Summary
A subpopulation of nociceptors, the glial cell-line derived neurotrophic factor (GDNF)-dependent, non-peptidergic C-fibers, express a cell-surface glycoconjugate that can be selectively labeled with isolectin B4 (IB4), a homotetrameric plant lectin from Griffonia simplicifolia. We show that versican is an IB4-binding molecule in rat dorsal root ganglion (DRG) neurons. Using reverse transcriptase polymerase chain reaction (RT-PCR), in situ hybridization and immunofluorescence experiments on rat lumbar DRG, we provide the first demonstration that versican is produced by neurons. In addition, by probing Western blots with splice variant specific antibodies we show that the IB4-binding versican contains only the glycosaminoglycan alpha (α GAG) domain. Our data support V2 as the versican isoform that renders this subpopulation of nociceptors IB4-positive (+).
Introduction
Nociceptors are sensory neurons that transmit electrical impulses, triggered by noxious stimuli, from the periphery to the trigeminal or spinal dorsal horn (Willis and Westlund 1997). The vast majority of nociceptors are either thinly myelinated Aδ- or unmyelinated C-fiber neurons whose activity is particularly important in the setting of inflammation or peripheral neuropathy (Cline et al. 1989; Woolf 2007; Ferrari et al. 2010; Serra et al. 2014). Based on differences in phenotype and neurotrophin dependence, C-fibers have been divided into nerve growth factor (NGF)-dependent, peptidergic, and glial cell line derived neurotrophic factor (GDNF)-dependent, non-peptidergic nociceptors (Snider and McMahon 1998). The latter class of nociceptors can also be characterized by their unique expression of glycoconjugates that are selectively labeled with isolectin B4 (IB4) (Streit et al. 1985; Silverman and Kruger 1990), a homotetrameric carbohydrate binding protein derived from Griffonia simplicifolia (Hayes and Goldstein 1974). The specificity for GDNF-dependent, non-peptidergic C-fiber nociceptors suggest that the IB4-binding glycoconjugates are critical for the biological function of these nociceptors (Bogen et al. 2008) (Bogen et al. 2009). We have previously demonstrated that the V2 splice variant of versican is the IB4-binding molecule in porcine spinal cord (Bogen et al. 2005). Although being the dominant splice variant of versican in nervous tissue, versican V2 is thought to be the product of glial cells (Asher et al. 2002; Melendez-Vasquez et al. 2005). However, if versican is responsible for the IB4-reactivity of GDNF-dependent, non-peptidergic C-fibers it should be expressed by sensory neurons. Therefore, the aim of our study was to: a) prove the neuronal expression of versican, and given that this study is done in rats b) confirm earlier results in pig and show that it is versican V2 that accounts for the IB4-reactivity of this population of nociceptors. Here we show that a single IB4-binding molecule can be immunoprecipitated anti-versican antibody from a subcellular preparation of rat spinal cord tissue. Using in situ hybridization on sections of rat dorsal root ganglia (DRG) with a riboprobe antisense to versican mRNA, we demonstrate, for the first time, a neuronal origin of versican. Immunoflurescence experiments on rat DRG demonstrate co-localization of IB4-binding and anti-versican immunoreactivity. Finally, analysis of the GAG domain structure of the IB4-binding versican reveals that it contains the GAG alpha but not the GAG beta domain. Our results suggest that versican V2, made by IB4 (+)-nociceptors contribute to the IB4-reactivity of GDNF-dependent, non-peptidergic C-fiber nociceptors.
Material and Methods
The monoclonal anti-versican antibody 12C5, developed by Asher and colleagues (Asher et al. 1991), was obtained from the Developmental Studies Hybridoma Bank founded under the auspices of the National Institute of Child Health and Human Development (NICHD) and maintained by the University of Iowa (Department of Biological Sciences, Iowa City, IA, USA).
Animals
All experiments were performed on adult male Sprague Dawley rats (obtained from either Charles River Laboratories, Hollister, CA or Janvier Labs, Le Genest Saint Isle, France). Animals were housed, three per cage, under a 12 h light/dark cycle in a temperature and humidity controlled room in the animal care facility of the University of California, San Francisco or at the Grünenthal GmbH, Aachen. Food and water were available ad libitum. Experimental protocols for experiments that were done in San Francisco were approved by the Institutional Animal Care and Use Committee at UCSF and adhered to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Experimental protocols for experiments done at Grünenthal, Aachen were approved by the local government committee for animal research and adhered to the German Animal Welfare Law. All effort was made to minimize the number of animals used and their suffering.
