Abstract
Reversible post-translation modifications of proteins are common in all cells and appear to regulate many processes. Nevertheless, the enzyme(s) responsible for the alterations and the significance of the modification are largely unknown. Succinylation of proteins occurs and causes large changes in the structure of proteins; however, the source of the succinyl groups, the targets, and the consequences of these modifications on other proteins are unknown. These studies focused on succinylation of mitochondrial proteins. The results demonstrate that the α-ketoglutarate dehydrogenase complex (KGDHC) can serve as a trans-succinylase that mediates succinylation in an α-ketoglutarate-dependent manner. Inhibition of KGDHC reduced suc-cinylation of both cytosolic and mitochondrial proteins in cultured neurons and in a neuronal cell line. Purified KGDHC can succinylate multiple proteins including other enzymes of the tricarboxylic acid (TCA) cycle leading to modification of their activity. Inhibition of KGDHC also modifies acetylation by modifying the pyruvate dehydrogenase complex. The much greater effectiveness of KGDHC than succinyl CoA suggests that the catalysis due to the E2k suc-cinyltransferase is important. Succinylation appears to be a major signaling system and it can be mediated by KGDHC.
Keywords: Acetylation, Alzheimer’s disease, brain metabolism, α-ketoglutarate dehydrogenase complex, mitochondria, succinylation
INTRODUCTION
Post-translational modifications are an efficient mechanism for regulating cellular physiology. Lysine residues are particularly good targets for a variety of modifications including acetylation, methylation, ubiquitination, biotinylation, propionlylation and butyrlation (Zhang et al. 2011). Acetylation is perhaps the best studied modification. Acetylation of hundreds of proteins occurs in the nucleus, cytosol and mitochondria. Acetylation may couple metabolism to regulation through post-translational modifications of proteins. Although the significance of acetylation has begun to be understood for nuclear proteins, its significance for cytosolic and mitochondrial proteins is largely unknown. Sixty five percent of mitochondrial proteins are acetylated at 2200 sites (Hebert et al. 2013). Acetylation seems to be a slow process, and the factors initiating protein acetylation in the mitochondria are unknown (Wagner & Payne 2013). The nucleus and cytosol have opposing acetyltransferases and de-acetylases. The acetylase in the mitochondria is unknown.
Succinylation of proteins is similar to acetylation, and likely as widespread, but it is much less studied. Succinylation of lysine groups induces more substantial changes to a protein’s chemical properties than does lysine methylation or acetylation, since it adds a bigger structural moiety and changes the charge status (Zhang et al. 2011). In non-neuronal systems, the suc-cinylation of three proteins have been studied in detail: isocitrate dehydrogenase (ICDH) (EC 1.1.1.42; EC 1.1.1.41), serine hydroxyl-methyltransferase (EC 2.1.2.1) and glyceralde-hyde-3-phosphate dehydrogenase (EC 1.2.1.12). Lysine modifications of ICDH have been documented by mass spectrometry as well as Western blot and co-elution by HPLC, but the consequences of succinylation have not been tested. Furthermore, many other proteins are suc-cinylated as shown by Western blots of E Coli or HELA cells (Zhang et al. 2011) . Recent studies that examined the mammalian succinylome identified 2565 succinylation sites on 779 proteins. Lysine succinylation is particularly enriched in cellular metabolic processes (Park et al. 2013). Examination of purified liver mitochondria revealed 1190 unique succinylation sites as well as 340 sites across 140 proteins representing multiple metabolic pathways (Rardin et al. 2013). The current studies reveal the source of succinyl groups in neuronal cell lines or cultured neurons and test whether succinylation of tricarboxylic acid (TCA cycle) enzymes occurs.
The effects of succinylation on activity have been examined in only a few proteins. For example, classic studies demonstrate that 3-hydroxy-3-methyl-glutaryl-CoA synthase (HMG-CoA) is inhibited by succinyl-CoA (Lowe & Tubbs 1985) and the exact sites of the inhibition have been identified (Rardin et al. 2013). The inhibition is due to the enzyme catalysis of its own succinylation. Other studies implicate succinylation in activity by comparing activity normal and SIRT5−/− mice. They suggest that SIRT5 represses biochemical activity and cellular respiration through two protein complexes identified as PDHC (pyruvate dehydrogenase complex) and SDH (Park et al. 2013). Succinylation of SDH has also been observed in potato mi-tochondria.(Millar et al. 1999).
Few studies have examined the source of the succinyl CoA (SC) groups in mammalian systems. In yeast, lysine succinylation depends on SC formed through the TCA cycle. The multi-protein KGDHC is made up of three components: E1k [α-Ketoglutarate Dehydrogenase (KGDH) (EC 1.2.4.2), E2k [Dihydrolipoyl Succinyltransferase (EC 2.3.1.61) (DLST)] and E3 [Dihydrolipoyl Dehydrogenase (DLDH) (EC 1.8.1.4)]. Thus, E2k is a succinyltransferase and these experiments test whether E2k can provide SC for succinylation of proteins without the need for other enzymes. In yeast, the mutated loss of E1k leads to a 6-fold reduction in global succinylation. On the other hand, the induction of E1k resulted in a 3- to 5-fold enhancement of succinylation (Weinert et al. 2013). These studies indicated that succinylation could also occur non-enzymatically and that it was likely at the same sites. Thus, our experiments tested whether KGDHC could serve as a donor and compared it to non-enzymatic succinylation by SC. The experiments also tested the impact of succinylation on enzyme activities.
An understanding of the multiple roles of KGDHC is important in understanding Alz-heimer’s disease (AD). KGDHC is diminished in AD (Bubber et al. 2005), but the consequences of the change are not resolved.
MATERIALS AND METHODS
KGDHC inhibition
The carboxy ethyl ester of succinylphosphonate (CESP) and diethy-lester succinylphosphonate were prepared exactly as previously (Bunik et al. 2005). CESP is highly selective for KGDHC and does not inhibit other thiamine dependent enzymes dehydro-genase or a variety of other enzymes that use alpha-keto acids as substrates (Bunik et al. 2005). The diethyl-ester analogue does not inhibit cellular KGDHC under the conditions of these experiments.
Protein determination
All proteins were determined by Bicinchoninic acid (BCA) Pierce™ BCA Protein Assay Kit for protein.
Cell culture and buffers for incubating cells
Neurons from embryos were prepared from the cerebral cortices of E15.5 C57BL/6 mice without selecting for sex (Charles River, Wilmington MA) exactly as described previously (Gibson et al. 2012b, Brewer & Torricelli 2007). The use of mice to prepare the cultures was approved by Weill Cornell Medical College Institutional Care and Use Committee.
