Abstract
Objective
Foot deformities, neuropathy, and dysfunction in the lower extremities are known risk factors that increase plantar peak pressure (PP) and, as a result, the risk of developing foot ulcers in patients with diabetes. However, knowledge about the prevalence of these factors is still limited. The aim of the present study was to describe the prevalence of risk factors observed in patients with diabetes without foot ulcers and to explore possible connections between the risk factors and high plantar pressure.
Patients and methods
Patients diagnosed with type 1 (n=27) or type 2 (n=47) diabetes (mean age 60.0±15.0 years) were included in this cross-sectional study. Assessments included the registration of foot deformities; test of gross function at the hip, knee, and ankle joints; a stratification of the risk of developing foot ulcers according to the Swedish National Diabetes Register; a walking test; and self-reported questionnaires including the SF-36 health survey. In-shoe PP was measured in seven regions of interests on the sole of the foot using F-Scan®. An exploratory analysis of the association of risk factors with PP was performed.
Results
Neuropathy was present in 28 (38%), and 39 (53%) had callosities in the heel region. Low forefoot arch was present in 57 (77%). Gait-related parameters, such as the ability to walk on the forefoot or heel, were normal in all patients. Eighty percent had normal function at the hip and ankle joints. Gait velocity was 1.2±0.2 m/s. All patients were stratified to risk group 3. Hallux valgus and hallux rigidus were associated with an increase in the PP in the medial forefoot. A higher body mass index (BMI) was found to increase the PP at metatarsal heads 4 and 5. Pes planus was associated with a decrease in PP at metatarsal head 1. Neuropathy did not have a high association with PP.
Conclusions
This study identified several potential risk factors for the onset of diabetic foot ulcers (DFU). Hallux valgus and hallux rigidus appeared to increase the PP under the medial forefoot and a high BMI appeared to increase the PP under the lateral forefoot. There is a need to construct a simple, valid, and reliable assessment routine to detect potential risk factors for the onset of DFU.
Keywords: diabetic foot, foot deformities, neuropathy, prevention, foot anthropometrics, plantar pressure, risk factors
Foot deformities, neuropathy, and high peak pressure (PP) on the foot have been identified as potential risk factors for the onset of diabetic foot ulcers (DFU) (1–4). Diabetes complications, such as DFU and amputation, have a negative impact on quality of life (QOL) (5, 6), and preventing DFU has been proven to increase QOL and to be cost-effective (7–9). The prevention of DFU is, therefore, an important research question for millions of people with diabetes in the world (10). In terms of prevention strategies, insoles and shoes are widely prescribed with the aim of protecting the feet and redistributing the plantar pressure to prevent the development of DFU (11, 12). There is, however, a need for better documentation and improved understanding with regard to the potential risk factors, such as foot deformities and dysfunction in the lower extremities, and the association of these risk factors with higher PP.
The severity and type of neuropathy play a central role in the onset of DFU. Whereas autonomous neuropathy leads to dry skin and fissures, motor neuropathy causes weakness in muscles (13). Due to the lack of sensitivity in the foot, patients’ awareness of their feet and the ability to protect them from trauma is reduced (11, 14–16). These aspects of neuropathy may also have an impact on walking mobility. Moreover, muscle hypotrophy and stiffness of the tendons in the lower extremity, leading to limited range of motion (ROM), have been shown to increase plantar pressure and thus increase the risk of ulceration (17).
At present, there is no globally accepted definition of the term ‘foot deformities’ and a variety of definitions have been used. Abbot et al. (1) defined deformity as the presence of three or more of the following findings: hallux valgus, hammer toes, bony prominences, prominent metatarsal heads, Charcot arthropathy, limited joint mobility, and small muscle wasting. Lavery et al. defined foot deformity as the presence of hallux valgus, hammer or claw toes, tailor's bunions, or hallux rigidus (18, 19). These authors also found that, with increasing numbers of foot deformities, there was a trend towards increased PP.
Some studies consider the simultaneous occurrence of neuropathy and foot deformities, as well as their combined effect on PP. For instance, in a recent study of patients with diabetes (n=243, 21% had neuropathy), 49% were reported to have hallux valgus and 39% had hammer toes (20). The corresponding proportion of hammer toes was 32% in another study (n=100 men, 34% had neuropathy) (21). Furthermore, an association between high PP and plantar tissue thickness in the forefoot has been described in patients with diabetes and neuropathy (22). At the present time, the number of these risk factors is unknown in Sweden, and knowledge in terms of the physical health status of patients with diabetes at risk of developing foot ulcers is sparse.
The primary aim of this cross-sectional study was to describe the type and frequencies of risk factors related to the development of DFU, such as foot deformities and dysfunction in the lower extremities, in patients with diabetes without foot ulcers in Sweden. The second aim was to explore the association of risk factors with PP.
Patients and methods
Patients with diabetes and at risk of developing foot ulcers were recruited from an RCT reported in a previous publication, which included an evaluation of three different types of insoles inserted in walking shoes (23). The current cross-sectional study is based on the assessment that was made at the 24-month follow-up of the original study. The original study had 114 participants. At this time, the remaining patient cohort (n=74) had used their prescribed shoes and insoles for 2 years. To meet the inclusion criteria in the original RCT, patients had to be aged 18 years or more, understand and follow given instructions, and be independent walkers. Moreover, they were all referred to the Department of Prosthetics and Orthotics being identified with at least one risk factor to develop DFU. The exclusion criterion was the presence of foot ulcers. This means that there were no patients at low risk to develop foot ulcer or patients with ongoing foot ulcers in the current study. The regional ethical review board approved the study (No 299-07), and all the patients gave their written consent prior to study entrance.
Measurements
All assessments were made at the Lundberg Laboratory for Orthopaedic Research, Gothenburg, Sweden. A certified prosthetist and orthotist assessed the feet and made a visual assessment of malalignment in the lower extremities when the patients were standing. In the sitting position, the feet were evaluated manually. Following a structured routine, foot deformities, classified as risk factors, were registered as being present or absent. They included hallux valgus, hallux rigidus, pes planus, pes cavus, low forefoot arch, and hammer toes. The height of the hammer toes was measured with a ruler. Skin callosities, heel fissures, a hypotrophic fat pad, and nail deformities were assessed and registered as present or absent. The maximum dorsiflexion angle at the ankle joint was measured with a goniometer in a standing position with the knee extended (24). Foot length and foot width were measured using a calliper (Fotmått, model Hyssna, Jerndahls Skinn & Läder; Kumla, Sweden). Foot width was measured using a line perpendicular to the projected length axis through the centre of the first metatarsal head. In five patients, the foot evaluation was not assessed as described above due to administrative problems. Those patients’ feet, therefore, had to be evaluated based on photos of the plantar surface (25). Two independent observers evaluated the photos to identify a hypotrophic fat pad; hallux valgus; heel fissure; callosities at the hallux; and metatarsal head 1 (MTH1), MTH2, MTH4, MTH5, midfoot, or heel, respectively. Only findings with total agreement between observers were classified as a foot deformity or callosity.
Finally, based on the findings, the patients were classified into risk groups 1–4 as follows: 1) diabetes without foot complications; 2) neuropathy and/or angiopathy; 3) foot deformities, severe callosities, previous amputation/foot ulcer; and 4) foot ulcer, critical ischemia, infection, and severe osteoarthropathy (Charcot foot); according to the grading system used in the Swedish National Diabetes Register (NDR) (26). To be able to compare the results with data from the NDR, a request for information from the NDR was made, regarding data from 2013 relating to the number of patients registered as having had their feet checked during the last 12 months and the stratification of those patients into risk groups 1–4.
