Abstract
Background
Transient outward K currents (Ito) have been reported both to suppress and facilitate early afterdepolarizations (EADs) when repolarization reserve is reduced. Here we used the dynamic clamp technique to analyze how Ito accounts for these paradoxical effects on EADs by influencing the dynamic evolution of repolarization reserve during the action potential.
Methods and Results
Isolated patch-clamped rabbit ventricular myocytes were exposed to either oxidative stress (H2O2) or hypokalemia to induce bradycardia-dependent EADs at a long pacing cycle length (PCL) of 6 s, when native rabbit Ito is substantial. EADs disappeared when the PCL was shortened to 1 s, when Ito becomes negligible due to incomplete recovery from inactivation. During 6-s PCL, EADs were blocked by the Ito blocker 4-aminopyridine, but reappeared when a virtual current with appropriate Ito-like properties was reintroduced using the dynamic clamp (n=141 trials). During 1-s PCL in the absence of 4-aminopyridine, adding a virtual Ito-like current (n=1,113 trials) caused EADs to reappear over a wide range of Ito conductance (0.005–0.15 nS/pF), particularly when inactivation kinetics were slow (τinact≥20 ms) and the pedestal (non-inactivating component) was small (<25% of peak Ito). Faster inactivation or larger pedestals tended to suppress EADs.
Conclusions
Repolarization reserve evolves dynamically during the cardiac action potential. Whereas sufficiently large Ito can suppress EADs, a wide range of intermediate Ito properties can promote EADs by influencing the temporal evolution of other currents affecting late repolarization reserve. These findings raise caution in targeting Ito as an antiarrhythmic strategy.
Keywords: arrhythmia, transient outward potassium current, repolarization reserve, early afterdepolarization, dynamic clamp
Introduction
Normal cardiac repolarization relies on a critical balance between depolarizing inward currents and repolarizing outward currents during the action potential (AP) plateau. Repolarization has built-in redundancy, or ‘reserve’, to protect against excessive AP duration (APD) lengthening and consequent QT interval prolongation. Repolarization reserve protects the heart against early afterdepolarizations (EADs) and triggered activities—both of which can promote ventricular arrhythmias such as torsade de pointes, polymorphic ventricular tachycardia and ventricular fibrillation. The concept of ‘reduced repolarization reserve’, originally formulated by Roden,1 summarizes conditions in which vulnerability to EAD-related arrhythmias increases due to a net decrease in repolarizing current—whether related to increased inward currents, decreased outward currents, or both. An intuitive commonly-held assumption is that all outward currents during the plateau phase increase repolarization reserve and thereby suppress EAD formation. However, recent experimental studies have shown that this is not always true for transient outward K currents (Ito). Although Ito suppressed EADs in atrial myocytes,2 Ito exacerbated EADs in ventricular myocytes with repolarization reserve reduced by oxidative stress.3 This seemingly paradoxical effect that an outward K current, which increases repolarization reserve, can exacerbate EADs has been explained theoretically3,4 as follows. By lowering the plateau voltage during the early phase 1 of the AP plateau, Ito delays the subsequent activation of other, slower time- and voltage-dependent outward currents (such as IKs), thus diminishing their contribution to repolarization reserve during phases 2 and 3 of the AP, and thereby facilitating EADs. The specific biophysical properties of Ito that determine whether it suppresses or promotes EADs, however, have not been systematically defined. This is an important concern given that drug therapy targeting Ito has been proposed as an antiarrhythmic and anti-heart failure therapy.
In this study, we used the dynamic clamp technique to experimentally define the arrhythmogenic ranges of three Ito properties—maximum conductance, inactivation kinetics, and pedestal (here defined as the Ito non-inactivating component). The dynamic clamp technique allows an Ito-like current with programmable properties to be introduced into a patch-clamped myocyte after the endogenous Ito is blocked. In this fashion, we could systematically analyze how each specific biophysical characteristic of Ito-like currents promotes or suppresses EAD formation. This systematic analysis is an important advantage of the dynamic clamp because the properties of Ito currents are quite diverse, both within and across species, with fast and slow voltage-dependent subtypes (Ito1,f and Ito1,s) and a Ca-dependent current (Ito2), all with differing kinetics. The dynamic clamp affords the opportunity to create virtual Ito currents with properties covering this full spectrum, including human Ito characteristics.
Our findings indicate that when overall repolarization reserve is reduced, Ito-like currents can promote EADs in rabbit ventricular myocytes over a wide range of conductances, particularly when the time constant of inactivation of Ito is relatively slow (>20 ms) and its pedestal is small. Large Ito conductances or large pedestals can also shorten APD and suppress EAD formation. These findings indicate that the repolarization reserve is not pre-determined at the onset of the action potential, but is a process that evolves dynamically during the entire AP plateau. This factor must be taken into account when designing antiarrhythmic strategies targeting Ito or preventing EAD-mediated arrhythmias, particularly because we find that human Ito properties fall within the range that can promote EADs.
Methods
An expanded methods section is available in the Supplemental Material.
