of nonmigrating cells, resulting in a significantly delayed healing phase.7 Cx43 mediated cell apoptosis, regulation of inflammatory signals/immunocompetent cells, growth factors, release of pro- or anti-oxidant molecules, and Cx43 regulation via the AMPK/mTOR signaling pathway may all have roles in delayed diabetic wound healing.33–35
In normal and diabetic animal models ACT1 treatment shortened and reduced the amplitude of the initial inflammatory phase of wound healing, reduced wound gape and edema, and accelerated the rate of wound closure.12 Targeting Cx43 with a dominant-negative construct prevents Cx43 upregulation and keratinocyte clumping in streptozotocin diabetic rat wound models, serving to accelerate reepitheliazation.7 Chronic wounds like DFUs tend to remain in an inflammatory phase of wound healing, that contributes to the their chronic phenotype.36,37 ACT1’s efficacy in the promotion of DFU reepitheliazation is likely linked to the inhibition of Cx43 interaction with its binding partners, resulting in the mediation of hemichannel function and inflammatory responses.15,16 Additional investigation into the relationship between connexin expression and hypoglycemic drugs, cytokine expression, oxidative environment, and inflammatory responses in the context of diabetic wounds will aid in further elucidating ACT1’s mechanism of action.
In summary, clinical incorporation of ACT1 in chronic DFU treatment protocols represents a non-toxic, effective therapeutic strategy that expedites the reepitheliazation of chronic DFUs by treating the underlying ulcer pathophysiology. These results warrant additional clinical studies that directly compare ACT1 to alternative advanced therapies.
Supplementary Material
Acknowledgments
The corresponding author had full access to all of the data in the study, takes responsibility for the integrity of the data as well as the accuracy of the data analysis, and had final responsibility for the decision to submit for publication. We gratefully acknowledge the patients who participated in the study, as well as the medical staff involved in patient care. We would like to also acknowledge Stefanie Cuebas who contributed to figure design and Dr. Jayashri Krishnan who served as a medical monitor. The support of the NIH (1R43DK080567-01 and 2R44DK080567-02 to GSG) is gratefully acknowledged for funding the research that led to the current trial.
We also thank the investigators who enrolled patients for the study: Dr. Qureshi Mohammad Asif Haji Pyare Saheb, Crescent Hospital and Heart Center, Nagpur, Maharashtra, Dr. Snehal Purandare Shrimati, Kashibai Navale General Hospital, Pune, Maharashtra, Dr. Ajay Yadhav, Sir Ganga Ram Hospital, New Delhi, Delhi, Dr. Shivakumar B R, Cosmopolitan Medical Center, Bengaluru, Karnataka, Dr. G.M. Prasad, Pace Clinical Research Center, Bengaluru, Karnataka, Dr. Vijay Viswanathan, M.V. Diabetes and Research Center, Chennai, Tamil Nadu, Dr. Puneet Agrawal, Chopra Superspeciality Hospital, Agra, Uttar Pradesh, and Dr. Vishwanath Pai, Sri Ramachandra Medical Center, Chennai, Tamil Nadu.
Role of the Funding Source: This study was entirely funded by FirstString Research, Inc., Mount Pleasant, SC.
Abbreviations
- AE
adverse event
- Cx43
connexin43
- DFU
diabetic foot ulcer
- GJ
gap junction
- ITT
intent-to treat
- IWRS
Interactive Web Response System
- PP
per protocol
- SOC
standard of care
Footnotes
CONFLICT OF INTEREST
Dr. Ghatnekar is an employee of FirstString Research and has ownership in the company and stock options issued by the company. Dr. Grek is an employee of FirstString Research and has stock options issued by the company. Drs. Armstrong and Gourdie are members of the Scientific Advisory Board of FirstString Research and have stock options issued by the company.
References
- 1.International Working Group of the Diabetic Foot (IWGDF) 2014 http://iwgdf.org.
- 2.Game FL, Hinchliffe RJ, Apelqvist J, Armstrong DG, Bakker K, Hartemann A, et al. A systematic review of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2012;28 (Suppl 1):119–41. doi: 10.1002/dmrr.2246. [DOI] [PubMed] [Google Scholar]
- 3.Stockl K, Vanderplas A, Tafesse E, Chang E. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care. 2004;27:2129–34. doi: 10.2337/diacare.27.9.2129. [DOI] [PubMed] [Google Scholar]
- 4.Frykberg RG, Zgonis T, Armstrong DG, Driver VR, Giurini JM, Kravitz SR, et al. Diabetic foot disorders. A clinical practice guideline (2006 revision) J Foot Ankle Surg. 2006;45:S1–66. doi: 10.1016/S1067-2516(07)60001-5. [DOI] [PubMed] [Google Scholar]
- 5.Margolis DJ, Kantor J, Berlin JA. Healing of diabetic neuropathic foot ulcers receiving standard treatment. A meta-analysis. Diabetes Care. 1999;22:692–5. doi: 10.2337/diacare.22.5.692. [DOI] [PubMed] [Google Scholar]
- 6.Hinchliffe RJ, Valk GD, Apelqvist J, Armstrong DG, Bakker K, Game FL, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2008;24 (Suppl 1):S119–44. doi: 10.1002/dmrr.825. [DOI] [PubMed] [Google Scholar]
- 7.Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56:2809–17. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- 8.Mendoza-Naranjo A, Cormie P, Serrano AE, Wang CM, Thrasivoulou C, Sutcliffe JE, et al. Overexpression of the gap junction protein Cx43 as found in diabetic foot ulcers can retard fibroblast migration. Cell Biol Int. 2012;36:661–7. doi: 10.1042/CBI20110628. [DOI] [PubMed] [Google Scholar]
- 9.Pollok S, Pfeiffer AC, Lobmann R, Wright CS, Moll I, Martin PE, et al. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J Cell Mol Med. 2011;15:861–73. doi: 10.1111/j.1582-4934.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandner JM, Houdek P, Husing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122:1310–20. doi: 10.1111/j.0022-202X.2004.22529.x. [DOI] [PubMed] [Google Scholar]