Tests of gross motor function in the lower extremities and the presence of neuropathy were assessed by a registered physiotherapist. Four techniques for measuring distal peripheral neuropathy were used: a tuning fork C128 Hz, a 10 g monofilament, the slight touch of a pencil, and different positioning of the hallux (23). A positive result from one of these assessments was defined as a sign of peripheral neuropathy (27). Foot pulses were palpated at the arteria dorsalis pedis and the arteria tibialis posterior. The evaluation of dysfunctions in the lower extremities was based on a routine similar to that used in clinical practice. Self-reported pain at the hip and knee joints, together with the passive joint motion, assessed in a supine position, was used to classify the patients into four categories at hip and knee joints: no dysfunction, mild dysfunction, moderate dysfunction, and severe dysfunction (28). The ability to walk on toes and heels was assessed and registered as ‘yes’ if the patient was able to walk approximately five steps on his/her toes, followed by five steps on the heels, respectively. In a sitting position, the patient was asked to make an active dorsiflexion of the ankle joint and the ability was registered as yes/no. Balance was classified as normal if the patient was able to stand on one leg in a balanced stable position, with 90° hip/knee flexion of the contralateral leg, for approximately 5 s. A 5-min walking test on level ground indoors, with the patients walking at their self-chosen speed, was used to produce data for calculations of gait speed.
Self-reported assessments included duration of diabetes, type of diabetes, level of HbA1c, medication for high blood pressure and/or heart disease, nicotine use (yes/no), and a question about whether the patient perceived that he/she had the ability to walk normally (yes/no). Furthermore, self-perceived health-related QOL was assessed using the SF-36 questionnaire (29–32).
In-shoe plantar pressure was recorded with the F-Scan® 6.10 (Tekscan®, Boston, MA, USA), as previously described in detail by Hellstrand Tang et al. (23). The patients were randomised to use one of the three types of insole, 35 EVA or 55 EVA custom-made insoles (35 and 55 shore ethylene vinyl acetate insoles) or prefabricated insoles. The PP between the sole of the foot and the insole was analysed in seven regions of interest (ROI; Fig. 2). These regions are exposed to pressure during normal walking and include the heel, the midfoot, MTH5, MTH4, MTH2, MTH1, and the hallux.
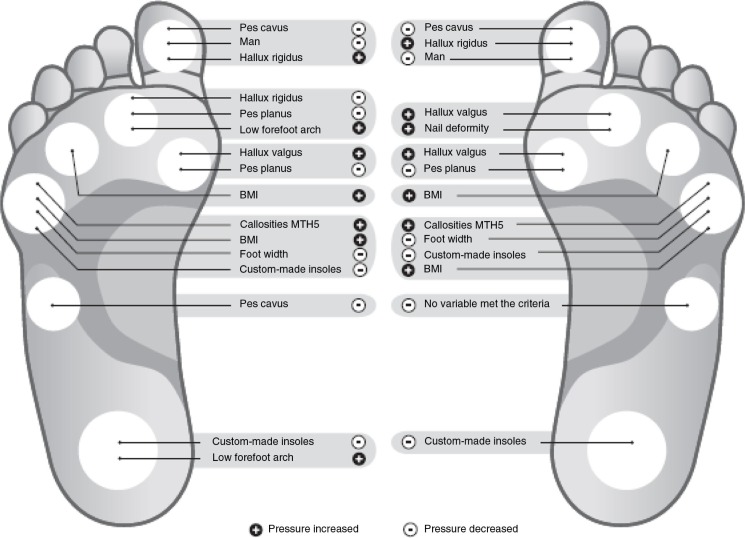
Fig. 2.
Illustration of variables associated with peak pressure (PP). The foot on the left represents the variables selected based on logarithmic PP, and the foot on the right illustrates the variables selected based on untransformed PP. The threshold value for a factor to be presented from the list was set at >0.70 (Appendix 1). In Appendices 2 and 3, the complete factor list and the direction and magnitude of their association can be found. The ranking procedure was performed for each separate region of interest (ROI). The seven ROI that were analysed were the hallux, metatarsal heads (1, 2, 4 and 5), the midfoot and the heel. The variable ‘Custom-made insoles’ is the effect this variable has on PP as compared with prefabricated insoles. The variable ‘Man’ is the effect this variable has on PP as compared with women. +, Factors that were shown to increase PP. –, Factors that were shown to decrease PP.
Statistical analysis
Descriptive results are presented as the number and/or percentage of observations for which a property was observed for discrete factors and as the mean±(SD) for continuous variables.
Several factors may have an association with PP and were included in the exploratory statistical analysis: type of diabetes; duration of the disease; age; sex; neuropathy; foot length; foot width; index foot length/foot width; body weight; height; body mass index (BMI); pes planus; pes cavus; hallux rigidus; hallux valgus; nail deformity; active dorsiflexion at the ankle joint,; low forefoot arch; hypotrophic fat pad; callosities at MTH2, MTH5, or heel; heel fissures; degree of ankle joint dorsiflexion; the ability to walk normally; type of insoles; and foot (right or left) (18, 33, 34). The patients with missing data for at least one of these factors were excluded, resulting in a data set consisting of 122 observations (61 patients, two feet). The exploratory statistical analysis was performed using a combination of a linear mixed model with random effects (35) and re-sampling. Both the untransformed PP and the logarithm of PP were considered. The approach is described in detail in Appendix 1 and the order of the 27 factors with an association with PP is listed in Appendix 2. An explanatory analysis was conducted for seven ROI on the sole of the foot. SPSS version 19, Microsoft Excel 2010, and software R were used for analysis and statistical calculations.
Results
Patient characteristics are described in Table 1 and are divided into three subgroups defined by the different types of insole they had used. The prevalence of foot deformities and data on foot anthropometrics for the 74 patients are presented in Tables 2 and 3. Of these, 55 (74%) used medication for high blood pressure or cardiac disease, 9 (12%) were cigarette smokers, and 4 (5%) used other nicotine products.
Table 1.
Characteristics of patients included in the study
| Type of insole | n | Age (years) | Women,% (n) | Diabetes type 1 (%) | Duration of diabetes (years) | BMI (kg/m2) | HbA1c (%) | Neuropathy % (n) |
|---|---|---|---|---|---|---|---|---|
| 35 EVAa | 24 | 56 (17) | 54 (13) | 42 (10) | 18 (15) | 27 (5) | 5.7 (0.7) | 42 (10) |
| 55 EVAa | 22 | 57 (15) | 41 (9) | 9 (2) | 10 (6) | 27 (4) | 5.6 (1.0) | 36 (8) |
| Prefabb | 28 | 63 (15) | 54 (15) | 29 (8) | 17 (12) | 27 (4) | 5.6 (1.0) | 36 (10) |
| Total | 74 | 60 (15) | 50 (37) | 27 (20) | 15 (12) | 27 (5) | 5.8 (0.8) | 38 (28) |
35 EVA and 55 EVA are 35 and 55 shore ethylene vinyl acetate custom-made insoles respectively
Prefab means prefabricated insoles.
Details of the characteristics of the patients in the original longitudinal RCT are found in Hellstrand Tang et al. (23).
Table 2.
Results of foot findings in patients with diabetes without foot ulcers
| Right | Left | |||
|---|---|---|---|---|
|
|
|
|||
| Variable | Number of feet, n (%) | Totala (n) | Number of feet, n (%) | Totala (n) |
| Pes planus | 25 (34) | 68 | 43 (58) | 68 |
| Pes cavus | 7 (10) | 68 | 6 (8) | 68 |
| Lower forefoot arch | 57 (77) | 68 | 62 (84) | 68 |
| Hallux valgus | 17 (23) | 73 | 28 (21) | 73 |
| Hallux rigidus/limitus | 13 (18) | 69 | 10 (13) | 69 |
| Active dorsiflexion at ankle joint | 66 (89) | 68 | 65 (88) | 68 |
| Hypotrophic fat pad | 35 (47) | 72 | 35 (47) | 72 |
| Callosities, MTH1 | 28 (38) | 74 | 32 (43) | 74 |
| Callosities, MTH2 | 18 (24) | 74 | 15 (20) | 74 |
| Callosities, MTH5 | 14 (19) | 74 | 11 (15) | 74 |
| Callosities, heel | 39 (53) | 74 | 39 (53) | 74 |
| Heel fissures | 49 (66) | 72 | 50 (68) | 72 |
| Nail deformity | 24 (32) | 67 | 27 (36) | 67 |
Total number of valid measurements.
Table 3.