Experimental animals and patch clamping
This study was approved by the UCLA Chancellor’s Animal Research Committee (ARC 2003-063-23C) and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996) and with UCLA Policy 990 on the Use of Laboratory Animal Subjects in Research (revised 2010). From young adult (3- to 4-month-old) New Zealand white male rabbits (1.7–2.0 kg), single ventricular myocytes were freshly isolated for whole-cell patch clamp with unbuffered intracellular Ca and dynamic clamp studies as described previously.5 To induce EADs, H2O2 (0.2 or 1 mmol/L) was added to the superperfusate or the extracellular [K] was reduced from 5.4 to 2.7 mmol/L. To inhibit Ito, 4-aminopyridine (4-AP; 2 mmol/L) was added to the perfusate.
Dynamic clamp technique and virtual Ito formulation
Patch-clamped rabbit ventricular myocytes were injected with a programmable virtual Ito using the dynamic clamp5,6 (10-kHz sampling frequency; real-time Linux-based software; www.rtxi.org). The virtual Ito, with instantaneous recovery from inactivation at −80 mV, was formulated as follows:
| (Eq.1) |
| (Eq.2) |
Three parameters in the virtual Ito—the maximum conductance Ḡto, the inactivation time constant τinact, and the pedestal α—were varied to simulate a wide range of features encompassing various Ito subtypes. Pedestal is the non-inactivating Ito component controlled by parameter α (Eq.1).
Data and statistical analysis
Electrophysiological data were analyzed using Clampfit 10.4 (Axon instruments, Inc.) and OriginPro 9.0 SR2 (Microcal software, Inc.). In the statistical analysis of the contingency table, measures of association (odds ratio) and accuracy (sensitivity, specificity, positive and negative predictive values, positive and negative likelihood ratios) were obtained under a logistic regression model using generalized estimating equation (GEE) methods. GEE allows for non-independent (correlated) binary observations due to multiple observations from the same myocyte and rabbit (hierarchical structure). The 95% confidence intervals ([95% CI]) were computed using resampling (bootstrap) methods under this model. A P value of <0.05 was considered significant.
Results
To investigate the biophysical properties that determine whether Ito suppresses or promotes EADs, we formulated a virtual Ito current to introduce into isolated patch-clamped rabbit ventricular myocytes using the dynamic clamp technique. The virtual Ito has three independently-adjustable parameters (Supplemental Figure 1): maximal conductance Ḡto, a single inactivation time constant τinact, and a pedestal (a very slowly-inactivating or non-inactivating component defined here as % of peak Ito that remains after 300 ms). Table 1 provides a literature review of experimentally-measured ranges of these Ito parameters in normal and failing hearts from different species, including humans. These data include both the fast subtype Ito1,f encoded by Kv4.3/Kv4.2/KCHiP2 and the slow subtype Ito1,s encoded by Kv1.4. To cover the spectrum of the three parameter ranges in Table 1, as well as to approximate aggregate currents composed of multiple Ito subtypes in the same cell or additional time-independent K currents that confer the equivalent of a pedestal, we varied the virtual Ito over a wide range, as follows: Ḡto from 0.0005 to 5.0 nS/pF, τinact from 5 to 1200 ms, and the pedestal from 0 to 100% of peak Ito. The parameter ranges of the virtual Ito also cover the reported features of both the human and rabbit Ito currents.
Table 1.
Literature review of ventricular Ito1 parameters measured from different species. Ito1 density was reported either as peak or as difference between peak and pedestal. Inactivation kinetics were reported as a mono- (τ1) or bi-exponential (τ1, τ2) decay time course.
| Species | Ito1 Subtype | Temp (°C) | Density (pA/pF) | Gto (nS/pF) | τinact (ms) | Pedestal (% of peak) | References |
|---|---|---|---|---|---|---|---|
| Human | |||||||
| Normal | Ito1,f | 35 | - | - | 8 | - | 7 |
| Ito1,f | 21–24 | 8–14 | 0.06–0.11 | 46–75 | 16–22 | 7–10 | |
| Heart failure | Ito1,f | 21–24 | ↓ ↔ | ↓ ↔ | - | - | 8–12 |
| Ito1,s | 21–24 | 5–10 | 0.04–0.08 | 59–73 | 0–17 | 8–12 | |
| Rabbit | |||||||
| Normal | Ito1,s | 34–37 | 10–18 | 0.08–0.14 | 10–20 | 4–25 | 3,13–17 |
| Ito1,s | 25 | 33–38 | 0.25–0.29 | 30–35 | 15 | 17 | |
| Heart failure | Ito1,s | 34–37 | 6–27 | 0.05–0.21 | τ1: 7–8, τ2: 70–118 | 5–21 | 13–17 |
| Ito1,s | 25 | 8 | 0.06 | 38–50 | 24 | 17 | |
| H2O2 | Ito1,s | 37 | 13 | 0.10 | τ1: 65–80, τ2: 350 | 35 | 3 |
| Canine | |||||||
| Normal | Ito1 | 36–37 | 17–46 | 0.13–0.35 | 18–36 | - | 3,9,18–23 |
| Ito1 | 24 | 20–22 | 0.15–0.17 | 34–49 | 0–16 | 9 | |
| Heart failure | Ito1 | 36–37 | 5–13 | 0.04–0.10 | 22–43 | 0–69 | 18–20,23 |
| Rat | |||||||
| Normal | Ito1,f | 37 | 20–22 | 0.15–0.17 | 45–97 | - | 24 |
| Ito1,f | 20–25 | 9–39 | 0.07–0.30 | 16–55 | 7–40 | 21,25–28 | |
| LVH | Ito1,f | 20–25 | 2–40 | 0.02–0.31 | 35–81 | 11–36 | 25–27,29,30 |
| Chronic MI | Ito1,f | 37 | 11–12 | 0.08–0.09 | 45–97 | - | 24 |
Temp: recording temperature; LVH: left ventricular hypertrophy; MI: myocardial infarction
Blockade of endogenous Ito suppresses EADs
To induce EADs, patch-clamped rabbit ventricular myocytes were exposed to either oxidative stress with H2O2 (1 mmol/L) or ionic stress with moderate hypokalemia (2.7 mmol/L). With either stress, bradycardia-dependent EADs were consistently observed at a slow pacing cycle length (PCL) of 6 s (Figure 1B–C row 1).