- 11.Abdullah KM, Luthra G, Bilski JJ, Abdullah SA, Reynolds LP, Redmer DA, et al. Cell-to-cell communication and expression of gap junctional proteins in human diabetic and nondiabetic skin fibroblasts: effects of basic fibroblast growth factor. Endocrine. 1999;10:35–41. doi: 10.1385/ENDO:10:1:35. [DOI] [PubMed] [Google Scholar]
- 12.Ghatnekar GS, O'Quinn MP, Jourdan LJ, Gurjarpadhye AA, Draughn RL, Gourdie RG. Connexin43 carboxyl-terminal peptides reduce scar progenitor and promote regenerative healing following skin wounding. Regen Med. 2009;4:205–23. doi: 10.2217/17460751.4.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gourdie RG, Ghatnekar GS, O'Quinn M, Rhett MJ, Barker RJ, Zhu C, et al. The unstoppable connexin43 carboxyl-terminus: new roles in gap junction organization and wound healing. Ann N Y Acad Sci. 2006;1080:49–62. doi: 10.1196/annals.1380.005. [DOI] [PubMed] [Google Scholar]
- 14.Rhett JM, Ghatnekar GS, Palatinus JA, O'Quinn M, Yost MJ, Gourdie RG. Novel therapies for scar reduction and regenerative healing of skin wounds. Trends Biotechnol. 2008;26:173–80. doi: 10.1016/j.tibtech.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–98. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–28. doi: 10.1091/mbc.E10-06-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004;12:163–8. doi: 10.1111/j.1067-1927.2004.012207.x. [DOI] [PubMed] [Google Scholar]
- 18.Balingit PP, Armstrong DG, Reyzelman AM, Bolton L, Verco SJ, Rodgers KE, et al. NorLeu3-A(1–7) stimulation of diabetic foot ulcer healing: results of a randomized, parallel-group, double-blind, placebo-controlled phase 2 clinical trial. Wound Repair Regen. 2012;20:482–90. doi: 10.1111/j.1524-475X.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehan P, Jones P, Caselli A, Giurini JM, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. 2003;26:1879–82. doi: 10.2337/diacare.26.6.1879. [DOI] [PubMed] [Google Scholar]
- 20.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic neuropathic foot ulcers: the association of wound size, wound duration, and wound grade on healing. Diabetes Care. 2002;25:1835–9. doi: 10.2337/diacare.25.10.1835. [DOI] [PubMed] [Google Scholar]
- 21.Greer N, Foman NA, Macdonald R, Dorrian J, Fitzgerald P, Rutks I, et al. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med. 2013;159:532–42. doi: 10.7326/0003-4819-159-8-201310150-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–7. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 23.Veves A, Falanga V, Armstrong DG, Sabolinski ML Apligraf Diabetic Foot Ulcer S. Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: a prospective randomized multicenter clinical trial. Diabetes Care. 2001;24:290–5. doi: 10.2337/diacare.24.2.290. [DOI] [PubMed] [Google Scholar]
- 24.Marston WA, Hanft J, Norwood P, Pollak R Dermagraft Diabetic Foot Ulcer Study G. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701–5. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 25.Pfenniger A, Wohlwend A, Kwak BR. Mutations in connexin genes and disease. Eur J Clin Invest. 2011;41:103–16. doi: 10.1111/j.1365-2362.2010.02378.x. [DOI] [PubMed] [Google Scholar]
- 26.O'Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. 2011;108:704–15. doi: 10.1161/CIRCRESAHA.110.235747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bobbie MW, Roy S, Trudeau K, Munger SJ, Simon AM, Roy S. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci. 2010;51:3758–63. doi: 10.1167/iovs.09-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acosta JB, del Barco DG, Vera DC, Savigne W, Lopez-Saura P, Guillen Nieto G, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5:530–9. doi: 10.1111/j.1742-481X.2008.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, et al. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol. 2003;13:1697–703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Mori R, Power KT, Wang CM, Martin P, Becker DL. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci. 2006;119:5193–203. doi: 10.1242/jcs.03320. [DOI] [PubMed] [Google Scholar]
- 31.Kretz M, Maass K, Willecke K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur J Cell Biol. 2004;83:647–54. doi: 10.1078/0171-9335-00422. [DOI] [PubMed] [Google Scholar]
- 32.Coutinho P, Qiu C, Frank S, Tamber K, Becker D. Dynamic changes in connexin expression correlate with key events in the wound healing process. Cell Biol Int. 2003;27:525–41. doi: 10.1016/s1065-6995(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 33.Contreras JE, Sanchez HA, Veliz LP, Bukauskas FF, Bennett MV, Saez JC. Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res Brain Res Rev. 2004;47:290–303. doi: 10.1016/j.brainresrev.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goubaeva F, Mikami M, Giardina S, Ding B, Abe J, Yang J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem Biophys Res Commun. 2007;352:97–103. doi: 10.1016/j.bbrc.2006.10.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo YN, Wang JC, Cai GY, Hu X, Cui SY, Lv Y, et al. AMPK-mediated downregulation of connexin43 and premature senescence of mesangial cells under high-glucose conditions. Exp Gerontol. 2014 doi: 10.1016/j.exger.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Mustoe T. Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. Am J Surg. 2004;187:65S–70S. doi: 10.1016/S0002-9610(03)00306-4. [DOI] [PubMed] [Google Scholar]
- 37.Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61:2937–47. doi: 10.2337/db12-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.