Description of foot anthropometrics and maximum dorsiflexion angle at the ankle for men and women with diabetes without foot ulcers
| Foot length (mm) | Foot width (mm) | Foot index (length/width) | Maximum dorsiflexion anglea (degree) | |||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Gender (n) | Right | Left | Right | Left | Right | Left | Right | Left |
| ♀ (37) | 247 (12) | 248 (12) | 97 (6) | 97 (6) | 2.56 (0.15) | 2.56 (0.17) | 28 (7) | 27 (8) |
| ♂ (37) | 265 (15) | 267 (15) | 102 (5) | 104 (6) | 2.59 (0.14) | 2.57 (0.13) | 27 (7) | 26 (7) |
| Total (74) | 257 (17) | 258 (16) | 100 (6) | 101 (7) | 2.57 (0.14) | 2.56 (0.15) | 27 (7) | 26 (7) |
Values shown for continuous variables are the mean±(SD). Numbers (n) of patients.
Missing value, n=2.
Of the total number of 74 patients, a low forefoot arch was found in 57 (77%) of the right feet and 62 (84%) of the left feet, respectively. Pes planus was present in 25 (34%) of the right feet and 43 (58%) of the left feet, respectively. Callosities of the heel were present in 39 (53%) of each of the feet (Table 2). Forty-three patients had hammer toes, and the maximum toe height for the right and left foot was 28±4 and 30±5 mm for men and 25±7 mm for women, the same for both feet. Four patients had missing measurements and were excluded when the stratification into risk groups was made. All the remaining patients (n=70) were classified in risk group 3. All patients were able to walk on their forefoot and heel, respectively. In the self-reported assessment, 55 (74%) answered that they had the ability to walk normally. Sixty-two patients (84%) were able to stand on one leg and 12 had some dysfunction (n=10 mild dysfunction, n=1 moderate dysfunction, n=1 severe dysfunction). Gait velocity during 5 min of walking was 1.2±0.2 m/s (0.5–1.9 m/s).
Fifty-seven (77%) had normal function in both the hip and knee joints. Normal function in the right hip and knee joints was found in 64 and 68 (86 and 92%) patients, respectively. The corresponding numbers for the left hip and knee joints were 70 and 68 (95 and 92%). None had severe dysfunction. Mild and moderate dysfunction in the right hip and knee joint was found in 10 (14%) and 6 (8%) of the patients, respectively, whereas the corresponding numbers for the left side were four (5%) and six (8%).
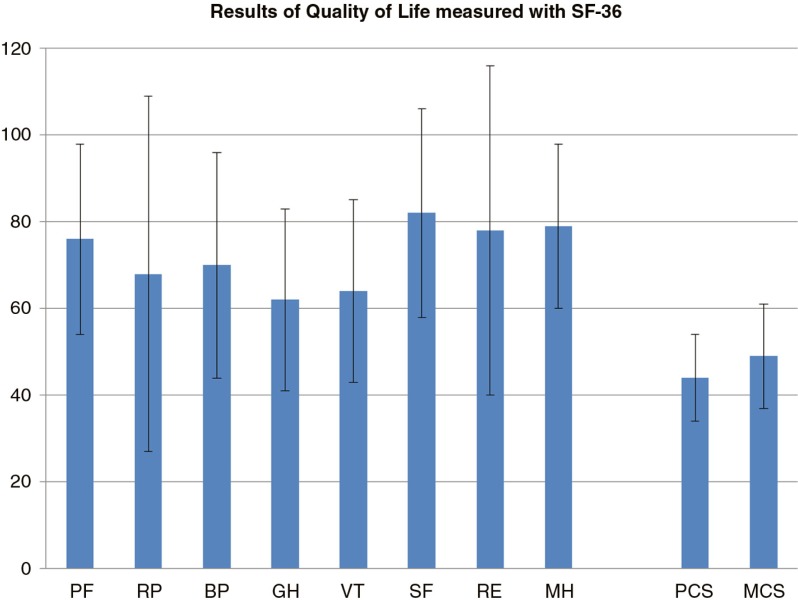
In Fig. 1, the results of the SF-36 are reported.
Fig. 1.
Results of the SF-36 with bars (mean±SD) showing the eight domains of the SF-36 Version 1 Scale Scores for the 74 patients. PF, physical functioning; RP, role physical; BP, bodily pain; GP, general health; VT, vitality; SF, social role functioning; RE, emotional role functioning; MH, mental health. The two summary scores are presented on the right: the Physical Component Score (PCS) and the Mental Component Score (MCS). These results are normalised against a Swedish population (n=8,000) with a mean of 50 SD 10.
In the explorative part of the study, all variables that remained in the models were explored (Fig. 2 and Appendices 2 and 3). The exploration was based both on logarithmic PP and on untransformed PP after the model selection was performed (Fig. 2 and Appendices 2 and 3). Hallux rigidus and hallux valgus increased the PP under the hallux and the MTH1. The variables ‘man’, pes planus, and pes cavus were associated with a decreasing PP at these ROI. Furthermore, BMI was associated with an increase in the PP at MTH4 and MTH5. Custom-made insoles reduced the PP under MTH5 and the heel. Neuropathy was not a factor, which had a high association with PP.
Discussion
To our knowledge, this is the first study that describes foot pathologies in patients with diabetes with high risk to develop DFU, but without foot ulcers, visiting a Department of Prosthetics and Orthotics. The most important finding is the high prevalence of foot deformities and callosities in the group that was studied. These findings are of importance, as foot deformities have been shown to increase plantar pressure and thus probably increase the risk of developing plantar foot ulcers (16, 19, 36). The high prevalence of plantar callosities, hypotrophic fat pads, and low forefoot arches also clearly shows that this group of patients is in need of protective footwear as an essential part of DFU preventive care. The group is representative of patients with diabetes in Sweden according to age, sex, duration, and proportion of type 1 diabetes as compared with figures from the NDR (26).
Compared with previous publications, the present study shows some differences. The prevalence of plantar callosities in the heel region is higher (53 vs. 38%) and the prevalence of hallux valgus is lower (<25 vs. 49%) than in the study presented by Formosa et al. (20). In a study by Guiotto et al. (three groups, 40 feet in each group), the prevalence of plantar callosities and hallux valgus was higher in patients with diabetes. They compared a control group with groups of patients with diabetes (with and without neuropathy). They found that the prevalence of callosities for those with and without neuropathy was 19 versus 21, whereas the corresponding numbers for hallux valgus were 18 and 13. In the control group, the authors found five callosities and 11 hallux valgus (37). The reasons for these differences might be different study populations, and the fact that the evaluation of callosities and hallux valgus varies between clinicians.
In the present study, patients with foot ulcers were excluded and no patient was therefore classified in risk group 4 according to the definition of the NDR (26). The proportion of patients being registered to risk group 4 in the 2013 annual report from the NDR was 1% (n=3,373). This proportion is low when compared with other studies reporting a prevalence of foot ulcers between 3 and 8% (38, 39). The NDR showed that 76% (n=210,571) of the patients that had being ‘foot-checked during the last year’ were classified in risk group 1. In the current study, none of the participants were classified in risk group 1 due to the inclusion criteria. We conclude that the risk group distribution reported in the report from the NDR does not properly reflect the results from other publications regarding the prevalence of foot deformities (20, 21, 37) and neuropathy (30–50%) (11, 38, 40). It appears that the result from the NDR gives a false positive picture of the foot status exemplified by the large proportion of patients with no presence of neuropathy and/or foot deformities (76% of the total amount being registered). Furthermore, the proportion of patients in the NDR being registered as ‘foot-checked’ was only 79% (n=274,834/n=351,177). In the current study, all participants were classified in risk group 3. The corresponding data from the NDR were 2% (n=5,349) in risk group 3 and 21% (n=56,947) in risk group 2. This trend in the NDR report towards a classification into the lower risk groups is supported by results from Leese et al. (41). They showed that 63% had low risk to develop DFU and 24% had a moderate risk (comparable with risk group 2).
Relevant to the interpretation of our results is that only one of following risk factors had to be present in the foot assessment for a foot to be classified as ‘high risk to develop DFU’, corresponding to risk group 3 (26): presence of foot deformities, skin pathologies, earlier ulcers/amputation regardless signs of neuropathy, angiopathy. However, Leese et al. defined a foot to be at high risk with a different set of the risk factors: (1) previous ulceration or amputation; (2) absent pulses and unable to feel the 10 g monofilament; or (3) ‘(1)’ or ‘(2)’ with callus or foot deformity. An issue of interest is to get more evidence of the criteria for a foot to be at high risk.