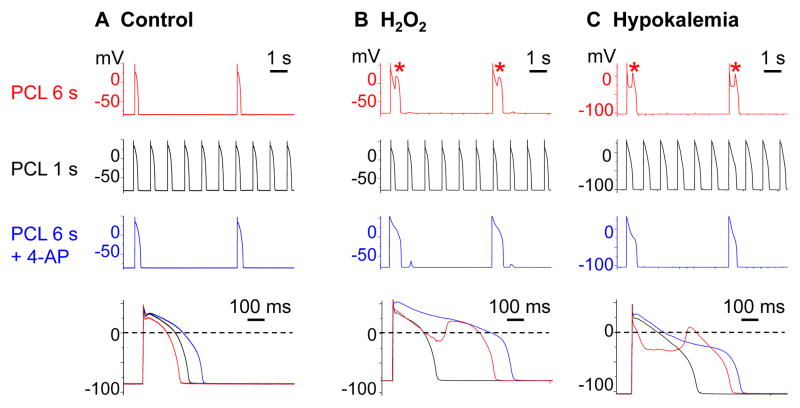
Figure 1.
Ito blockade by rapid pacing at PCL 1 s or by 4-AP suppresses H2O2-induced and hypokalemia-induced EADs in rabbit ventricular myocytes. A. No EADs arose under control conditions at PCL 6 s, 1 s, or 6 s in the presence of 4-AP (2 mmol/L). B–C. Following exposure to H2O2 (1 mmol/L; B) or hypokalemia (2.7 mmol/L; C), EADs (*) occurred at PCL 6 s (row 1), but were suppressed by rapid pacing at PCL 1 s (row 2) or by adding 4-AP (row 3). Superimposed action potentials under the 3 conditions are shown below.
EADs disappeared when Ito was blocked, either by shortening the PCL to 1 s (Figure 1B–C row 2) or by application of the Ito blocker 4-AP (2 mmol/L) during pacing at 6 s (Figure 1B–C row 3 & Figure 2). Suppression of EADs by Ito blockade suggests that Ito contributes to EAD formation under both stressed conditions. However, the evidence of EAD suppression by 4-AP is not definitive because 4-AP is not completely selective for Ito31 such that 4-AP off-target effects could have also been responsible.
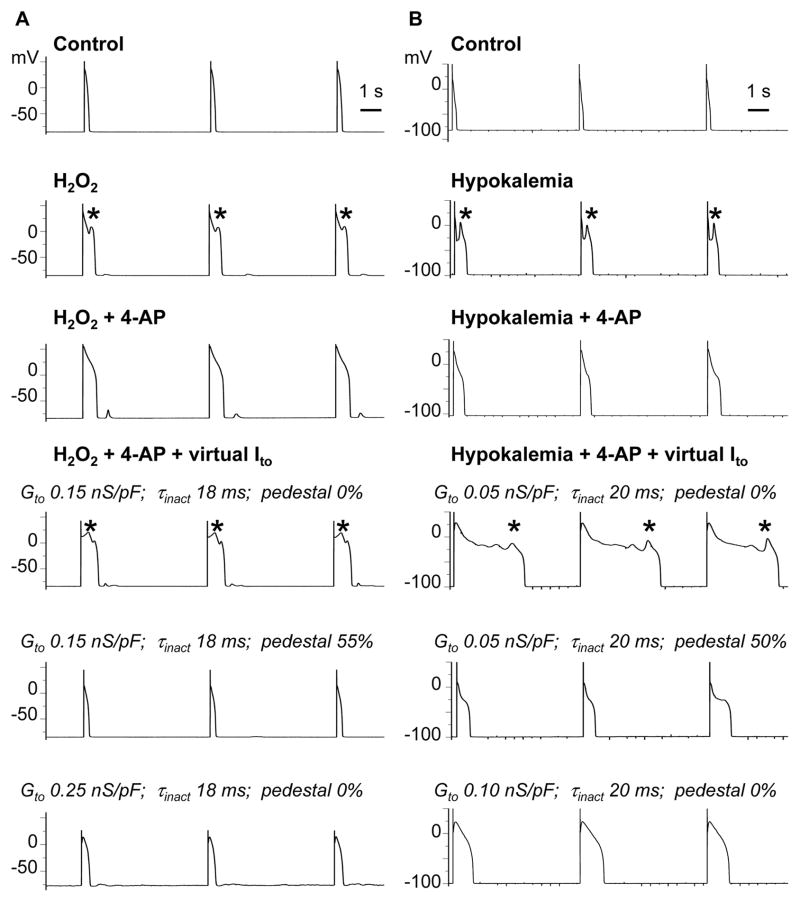
Figure 2.