Preparation of the cytosolic and mitochondrial fraction
Neurons were treated with or without 100 μM CESP in balanced salt solution (BSS) (140 mM NaCl, 5 mM KCl, 1.5 mM pH 7.4) for 60 min at 37°C. Cells MgCl2, 5 mM glucose, 10 mM HEPES and 2.5 mM CaCl2 were then washed twice with cold PBS (phosphate buffered saline) (Invitrogen Cat # 14190-250). Cold PBS /1 mM EGTA was added to the dish and cells transferred into tubes. The tubes were centrifuged at 1000 g for 1 min at 4°C. The pellets were suspended with K-EGTA buffer (125 mM KCl, 20 mM Hepes pH 7.4, 1 mM EGTA) and homogenized with a glass pestle B (40 strokes). The homogenates were centrifuged at 60,000 g for 30 min at 4°C (Beckman Optima Ultracentrifuge, rotor TLA100.1). The pellet contained mitochondria and the supernatant was defined to be the cytosolic fraction. The pellet (crude mitochondrial fraction was homogenized in K+−EGTA buffer with a Dounce homogenizer (B) and centrifuged 1,000 g for 10 min at 4°C. The supernatants were transferred into new tubes and centrifuged at 8,000 g at 4°C for 10 min. The pellet contained the mitochondria.
Concentration of the cytosolic fraction
The supernatant from the ultracentrifugation was transferred to an Amicon Ultra-0.5 ml 3 K centrifugal filter (Millipore; Billerca, MA) and centrifuged at 14,000 x g for 30 min at 4°C (Eppendorf centrifuge 5415 R, rotor FA-45-24-11).
Immunoprecipitation (IP)
The mitochondrial pellet, cell pellet or concentrated cyto-solic fraction was suspended in chilled lysate buffer [10 mM Tris-HCl; pH 8.0 100 mM NaCl, 1 mM EDTA, 0.5 % (w/v) NP-40], sonicated and centrifuged at 10,000 g for 10 min at 4°C. The supernatant was saved for IP. Protein was determined and diluted to 200 μg protein/ml. The anti-succinyl-lysine antibody or anti-acetyllysine antibody was diluted 1:200 in the supernatant and protein A agarose beads (20 μl/ml) were added. They were incubated overnight at 4°C with gentle shaking. The beads were washed three times with one ml of NETN buffer [100 mM NaCl, 1 mM EDTA, 20 mM Tris pH 8.0 and 0.5 % (w/v) NP-40] and three times with ETN (50 mM Tris –HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA). The bound peptides were eluted from beads by washing three times with 100 μl of 0.1 M glycine (pH 2.5) (Zhang et al. 2011).
Western blots
Samples were diluted 1:1 with loading buffer (2 x SDS non-reducing) and 10 μg protein was loaded on each lane. Electrophoresis was done on a 4–20 % Tris –Glycine gel (In Vitrogen; Grand Island, NY) and then proteins were transferred to PVDF membranes (Amersham Biosciences) using 80 volts for 4 hours at 4°C. Blot membranes were blocked with TBS (Tris buffered saline) and Odye blocking buffer (1:1)( LI-COR Biosciences, Lincoln, NE). The anti-succinyl-lysine or acetyl-lysine antibody (PTM Bio Labs, INC Chicago, IL) were diluted 1:1000 in 1:1 blocking buffer /TBS/0.1% Tween -20 and incubated overnight at 4°C with gentle shaking. For polyclonal antibodies Odyssey Goat –rabbit IRDye680 was used as the secondary antibody (1:10000). For monoclonal antibodies goat anti-mouse IRDye 800CW antibody was used (1:10,000 dilution; Li-COR Biosciences, Lincoln, NE). Membranes were incubated at room temperature for 40 min. Membranes were then washed with TBS/0.1 % Tween-20 three times. Membranes were shaken 5 min for each wash. The membranes were then washed three more times with TBS. The membranes were scanned with the Odyssey Infrared Imaging System (LICOR Biosciences, Lincoln, NE). The signal is directly proportional to the amount of target protein over a large dynamic range
The antibodies and dilutions for the other measures included: cytochrome C antibody (1:1000) BD BioSciences, San Jose CA cat# 556433 ) or COX-IV antibody (1:1000; Life Technologies, Grand Island NY); pyruvate dehydrogenase (PDH) antibody cocktail (1:1000 ABCAM Cat # AB110416) which includes anti E2 (69kD), E3bp (54KD), E1a (43.3kD), E1B (39.4 KD) and E1a; fumarate hydratase (Santa Cruz Cat number Sc-393992); ICDH (Isocitrate dehydrogensase) and fumarate hydratase (EC 4.2.1.2; fumarase) (Santa Cruz, Dallas, Texas); KGDHC E2k (Rockland. Limerick, PA), PDHC cocktail (ABCAM, Cambridge MA).
Activity measures
KGDHC and ICDH activities were measured by following the increase in fluorescence as NAD(P) was converted to NAD(P)H at 30°C (Bubber et al. 2005). Measurements were made using a SPECTRAmax GEMINI EM fluorescence micro-plate reader (Molecular Devices; Sunnyvale, CA) at an excitation wavelength of 340 nm and an emission wavelength of 460 nm. Samples were diluted in KGDHC lysate buffer (50 mM Tri-HCl pH 7.2, 1 mM DTT, 50 μM leupeptin, 0.2 mM EGTA, 0.4 % TritonX-100) with SC (100 μM final) and incubated at 30°C for 0 or 30 min. Activity of KGDHC was measured in 25 μl of sample per well of a 96-well plate followed by addition of 100 μl of KGDHC reaction buffer, 50 μl 4 mM NAD/0.665 mM CoA, 25 μl of KG (8 mM) for total volume of 200 μl.
The ICDH assay buffer included 8 mM isocitric acid trisodium salt, 20 mM manganese sulfate monohydrate 100 mM Tris-HCl, pH 7.6, 0.5 mM NADP. The sample was read at an excitation wavelength of 340 nm and an emission wavelength of 460 nm at 30°C for 15 min.
A positive control with commercial enzyme was run in parallel with each sample. ICDH (Sigma Cat #I-2002), pyruvate dehydrogenase (Sigma P-7032), fumarase (SigmaF-1757 ) and KGDHC (Sigma K1502) were from Sigma (St. Louis, MO).
Conditions to test succinylation of proteins by KGDHC
KGDHC (0.4 mU), ICDH (8 mU) and/or SC (200 μM) were mixed together in a total 200 μl of KGDHC lysate buffer (50 mM Tri-HCl, pH 7. 2, 14 mM DTT, 50 μM leupeptin, 0.2 mM EGTA, 0.4 % TritonX-100). Another 600 μl of KGDHC reaction buffer (1mM MgCl2, 1 mM CaCl2, 1 mM DTT, 0.1 % Triton X-100, 0.5 mM EDTA, 300 μM TPP, 50 mM Tris pH 7.8) and 400 μl of NAD/CoA were added. Then 200 μl of KG (8 mM final) was added for a total volume of 1400 μl. Final is KGDHC (0.2857 mU/ml); ICDH (5.7 mU/ml) SC (28.6 μM final). Samples were pooled and Westerns run as described above.
Conditions to test the effects of succinylation on KGDHC activity
KGDHC (7.8 mU) was diluted in KGDHC lysate buffer in a total of 500 μl. Where indicated, SC was added and incubated at 30°C for 0 or 30 min. At the end of the incubation, KGDHC was determined as described above.