Subcellular fractionation
Synaptosomes were prepared based on the method originally reported by Gray and Whittaker (Gray and Whittaker 1962), with minor modifications. Frozen rat spinal cord was homogenized in homogenization buffer (10 mM Hepes, pH 7.4,1 mM EDTA, 320 mM sucrose) containing a protease inhibitor cocktail (Roche Diagnostics Corp., Indianapolis, IN, USA), using a motor-driven Glass-Teflon homogenizer (0.2 mm clearance) by 12 up and down strokes at 800 rpm. The homogenate was centrifuged at 1.000 g for 10 min, and the supernatant (S1) removed and placed on ice. The pellet (P1) was resuspended in homogenization buffer and further homogenized as described above. This homogenate was centrifuged at 1.000 g for 10 min, and the resulting pellet (P1′, cell debris and nuclei) discarded. The supernatant S1′ was combined with supernatant S1 and centrifuged at 12.000 g for 15 min. The supernatant (S2) was discarded and the pellet (P2, crude membrane fraction) resuspended in homogenization buffer and again homogenized with six up and down strokes, at 800 rpm, using the motor-driven Glass-Teflon homogenizer. The homogenate was centrifuged at 12.000 g for 20 minutes. The supernatant (S2) was discarded, the pellet (P2′) resuspended with 0.32 M sucrose in 5 mM Tris/HCl, pH 8.1, and layered onto a discontinuous sucrose gradient (0.85/1.0/1.2 M sucrose) and centrifuged at 85.000 g for 2 h. The resulting subcellular fractions were harvested with a widened Pasteur pipette: myelin accumulates at the 0.32/0.85 M sucrose interface, light membranes at the 0.85/1.0 M sucrose interface, synaptosomes at the 1.0/1.2 M sucrose interface, and mitochondria at the bottom of the centrifugation tube (Gray and Whittaker 1962). All subcellular fractions were diluted to a final sucrose concentration of less than 0.3 M with protease inhibitor containing phosphate buffered saline (PBS), centrifuged at 12.000 g for 10 minutes, and recovered from the bottom of the tube with protease inhibitor containing PBS. The protein concentration was determined using the Bradford assay (Bradford 1976) with BSA as standard.
Western blot analysis
Samples (30 - 40 μg of protein) were combined with sample buffer [62.5 mM Tris/HCl, pH 6.8, 3% SDS, 10% (v/v) glycerol, 5% (v/v) β-mercaptoethanol, 0.025% Bromophenol blue], heated for 10 min at 60°C and electrophoresed on 7.5% polyacrylamide gels in 25 mM Tris containing 192 mM glycine and 0.1% SDS (Laemmli 1970). Proteins were electrophoretically transferred to nitrocellulose using the semidry method [transfer time 2 h at 1.5 mA/cm2, with 47.9 mM Tris, 38.9 mM glycine, 0.038% SDS and 20% (v/v) methanol].
Blots used for the analysis of the IB4-reactivity were blocked overnight with 1% BSA in Tris-buffered saline (20 mM Tris, 150 mM NaCl), incubated for 2 h at room temperature (RT) with IB4-HRP (Sigma-Aldrich, Saint Louis, MO, USA, 1:5.000) in Tris-buffered saline containing 0.1% (v/v) Tween 20 and 0.1 mM CaCl2, 0.1 mM MnCl2, and 0.1 mM MgCl2. IB4-reactivity was visualized using the enhanced chemiluminescence detection system (GE Healthcare, Piscataway, NJ, USA). Blots used for the analysis of the GAG domain structure of the IB4-binding versican were blocked with 5% non-fat milk in 0.1% (v/v) Tween 20 containing Tris-buffered saline (TBS-T) overnight and incubated with the respective anti-GAG alpha or anti-GAG beta specific antibodies (1:1.000; in 5% non-fat milk containing TBS-T) for 2 h at RT (Milev et al. 1998). After rinsing with TBS-T (3 times; 10 min each) blots were probed with an HRP-conjugated anti-rabbit antibody (1:5.000; in 5% non-fat milk containing TBS-T) for 1 h and rinsed with TBS-T (3 times; 10 min each). Immunoreactivities were visualized using the ECL detection system (GE Healthcare).
Hyaluronidase extraction
Protein from combined light membrane and synaptosome preparations was pelleted by centrifugation (30 min, 4°C, 436.000 g). This pellet was resuspended in protease inhibitor and 150 mM NaCl containing 50 mM NaxHxPO4 (prepared from stock solutions of NaH2PO4 and Na2HPO4), pH 5.3, and homogenized with a Glass/Glass homogenizer (0.1 mm clearance). A total of 250 μg of protein was combined with 50 units of hyaluronidase (Sigma-Aldrich) and incubated for 2 h at 37°C. The extracted proteins were separated from the insoluble pellet by centrifugation (10 min, 10.000 g) and concentrated in microconcentrators with a molecular cut-off of 3 kDa (EMD Millipore, Billerica, MA, USA).
Immunoprecipitation
The supernatant of hyaluronidase extracted light membranes and synaptosomes (500 μg of protein in total) was equilibrated for immunoprecipitation by adding an equal volume of 150 mM NaCl containing Tris/HCl, pH 7.4, supplemented with the protease inhibitor cocktail. Anti-versican antibody (12C5, 5 μg) was added and the mixture incubated for 30 min under vigorous shaking at 4°C. About 50 μg of protein G sepharose (GE Healthcare) was equilibrated in 150 mM NaCl containing Tris/HCl, pH 7.4. The protein G sepharose was added and the mixture incubated under continuous rotation for 2 h at 4°C. The sepharose beads were washed twice by 15 min incubation under powerful shaking with Tris/HCl, pH 7.4, containing 150 mM NaCl, 0.2% dodecylmaltosid, and a protease inhibitor cocktail (Roche). Bound proteins were eluted by incubation for 30 min with sample buffer at room temperature. All fractions were concentrated using microconcentrators with a molecular cut-off of 3 kDa and analyzed by Western blotting using IB4-HRP or the GAG-domain specific antibodies (Milev et al. 1998).