Virtual Ito reconstitutes H2O2-induced (A) and hypokalemia-induced (B) EADs blocked by 4-AP. Row 1: Action potentials were elicited during pacing at PCL 6 s under control conditions. Row 2: H2O2 (1 mmol/L) or hypokalemia (2.7 mmol/L) induced EADs (*) during slow pacing at 6 s. Row 3: Ito blockade with 4-AP (2 mmol/L) suppressed EADs. Row 4: Representative parameter combinations of the virtual Ito that caused EADs to reappear. Rows 5–6: Representative parameter combinations of the virtual Ito that shortened APD and suppressed EAD reappearance by increasing the pedestal or Ito conductance.
Ito reconstitution reverses EAD suppression by 4-AP
To determine whether EAD suppression by 4-AP was related primarily to its blockade of Ito rather than its off-target effects, we injected a virtual dynamic-clamp Ito resembling the native rabbit Ito. In myocytes superfused with control Tyrode’s solution and paced at PCL 6 s or 1 s, EADs were never observed in the absence or presence of an injected virtual Ito for any parameter combinations tested (46 trials, 6 myocytes, 5 rabbits). Thus, when repolarization reserve was normal, Ito reconstitution did not promote EADs de novo.
However, after EADs had already been induced by either H2O2 or hypokalemia and subsequently suppressed by 4-AP, injection of a virtual Ito with properties approximating the native rabbit Ito1,s (Table 1) caused EADs to reappear (representative illustration in Figure 2, row 4). For the same value of Ḡto, increasing the pedestal current from 0 to 50–55% of peak Ito caused EADs to disappear and APD to shorten markedly (Figure 2 row 5). Likewise, increasing Ḡto without increasing the pedestal had the same effect (Figure 2 row 6).
Ito reconstitution reverses EAD suppression by rapid pacing
To eliminate possible confounding off-target effects of 4-AP unequivocally, we took advantage of the fact that the native rabbit ventricular Ito1 (chiefly Ito1,s) has an unusually long time constant of recovery from inactivation, averaging 6 s at −80 mV.3 Thus, whereas Ito1 amplitude is substantial at a PCL of 6 s due to the long diastolic recovery interval between beats, Ito1 is almost completely inactivated and makes a negligible contribution to the AP at a PCL of 1 s. Coincidentally, EADs induced by either oxidative stress or hypokalemia at PCL 6 s disappeared at PCL 1 s (Figure 1). These features allowed us to introduce a dynamic-clamp Ito programmed with instantaneous recovery from inactivation kinetics at −80 mV during PCL 1 s to determine if EADs reappear, and, if so, to evaluate what properties of Ito are required.
To assess the independent contribution of each of the three Ito parameters to EAD formation, we introduced a virtual Ito, varying only one parameter at a time (Figs 3–5), into myocytes in which stress-induced EADs at PCL 6 s had been suppressed by decreasing the PCL to 1 s.
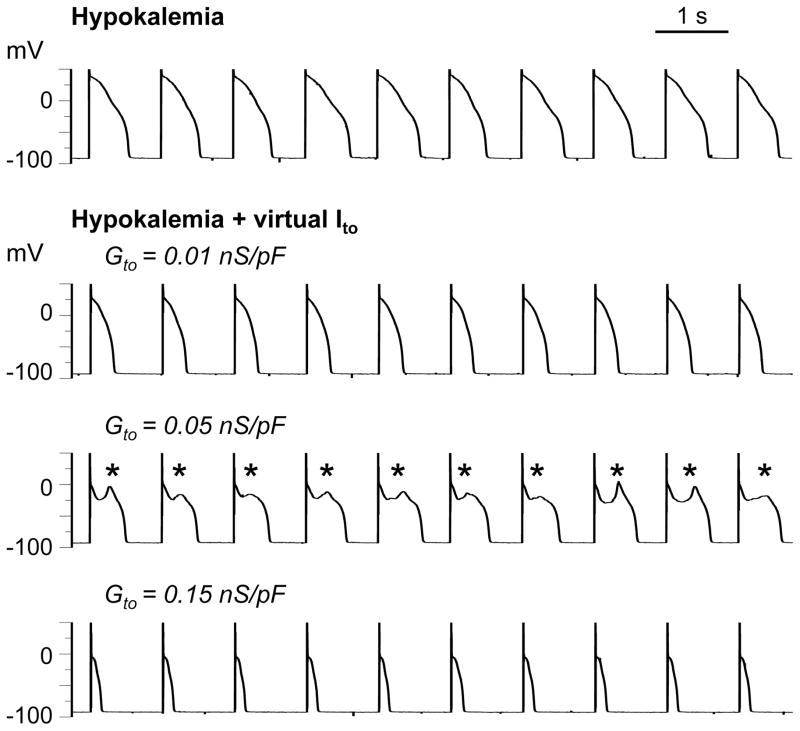
Figure 3.