Furthermore, we put into question whether health care professionals have the proper tools to accurately assess the risk group and the knowledge to report the true risk group classification to the NDR. These uncertainties need to be investigated and if weakness is found in the current routine, we suggest that a standardised routine should be implemented for foot assessment and risk classification (42–44). Good experience has been reported from Scotland and studies showed that using a web-based assessment tool is preferable (45, 46) and gives the decision-makers the option to monitor and evaluate long-term incidence of foot ulcers and amputation. The overall goal in the management of patients with diabetes at risk to develop foot ulcers is to offer these patients a prevention programme including adequate footwear, podiatry, education, and information and, finally, in the presence of acute foot ulcers, treatment by a multidisciplinary team (11, 12, 47).
The walking ability in the current study was expected to be good, as one of the inclusion criteria was ‘to be independent walkers’. Nevertheless, a large inter-individual variation in gait speed was shown in the walking test, where the gait velocity varied from a minimum of 0.5 m/s to a maximum of 1.9 m/s. One explanation for the variation in speed might be the tendency to walk slower with higher age as earlier reported (48, 49). Another explanation is the impact that the disease has on gait, which has been described in a systematic review (50), showing that patients with diabetes walked slower than a group of controls, with a range from 0.7 to 1.24 m/s versus 0.9 to 1.47 m/s.
All patients were able to walk on the forefoot and on the heels, but only 74% of the patients reported that they had the ability to walk normally. The gait-related parameters, such as the function of the hip and knee joints, were classified as normal in more than 77% of the patients. These results are reflected in the results from the domain of physical functioning (PF) in the SF-36, showing good self-experienced physical function (mean 76±22); however, the variation (±22) reflects that some individuals experienced low PF (Fig. 1). Moreover, the SF-36 showed that the patients generally had a normal level of health in the eight domains, but once again we found a large individual variation. This variation can be explained by the fact that the group comprised patients with a wide range of physical and mental abilities at different ages (22–85 years old). Compared with a general Swedish population, the Physical Component Score (44±10) in the SF-36 was lower than the mean value of 50, which is in line with studies from the literature (51). However, the Mental Component Score was close to the value for the general population (49±12 vs. 50±10), indicating good self-experienced mental health in general, which can be reasonably explained by the absence of DFU. Patients with DFU have been shown to experience poorer QOL (5, 7).
The second aim of this study was to explore the association of different factors with PP. This was done for seven ROI. In the medial aspect of the forefoot, the deformities of hallux valgus and hallux rigidus were associated with an increase in PP. These deformities have previously been found to produce increased plantar pressure (36). Another factor that increased PP was a higher BMI, which is intuitive and relates to the fact that the numerator in the equation for pressure is force (pressure=force/area) and a high BMI is strongly related to force. The same relationship between BMI and PP has previously been described by Ahroni et al. (36). Pes planus produced decreased pressure at MTH1 and MTH2 (MTH2 based only on the logarithmic PP), which can be explained by the fact that the total area loaded on the sole of the foot increases in the presence of pes planus. It should be observed that the relationship between risk factors and PP may not always be causal. It is reasonable to state that the presence of some factors, such as callosities, is caused by high PP and not vice versa. The two regions, MTH2 and midfoot, produced different results for untransformed and logarithmic PP. There may be several reasons for this, such as the fact that no factor among the ones tested is clearly superior to others with regard to predicting PP for these particular ROI. Another explanation is that logged and unlogged PP captures different aspects of pressure distribution. The logarithmic transformation has the effect of downplaying the importance of the very high PP values, while, at the same time, leading to better model fit and more reliable results. This means that the difference in the models may be due to the fact that a different set of factors is chosen in non-logged data to capture the high PP, the influence of which is not as prominent in the logged data.
Surprisingly, neuropathy did not emerge as an important factor with an association with PP in any of the seven ROI. The same finding was reported in the original study comparing PP for the three different types of insole (23). One explanation might be that the severity of neuropathy was mild in the group of patients studied and, consequently, one limitation is that the simple clinical tests that were used did not assess the degree of neuropathy accurately. Neuropathy is elsewhere stated to cause areas of high pressure due to the development of foot deformities (11, 14, 15), and Lavery et al. (18) have shown that higher PP was found in patients with severe neuropathy.
The analysis in the present study also showed that custom-made insoles reduced the pressure in the heel region. In the original study, the difference between insoles was found to be statistically significant, showing that the 35 EVA custom-made insoles produced lower PP than prefabricated insoles (171±13 vs. 234±10 kPa) and the 55 EVA custom-made insoles had 161±13 kPa (23). For the other six ROI analysed in that study, no statistically significant difference could be detected.
Limitations
Although the patients included in this study are representative for the general population of patients in Sweden with diabetes according to age, sex, and duration and proportion of type 1 diabetes, there is a risk for bias depending on the fact that they initially were identified to have raised risk to develop foot ulcers and were accordingly referred to the Department of Prosthetics and Orthotics. An optimal study design would have been to include control groups of patients with diabetes without loss of protective sensation and without deformities and also a group consisting of participants representing the general population. In the current study, no patients with foot ulcers were represented because those developing foot ulcer discontinued the study and left the intervention group they original were assigned to. This procedure was based on ethical considerations as they needed to receive specialist treatment appropriate for ulcer healing.
Further limitation of the current study is the lack of a globally standardised protocol that could be used for foot assessments in patients with diabetes. For this reason, we introduced a protocol based on regional guidelines (47) and clinical experience, yet not tested for validity or reliability. We suggest that there is a need for valid and reliable methods in the examination of risk factors such as foot deformities, limited ROM, and callosity formation. Using this kind of protocol, which quantifies the structural features of the foot, would improve the quality of future studies. The number of patients in the study was based on the calculation of sample size in the original study (n=114) (23). During the 2-year study period, patients dropped out and the remaining 74 patients could be included in the current study. However, for a confirmatory analysis of the results, a control group should be included and, furthermore, a sample size calculation is recommended prior to study start.
Finally, in the explorative analyses made, the Akaike information criteria were used in model selection, and it is possible that the use of other criteria (e.g. Bayesian information criteria) could have led to somewhat different results. All measurements of PP were taken as the patients walked with their dedicated footwear including the insoles and no reference value of PP was taken when walking barefoot. Without a barefoot measurement, the effect of footwear on PP is unknown and also the interaction of footwear and foot structure.
Conclusion
This study demonstrates that patients with diabetes without foot ulcers have several potential risk factors for the onset of DFU and are in need of preventive strategies. More than 50% had one or more of the following risk factors: low forefoot arch, callosities at the heel, heel fissures, and pes planus. Hallux valgus and hallux rigidus appeared to increase the PP under the medial forefoot and a high BMI appeared to increase the PP under the lateral forefoot.
Based on the findings in this study and the comparison with figures from the NDR, it is reasonable to assume that some risk factors are not detected and registered in the NDR. In a future strategy to prevent DFU and to give equal health care, a simple, valid, and reliable assessment routine should be used for the annual foot check and risk stratification.
Acknowledgements
Ulla Hellstrand Tang (U.H.T) is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. UHT designed the study, researched the data, contributed to discussions, and wrote the manuscript. RZ and RT designed the study, researched the data, contributed to discussions, and reviewed and edited the manuscript. VL made statistical analyses, contributed to discussions, and wrote the manuscript. KH and JK contributed to discussions and reviewed and edited the manuscript. Finally, we thank all the patients for their contribution to the study, as well as all the co-workers at the Department of Prosthetics & Orthotics, Sahlgrenska University Hospital, Gothenburg, Sweden. The illustration was created by Pontus Andersson. Finally, we thank the Swedish National Diabetes Register for the contribution of data on foot assessments.
Appendix 1: Exploratory statistical analysis
The statistical analysis we performed was of an exploratory nature. We conducted no formal hypothesis testing and do not claim to prove that BMI, for example, has an impact on PP. Instead, we have studied these statements to see whether they are reflected in our data, with the caveat that, to make claims of ‘statistical significance’, an additional analysis should be performed using a different data set.