ICDH treatment with varying SC (Sigma #S1129)
Twenty μl containing 200 mU ICDH was added to 430 MES (Morpholinoethane sulfonic acid monohydrate) buffer. Different concentration of SC in 20 μl were added to 230 μl of this diluted ICDH and incubated for 40 min at 30°C. To determine the effects on activity 20 μl of each was added to the ICDH assay buffer (see above).
ICDH treatment with varying ICDH
CDH was diluted in 230 ul MES Buffer, pH 6. In the treated samples, 25 μl of 2.5 mM Succinyl CoA was added for a total volume of 250 μl. The combination was incubated at 30°C for 50 min. Aliquots (20 μl) were used to measure ICDH activity. Final ICDH concentrations were 4, 8, 16, 32 mU SC with 200 μM to ICDH assay buffer (see above).
PDHC
These two enzymes were treated exactly like ICDH. The activities were measured by our previous methods (Bubber et al. 2005). PDHC (0.4 mU) and KGDHC (0.4 mU) or SC (200 μM) were mixed together in KGDHC lysate buffer in a total of 200 μl. Another 600 μl of reaction buffer and 400 μl of assay buffer were added. Then 200 μl of KG (8 mM, final concentration) or reaction buffer was added for a total volume of 1400 μl. For activity measurements, 200 μl was transferred to a plate reader for 30 min at 30 degrees. [Final is (KGDHC; 0.2857 mU/ml); PDHC (0.29 mU/ml) SC (28.6 μM final)].
Fumarase
KGDHC (0.4 mU), fumarase (8 mU) and SC (200 μM) were mixed together in KGDHC lysate buffer in a total of 200 μl. Another 600 μl of reaction buffer and 400 μl of assay buffer were added. KG reaction buffer (200 μl) was added for a total volume of 1400 μl. For activity measures, 200 μl was added to the plate reader for 30 min at 30 degrees. [Final is (KGDHC; 0.2857 mU/ml); Fumarase (5.7 mU/ml) SC (28.6 μM final)] Western blot use fumarate hydratase (Santa Cruz Cat number Sc-393992). Fumarate and succinyllysine at 46 kDa overlapped.
Statistical analysis
All values are presented as mean plus or minus standard error of the mean. Comparison of two values were by Student’s t test. Multiple comparisons were done by Analysis of Variance followed by Student-Newman-Keuls test.
Mass spectrometry
In-gel trypsin digestion of SDS gel bands
The protein bands from an SDS-PAGE gel were cut into ~1 mm cubes and subjected to in-gel tryptic digestion followed by extraction of the tryptic peptide as reported previously (Yang et al. 2007). Extracts from each sample were lyophilized.
Protein Identification by nano LC/MS/MS Analysis
The in-gel tryptic digests were reconstituted in 20 μl of 0.5% FA for nanoLC-ESI-MS/MS analysis, which was carried out by a LTQ-Orbitrap Elite mass spectrometer (Thermo-Fisher Scientific, San Jose, CA) equipped with a “CorConneX” nano ion source device (CorSolutions LLC, Ithaca, NY). The Orbitrap was interfaced with a Dionex UltiMate3000RSLCnano system (Thermo, Sunnyvale, CA). The samples (5 μl) were injected onto a PepMap C18 trap column-nano Viper (5 μm, 100 μm × 2 cm, Thermo) at 20 μl/min flow rate and separated on a PepMap C18 RP nano column (3 μm, 75 μm x 15 cm, Thermo). The peptides were eluted with a 90 min gradient of 5% to 38% ace-tonitrile (ACN) in 0.1% formic acid at a flow rate of 300 nl/min, followed by a 5-min ramping to 95% ACN-0.1% FA and a 7-min hold at 95% ACN-0.1% FA. The Orbitrap Elite was operated in positive ion mode with nano spray voltage set at 1.5 kV and source temperature at 250°C.
The instrument was operated in parallel data-dependent acquisition (DDA) mode using fourier transform (FT) mass analyzer for one precursor ion MS scan from m/z 375 to 1800 with a resolution of 120,000 (fwhm at m/z 400) followed by MS/MS scans at resolution 15,000 on the 15 most intensive peaks subjected to high energy collision dissociation (HCD) with normalized collision energy of 35%. Dynamic exclusion parameters were set same as previously (Yang et al. 2011). All data are acquired under Xcalibur 2.2 operation software (Thermo).
Data analysis
All MS and MS/MS raw spectra were output as MGF files by Proteome Discoverer 1.4 (PD1.4, Thermo) for subsequent database search using Mascot searching engine (2.3.02, Matrix Science, Boston, MA). The porcine RefSeq sequence database (39,777 entries, downloaded on 3/26/2013 from NCBInr) was used for database searches. The database search was performed with two-missed cleavage site by trypsin. The peptide tolerance was set to 10 ppm and MS/MS tolerance was set to 0.05 Da. A fixed carbamidomethyl modification of cys-teine was set along with the variable modifications: methionine oxidation, deamidation on as-paragines/glutamine residues, acetylation on N-terminal peptide and lysine residue, and suc-cylation of lysine. Data filtering parameters were as follows: (a) ≤1% FDR; (b) the peptide identity probability is 99% CI with 35 peptide score cutoff; (c) at least two distinct peptides met above criteria hit for each proteins as a finally identified protein list. All MS/MS spectra for possibly identified acetylated and succinylated peptides from initial database searching were manually inspected and validated using Xcalibur 2.2.
For relatively quantitative analysis of succinylated peptides across samples, the peak areas of detected precursor ions at each specific m/z corresponding to the succinylated peptides were determined by extracted ion chromatogram (XIC) as reported previously (Yang et al. 2011). The XIC of two independent (non-target), tryptic peptides identified from the same proteins in the same LC-MS/MS runs were also used as reference control for normalization of loaded sample digests.
RESULTS
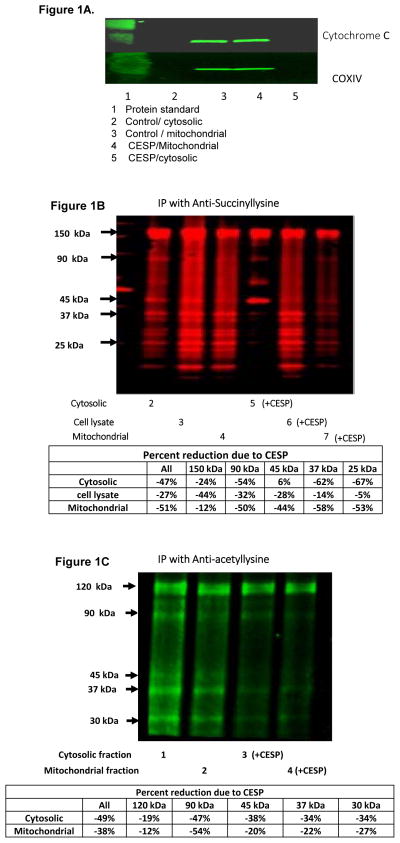
To test for the presence of succinylation in neurons, cultured neurons were lysed and separated into mitochondrial and cytosolic fractions. The cytosolic fraction did not have any detectable mitochondrial contamination as measured with cytochrome C or COX-IV whether or not CESP was present (Figure 1A). Strong succinylation signals were observed in the cell lysate as well as in the mitochondrial and cytosolic fractions (see Figure 1B lanes 2, 3, 4). Primary cultured neurons also showed a strong acetylation signal in the mitochondrial and cyto-solic fractions (Figure 1C, Lanes 1 and 2). The results clearly show strong succinylation and acetylation in both cellular compartments.