Effects of Ḡto on reappearance of pacing-suppressed EADs. Row 1: EADs induced by hypokalemia (2.7 mmol/L) at PCL 6 s (not shown) were suppressed by shortening PCL to 1 s. Rows 2–4: A virtual Ito with τinact = 25 ms and no pedestal reconstituted EADs (*) at an intermediate Ito conductance (Ḡto = 0.05 nS/pF, row 3), whereas smaller (0.01 nS/pF, row 2) or larger conductances (0.15 nS/pF, row 4) caused APD shortening.
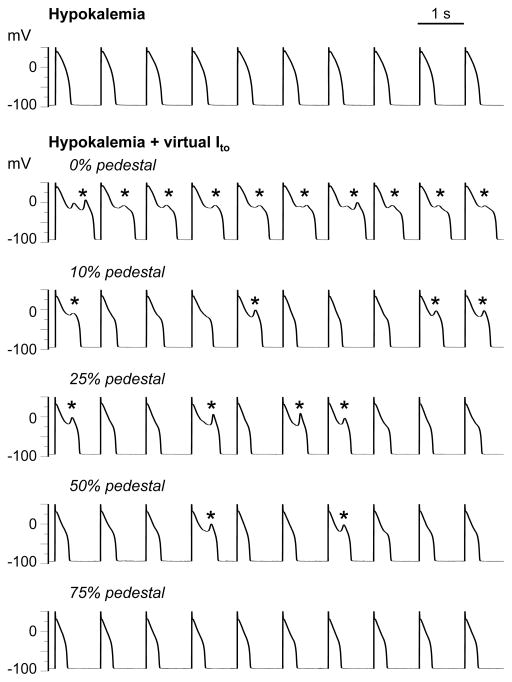
Figure 5.
Effects of the pedestal component on the reappearance of pacing-suppressed EADs. Row 1: EADs induced by H2O2 (1 mmol/L) at PCL 6 s (not shown) were suppressed by shortening PCL to 1 s. Rows 2–6: A virtual Ito with Ḡto = 0.025 nS/pF and τinact = 80 ms reconstituted EADs (*) for pedestals up to 50% (rows 2–5), but not for a pedestal of 75% (row 6).
Figure 3 illustrates the contribution of Ḡto, assessed by altering Ḡto while holding τinact and the pedestal constant. In this representative myocyte, after hypokalemia-induced EADs were suppressed by pacing at 1 s (row 1), introducing a small virtual Ito (Ḡto = 0.01 nS/pF, τinact = 20 ms, 0% pedestal) caused APD to shorten (row 2). When Ḡto was increased to 0.05 nS/pF, EADs reappeared (row 3). Further increasing Ḡto to 0.15 nS/pF caused EADs to disappear and the APD to shorten markedly (row 4). These findings demonstrate that for a given τinact and pedestal, Ito promotes EADs over a critical range of Ḡto and shortens APD at smaller or larger Ḡto values beyond that critical range.
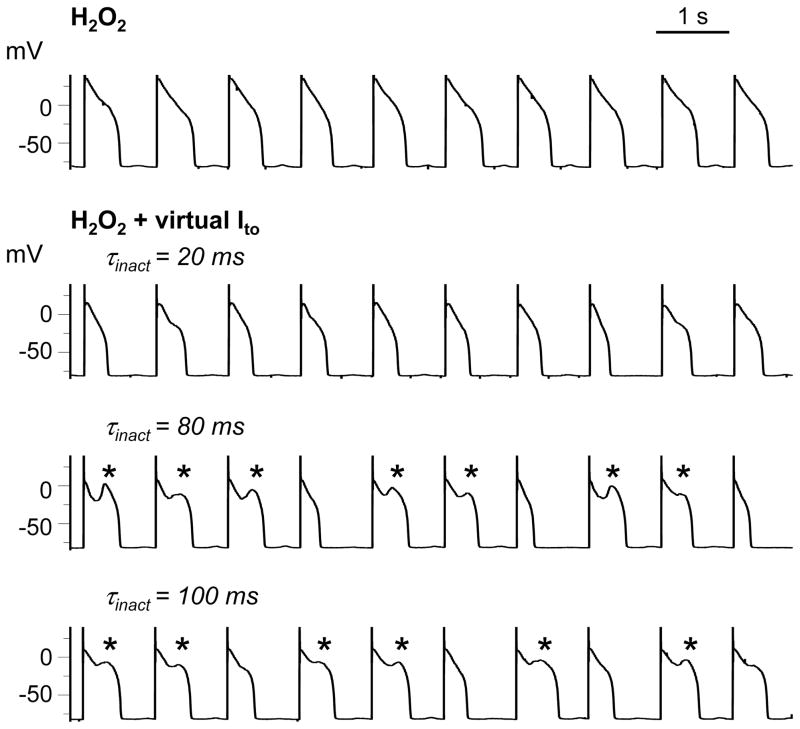
Figure 4 illustrates the contribution of τinact, assessed by altering τinact while holding Ḡto and the pedestal constant. In another myocyte, after H2O2-induced EADs were suppressed by pacing at 1 s (row 1), Ito was reconstituted using the dynamic clamp (Ḡto = 0.05 nS/pF, τinact = 20 ms, 0% pedestal). In this myocyte, τinact of 20 ms did not cause EADs to reappear, but shortened APD (row 2). Prolonging τinact to 80 or 100 ms, however, caused EADs to reappear (rows 3–4).
Figure 4.