Immunohistochemistry
Male Sprague-Dawley rats (170-310 g, obtained from Janvier Labs, Le Genest Saint Isle, France) were deeply anesthetized with sodium pentobarbital and transcardially perfused with PBS, pH 7.4, until the exudate ran clear. L4 to L6 DRG were dissected out and fixed by storage in ice-cold acetone at -20°C for 10 minutes before they were embedded in Tissue Tek (OCT compound).
Dual-labeling immunofluorescence experiments were performed with 20 μm thick cryostat sections. All sections were treated with 1% periodic acid for 3 minutes, and blocked and permeabilized by a 30 minute incubation in 0.3% Triton X-100 and 5% normal goat serum in PBS (antibody dilution buffer). Anti-versican antibody (12C5; 1:100) and IB4-FITC (1:200; Sigma-Aldrich; L2895) were applied in 0.1 mM CaCl2, 0.1 mM MgCl2, 0.1 mM MnCl2 containing antibody dilution buffer at 4°C overnight. After rinsing with PBS (3 times at RT, 10 minutes each) supplied with the mixture of bivalent cations, tissue sections were incubated with rabbit anti-mouse Cy3 antibody (1:100; Jackson Immunoresearch, West Grove, PA, USA) in antibody dilution buffer supplied with the bivalent cations for 2 h at RT. After rinsing with 0.1 mM CaCl2, 0.1 mM MgCl2, 0.1 mM MnCl2 containing PBS the slides were dried at RT, mounted with mounting media and stored at 4°C.
Tissue sections were examined with an epifluorescence microscope (Zeiss Axiophot, Oberkochen, Germany) equipped with a CCD camera (Olympus DP50, Hamburg, Germany). Appropriate filter sets were used to detect FITC and Cy3 fluorescence. Images were captured and processed using an image analysis software (analySIS®, Soft Imaging System, Münster, Germany). Double labeling was visualized by image superposition.
Reverse transcriptase polymerase chain reaction
Total RNA from 20 rat DRG was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) with the PureLink™ RNA mini kit (Life technologies, Grand Island, NY, USA) according to the manufacturers instructions. The amount of RNA was quantified with a spectrophotometer, and cDNA preparation was carried out with 1 μg of total RNA/sample and the superscript III platinum 1-step RT-PCR system (Life technologies). The PCR primers (Invitrogen) used for the amplification of the different versican splice variants according to the National Center for Biotechnology Information database entry NM_001170558 were: Vcan_exon4_for = 5′-GCG ACC AGC AGA TAC ACT CT-3′; Vcan_exon7_for = 5′-CCA TTC ACT GAG GAA CCA CAC AT- 3′; Vcan_exon8_rev = 5′-GGG TGT CAG TTG CGG AAG TAT TTG-3′; Vcan_exon11_rev = 5′-CAT GTA CGG CGA TGA GCA AAG TA-3′.
In situ-hybridization
Hybridization to cryosections of rat DRG was performed with dioxigenin-labelled riboprobes as described previously (Schlenstedt et al. 2006). Antisense and sense probes were transcribed by using T7 RNA polymerase (Roche) from a 435 bp cDNA fragment encoding a sequence of exon 4-6 that is common to all 4 different versican splice variants [position 748 (Vcan_exon4_for = 5′-GCG ACC AGC AGA TAC ACT CT-3) to 1163 (Vcan_exon6_rev = 5′-ATC CGA CAG CCA GCC GTA AT-3′) within NM_001170558].
Hybridization was for 12 h at 42°C in a solution containing 50% formamide, 5 × saline sodium citrate (SSC; 20 × SSC = 3 M NaCl, 0.3 M sodium citrate, pH 7.4), 100 μg/ml salmon sperm DNA, 50 μg/ml heparin, 0.1% Tween 20, and 0.5 μg/ml digoxigenin-labeled probe. Washing was done three times in 50% formamide and 2 × SSC at 37°C for 1 h each. For the detection of hybrids, sections were incubated with anti-digoxigenin antibody conjugated with alkaline phosphatase (1:500; Roche) and stained with NBT/BCIP solution following the instructions of the DIG Nucleic Acid Detection Kit (Roche).
Results
1) IB4-reactivity in rat spinal cord
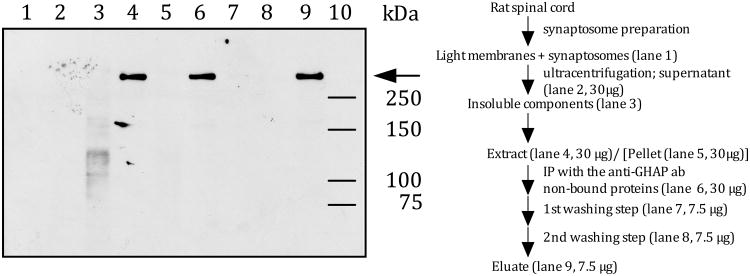
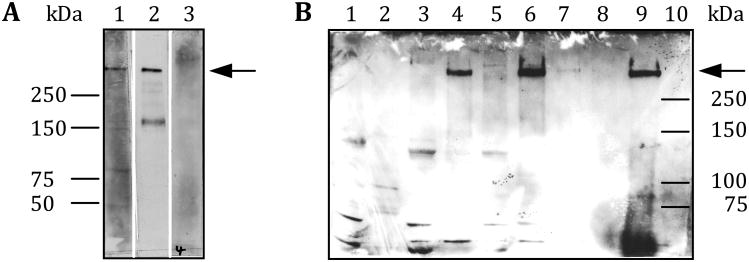
To determine whether or not versican is an IB4-binding molecule in rat spinal cord, a subcellular preparation composed of light membranes and synaptosomes was treated with hyaluronidase. The extract was immunoprecipitated with a monoclonal anti-versican (12C5) antibody, which is known to bind all versican splice variants (Westling et al. 2004), and analyzed by Western blotting, using IB4-conjugated horseradish peroxidase (HRP). As shown in Figure 1, only one IB4-binding molecule could be immunoprecipitated with the anti-versican antibody (lane 9). The apparent molecular weight of the IB4-binding molecule is > 250 kDa.