Figure 1. Succinylation and acetylation of proteins in the cytosol and mitochondria are sensitive to inhibition of KGDHC.
Figure 1A. Characterization of the mitochondrial and cytosolic fractions. The media of cultured neurons was changed to BSS and the cells were incubated with or without 100 μM-CESP in BSS buffer for one hour at 37°C. Mitochondrial and cytosolic fractions were prepared. Ten μg protein were loaded on each lane and were separated on 4–20 % Tris-glycine gel and transferred to blotting membrane.
Figure 1B. Succinylation is sensitive to inhibition of KGDHC. Aliquots (10 μg protein) were added to each lane. Similar results were observed in three independent experiments.
Figure 1C. Acetylation is sensitive to inhibition of KGDHC. Aliquots (10 μg protein) were added to each lane.
To test whether the succinyl groups can be derived from KGDHC, neurons were incubated for one hour before harvesting with CESP, the very specific inhibitor of KGDHC (Bunik et al. 2005) (Figure 1B). CESP inhibited succinylation in the cell lysate by 26.9% (Compare lanes 3 and 6), in the cytosolic fraction by 47% (compare lanes 2 and 5) as well as in the mito-chondria by 51.2% (compare lanes 4 and 7). Furthermore, comparison of the bands in each lane make it clear that the reductions in succinylation with KGDHC varied between bands. The diethylester of succinylphosphonate that does not inhibit cellular KGDHC under these conditions did not alter succinylation (data not shown).
Acetylation was also sensitive to inhibition of KGDHC (Figure 1C). CESP diminished acetylation in cytosolic fraction (compare lanes 1 and 3) and the mitochondrial fraction (compare lanes of 2 and 4). Some bands were relatively insensitive to CESP. For example, the 200 kDa band increased by 3 and 4% in cytosol and mitochondria, respectively (Data not shown). However, CESP caused a reduction in most bands (cytosol, mitochondria): 120 kDa (−19, −12), 90 kDa (−47, −54), 80 (−31, −20), 45 kDa (−38, −20), 37 kDa (−34, −22) and 30 kDa (−34, −27). The results suggest that inhibition of KGDHC also alters PDHC activity, which can act as a trans-acetylase. This possibility is tested in subsequent experiments that are describe with Figure 5.
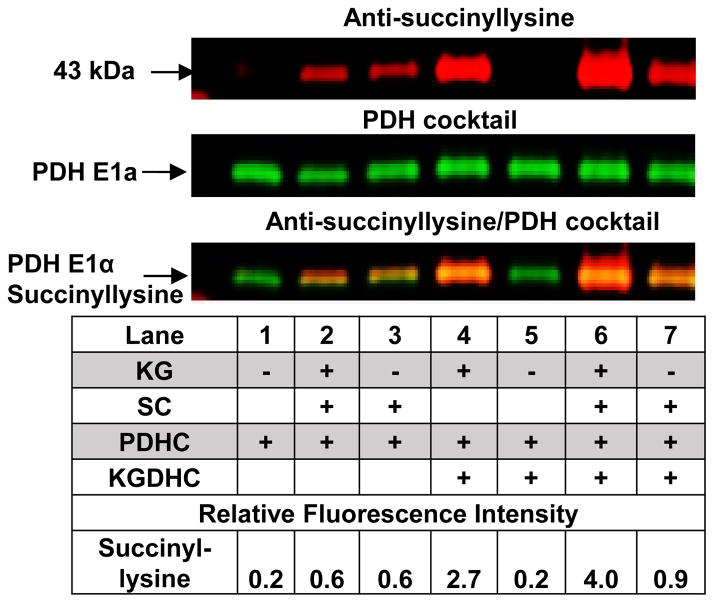
FIGURE 5.
PDHC is succinylated in KG dependent manner. See methods for the treatment paradigm. For Western blots, 10 μg of protein were added on each lane.
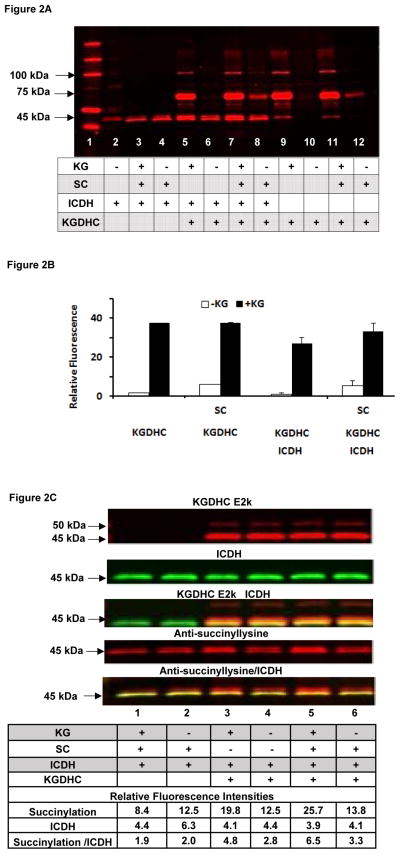
The next experiments tested whether KGDHC can donate SC groups to other proteins (Figure 2A with quantification in Figures 2B, 2C and Table 1). Commercial KGDHC was incubated with commercial ICDH with or without succinyl-CoA and KG. The products were separated by gel electrophoresis and probed with the antibodies to lysine succinylation (Figure 2A). Although ICDH had considerable succinylation without any manipulation (lane 2), KGDHC did not. The results clearly show a KG dependent KGDHC succinylation. KGDHC consists of three subunits (E1k, E2k and E3). Immunostaining revealed that the 100 kDa protein in Figure 2A is the E1k component of KGDHC (Immunostaining not shown). In the presence of KGDHC, comparison of the lanes without KG (lanes 6, 8, 10, 12) to the corresponding lanes with KG (lanes 5, 7, 9, 11, respectively) reveals KG-dependent succinylation in the presence of KGDHC. Succinyl-CoA also increased succinylation (e.g., compare lanes 10 and 12), but the effect was not as striking as with KGDHC. The presence of succinylation bands at 45–50 kDa in lanes 2–4 and the lack of a band in lanes 9–12 in Figure 2A suggest that the succinyl-ation in those bands is primarily ICDH. The results are characterized and quantified in Figure 2B and 2C.
FIGURE 2. KG dependent succinylation of proteins by KGDHC.
Figure 2A. KG dependent succinylation of ICDH by KGDHC. Various combinations of purified KGDHC, purified ICDH, KG or SC were used to test whether KGDHC could donate succinyl groups to other proteins or if they could be non-enzymatically succinylated. The details are in the Methods section. KGDHC, ICDH and/or SC were mixed together in media in which KGDHC generated SC. Ten μg protein was added to each lane of a 4–20 % TG gel. Each condition was run in triplicate with similar results.