Effects of τinact on reappearance of pacing-suppressed EADs. Row 1: EADs induced by H2O2 (1 mmol/L) at PCL 6 s (not shown) were suppressed by shortening PCL to 1 s. Rows 2–4: A virtual Ito with Ḡto = 0.05 nS/pF and no pedestal did not reconstitute EADs (*) for τinact =20 ms (row 2), but did when τinact was prolonged to 80 (row 3) or 100 ms (row 4).
Figure 5 illustrates the contribution of the pedestal, assessed by altering the pedestal while holding Ḡto and τinact constant. In this myocyte, after H2O2-induced EADs were suppressed by pacing at 1 s (row 1), introducing a virtual Ito (Ḡto 0.025 nS/pF, τinact = 80 ms) with no pedestal caused EADs to re-emerge (row 2). EADs persisted (albeit with decreased frequency) when the pedestal was increased to 10, 25, and then 50% (rows 3–5). However, EADs disappeared when the pedestal was further increased to 75% (row 6).
Ito properties that reverse EAD suppression by rapid pacing
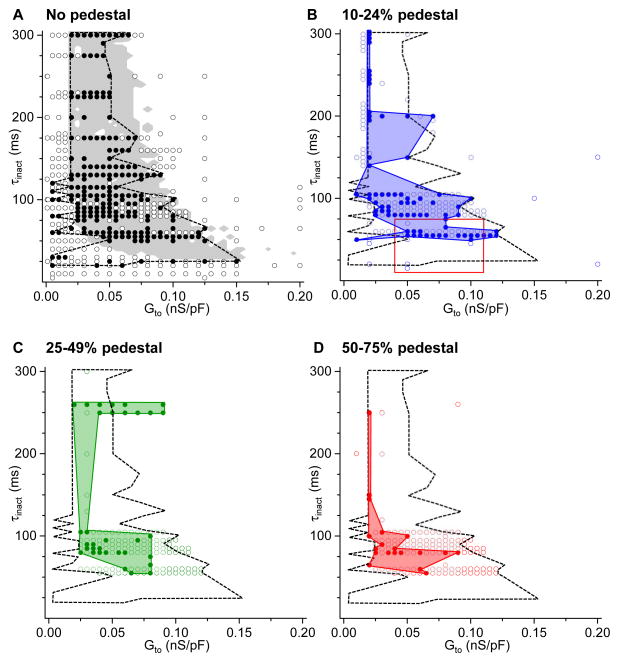
Figure 6 summarizes results from 772 parameter combinations of Ḡto, τinact, and pedestal values obtained in 1,113 trials using 131 rabbit ventricular myocytes isolated from a total of 46 rabbits. Using the protocols illustrated in Figures 3–5, we exposed myocytes to either H2O2 or hypokalemia at PCL 6 s to induce EADs, then suppressed EADs by shortening the PCL to 1 s, prior to injecting a virtual Ito. In the plots of Ḡto vs. τinact, parameter combinations of the virtual Ito that caused EADs to reappear at PCL 1 s are indicated by solid symbols, while those that did not are indicated by open symbols. The four plots, labeled A–D, correspond to the size of the pedestal, which was 0% in A, 10–24% in B, 25–49% in C, and 50–75% in D.
Figure 6.
Virtual Ito parameter combinations causing pacing-suppressed H2O2-induced or hypokalemia-induced EADs to reappear. Graphs show (Ḡto, τinact) combinations that did (solid circles) or did not (open circles) cause EADs to reappear at PCL 1 s, using the protocols shown in Figures 3–5, for the different ranges of Ito pedestal components as indicated in A–D. A. Dashed black line outlines the border of the experimental region in parameter space causing EADs to reappear, compared to the predictions from a computer model (gray shaded area), adapted from Zhao et al.3 B–D. Solid colored regions outline the experimental region in parameter space causing EADs to reappear for pedestals ranging from 10–24% (B), 25–49% (C), and 50–75% (D), compared to the no-pedestal case (black line reproduced from A). In B, the red box indicates the typical parameter values for human ventricular Ito1,f in normal and failing hearts (see Table 1). No EADs re-emerged with pedestals>75%.
Figure 6A shows that a wide range of (Ḡto, τinact) parameter combinations (outlined by the dashed black line) caused EADs to reappear when the virtual Ito had no pedestal component. The distribution of these parameter combinations agrees well with theoretical predictions from computer modeling simulating the effects of an Ito with 0% pedestal on EAD formation.3 The gray-shaded area indicates the region in (Ḡto, τinact) parameter space that caused EADs in the computer model. Note that the parameter combinations that caused EADs to reappear at PCL 1 s (the solid symbols) are mostly clustered inside the gray region, whereas those that did not (the open symbols) are mostly outside this gray area. This preferential clustering was statistically significant (P<0.0001, Table 2). The mismatches, indicated by the fraction of open symbols falling within the gray area or solid symbols outside the gray area, most likely reflect biological variability that does not exist in the deterministic computer AP model, as different patch-clamped myocytes came from different hearts and different ventricular regions in the same heart.
Table 2.