Figure 1. IB4-reactivity in rat spinal cord.
Rat spinal cord tissue was fractionated according to a synaptosome preparation scheme (Gray and Whittaker 1962). A combined light membrane and synaptosome fraction was treated with hyaluronidase to release hyaluronan-bound proteins, such as versican, into the supernatant. Proteins in the hyaluronidase-extract were immunoprecipitated with the monoclonal anti-versican antibody (12C5), which binds all known versican splice variants (Westling et al. 2004).
Different amounts of proteins (see fractionation scheme on the right) from each fraction were separated by SDS-PAGE and electroblotted onto a nitrocellulose membrane. IB4-reactivity (arrow) was visualized using HRP-conjugated IB4 and ECL as the detection system.
2) Versican mRNA in rat DRG
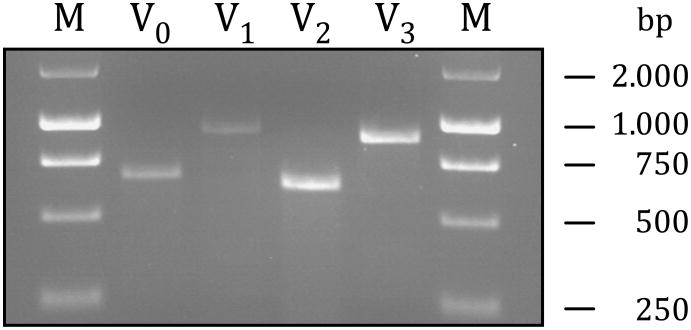
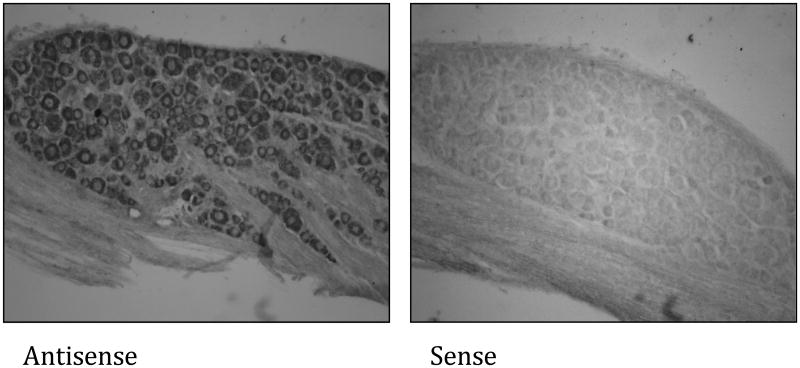
If versican is the molecule that renders non-peptidergic C-fiber nociceptors IB4-positive, it must be expressed in DRG. To determine whether versican transcripts are present in rat DRG, a RT-PCR on RNA extracts was performed. As shown in Figure 2 all four known splice variants of versican could be detected in RNA extracts derived from rat DRG. Given that DRG also contain non-neuronal cells, such as glia, the RT-PCR results do not prove a neuronal origin of any of the versican transcripts. To determine whether versican is expressed by sensory neurons in the DRG we performed an in situ hybridization using a riboprobe complementary to a nucleotide sequence of exons 4 to 6, which are present in the mRNA of all versican isoforms (748 to 1163 within NM_001170558). As shown in Figure 3, versican transcripts could be detected in small-, medium- and large-diameter sensory neurons but not in any other cells in the ganglion.
Figure 2. Versican transcripts in rat DRG.
To analyze for the presence of versican transcripts in rat DRG an RT-PCR for each splice variant was performed. Primer pairs were chosen to determine the presence or absence of a certain splice variant in the DRG [V0: Exon 7_for - Exon 8_rev = 673 bp; V1: Exon 4_for - Exon 8_rev = 925 bp; V2: Exon 7_for -Exon 11_rev = 626 bp; V3: Exon 4_for - Exon 11 _rev = 878 bp; M = Marker]. PCR amplification products were separated on 2% agarose gels and visualized by ethidium bromide intercalation.
Figure 3. Versican mRNAs in rat DRG.
In situ hybridization analysis of versican expression in rat L6 DRG. 20 μm thick tissue sections were exposed to digoxigenin (DIG)-labeled riboprobes for the highly conserved N-terminus of versican (exon 4 to exon 6). For detection of hybrids, the sections were incubated with anti-DIG antibody conjugated with alkaline phosphatase and stained through exposure with nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate, 4-toluidine salt solution.