Figure 2B. KG dependent succinylation of the 70 kDa protein and smaller succinylation with SC. KGDHC increases the succinyl-lysine of the 70 kDa protein band in KG dependent manner. The bars are the quantitation of the signals for the 70 kDa bands in Figure 2A from multiple experiments.
Figure 2C. KG dependent succinylation of ICDH and KGDHC. The conditions are described above for 2A except the Western blot was probed with antibodies against KGDHC (E1k; E2k) and ICDH. The quantification of the intensities are shown below the Western.
TABLE 1. KG-dependent succinylation of KGDHC.
The 70 kDa bands in Figure 2A were cut and processed for mass spectrometry. This protein band contains many proteins. The main protein was albumin. The sequence of the residues that were succinylated are in Table 1. Each value was divided by an internal standard (the bottom line of the table) and then the plus KG was divided by the minus KG. We then divided the KG+ by KG-. When this is done for the standard the ratio is one.
| Peptide sequence
|
Increased succinylation
|
|---|---|
| TPVSEKVTK | 5.4 |
| KQTALVELLK | 3.1 |
| LCVLHEKTPVSEK | 7.1 |
| LCAIPSLR | 1.0 |
The strongest succinylated band in Figure 2A is the 70 kDa protein, which is the albumin that is added to the purified KGDHC to stabilize it. Therefore, these numbers were quantified (Figure 2B). The KG dependent succinylation in the presence of KGDHC is striking (Figure 2B). SC provides only slight increases in succinylation. Clearly, KGHDC can readily donate succinyl groups to albumin. SC increased succinylation in the presence of KGDHC, but not near as much KG and the effects were not additive. The presence of ICDH slightly decreased the succinylation perhaps it served as an SC sink. The much greater effectiveness of KG than SC suggests that the catalysis due to the E2k succinyltransferase is important.
Since the largest changes in succinylation occurred in the 70 kDa band. This band was cut and used for mass spectrometry analysis to verify that succinylation was occurring and the sites of succinylation (Table 1). The data show that KG/KGDHC dependent increases in suc-cinylation occur at very specific sites.
Detailed analysis of the 45–50 kDa succinylated bands in Figure 2A distinguished the succinylation of E2k and ICDH (Figure 2C). As shown in the top row, E2k consists of one band at 50 kDa and a second band at 45 kDa. ICDH is a single band at 45 kDa. The relation of E2k and ICDH is shown in the third row. The second band of E2k is immediately above the ICDH. The fourth row reveals the response of the antisuccinyl-lysine and the final row shows the overlap of the 45 kDa band ICDH and succinylation. These basic relationships were confirmed in three other experiments.
Comparison of bands shows a striking dependence of succinylation of the 45 kDa band of ICDH on KG and KGDHC (See the numbers on the bottom of Figure 2C). The signal for succinylation (Fourth row; Figure 2C) in the absence of KG and SC (lane 4) is 12.5 and the addition of KG increases it by 58 percent to 19.8 (Lane 3). On the other hand, the addition of only SC (lane 6) hardly increases the signal (13.8). The combination of SC and KG (Lane 5) increases the signal by 105 percent to 25.66. Clearly, KG-KGDHC dependent succinylation of ICDH can occur. A similar conclusion follows if the signal for succinylation of ICDH is divided by the signal for ICDH (Figure 2C). The signal (2.83) in the absence of KG and SC (lane 4) increased by 69% to 4.79. By comparison, the addition of just SC (Lane 6) increased the signal to only 3.34. The combination of SC and KG increased the signal by 130% to 6.53 (Lane 5). At the same time, the amount of ICDH immune-detected protein did not change in the presence or absence of KG (Row 2).
To test whether the succinylation has an impact on these other proteins, the effects of SC on ICDH activity was determined (Figure 3). In the first set of experiments, the amount of ICDH was varied with a constant amount of SC (16 μM) (Figure 3A). A forty minute incubation with SC inhibited varying activities of ICDH (4, 8, 16 mU) by variable amounts (47%, 31% or 13%, respectively) (Figure 3A). The inhibition was stronger at lower enzyme concentrations. In the second set of experiments, a constant amount of enzyme (8 mU) was incubated with varying concentrations of SC (Figure 3B). Concentrations as low as 16 μM SC produced a nearly maximal effect on ICDH activity, and no further inhibition was observed even with concentrations as high as 200 μM (Figure 3B). Succinyl-CoA decreased ICDH activity by 36 % (16 μM), 32 % (32 μM), 45% (100 μM ) and 48% (200 μM). These concentrations are below those used by others to examine succinylation (Wagner & Payne 2013).
FIGURE 3. SC inhibits isocitrate dehydrogenase (ICDH) activity.
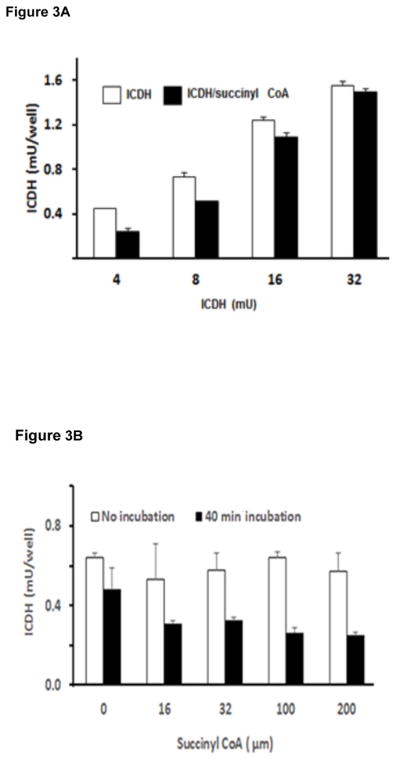
Figure 3A. The inhibitory effects of SC varied with the amount of ICDH. The treatments were at 30°C for 50 min. The final ICDH activities were 4, 8, 16 or 32 mU/well. Values are means of two independent experiments.
Figure 3B. SC concentration dependent changes in ICDH. The amount of enzyme was kept at 8 mU and the amount of succinyl Co A was varied. ICDH was either not incubated or incubated for 40 min with the indicated concentration of SC and incubated at 30°C for 0 or 40 min. After incubation, the ICDH activity was assayed in a 96 well plate.
SC alters the activity of KGDHC (Figure 4). The effects SC on two different activities of KGDHC were determined with or without a pre-incubation. With 0.39 mU and in absence of a pre-incubation, SC (100 μM) increased activity by 46% (left columns of each pair Figure 4). Although a thirty minute pre-incubation reduced KGDHC activity by 46% (Figure 4 Left two columns), SC still activated KGDHC (right column of each pair Figure 4).
FIGURE 4.
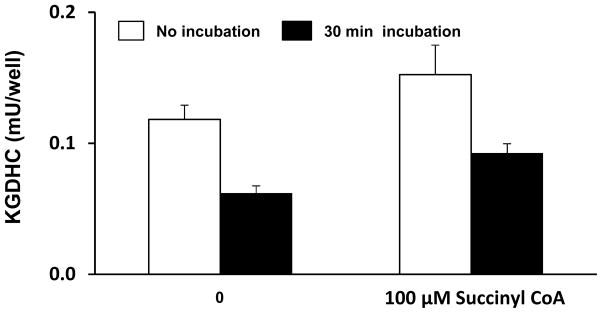
Succinylation alters the activity of KGDHC. KGDHC was incubated with SC (100 μM) for either 0 min or 30 min in KGDHC lysate buffer. KGDHC (7.8 mU) was diluted in KGDHC lysate buffer and were read at 340–460 nm (cut off 455 nm) at 30°C for 30 min.