We regard each foot as a separate observation rather than each patient. Not all the variables described earlier were used for the analysis, due to the large number of missing values for some of them. The exact factors are listed in the ‘statistical section’ and in Appendix 2. The observations that had missing values for at least one of these factors were removed, which led to a data set consisting of 122 feet and 27 possible factors with an influence on PP. Our objective was to find which of the possible factors (explanatory variables) have the most effect on PP. To accomplish this, we considered different models that connected PP for the ROI to some, or all, of the factors and attempted to determine which of these models has/have the greatest support in our data.
Due to the structure of the data, the observations have a natural grouping, namely the right and the left feet that belong to the same patient. It is reasonable to assume that the two measurements in each such group would exhibit some sort of dependence. A statistical modelling approach that ignores this possible dependence, such as simple linear regression, is therefore not appropriate. Instead, we made use of mixed models, where the pressure measurements for feet belonging to the same person were assumed to be correlated. The only random effect in these models was the intercept and this parameter corresponded to the pressure associated with prefabricated insoles, while all the other factors were set at 0. This allowed the two grouped feet simultaneously to have either higher or lower pressure.
To determine which of the factors appears to have an effect on plantar pressure, fairly complex model selection was performed. During this process, subsamples were drawn from the data. For each subsample, a classical stepwise model selection was performed, using Akaike information criteria (AIC). This process was iterated 1,000 times on both untransformed PP and the logarithm of the PP. A note was then made of the factors remaining after the AIC selection, which resulted in a table describing the proportion of the re-sampled data sets for which a particular variable was chosen (Appendix 2). This was done to make the results more reliable, as we can see which of the variables persistently remain/s in the models even when the data set is perturbed.
Appendix 2: Factors associated with peak pressure
Factors associated with peak pressure (PP) expressed as the logarithm of PP and untransformed PP. The factors with a high association are listed first and those with a low association are listed at the end. This is done for each of the seven regions of interest (ROI). The ROI analysed here were the hallux, metatarsal heads (1, 2, 4, and 5), the midfoot, and the heel. The variable ‘Foot’ is the effect the left foot has on PP as compared with the right foot. The variable ‘Type of insoles 35 and 55 EVA’ is the effect custom-made insoles have on the PP as compared with prefabricated insoles. The variable ‘Gender’ is the effect the group ‘men’ has on the PP as compared with the group ‘women’.
| Results based on logarithmic PP | Results based on untransformed PP | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Region of interest | Order of priority | Factors with a potential association with PP | Value | Factors with a potential association with PP | Value |
| Hallux | |||||
| 1 | Pes cavus | 0.97 | Pes cavus | 0.89 | |
| 2 | Gender | 0.83 | Hallux rigidus | 0.77 | |
| 3 | Hallux rigidus | 0.79 | Gender | 0.74 | |
| 4 | Heel fissures | 0.55 | Heel fissures | 0.45 | |
| 5 | Foot | 0.43 | Active dorsiflexion at ankle foot joint | 0.41 | |
| 6 | Neuropathy | 0.41 | Foot | 0.35 | |
| 7 | Active dorsiflexion at ankle foot joint | 0.36 | Neuropathy | 0.33 | |
| 8 | Ability to walk normally | 0.30 | Hallux valgus | 0.30 | |
| 9 | Hallux valgus | 0.29 | Low forefoot arch | 0.27 | |
| 10 | BMI | 0.28 | Hypotrophic fat pad | 0.26 | |
| 11 | Nail deformity | 0.23 | Nail deformity | 0.22 | |
| 12 | Low forefoot arch | 0.20 | BMI | 0.17 | |
| 13 | Hypotrophic fat pad | 0.18 | Ability to walk normally | 0.16 | |
| 14 | Type of insoles | 0.09 | Type of insoles | 0.06 | |
| 15 | Callosities at heel | 0.08 | Weight | 0.06 | |
| 16 | Duration | 0.06 | Index foot length/foot width | 0.05 | |
| 17 | Type of diabetes | 0.05 | Foot width | 0.05 | |
| 18 | Callosities at MTH2 | 0.05 | Callosities at MTH5 | 0.05 | |
| 19 | Callosities at MTH5 | 0.05 | Callosities at MTH2 | 0.05 | |
| 20 | Index foot length/foot width | 0.05 | Type of diabetes | 0.04 | |
| 21 | Weight | 0.04 | Duration | 0.02 | |
| 22 | Degree of dorsiflexion at ankle joint | 0.04 | Degree of dorsiflexion at ankle joint | 0.02 | |
| 23 | Foot width | 0.04 | Callosities at heel | 0.02 | |
| 24 | Height | 0.02 | Age | 0.02 | |
| 25 | Age | 0.02 | Pes planus | 0.01 | |
| 26 | Foot length | 0.02 | Foot length | 0.00 | |
| 27 | Pes planus | 0.01 | Height | 0.00 | |
| Metatarsal head 1 | |||||
| 1 | Hallux valgus | 0.95 | Hallux valgus | 0.92 | |
| 2 | Pes planus | 0.85 | Pes planus | 0.75 | |
| 3 | Low forefoot arch | 0.58 | Nail deformity | 0.61 | |
| 4 | Hallux rigidus | 0.53 | Pes cavus | 0.57 | |
| 5 | Degree of dorsiflexion at ankle joint | 0.34 | Low forefoot arch | 0.49 | |
| 6 | Nail deformity | 0.34 | Degree of dorsiflexion at ankle joint | 0.45 | |
| 7 | Foot length | 0.31 | Hallux rigidus | 0.38 | |
| 8 | Type of insoles | 0.31 | Type of insoles | 0.33 | |
| 9 | Callosities at heel | 0.26 | Weight | 0.24 | |
| 10 | Index foot length/foot width | 0.25 | Duration | 0.23 | |
| 11 | Pes cavus | 0.21 | Foot length | 0.21 | |
| 12 | Duration | 0.20 | BMI | 0.20 | |
| 13 | BMI | 0.18 | Callosities at MTH2 | 0.17 | |
| 14 | Neuropathy | 0.18 | Age | 0.13 | |
| 15 | Callosities at MTH2 | 0.18 | Callosities at heel | 0.11 | |
| 16 | Age | 0.15 | Hypotrophic fat pad | 0.10 | |
| 17 | Ability to walk normally | 0.14 | Active dorsiflexion at ankle foot joint | 0.10 | |
| 18 | Weight | 0.12 | Index foot length/foot width | 0.09 | |
| 19 | Height | 0.08 | Callosities at MTH5 | 0.08 | |
| 20 | Active dorsiflexion at ankle foot joint | 0.07 | Neuropathy | 0.05 | |
| 21 | Gender | 0.06 | Foot | 0.03 | |
| 22 | Type of diabetes | 0.05 | Type of diabetes | 0.03 | |
| 23 | Foot width | 0.04 | Heel fissures | 0.03 | |
| 24 | Callosities at MTH5 | 0.03 | Height | 0.02 | |
| 25 | Hypotrophic fat pad | 0.03 | Gender | 0.02 | |
| 26 | Foot | 0.03 | Ability to walk normally | 0.02 | |
| 27 | Heel fissures | 0.01 | Foot width | 0.02 | |
| Metatarsal head 2 | |||||
| 1 | Hallux rigidus | 0.79 | Hallux valgus | 0.86 | |
| 2 | Pes planus | 0.76 | Nail deformity | 0.80 | |
| 3 | Low forefoot arch | 0.70 | Hallux rigidus | 0.47 | |
| 4 | Nail deformity | 0.63 | Pes planus | 0.40 | |
| 5 | Hallux valgus | 0.58 | Foot | 0.40 | |
| 6 | BMI | 0.42 | Heel fissures | 0.32 | |
| 7 | Foot | 0.36 | Low forefoot arch | 0.21 | |