Statistical analysis of predicted vs. observed (Gto, τinact) parameter combinations causing EADs to reappear. A total of 480 observed (Gto, τinact) combinations of a virtual Ito with no pedestal (787 trials in 79 ventricular myocytes from 25 rabbit hearts) that did (EAD+) or did not (EAD−) cause H2O2-induced or hypokalemia-induced EADs to reappear at PCL 1 s are compared to theoretical predictions from a computer model.
| No. of Gto-τinact Combinations | Observed
|
|||
|---|---|---|---|---|
| EAD+ | EAD− | Total | ||
| Predicted | EAD+ | 186 | 49 | 235 |
| EAD− | 22 | 223 | 245 | |
| Total | 208 | 272 | 480 | |
|
| ||||
| Positive Predictive Value [95% CI] | 0.77 [0.67, 0.87] | |||
| Negative Predictive Value [95% CI] | 0.93 [0.86, 0.99] | |||
| Sensitivity [95% CI] | 0.92 [0.82, 0.99] | |||
| Specificity [95% CI] | 0.72 [0.55, 0.89] | |||
| Positive Likelihood Ratio | 3.3 [1.21, 5.41] | |||
| Negative Likelihood Ratio | 0.11 [0.01, 0.26] | |||
| Odds Ratio [95% CI] | 41.5 [1.8, 84.9] | |||
| P | <0.0001 | |||
Figure 6B–D reveal the EAD-suppressing effects of pedestal current. As the pedestal component became larger, the region in the (Ḡto, τinact) parameter space causing EADs to reappear (outlined by the colored regions) became progressively smaller, consistent with previously reported theoretical predictions.3 No EADs reemerged with pedestal current>75% (data not shown).
Finally, the red box in Figure 6B encloses parameter combinations representative of the human ventricular Ito (predominantly Ito1) reported in the literature for both normal and failing human hearts (Table 1). Note that the human Ito range falls within the virtual Ito parameter region causing EADs to re-emerge.
In summary, the data in Figure 6 demonstrate that virtual Ito-like currents can promote EADs over a wide range of peak conductances (Ḡto over a 30-fold range from 0.005–0.15 nS/pF), especially when inactivation kinetics are slow (τinact>20 ms) and the pedestal is small (<25% of peak Ito), in good overall agreement with theoretical predictions.3
Discussion
Ito as friend and foe in EAD genesis
Transient outward currents have been reported to both suppress and promote EADs.2,3 Because of the wide diversity of Ito properties between different subtypes (Ito1,s, Ito1,f, and Ito2), marked differences in regional expression profiles within atrial and ventricular tissue in the same species, as well as marked interspecies differences (Table 1), the dynamic clamp technique offers a powerful tool to analyze how variations in Ito properties affect EAD formation in diverse experimental settings. Moreover, the previous experimental evidence that Ito promotes EADs has relied solely on the disappearance of EADs after applying 4-AP to block Ito. However, 4-AP is not completely selective for Ito and has significant off-target effects on other ionic currents.31,32 The dynamic clamp allows the effects of Ito on EADs to be tested directly without this complication.
The role of Ito in EAD formation is a critical issue to understand because derangements in Ito physiology have been linked to increased susceptibility to malignant arrhythmias and pharmacological strategies targeting this current are under development. In this context, both selective Ito blockade and activation have been suggested as potential antiarrhythmic strategies.2,18 Downregulation of Ito1 contributes to reduced repolarization reserve in heart failure,33 which has been linked to higher risk of triggered ventricular arrhythmias.34 Genetic ablation of Ito1 in transgenic mice by Kv1.4−/− and Kv4.2W362F crossbreeding35 or KChIP2 knockout36 significantly prolonged APD, promoting EADs in single ventricular myocytes35 and markedly prolonged QT interval, promoting spontaneous ventricular tachycardia in intact tissue in the absence of ventricular hypertrophy or heart failure.35,36 Potentiation of Ito to increase repolarization reserve has therefore been suggested as a potential antiarrhythmic strategy in heart failure, with the caveat that excessive Ito can cause arrhythmias by a different mechanism, namely phase 2 reentry as in Brugada syndrome 37,38 and acute ischemia.39
Our findings indicate that additional caution is warranted, since in addition to these potential pro-arrhythmic effects of excessive Ito, even mild to moderate augmentation of Ito may be proarrhythmic by promoting EADs when repolarization reserve is already compromised. However, we emphasize that when repolarization reserve was normal, adding a virtual Ito to the normal rabbit ventricular AP never induced EADs. Hence, for Ito to promote EADs, overall repolarization reserve must be reduced by additional factors, such as oxidative stress or hypokalemia. Also, we do not mean to imply that Ito is an absolute requirement for EADs. Rather Ito is a current whose properties can enable EADs that otherwise would not occur depending on specific (but common) electrophysiological conditions in multiple species.
Our systematic analysis of the Ito properties promoting EADs in this study also provides novel insights into the apparent discrepancy between Zhao et al’s study,3 which concluded that Ito promotes EADs in ventricular and Purkinje myocytes from multiple species, and Workman et al’s dynamic clamp study,2 which concluded that Ito suppresses EADs in rabbit and human atrial myocytes. Atrial tissue has a very large endogenous Ito that accounts for the triangular shape of its action potential. This large Ito is in the range that typically suppresses EADs (>0.10 to 0.15 nS/pF in Figure 6), such that reducing Ito by dynamic clamp subtraction brought Ḡto into the range that frequently promotes EADs (<0.10 nS/pF). Indeed, in Workman et al study,2 Ḡto averaged 0.34 nS/pF in rabbit atrial myocytes and 0.12 nS/pF in human atrial myocytes. In contrast, ventricular myocytes from most non-rodent mammals have a smaller Ito density (Table 1) that may place them in the range of Ḡto that facilitates EAD formation such that Ito block with 4-AP or other agents will suppress EADs. Rat and mouse ventricular myocytes, on the other hand, have higher Ito densities than larger mammals, but may also develop EADs that are suppressed by 4-AP,3 suggesting that other differences between ventricular and atrial electrophysiology may also be important.