3) Anti-versican- and IB4-reactivity in rat DRG
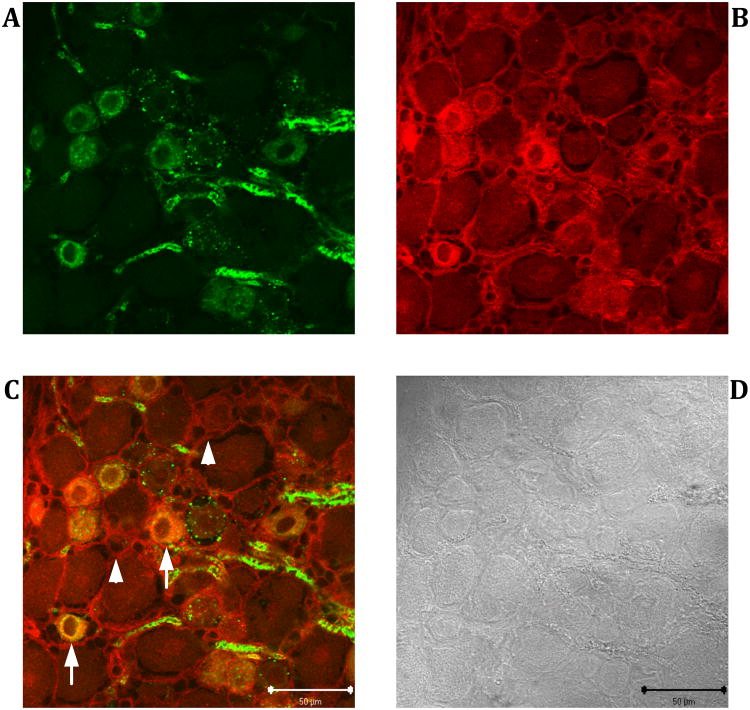
If versican is responsible for the IB4-reactivity of C-fiber nociceptors, its immunoreactivity should co-localize with the IB4-reactivity in the DRG. As shown in Figure 4, dual-labeling immunofluorescence experiments on cryosections of L4 DRGs showed that versican immunoreactivity and IB4-reactivity did indeed co-localize in small-diameter neuronal cell bodies (arrows in Fig. 4C). In addition, prominent versican immunoreactivity was observed in the extracellular matrix (arrowheads in Fig. 4C). Versican labeling was absent when cryosections were exposed only to the secondary antibody while omitting the primary anti-versican antibody (data not shown).
Figure 4. Co-localization of IB4- and anti-versican immunoreactivity in rat L4 DRG.
Dual-labeling immunofluorescence experiments were performed on 20 μm thick cryostat sections. IB4-reactivity (green) was revealed with FITC conjugated IB4 (A), anti-versican immunoreactivity (red) by probing the tissue sections with the mouse monoclonal anti-versican antibody followed by a Cy3-labeled rabbit anti-mouse antibody (B). Subcellular areas in which both immunoreactivities co-localize appear yellow in the merged image (C). Corresponding bright field image captured with differential interference contrast optics (D). Arrows: IB4-binding sensory neurons that also express versican. Arrowheads: Versican immunoreactivity in the extracellular matrix. Scale bar: 50 μm.
4) Domain structure of the IB4-binding versican
The four versican splice variants differ in the composition of their glycosaminoglycan (GAG) attachment domains. Versican V0 carries both, the alpha and beta GAG domains, V1 just the beta domain, V2 just the alpha domain and V3 neither GAG domain (Wight 2002). To determine which of the four versican splice variants carries the IB4-binding epitopes a subcellular preparation of rat spinal cord (light membranes and synaptosomes) was extracted with hyaluronidase. The extract was then analyzed by Western blotting using antibodies selective for either of the two GAG domains (Milev et al. 1998). As shown in Figure 5A, the IB4-binding versican reacted only with the anti-GAG alpha antibody suggesting that it is versican V2 that carries the IB4-binding sugar epitopes. In parallel, we repeated the co-immunoprecipitation experiment with the monoclonal anti-versican antibody (12C5) as illustrated in the scheme to Figure 1 and probed the corresponding Western blot with the anti-GAG alpha specific antibody. As shown in Figure 5B the IB4-binding versican could be detected by the GAG alpha specific antibody suggesting - once again- that it is V2 that is responsible for the IB4-reactivity of the GDNF-dependent, non-peptidergic nociceptors in the rat.
Figure 5. Versican V2 is the IB4-binding splice variant.
A subcellular fraction of rat spinal cord tissue was treated with hyaluronidase to release hyaluronan-associated proteins such as versican into the supernatant. The extract was then either directly analyzed by Western blotting using anti-GAG alpha or GAG beta specific antibodies (A) or first immunoprecipitated with a monoclonal anti-versican antibody and subsequently analyzed by Western blotting using the anti-GAG alpha selective antibody only (B). A) A hyaluronidase extracted subcellular fraction of rat spinal cord tissue only contains V2. Lane 1: Hyaluronidase extract probed with the anti-GAG alpha antibody; Lane 2: Hyaluronidase extract probed with IB4-HRP; Lane 3: Hyaluronidase extract probed with the anti-GAG beta antibody. B) The IB4-binding molecule is versican V2. Proteins derived from the same subcellular fractions as those that were used to demonstrate the enrichment of the IB4-binding molecule in Figure 1 were separated by SDS-PAGE and analyzed by Western blotting with the anti-GAG alpha specific antibody. See fractionation scheme on the right in Fig. 1 for more detailed information about the different subcellular fractions that were analyzed and the amount of protein that was loaded onto each lane. Arrow: Position of the IB4-binding molecule/anti-GAG alpha immunoreactivity on the Western blot.