| 8 | Heel fissures | 0.35 | Gender | 0.21 | |
| 9 | Hypotrophic fat pad | 0.29 | Hypotrophic fat pad | 0.18 | |
| 10 | Gender | 0.27 | Foot width | 0.15 | |
| 11 | Callosities at heel | 0.19 | BMI | 0.14 | |
| 12 | Weight | 0.18 | Index foot length/foot width | 0.10 | |
| 13 | Foot width | 0.18 | Duration | 0.10 | |
| 14 | Duration | 0.17 | Callosities at heel | 0.09 | |
| 15 | Index foot length/foot width (??) | 0.15 | Neuropathy | 0.08 | |
| 16 | Ability to walk normally | 0.09 | Type of insoles | 0.06 | |
| 17 | Type of insoles | 0.05 | Ability to walk normally | 0.06 | |
| 18 | Neuropathy | 0.05 | Weight | 0.05 | |
| 19 | Type of diabetes | 0.05 | Pes cavus | 0.04 | |
| 20 | Pes cavus | 0.03 | Age | 0.03 | |
| 21 | Callosities at MTH2 | 0.03 | Degree of dorsiflexion at ankle joint | 0.02 | |
| 22 | Age | 0.03 | Active dorsiflexion at ankle foot joint | 0.02 | |
| 23 | Callosities at MTH5 | 0.03 | Type of diabetes | 0.02 | |
| 24 | Active dorsiflexion at ankle foot joint | 0.02 | Callosities at MTH2 | 0.02 | |
| 25 | Height | 0.02 | Height | 0.01 | |
| 26 | Foot length | 0.02 | Foot length | 0.01 | |
| 27 | Degree of dorsiflexion at ankle joint | 0.02 | Callosities at MTH5 | 0.00 | |
| Metatarsal head 4 | |||||
| 1 | BMI | 0.82 | BMI | 0.88 | |
| 2 | Pes planus | 0.68 | Type of insoles | 0.59 | |
| 3 | Foot length | 0.55 | Callosities at MTH5 | 0.39 | |
| 4 | Type of insoles | 0.49 | Foot width | 0.38 | |
| 5 | Callosities at MTH5 | 0.44 | Weight | 0.32 | |
| 6 | Foot width | 0.31 | Foot length | 0.32 | |
| 7 | Age | 0.24 | Degree of dorsiflexion at ankle joint | 0.31 | |
| 8 | Type of diabetes | 0.20 | Age | 0.30 | |
| 9 | Low forefoot arch | 0.18 | Hallux rigidus | 0.24 | |
| 10 | Weight | 0.16 | Height | 0.22 | |
| 11 | Hallux rigidus | 0.15 | Pes planus | 0.21 | |
| 12 | Hypotrophic fat pad | 0.30 | Hypotrophic fat pad | 0.19 | |
| 13 | Gender | 0.12 | Pes cavus | 0.18 | |
| 14 | Nail deformity | 0.11 | Duration | 0.15 | |
| 15 | Neuropathy | 0.11 | Neuropathy | 0.14 | |
| 16 | Callosities at heel | 0.10 | Nail deformity | 0.14 | |
| 17 | Duration | 0.09 | Type of diabetes | 0.12 | |
| 18 | Pes cavus | 0.06 | Hallux valgus | 0.11 | |
| 19 | Degree of dorsiflexion at ankle joint | 0.04 | Low forefoot arch | 0.07 | |
| 20 | Height | 0.04 | Ability to walk normally | 0.06 | |
| 21 | Index foot length/foot width | 0.03 | Gender | 0.06 | |
| 22 | Ability to walk normally | 0.03 | Callosities at MTH2 | 0.05 | |
| 23 | Callosities at MTH2 | 0.02 | Index foot length/foot width | 0.04 | |
| 24 | Hallux valgus | 0.02 | Foot | 0.03 | |
| 25 | Heel fissures | 0.02 | Callosities at heel | 0.02 | |
| 26 | Foot | 0.01 | Heel fissures | 0.02 | |
| 27 | Active dorsiflexion at ankle foot joint | 0.01 | Active dorsiflexion at ankle foot joint | 0.01 | |
| Metatarsal head 5 | |||||
| 1 | Callosities at MTH5 | 0.89 | Callosities at MTH5 | 0.96 | |
| 2 | BMI | 0.85 | Foot width | 0.87 | |
| 3 | Foot width | 0.82 | Type of insoles | 0.85 | |
| 4 | Type of insoles | 0.82 | BMI | 0.77 | |
| 5 | Nail deformity | 0.39 | Nail deformity | 0.59 | |
| 6 | Neuropathy | 0.38 | Neuropathy | 0.36 | |
| 7 | Pes planus | 0.21 | Weight | 0.23 | |
| 8 | Hallux valgus | 0.21 | Hallux valgus | 0.16 | |
| 9 | Weight | 0.19 | Degree of dorsiflexion at ankle joint | 0.16 | |
| 10 | Heel fissures | 0.18 | Foot length | 0.12 | |
| 11 | Pes cavus | 0.18 | Pes planus | 0.12 | |
| 12 | Age | 0.15 | Heel fissures | 0.07 | |
| 13 | Callosities at heel | 0.13 | Age | 0.05 | |
| 14 | Height | 0.09 | Index foot length/foot width | 0.05 | |
| 15 | Foot length | 0.09 | Hypotrophic fat pad | 0.04 | |
| 16 | Ability to walk normally | 0.09 | Callosities at heel | 0.03 | |
| 17 | Duration | 0.08 | Low forefoot arch | 0.03 | |
| 18 | Index foot length/foot width | 0.07 | Gender | 0.02 | |
| 19 | Hypotrophic fat pad | 0.06 | Pes cavus | 0.02 | |
| 20 | Degree of dorsiflexion at ankle joint | 0.06 | Callosities at MTH2 | 0.02 | |
| 21 | Type of diabetes | 0.06 | Ability to walk normally | 0.02 | |
| 22 | Low forefoot arch | 0.04 | Height | 0.01 | |
| 23 | Gender | 0.03 | Foot | 0.02 | |
| 24 | Active dorsiflexion at ankle foot joint | 0.03 | Duration | 0.01 | |
| 25 | Callosities at MTH2 | 0.03 | Type of diabetes | 0.01 | |
| 26 | Hallux rigidus | 0.02 | Hallux rigidus | 0.01 | |
| 27 | Foot | 0.01 | Active dorsiflexion at ankle foot joint | 0.00 | |
| Midfoot | |||||
| 1 | Pes cavus | 0.88 | Active dorsiflexion at ankle foot joint | 0.59 | |
| 2 | Callosities at heel | 0.63 | BMI | 0.57 | |
| 3 | BMI | 0.62 | Hallux valgus | 0.54 | |
| 4 | Active dorsiflexion at ankle foot joint | 0.62 | Weight | 0.45 | |
| 5 | Ability to walk normally | 0.53 | Gender | 0.40 | |
| 6 | Weight | 0.41 | Hypotrophic fatpad | 0.36 | |
| 7 | Hallux valgus | 0.39 | Callosities at MTH5 | 0.34 | |
| 8 | Gender | 0.38 | Foot | 0.33 | |
| 9 | Height | 0.27 | Callosities at heel | 0.29 | |
| 10 | Type of insoles | 0.27 | Ability to walk normally | 0.28 | |
| 11 | Type of diabetes | 0.26 | Type of insoles | 0.26 | |
| 12 | Callosities at MTH5 | 0.25 | Pes cavus | 0.24 | |
| 13 | Foot length | 0.15 | Hallux rigidus | 0.24 | |
| 14 | Hallux rigidus | 0.11 | Nail deformity | 0.23 | |
| 15 | Neuropathy | 0.11 | Degree of dorsiflexion at ankle joint | 0.13 | |
| 16 | Hypotrophic fat pad | 0.10 | Duration | 0.11 | |
| 17 | Nail deformity | 0.07 | Height | 0.09 | |
| 18 | Heel fissures | 0.05 | Foot length | 0.09 | |
| 19 | Age | 0.04 | Type of diabetes | 0.05 | |
| 20 | Index foot length/foot width | 0.04 | Foot width | 0.05 | |
| 21 | Callosities at MTH2 | 0.03 | Heel fissures | 0.05 | |
| 22 | Low forefoot arch | 0.03 | Age | 0.05 | |
| 23 | Foot width | 0.03 | Pes planus | 0.05 | |
| 24 | Duration | 0.03 | Callosities at MTH2 | 0.05 | |
| 25 | Degree of dorsiflexion at ankle joint | 0.02 | Neuropathy | 0.03 | |
| 26 | Pes planus | 0.01 | Index foot length/foot width | 0.02 | |
| 27 | Foot | 0.01 | Low forefoot arch | 0.02 | |
| Heel | |||||
| 1 | Type of insoles | 1.00 | Type of insoles | 1.00 | |
| 2 | Low forefoot arch | 0.71 | Low forefoot arch | 0.53 | |
| 3 | Hypotrophic fat pad | 0.34 | Callosities at MTH5 | 0.39 | |
| 4 | Callosities at MTH2 | 0.30 | Hypotrophic fat pad | 0.28 | |
| 5 | Callosities at MTH5 | 0.30 | Pes cavus | 0.27 | |
| 6 | Pes cavus | 0.23 | Callosities at MTH2 | 0.23 | |
| 7 | Pes planus | 0.19 | Foot length | 0.15 | |
| 8 | Gender | 0.14 | Gender | 0.12 | |
| 9 | Age | 0.13 | Pes planus | 0.09 | |
| 10 | Foot length | 0.11 | Duration | 0.08 | |
| 11 | Hallux rigidus | 0.08 | Active dorsiflexion at ankle foot joint | 0.08 | |
| 12 | Index foot length/foot width | 0.08 | Age | 0.07 | |
| 13 | BMI | 0.05 | Index foot length/foot width | 0.07 | |
| 14 | Height | 0.05 | Neuropathy | 0.04 | |
| 15 | Type of diabetes | 0.03 | Foot width | 0.03 | |
| 16 | Foot width | 0.03 | Hallux valgus | 0.03 | |