Inhibition of KGDHC with CESP also altered acetylation (Figure 1B), which we postulate to be accomplished by the acetyltransferase component of PDHC. Therefore we tested whether PDHC could be succinylated. Although E1p appeared succinylated, the lack of appropriate antibodies prevented definitive measurement. KGDHC also succinylated PDHC E1alpha (Figure 5). In the absence of KGDHC, KG or succinlyl CoA no basal succinylation was observed (Lane 1; 0.2). The succinylation was increased by SC (Lane 2 or 3: 0.6). The increases were much greater in the presence of KGDHC. The addition of KG increased succinylation from 0.9 to 4 (compare Lane 7 to 6). The addition of just SC increased the signal from 0.2 (Lane 5) to 2.7 (Lane 4) and the combination increased the signal to 4 (Lane 6). Although E3 also appeared to be succinylated, the overlap with albumin made it difficult to make a clear conclusion. Under these precise conditions, PDHC activity was diminished by 25%.
KGDHC also promoted succinylation of fumarase and increased its activity (Figure 6). Fumarase had more baseline succinlyation than the other proteins. SC increased the signal from 1 (Lane 1) to 2.7 (Lane 3). In the presence of KGDHC, the signal increased from 1 (Lane 1) to 2 (Lane 5) and the presence of KG increased it to 3.3. The combination of KG and SC increased the signal to 5.2 (Lane 6). KGDHC dependent succinylation increased the activity of fumarase from 0.89 ±0.02 to 1.11 ± 0.02 (24.7%). SC increased fumarase activity from 0.90 ±0.01 to 0.97 ±0.01 (7.8%).
FIGURE 6.
Succinylation of fumarate hydratase. See methods for the treatment paradigm.
DISCUSSION
Current state of succinylation research
Succinylation is a well-documented post-translational modification (Zhang et al. 2011). In their initial reports, Zhang et al identified 69 succinyllysine sites among 14 E. coli proteins. Succinylation of serine hydroxymethyltransfer-ase, ICDH and GAPDH are particularly well documented. Systematic profiling of the mammalian succinylome identified 2565 succinylation sites on 779 proteins (Park et al. 2013). Twenty-three percent of liver mitochondrial proteins were succinylated. Succinylation profiles have been published for multiple cancer cell lines [HeLa, adenocarcinoma; HCT116, colorectal carcinoma; A549, lung carcinoma; A375, skin melanoma, HepG2 (hepatocellular carcinoma), MG-63 (osteosarcoma), Du145 (brain carcinoma), MDA-MB-231( adenocarcinoma), HEK293T) and human embryonic kidney cells (Zhang et al. 2011). Succinylation is dynamic, with experiments in mouse liver mitochondria or fibroblasts from SIRT 5 (an NAD+ de-cuccinylase) knockouts revealing succinylation of 80% of the proteins in the TCA cycle. The results provide further support to growing evidence that succinylation may be as common as other post-translational modifications. Our results show extensive succinylation in neurons and N2a cells in mitochondria, cytosol and nucleus. Thus, an understanding of the role of succinyl-ation in brain metabolism is essential.
The source of succinyl groups for succinylation
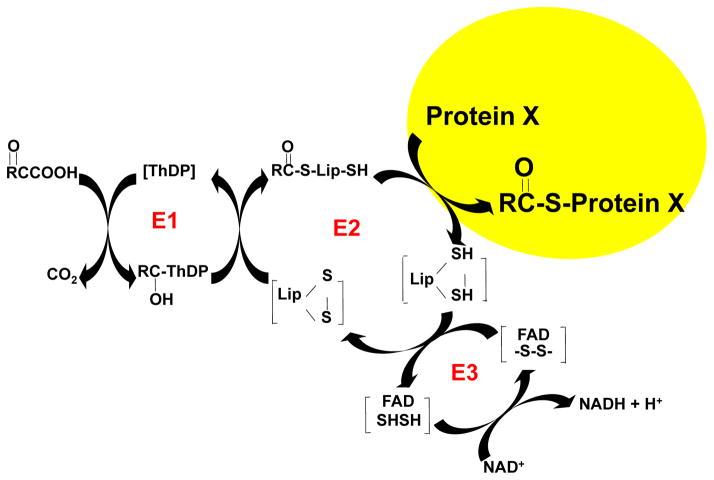
The source of the succinyl groups is unknown. The second enzyme of KGDHC is a trans-succinylase. Thus, KGDHC is a logical source of the succinyl groups (Figure 7). This possibility has been explored in non-mammalian systems (Weinert et al. 2013). KGD1 is required for KGDHC activity and is under control of catabolite repression, whereas growth in galactose-containing media induces KGD1 gene expression (Weinert et al. 2013). In yeast, induction of KGD1 increases cellular succinylation by about 1.7 fold and mitochondrial succinylation by 2.7 to 4.7 fold. Loss of KGD1 causes a four-fold reduction in succinylation and a six-fold reduction in mitochondrial succinylation. Nearly every enzyme of the liver mitochondria is succinylated (Weinert et al. 2013). The current studies using neurons or neuronal cell lines demonstrate that inhibiting KGDHC reductes succinylation of multiple proteins in both cultured neurons and a neuronal cell line. The reductions with KGDHC varied between the different bands, suggesting additional levels of control. The inhibitor experiments suggest that succinylation is rapid and that KGDHC can serve as a donor of succinyl groups for other enzymes. The results also suggest that turnover at different sites vary and/or that KGDHC is not the only donor of succinyl groups.
FIGURE 7.
E2k as a succinyltransferase to proteins. The picture shows the three components of KGDHC: E1k (α-ketoglutarate dehy-drogenase), E2k (dihyrolipoyul succinyltransferase (DLST) and E3 dihyrdolipoyl dehydrogen-ase. The results suggest that E2k can transfer succinyl groups to other proteins.
The much greater effectiveness of KG plus KGDHC than SC suggests that the enzymatic catalysis due to the E2k succinyltransferase is important. The current study and those of others suggest that if there is enough SC, non-enzymatic succinylation can occur as well (Wagner & Payne 2013). The data strongly suggest that the succinyltransferase of KGDHC is an enzymatic catalyst for succinylation.
The inhibition of succinylation by CESP in cells strongly suggests that this is caused by reduced KGDHC, but other explanations are possible. Reducing KGDHC activity with CESP may make the cells unhealthy, and sick cells may succinylate less from some other succinyl-transferase. CESP toxicity, as measured by cell death, is minimal. Furthermore, the experiments show that cytochrome C is not released from the mitochondria (Figure 1A). However, we know that reducing KGDHC either by CESP or siRNA alters cellular calcium homeostasis (Gibson et al. 2012a) and the response to oxidants (Chen HL et al. 2015, Shi et al. 2008). Thus, future experiments need to determine how succinylation and the roles of KGDHC vary with toxicity.