Discussion
A subset of small-diameter sensory afferents, the so-called GDNF-dependent, non-peptidergic C-fiber nociceptors, express cell-surface glycoconjugates that can be selectively labeled with isolectin B4 (Streit et al. 1985; Silverman and Kruger 1990), a homotetrameric lectin with high binding affinity for terminal α-D-galactosyl residues (Hayes and Goldstein 1974). The specificity of their expression suggests that the IB4-binding glycoconjugates are critical for the biological function of these nociceptors although nothing is known regarding their functions. We have previously demonstrated that versican V2 binds IB4 and that versican V2 is one of the molecules that accounts for the IB4-reactivity of GDNF-dependent, non-peptidergic C-fibers in pig spinal cord (Bogen et al. 2005). Although versican V2 is the most abundant versican isoform in nervous tissue (Schmalfeldt et al. 1998), it is thought to be the product of glial cells in the peripheral and central nervous system (Asher et al. 2002; Melendez-Vasquez et al. 2005). However, if versican V2 is the molecule that renders a subset of sensory afferent C-fiber nociceptors IB4-positive (+), it must also be expressed by sensory neurons in the DRG. The aim of this study was therefore to: a) demonstrate the neuronal expression of versican, and given that this study was performed in the rat b) identify the versican splice variant that renders C-fiber nociceptors IB4-positive (+).
Using splice variant specific primer pairs we were able to amplify all 4 known versican isoforms within RNA extracts of rat DRG. Because the results of the RT-PCR do not prove the neuronal origin of any of the versican transcripts, we additionally performed an in situ hybridization with a riboprobe antisense to an mRNA sequence present in all versican isoforms. As shown in Figure 3, versican transcripts could be detected in all sensory neurons in the DRG. However, the presence of versican mRNAs within the somata of sensory neurons does not necessarily prove that they are being translated into protein (Wang et al. 2010; Zhao et al. 2010). To determine whether the versican transcripts are translated into protein we analyzed the versican expression by immunofluorescence. As shown in Figure 4, versican could be detected within the extracellular matrix and - as indicated by the co-localization with the IB4-reactivity - in the cytoplasm of GDNF-dependent, non-peptidergic C-fibers suggesting that versican is expressed by sensory neurons and that at least part of it is being modified with the IB4-binding epitopes.
Based on the results of our in situ hybridization (Figure 3) one would have expected to detect anti-versican immunoreactivity within the cell bodies of many more neurons. However, not every cell that transcribed a certain protein encoding gene translates the resulting mRNA necessarily and immediately into the corresponding protein (Gebauer and Hentze 2004) (Hershey et al. 2012). Furthermore, versican is an extracellular matrix molecule. It is therefore quite likely that most of the sensory neurons within the DRG export versican into the surrounding extracellular matrix as soon as it is synthesized. This assumption is supported by our immunohistochemical analysis, which demonstrates prominent anti-versican immunoreactivity within the extracellular matrix of the DRG (Figure 4). Our immunohistochemical analysis also shows that there is only a partial overlap between anti-versican immunoreactivity and IB4-binding in the DRG. This result is supported by earlier findings by others and ourselves that versican is not the only IB4-binding molecule in the DRG or spinal cord (Fullmer et al. 2004) (Bogen et al. 2005) but that IB4-binding sensory neurons are the only neuronal cells within the DRG that do express the enzymes necessary to modify proteins with the IB4-binding sugar epitopes (Fullmer et al. 2007). These results are also consistent with our previous studies showing that intrathecal injections of antisense oligonucleotides for versican mRNA could attenuate GDNF- and MCP-1 induced inflammatory hyperalgesia in the rat hindpaw, both of which are known to depend on IB4-binding, non-peptidergic C-fiber afferents (Bogen et al. 2008, 2009).
To determine whether versican is an IB4-binding molecule in the rat we fractionated spinal cord tissue according to a synaptosome preparation scheme (Gray and Whittaker 1962). Subcellular fractions with high IB4-reactivity (light membranes and synaptosomes) were treated with hyaluronidase and the supernatant immunoprecipitated with a selective anti-versican antibody. Fractions of every single step of the procedure were analyzed by Western blotting using HRP conjugated IB4. The entire procedure was based on the idea that areas in the spinal dorsal horn with high IB4-reactivity contain many synapses that involve presynaptic terminals of GDNF-dependent, non-peptidergic C-fiber nociceptors (Light et al. 1979; Streit et al. 1985; Wang et al. 2003), and that versican association with the plasma membrane of IB4-binding fibers is mediated by its binding to hyaluronan (LeBaron et al. 1992; Bogen et al. 2005). As shown in Figure 1, we could only immunoprecipitate one IB4-binding molecule from the hyaluronidase-extracted subcellular fractions of rat spinal cord. Given that we used a highly selective versican antibody for the immunoprecipitation (Asher et al. 1991; Perides et al. 1995; Westling et al. 2004), it is very likely that the IB4-binding molecule on the Western blot represents versican. The apparent molecular weight of the IB4-binding versican variant is >250kDa.