| 17 | Nail deformity | 0.03 | Nail deformity | 0.03 | |
| 18 | Hallux valgus | 0.03 | Hallux rigidus | 0.03 | |
| 19 | Weight | 0.03 | Height | 0.02 | |
| 20 | Duration | 0.02 | BMI | 0.02 | |
| 21 | Ability to walk normally | 0.02 | Heel fissures | 0.02 | |
| 22 | Neuropathy | 0.02 | Callosities at heel | 0.02 | |
| 23 | Callosities at heel | 0.02 | Weight | 0.02 | |
| 24 | Heel fissures | 0.02 | Degree of dorsiflexion at ankle joint | 0.02 | |
| 25 | Active dorsiflexion at ankle foot joint | 0.02 | Ability to walk normally | 0.01 | |
| 26 | Degree of dorsiflexion at ankle joint | 0.02 | Type of diabetes | 0.01 | |
| 27 | Foot | 0.01 | Foot | 0.01 |
Appendix 3: Magnitude and directions of factors associated with peak pressure
The table shows the magnitude and the direction of factors that have an association with the peak pressure (PP) in seven regions of interest. The threshold value for a factor to be presented in this table is set at >0.70 and drawn from the list in Appendix 2 and is illustrated in Fig. 2. The effect is presented as the logarithm of peak pressure and untransformed PP. The variable ‘Type of insoles 35 and 55 EVA’ means the magnitude and direction as compared with prefabricated insoles for these two different custom-made insoles. The variable ‘Gender’ means men as compared with women. The intercept corresponds to the pressure associated with the PP for prefabricated insoles.
| Results based on logarithmic PP | Results based on untransformed PP | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Factors | Intercept | Effect | Factors | Intercept | Effect |
| Hallux | |||||
| Pes cavus | 5.37 | −0.61 | Pes cavus | 244 | −118 |
| Gender | 5.49 | −0.32 | Hallux rigidus | 225 | 61 |
| Hallux rigidus | 5.30 | 0.17 | Gender | 268 | −66 |
| Metatarsal head 1 | |||||
| Hallux valgus | 5.10 | 0.35 | Hallux valgus | 189 | 75 |
| Pes planus | 5.28 | −0.18 | Pes planus | 225 | −34 |
| Metatarsal head 2 | |||||
| Hallux rigidus | 5.52 | −0.27 | Hallux valgus | 239 | 84 |
| Pes planus | 5.53 | −0.09 | Nail deformity | 237 | 64 |
| Low forefoot arch | 5.29 | 0.22 | |||
| Metatarsal head 4 | |||||
| BMI | 4.48 | 0.02 | BMI | 62 | 4 |
| Metatarsal head 5 | |||||
| Callosities at MTH5 | 4.75 | 0.30 | Callosities at MTH5 | 134 | 57 |
| BMI | 3.78 | 0.04 | Foot width | 457 | −3 |
| Foot width | 6.80 | −0.02 | Type of insoles 35 EVA | 172 | −52 |
| Type of insoles 35 EVA | 4.98 | −0.34 | Type of insoles 55 EVA | 172 | −38 |
| Type of insoles 55 EVA | 4.98 | −0.21 | BMI | 28 | 4 |
| Midfoot | |||||
| Pes cavus | 4.44 | −0.72 | No variable selected | ||
| Heel | |||||
| Type of insoles 35 EVA | 5.47 | −0.38 | Type of insoles 35 EVA | 252 | −80 |
| Type of insoles 55 EVA | 5.47 | −0.26 | Type of insoles 55 EVA | 252 | −60 |
| Low forefoot arch | 5.10 | 0.20 |
Conflict of interest and funding
The authors have not received any funding or benefits from industry to conduct this study. Funding for this study was received from the following non-profit organisations: Stiftelsen Promobilia, Stiftelsen Skobranschens utvecklingsfond, the Local Research and Development Board for Gothenburg and Södra Bohuslän, stiftelsen Felix Neubergh, stiftelsen Gunnar Holmgrens Minne, IngaBritt & Arne Lundbergs Forskningsstiftelse, Adlerbertska forskningsstiftelsen, Diabetesfonden and the Gothenburg Diabetes Association.
References
- 1.Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, et al. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377–84. doi: 10.1046/j.1464-5491.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 2.Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21:1714–9. doi: 10.2337/diacare.21.10.1714. [DOI] [PubMed] [Google Scholar]
- 3.Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35:660–3. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- 4.Andersen H. Motor dysfunction in diabetes. Diabetes Metab Res Rev. 2012;28:89–92. doi: 10.1002/dmrr.2257. [DOI] [PubMed] [Google Scholar]
- 5.Ribu L, Hanestad BR, Moum T, Birkeland K, Rustoen T. A comparison of the health-related quality of life in patients with diabetic foot ulcers, with a diabetes group and a nondiabetes group from the general population. Qual Life Res. 2007;16:179–89. doi: 10.1007/s11136-006-0031-y. [DOI] [PubMed] [Google Scholar]
- 6.Ragnarson Tennvall G, Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabetes Complications. 2000;14:235–41. doi: 10.1016/s1056-8727(00)00133-1. [DOI] [PubMed] [Google Scholar]
- 7.Raspovic KM, Wukich DK. Self-reported quality of life and diabetic foot infections. J Foot Ankle Surg. 2014;53:716–9. doi: 10.1053/j.jfas.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–24. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 9.Ragnarson Tennvall G, Apelqvist J. Prevention of diabetes-related foot ulcers and amputations: a cost-utility analysis based on Markov model simulations. Diabetologia. 2001;44:2077–87. doi: 10.1007/s001250100013. [DOI] [PubMed] [Google Scholar]
- 10.Hirst M. Diabetes in 2013. The new figures. Diabetes Res Clin Pract. 2013;102:265. doi: 10.1016/j.diabres.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Bakker K, Apelqvist J, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28:225–31. doi: 10.1002/dmrr.2253. [DOI] [PubMed] [Google Scholar]
- 12.Ragnarson Tennvall G, Apelqvist J. Health-economic consequences of diabetic foot lesions. Clin Infect Dis. 2004;39:S132–9. doi: 10.1086/383275. [DOI] [PubMed] [Google Scholar]
- 13.Tesfaye S. Neuropathy in diabetes. Medicine. 2010;38:649–55. [Google Scholar]
- 14.Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22:1036–42. doi: 10.2337/diacare.22.7.1036. [DOI] [PubMed] [Google Scholar]
- 15.Crawford F, Young MJ, Abbott CA, Boulton AJM, Boyko EJ, Kastenbauer T, et al. Protocol for a systematic review and individual patient data meta-analysis of prognostic factors of foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS) BMC Med Res Methodol. 2013;13:22. doi: 10.1186/1471-2288-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veves A, Giurini JM, Logerfo FW. The diabetic foot: medical and surgical management. New York, NY: Humana Press; 2012. [Google Scholar]
- 17.Birke JA, Franks BD, Foto JG. First ray joint limitation, pressure, and ulceration of the first metatarsal head in diabetes mellitus. Foot Ankle Int. 1995;16:277–84. doi: 10.1177/107110079501600506. [DOI] [PubMed] [Google Scholar]
- 18.Lavery LA, Armstrong DG, Vela SA, Quebedeaux TL, Fleischli JG. Practical criteria for screening patients at high risk for diabetic foot ulceration. Arch Intern Med. 1998;158:157–62. doi: 10.1001/archinte.158.2.157. [DOI] [PubMed] [Google Scholar]
- 19.Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJ. Predictive value of foot pressure assessment as part of a population-based diabetes disease management program. Diabetes Care. 2003;26:1069–73. doi: 10.2337/diacare.26.4.1069. [DOI] [PubMed] [Google Scholar]