Cytosolic succinylation
The results demonstrating that inhibition of KGDHC also reduces cytosolic succinylation supports the suggestion that succinylation of cytosolic proteins depends upon SC in the mitochondria (Weinert et al. 2013). Nevertheless, the sensitivity of succinylation in the cytosol to inhibition of KGDHC is surprising. One possibility is that a product of KGDHC is a required substrate for an alternative succinyl CoA transferase in the cytosol. A second, but unlikely, possibility is that succinyl CoA is transported from the mito-chondria. A third possibility is that the cytosol has functional KGDHC. In the standard fractionation procedures employed in this manuscript, KGDHC activity and protein for E1k and E2k can be readily measured in these cytosolic fractions. In experiments with neurons, in which 1.4% of mtDNA was in the cytosolic fraction, 17.3% of the activity and 24.8% of KGDHC protein was in the cytosol. In experiments with N2a cells, in which 2.2% of the activity was in the cytosolic fraction, 32.3% of the KGDHC activity and 22.0% of the KGDHC protein were in that fraction. Others have postulated many components of the TCA cycle being in the cytosol (Panfoli et al.).
Succinylation alters the activities of tricarboxylic acid cycle enzymes
The results demonstrate that the KG-dependent provision of SC can regulate the activity of multiple enzymes through succinylation. Our data are consistent with SC from KGDHC inhibiting ICDH and activating fumarase. Previous studies with mutational analysis of the lysines suggest that particular lysines are critical for activity and postulate that succinylation of these lysines will alter activity, but activity measurements were not made (Zhang et al. 2011). Regulation of other enzymes by succinylation has been shown to have physiological relevance. Glucagon activates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in vivo by decreasing the extent of succinylation of the enzyme (Quant et al. 1990). Acetylation of many mitochondrial enzyme acetylation sites does not alter activities (Wagner & Payne 2013) and we have not shown if succinylation of only a few sites is responsible for the changes in activities. Succinylation of KGDHC appears regulatory, with succinylation of E1k being related to changes in activity. This is in agreement with previous results (Waskiewicz & Hammes 1984). KGDHC is also regulated by internally generated reactive oxygen species produced by E3 that inhibit E1k (Bunik & Sievers 2002). 3-Hydroxy-3-methylglutaryl-CoA reductase can promote its own suc-cinylation, which inhibits the enzyme (Quant et al. 1990).
Interactions of succinylation and SIRTUINS
Others have manipulated acetylation and succinylation state by varying de-acetylation and de-succinylation. SIRT 3 regulates deacetylation and SIRT 5 regulates desuccinylation. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells (Ozden et al. 2014). Those studies show that PDHE1alpha, the first step of the complex, is acetylated and activated by SIRT2. The current studies show that PDHE1alpha is highly succinylated. Just as with acetylation, the enzymatic removal of the succinyl groups provides another level of metabolic control. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks (Rardin et al. 2013). Studies in Sirt5−/− cells suggest that PDHC and SDH may be activated by succinylation and inactivated by desuccinylation (Park et al. 2013). SIRT5 also activates SOD1 to eliminate ROS (Lin et al. 2013). Sirt-5 is the only known enzyme to promote desuccinylation. Sirt-5 has been shown to repress activities of PDHC and SDH. Thus, it needs to be determined if the succinylation state varies with the bioenergetics and what regulates it. De-succinylation may also provide a level of control. Metformin reduces SIRT 5 levels and causes hypersuccinylation in the mitochon-dria (Buler et al. 2014). SIRT 5, which is under the control of PGC1alpha and AMPK, regulates mitochondrial energy metabolism.
The direct relevance of these changes to AD must be tested
KGDHC activity is diminished in multiple neurodegenerative diseases including Alzheimer’s disease (Gibson et al. 2000, Bubber et al. 2005, Gibson et al. 2013), although its impact on metabolism and brain function is unknown. A reduction in succinylation may be one of the consequences of the decline in KGDHC in the brain in neurodegenerative diseases. Succinylation has been implicated in plaque and tangle formation. SIRT5 increases during the progression of AD (Lutz et al. 2014). There are many reports in the chemical literature suggesting that succinylation may diminish aggregation. For example, succinylation keeps neuro-filaments from aggregating (Iqbal et al. 1975). Succinylation has been used to solubilize tangles (Wischik et al. 1988) and amy-loid plaque (Subasinghe et al. 2003, Vetri et al. 2007). These studies used high concentrations, so the relevance of those findings to the current results remains to be tested, but they are consistent with the suggestion that diminished succinylation related to a KGDHC deficit would promote aggregation Nevertheless, no measurements of succinylation have been made in brains from patients who died from neurodegenerative diseases.
Acknowledgments
Supported by NIH PPG AG14930 to GG; NIH SIG grant 1S10RR025449-01 to SZ. The authors would like to thank Mr. James A. McCardle for his technical support.
Abbreviations
- BSA
bovine serum albumin
- BSS
balanced salt solution
- CESP
carboxyethyl ester of succinylphophonate
- DMEM
Dulbecco’s Modified Eagles Medium
- ETN
Tris NaCl, EDTA
- ICDH
isocitrate dehydrogenase
- KG
α-ketoglutarate
- KGDHC
α-ketoglutarate dehydro-genase complex
- IP
immunoprecipitation
- MES
Morpholinoethane sulfonic acid monohydrate
- NETN
NaCl, EDTA Tris, NP-40
- PBS
phosphate buffered saline
- PDHC
pyruvate dehydrogen-ase complex
- SC
succinyl CoA
- TG
Tris Glycine
- TBS
Tris buffered saline
- TCA
tricarboxylic acid
Footnotes
CONFLICT-OF-INTEREST DISCLOSURE
The authors have no conflicts of interest.