To determine which of the four different splice variants of versican is the IB4-binding molecule we analyzed proteins derived from hyaluronidase extracted light membranes and synaptosomes by Western blotting using antibodies selective for the rat GAG alpha or GAG beta domain (Milev et al. 1998). As shown in Figure 5A, only the anti-GAG alpha specific antibody reacted with the IB4-binding molecule suggesting that the V2 splice variant of versican is the IB4-binding molecule. Finally, to confirm that the IB4-binding molecule that was co-immunoprecipitated with the monoclonal anti-versican antibody from hyaluronidase extracted subcellular fractions of spinal cord tissue is V2 a Western blot analogous to the one that is illustrated in the scheme to Figure 1 was probed with the anti-GAG alpha specific antibody. As shown in Figure 5B only one versican variant could be detected. Its apparent molecular weight is >250 kDa. Given that we used an antibody that is directed against the rat GAG alpha domain it is quite likely that the versican variant on the Western blot represents V2 suggesting again that V2 is responsible for the IB4-reactivity of the GDNF-dependent, non-peptidergic nociceptors in the rat. These results are in line with our previous findings showing that versican V2 renders GDNF-dependent, non-peptidergic C-fiber nociceptors IB4-positive (+) in the pig (Bogen et al. 2005).
Within recent years it has become clear how versican affects nociceptor biology: it a) protects nociceptive terminals against oxidative stress (Morawski et al. 2004; Canas et al. 2007), b) acts as a co-receptor for molecules that impact cell phenotype such as growth factors (Bogen et al. 2008) and chemokines (Hirose et al. 2001; Bogen et al. 2009), and c) mediates H+-dependent sensitization of mechanically activated inward currents under anaerobic, ischemic and inflammatory conditions (Kubo et al. 2012). Less clear, however, is the biological function of the IB4-binding epitopes on glycosylated proteins (Knibbs et al. 1989; Holzknecht and Platt 1995; Lin et al. 1998; Fullmer et al. 2004) and lipids (Chou et al. 1989). Work by Dodd and Jessell suggests that the IB4-binding sugar moieties are vital for the guidance of axons to their termination area and the formation of cell-matrix (in the periphery) and cell-cell (in the spinal dorsal horn) contacts during development in rodents (Dodd et al. 1984; Dodd and Jessell 1985). Interestingly, their findings are supported by several recent reports demonstrating that the intrathecal administration of the selective neurotoxin IB4-saporin attenuates the mechanical hyperalgesia in rodent models of chronic inflammatory and neuropathic pain (Ye et al. 2012) (Joseph and Levine 2010). However, given that humans do not express IB4-binding epitopes (Galili et al. 1988) and that the neuronal wiring of their nociceptive circuits in the spinal cord isn't much different from the one in mice or rats, additional factors need to be involved.
Taken together, our results show - for the first time - that versican is expressed by neurons. We also show that it is the V2 isoform of rat versican that binds to IB4. We suggest that versican V2 is the splice variant that renders GDNF-dependent, non-peptidergic C-fibers IB4-positive.
Acknowledgments
This work was financially supported by the BMBF (Federal Ministry for Education and Research in Germany), the Grünenthal GmbH, and the NIH (Grants AR063312; NS085831, and NS084545).
Abbreviations
- BCIP
5-Bromo-4-chloro-3-indolyl phosphate
- BSA
Bovine serum albumin
- DRG
Dorsal root ganglia
- GAG
Glycosaminoglycan
- GDNF
Glial derived neurotrophic factor
- HRP
Horseradish peroxidase
- IB4
Isolectin B4
- NBT
Nitroblue tetrazolium chloride
- NGF
Nerve growth factor
- PAGE
Polyacrylamide gel electrophoresis
- PBS
Phosphate buffered saline
- RT-PCR
Reverse transcriptase polymerase chain reaction
- SDS
Sodium dodecyl sulfate
- TBS
Tris-buffered saline
References
- Asher R, Perides G, Vanderhaeghen JJ, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate-protein complex. J Neurosci Res. 1991;28:410–421. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22:2225–2236. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen O, Dreger M, Gillen C, Schroder W, Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. FEBS J. 2005;272:1090–1102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. Eur J Neurosci. 2008;28:12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Canas N, Valero T, Villarroya M, Montell E, Verges J, Garcia AG, Lopez MG. Chondroitin sulfate protects SH-SY5Y cells from oxidative stress by inducing heme oxygenase-1 via phosphatidylinositol 3 -kinase/ Akt. J Pharmacol Exp Ther. 2007;323:946–953. doi: 10.1124/jpet.107.123505. [DOI] [PubMed] [Google Scholar]
- Chou DK, Dodd J, Jessell TM, Costello CE, Jungalwala FB. Identification of alpha-galactose (alpha-fucose)-asialo-GM1 glycolipid expressed by subsets of rat dorsal root ganglion neurons. J Biol Chem. 1989;264:3409–3415. [PubMed] [Google Scholar]
- Cline MA, Ochoa J, Torebjork HE. Chronic hyperalgesia and skin warming caused by sensitized C nociceptors. Brain. 1989;112:621–647. doi: 10.1093/brain/112.3.621. [DOI] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J Neurosci. 1985;5:3278–3294. doi: 10.1523/JNEUROSCI.05-12-03278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J, Solter D, Jessell TM. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature. 1984;311:469–472. doi: 10.1038/311469a0. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Levine JD. Nociceptor subpopulations involved in hyperalgesic priming. Neuroscience. 2010;165:896–901. doi: 10.1016/j.neuroscience.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer JM, Riedl M, Williams FG, Sandrin M, Elde R. Enzymes that synthesize the IB4 epitope are not sufficient to impart IB4 binding in dorsal root ganglia of rat. J Comp Neurol. 2007;501:70–82. doi: 10.1002/cne.21233. [DOI] [PubMed] [Google Scholar]