- 20.Formosa C, Gatt A, Chockalingam N. The importance of clinical biomechanical assessment of foot deformity and joint mobility in people living with type-2 diabetes within a primary care setting. Prim Care Diabetes. 2013;7:45–50. doi: 10.1016/j.pcd.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Holewski JJ, Moss KM, Stess RM, Graf PM, Grunfeld C. Prevalence of foot pathology and lower extremity complications in a diabetic outpatient clinic. J Rehabil Res Dev. 1989;26:35. [PubMed] [Google Scholar]
- 22.Abouaesha F, van Schie CH, Griffths GD, Young RJ, Boulton AJ. Plantar tissue thickness is related to peak plantar pressure in the high-risk diabetic foot. Diabetes Care. 2001;24:1270–4. doi: 10.2337/diacare.24.7.1270. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrand Tang U, Zügner R, Lisovskaja V, Karlsson J, Hagberg K, Tranberg R. Comparison of plantar pressure in three types of insole given to patients with diabetes at risk of developing foot ulcers – a two-year, randomized trial. J Clin Transl Endocrinol. 2014;1:121–32. doi: 10.1016/j.jcte.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munteanu SE, Strawhorn AB, Landorf KB, Bird AR, Murley GS. A weightbearing technique for the measurement of ankle joint dorsiflexion with the knee extended is reliable. J Sci Med Sport. 2009;12:54–9. doi: 10.1016/j.jsams.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Bus SA, Waaijman R, Arts M, de Haart M, Busch-Westbroek T, van Baal J, et al. Effect of custom-made footwear on foot ulcer recurrence in diabetes: a multicenter randomized controlled trial. Diabetes Care. 2013;36:4109–16. doi: 10.2337/dc13-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NDR. Nationella Diabetesregistret. Årsrapport 2013 års resultat. [Swedish National Diabetes Register. Annual report 2013].2014. [Google Scholar]
- 27.Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: a report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679–85. doi: 10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McRae R. Clinical orthopaedic examination. Edinburgh: Churchill Livingstone; 2010. [Google Scholar]
- 29.Sullivan M, Karlsson J, Taft C, Ware JE., Jr . SF-36 hälsoenkät: svensk manual och tolkningsguide [Swedish manual and interpretation guide] Gothenburg: Göteborg Sahlgrenska sjukhuset, Sektionen för vårdforskning; 2002. [Google Scholar]
- 30.Gandek B, Kaasa S, Leplège A, Sullivan M, Ware JE, Aaronson NK, et al. Tests of data quality, scaling assumptions, and reliability of the SF-36 in eleven countries. J Clin Epidemiol. 1998;51:1149–58. doi: 10.1016/s0895-4356(98)00106-1. [DOI] [PubMed] [Google Scholar]
- 31.Alonso J, Apolone G, Bucquet D, Keller S, Sullivan M, Wagner A. International quality of life assessment (IQOLA) project. Qual Life Res. 1992;1:349–51. doi: 10.1007/BF00434949. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 33.Zimny S, Schatz H, Pfohl M. The role of limited joint mobility in diabetic patients with an at-risk foot. Diabetes Care. 2004;27:942–6. doi: 10.2337/diacare.27.4.942. [DOI] [PubMed] [Google Scholar]
- 34.McPoil T, Vicenzino B, Cornwall M, Collins N. Can foot anthropometric measurements predict dynamic plantar surface contact area? J Foot Ankle Res. 2009;2:28. doi: 10.1186/1757-1146-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. New York, NY: Springer; 2000. [Google Scholar]
- 36.Ahroni JH, Boyko EJ, Forsberg RC. Clinical correlates of plantar pressure among diabetic veterans. Diabetes Care. 1999;22:965–72. doi: 10.2337/diacare.22.6.965. [DOI] [PubMed] [Google Scholar]
- 37.Guiotto A, Sawacha Z, Guarneri G, Cristoferi G, Avogaro A, Cobelli C. The role of foot morphology on foot function in diabetic subjects with or without neuropathy. Gait Posture. 2013;37:603–10. doi: 10.1016/j.gaitpost.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 38.Kärvestedt L, Mårtensson E, Grill V, Elofsson S, von Wendt G, Hamsten A, et al. The prevalence of peripheral neuropathy in a population-based study of patients with type 2 diabetes in Sweden. J Diabetes Complications. 2011;25:97–106. doi: 10.1016/j.jdiacomp.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot. Diabetes Metab Res Rev. 2000;16:S75–83. doi: 10.1002/1520-7560(200009/10)16:1+<::aid-dmrr139>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Majumder A, Chatterjee S, Maji D. Peripheral neuropathy in diabetes. J Indian Med Assoc. 2013;111:382, 384–6. [PubMed] [Google Scholar]
- 41.Leese G, Schofield C, McMurray B, Libby G, Golden J, MacAlpine R, et al. Scottish foot ulcer risk score predicts foot ulcer healing in a regional specialist foot clinic. Diabetes Care. 2007;30:2064–9. doi: 10.2337/dc07-0553. [DOI] [PubMed] [Google Scholar]
- 42.Wylie-Rosett J. Assessment of documented foot examinations for patients with diabetes in inner-city primary care clinics. Arch Fam Med. 1995;4:46–50. doi: 10.1001/archfami.4.1.46. [DOI] [PubMed] [Google Scholar]
- 43.Bailey TS, Yu HM, Rayfield EJ. Patterns of foot examination in a diabetes clinic. Am J Med. 1985;78:371–4. doi: 10.1016/0002-9343(85)90326-2. [DOI] [PubMed] [Google Scholar]
- 44.Baker N, Kenny C. Prevention, screening and referral of the diabetic foot in primary care. Diabetes Primary Care. 2014;16:307–16. [Google Scholar]
- 45.Leese GP, Reid F, Green V, McAlpine R, Cunningham S, Emslie-Smith AM, et al. Stratification of foot ulcer risk in patients with diabetes: a population-based study. Int J Clin Pract. 2006;60:541–5. doi: 10.1111/j.1368-5031.2006.00899.x. [DOI] [PubMed] [Google Scholar]
- 46.Kennon B, Leese GP, Cochrane L, Colhoun H, Wild S, Stang D, et al. Reduced incidence of lower-extremity amputations in people with diabetes in Scotland: a nationwide study. Diabetes Care. 2012;35:2588–90. doi: 10.2337/dc12-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Västra Götalandsregionen. Regionalt vårdprogram/riklinjer. Diabetesfoten 2008 [Regional guidelines on the Diabetic Foot 2008] Available from: https://alfresco.vgregion.se/alfresco/service/vgr/storage/node/content/3132/Diabetesfoten.pdf?a=false&guest=true&native=true [cited 10 April 2015].
- 48.Kozakai R, Tsuzuku S, Yabe K, Ando F, Niino N, Shimokata H. Age-related changes in gait velocity and leg extension power in middle-aged and elderly people. J Epidemiol. 2000;10:S77. doi: 10.2188/jea.10.1sup_77. [DOI] [PubMed] [Google Scholar]
- 49.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997;26:15–9. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 50.Allet L, Armand S, Golay A, Monnin D, de Bie RA, de Bruin ED. Gait characteristics of diabetic patients: a systematic review. Diabetes Metab Res Rev. 2008;24:173–91. doi: 10.1002/dmrr.809. [DOI] [PubMed] [Google Scholar]
- 51.Rubin RR, Peyrot M. Quality of life and diabetes. Diabetes Metab Res Rev. 1999;15:205–18. doi: 10.1002/(sici)1520-7560(199905/06)15:3<205::aid-dmrr29>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]