References
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nat Protocols. 2007;2:1490–1498. doi: 10.1038/nprot.2007.207. [DOI] [PubMed] [Google Scholar]
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Annals of Neurology. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- Buler M, Aatsinki S-M, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism. The FASEB Journal. 2014 doi: 10.1096/fj.13-245241. [DOI] [PubMed] [Google Scholar]
- Bunik VI, Denton TT, Xu H, Thompson CM, Cooper AJL, Gibson GE. Phosphonate Analogues of α-Ketoglutarate Inhibit the Activity of the α-Ketoglutarate Dehydrogenase Complex Isolated from Brain and in Cultured Cells†. Biochemistry. 2005;44:10552–10561. doi: 10.1021/bi0503100. [DOI] [PubMed] [Google Scholar]
- Bunik VI, Sievers C. Inactivation of the 2-oxo acid dehydrogenase complexes upon generation of intrinsic radical species. European Journal of Biochemistry. 2002;269:5004–5015. doi: 10.1046/j.1432-1033.2002.03204.x. [DOI] [PubMed] [Google Scholar]
- Chen HL, Denton TT, Xu H, Gibson GE. Reductions in the mitochondrial enzyme α-ketoglutarate dehydrogenase complex in neurodegenerative disease - beneficial or detrimental? Antioxidants and Redox Signalling. 2015 doi: 10.1111/jnc.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Chen HL, Xu H, Qiu L, Xu Z, Denton TT, Shi Q. Deficits in the mitochondrial enzyme α-ketoglutarate dehydrogenase lead to Alzheimer’s disease-like calcium dysregulation. Neurobiology of Aging. 2012a;33:1121.e1113–1121.e1124. doi: 10.1016/j.neurobiolaging.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Chen HL, Xu H, Qiu L, Xu Z, Denton TT, Shi Q. Deficits in the Mitochondrial Enzyme α-Ketoglutarate Dehydrogenase Lead to Alzheimer’s Disease-like Calcium Dysregulation. Neurobiology of Aging. 2012b;33:1121.e1113–1121.e1124. doi: 10.1016/j.neurobiolaging.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Hirsch JA, Cirio RT, Jordan BD, Fonzetti P, Elder J. Abnormal thiamine-dependent processes in Alzheimer’s Disease. Lessons from diabetes. Molecular and Cellular Neuroscience. 2013;55:17–25. doi: 10.1016/j.mcn.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GE, Park LCH, Sheu KFR, Blass JP, Calingasan NY. The α-ketoglutarate dehydrogenase complex in neurodegeneration. Neurochemistry International. 2000;36:97–112. doi: 10.1016/s0197-0186(99)00114-x. [DOI] [PubMed] [Google Scholar]
- Hebert Alexander S, Dittenhafer-Reed Kristin E, Yu W, et al. Calorie Restriction and SIRT3 Trigger Global Reprogramming of the Mitochondrial Protein Acetylome. Molecular Cell. 2013;49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Wisniewski HM, Grundke-Iqbal I, Korthals JK, Terry RD. Chemical pathology of neurofibrils. Neurofibrillary tangles of Alzheimer’s presenile-senile dementia. Journal of Histochemistry & Cytochemistry. 1975;23:563–569. doi: 10.1177/23.7.1141687. [DOI] [PubMed] [Google Scholar]
- Lin ZF, Xu HB, Wang JY, Lin Q, Ruan Z, Liu FB, Jin W, Huang HH, Chen X. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochemical and Biophysical Research Communications. 2013;441:191–195. doi: 10.1016/j.bbrc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Lowe DM, Tubbs PK. Succinylation and inactivation of 3-hydroxy-3-methylglutaryl-CoA synthase by succinyl-CoA and its possible relevance to the control of ketogenesis. Biochem J. 1985;232:37–42. doi: 10.1042/bj2320037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz M, Milenkovic I, Regelsberger G, Kovacs G. Distinct Patterns of Sirtuin Expression During Progression of Alzheimer’s Disease. Neuromol Med. 2014;16:405–414. doi: 10.1007/s12017-014-8288-8. [DOI] [PubMed] [Google Scholar]
- Millar AH, Hill SA, Leaver CJ. Plant mitochondrial 2-oxoglutarate dehydrogenase complex: purification and characterization in potato. Biochem J. 1999;343(Pt 2):327–334. doi: 10.1042/0264-6021:3430327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden O, Park SH, Wagner BA, Yong Song H, Zhu Y, Vassilopoulos A, Jung B, Buettner GR, Gius D. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radical Biology and Medicine. 2014;76:163–172. doi: 10.1016/j.freeradbiomed.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panfoli I, Calzia D, Ravera S, Bruschi M, Tacchetti C, Candiani S, Morelli A, Candiano G. Extramitochondrial tricarboxylic acid cycle in retinal rod outer segments. Biochimie. doi: 10.1016/j.biochi.2011.05.020. In Press, Uncorrected Proof. [DOI] [PubMed] [Google Scholar]
- Park J, Chen Y, Tishkoff Daniel X, et al. SIRT5-Mediated Lysine Desuccinylation Impacts Diverse Metabolic Pathways. Molecular Cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quant PA, Tubbs PK, Brand MD. Glucagon activates mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in vivo by decreasing the extent of succinylation of the enzyme. Eur J Biochem. 1990;187:169–174. doi: 10.1111/j.1432-1033.1990.tb15291.x. [DOI] [PubMed] [Google Scholar]
- Rardin Matthew J, He W, Nishida Y, et al. SIRT5 Regulates the Mitochondrial Lysine Succinylome and Metabolic Networks. Cell Metabolism. 2013;18:920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Xu H, Kleinman WA, Gibson GE. Novel functions of the alpha-ketoglutarate dehydrogenase complex may mediate diverse oxidant-induced changes in mitochondrial enzymes associated with Alzheimer’s disease. Biochim Biophys Acta. 2008;1782:229–238. doi: 10.1016/j.bbadis.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subasinghe S, Unabia S, Barrow CJ, Mok SS, Aguilar MI, Small DH. Cholesterol is necessary both for the toxic effect of Aβ peptides on vascular smooth muscle cells and for Aβ binding to vascular smooth muscle cell membranes. Journal of Neurochemistry. 2003;84:471–479. doi: 10.1046/j.1471-4159.2003.01552.x. [DOI] [PubMed] [Google Scholar]
- Vetri V, Librizzi F, Militello V, Leone M. Effects of succinylation on thermal induced amyloid formation in Concanavalin A. Eur Biophys J. 2007;36:733–741. doi: 10.1007/s00249-007-0181-z. [DOI] [PubMed] [Google Scholar]
- Wagner GR, Payne RM. Widespread and Enzyme-independent Nε-Acetylation and Nε-Succinylation of Proteins in the Chemical Conditions of the Mitochondrial Matrix. Journal of Biological Chemistry. 2013;288:29036–29045. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz DE, Hammes GG. Elementary steps in the reaction mechanism of the alpha-ketoglutarate dehydrogenase multienzyme complex from Escherichia coli: kinetics of succinylation and desuccinylation. Biochemistry. 1984;23:3136–3143. doi: 10.1021/bi00309a005. [DOI] [PubMed] [Google Scholar]
- Weinert Brian T, Schölz C, Wagner Sebastian A, Iesmantavicius V, Su D, Daniel Jeremy A, Choudhary C. Lysine Succinylation Is a Frequently Occurring Modification in Prokaryotes and Eukaryotes and Extensively Overlaps with Acetylation. Cell Reports. 2013;4:842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Wischik CM, Novak M, Thøgersen HC, et al. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proceedings of the National Academy of Sciences. 1988;85:4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Qiang X, Owsiany K, Zhang S, Thannhauser TW, Li L. Evaluation of Different Multidimensional LC–MS/MS Pipelines for Isobaric Tags for Relative and Absolute Quantitation (iTRAQ)-Based Proteomic Analysis of Potato Tubers in Response to Cold Storage. Journal of Proteome Research. 2011;10:4647–4660. doi: 10.1021/pr200455s. [DOI] [PubMed] [Google Scholar]
- Yang Y, Thannhauser TW, Li L, Zhang S. Development of an integrated approach for evaluation of 2-D gel image analysis: Impact of multiple proteins in single spots on comparative proteomics in conventional 2-D gel/MALDI workflow. ELECTROPHORESIS. 2007;28:2080–2094. doi: 10.1002/elps.200600524. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]