- Fullmer JM, Riedl MS, Higgins L, Elde R. Identification of some lectin IB4 binding proteins in rat dorsal root ganglia. Neuroreport. 2004;15:1705–1709. doi: 10.1097/01.wnr.0000136037.54095.64. [DOI] [PubMed] [Google Scholar]
- Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha- galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Hayes CE, Goldstein IJ. An alpha-D-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem. 1974;249:1904–1914. [PubMed] [Google Scholar]
- Hershey JW, Sonenberg N, Mathews MB. Principles of translational control: an overview. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276:5228–5234. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- Holzknecht ZE, Platt JL. Identification of porcine endothelial cell membrane antigens recognized by human xenoreactive natural antibodies. J Immunol. 1995;154:4565–4575. [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169:431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibbs RN, Perini F, Goldstein IJ. Structure of the major concanavalin A reactive oligosaccharides of the extracellular matrix component laminin. Biochemistry. 1989;28:6379–6392. doi: 10.1021/bi00441a034. [DOI] [PubMed] [Google Scholar]
- Kubo A, Katanosaka K, Mizumura K. Extracellular matrix proteoglycan plays a pivotal role in sensitization by low pH of mechanosensitive currents in nociceptive sensory neurones. J Physiol. 2012;590:2995–3007. doi: 10.1113/jphysiol.2012.229153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- Light AR, Trevino DL, Perl ER. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979;186:151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Lin SS, Parker W, Everett ML, Platt JL. Differential recognition by proteins of alpha-galactosyl residues on endothelial cell surfaces. Glycobiology. 1998;8:433–443. doi: 10.1093/glycob/8.5.433. [DOI] [PubMed] [Google Scholar]
- Melendez-Vasquez C, Carey DJ, Zanazzi G, Reizes O, Maurel P, Salzer JL. Differential expression of proteoglycans at central and peripheral nodes of Ranvier. Glia. 2005;52:301–308. doi: 10.1002/glia.20245. [DOI] [PubMed] [Google Scholar]
- Milev P, Maurel P, Chiba A, Mevissen M, Popp S, Yamaguchi Y, Margolis RK, Margolis RU. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochem Biophys Res Commun. 1998;247:207–212. doi: 10.1006/bbrc.1998.8759. [DOI] [PubMed] [Google Scholar]
- Morawski M, Bruckner MK, Riederer P, Bruckner G, Arendt T. Perineuronal nets potentially protect against oxidative stress. Exp Neurol. 2004;188:309–315. doi: 10.1016/j.expneurol.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Perides G, Asher RA, Lark MW, Lane WS, Robinson RA, Bignami A. Glial hyaluronate-binding protein: a product of metalloproteinase digestion of versican? Biochem J. 1995;312:377–384. doi: 10.1042/bj3120377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt J, Balfanz S, Baumann A, Blenau W. Am5-HT7: molecular and pharmacological characterization of the first serotonin receptor of the honeybee (Apis mellifera) J Neurochem. 2006;98:1985–1998. doi: 10.1111/j.1471-4159.2006.04012.x. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt M, Dours-Zimmermann MT, Winterhalter KH, Zimmermann DR. Versican V2 is a major extracellular matrix component of the mature bovine brain. J Biol Chem. 1998;273:15758–15764. doi: 10.1074/jbc.273.25.15758. [DOI] [PubMed] [Google Scholar]
- Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol. 2014;75:196–208. doi: 10.1002/ana.24065. [DOI] [PubMed] [Google Scholar]
- Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Schulte BA, Balentine DJ, Spicer SS. Histochemical localization of galactose-containing glycoconjugates in sensory neurons and their processes in the central and peripheral nervous system of the rat. J Histochem Cytochem. 1985;33:1042–1052. doi: 10.1177/33.10.4045182. [DOI] [PubMed] [Google Scholar]
- Wang R, Guo W, Ossipov MH, Vanderah TW, Porreca F, Lai J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience. 2003;121:815–824. doi: 10.1016/s0306-4522(03)00491-3. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu G, Zhou J. Repression of versican expression by microRNA- 143. J Biol Chem. 2010;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westling J, Gottschall PE, Thompson VP, Cockburn A, Perides G, Zimmermann DR, Sandy JD. ADAMTS4 (aggrecanase-1) cleaves human brain versican V2 at Glu405-Gln406 to generate glial hyaluronate binding protein. Biochem J. 2004;377:787–795. doi: 10.1042/BJ20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864–867. doi: 10.1097/01.anes.0000264769.87038.55. [DOI] [PubMed] [Google Scholar]
- Ye Y, Dang D, Viet CT, Dolan JC, Schmidt BL. Analgesia targeting IB4-positive neurons in cancer-induced mechanical hypersensitivity. J Pain. 2012;13:524–531. doi: 10.1016/j.jpain.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Lee MC, Momin A, Cendan CM, Shepherd ST, Baker MD, Asante C, Bee L, Bethry A, Perkins JR, Nassar MA, Abrahamsen B, Dickenson A, Cobb BS, Merkenschlager M, Wood JN. Small RNAs control sodium channel expression, nociceptor excitability, and pain thresholds. J Neurosci. 2010;30:10860–10871. doi: 10.1523/JNEUROSCI.1980-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]