Applicability to human Ito
Our study is the first systematic analysis demonstrating that the range of Ito properties capable of promoting EADs is wide and inclusive of Ito from other species than just rabbit. We show that EADs were promoted over a 30-fold range of Ito conductance, favored by an inactivation time constant τinact≥20 ms and a pedestal component <25% of peak Ito. This range includes the typical Ito1 characteristics of both healthy and failing human ventricles, which exhibited a single inactivation time constant τinact averaging 8–75 ms and a pedestal of 16–22% in normal and failing epicardial ventricular myocytes.10,12,40 As shown in Figure 6B (red box), these characteristics fall clearly within the parameter range promoting EADs.
In addition, our findings also establish that the EAD-promoting effect of Ito occurs not just when EADs are induced by oxidative stress with H2O2 (which significantly modified Ito properties3), but also when EADs are induced by the clinically relevant condition of moderate hypokalemia, a common complication of diuretic therapy in patients with heart failure. Hypokalemia induces EADs primarily by reducing outward K currents, whereas H2O2 augments the late Na current and Ca currents primarily through oxidative CaMKII activation (reflected in the more common appearance of DADs in association with H2O2-induced EADs, rather than with hypokalemia-induced EADs, as in Figure 2).41,42 Thus, the specific mechanism by which overall repolarization reserve is reduced does not appear to be critical to the ability of Ito to promote EADs. The overall implication is that Ito may play an important role in facilitating EAD formation in multiple settings and in multiple species, including humans.
Mechanism of EAD potentiation by Ito
The mechanism by which Ito potentiates EADs is consistent with the dynamic theory of EAD formation by a Hopf-homoclinic bifurcation mechanism.4,43 In this theory, EADs are generated by the opposing effects of inward ICaL, which is activated as the plateau voltage dips below 0 mV and outward K currents, particularly IKs, which is reactivated during the ICaL-mediated EAD upstroke. The activation-deactivation kinetics of IKs must be matched appropriately to ICaL recovery kinetics to achieve membrane potential oscillations,4,43 the defining feature of EADs. If IKs activates too rapidly, then repolarization rate is too fast for ICaL to reactivate and prevent repolarization. Ito can promote EADs by lowering the voltage during the early plateau, thereby slowing IKs activation (since IKs activation rate and its open probability are highly voltage-dependent) while giving ICaL enough time to reactivate and oppose full repolarization. Thus, although Ito always increases early repolarization reserve, the indirect effect of Ito on the temporal evolution of other voltage-dependent currents such as IKs and ICaL can paradoxically reduce late repolarization reserve, which is the critical phase during which EADs develop. This also explains why a larger Ito pedestal current tends to suppress EADs, since the outward pedestal current directly increases late repolarization reserve, compensating for the reduction in IKs.
The agreement in Figure 6A between the computer model and the experimental findings lends further support to the dynamic theory of EAD formation via ICaL reactivation, even though other factors such as Ca cycling dynamics may also contribute importantly to EAD formation in many settings.44 The effects of Ca cycling might also account for the lack of an exact overlap between the computer model predictions (gray-shaded area) and the observed Ito properties causing EADs to reappear in Figure 6A.
Finally, the findings in this study are also consistent with a previous study5 showing that fibroblast-myocyte coupling can promote EADs as a result of the Ito-like outward capacitive current introduced by the fibroblast into the myocyte through gap junctions during the early AP plateau phase. However, that myocyte-fibroblast gap junctional current also has a late sustained component that can become inward during the later phases of the action potential plateau, thus can further directly reduce late repolarization reserve.
Study limitations
To keep the number of parameters manageable, we simplified the virtual Ito formulation to include only a single inactivation time constant τinact, whereas two time constants have been reported in some studies, although not in humans (Table 1). A pedestal component was used to approximate long time constants of inactivation (>200 ms) as well as truly non-inactivating components. In addition, under some conditions, time-independent K currents, such as the plateau K current (IKp) or the ATP-sensitive K current (IKATP), can potentially contribute a sustained outward current during the plateau phase that may summate with the Ito pedestal current.
We did not explicitly test scenarios in which multiple virtual Ito currents with different parameter combinations were added together into the same myocyte, even though multiple Ito subtypes (Ito1f, Ito1s, and Ito2) can coexist in the same myocyte. However, the wide range of parameter combinations that we tested, which exceeded the experimentally measured range in Table 1, would approximate many of these potential cases. Unlike Ito1, Ito2 is not a K current, but a Ca-activated Cl current with time course that parallels the intracellular Ca transient.45,46 Nevertheless, since Ito2 activates rapidly and inactivates within 20–50 ms,45,46 its kinetics as an outward current fall within the range of virtual Ito parameter combinations that we tested. Similarly, recent evidence indicates that small Ca-activated K (SK) channels are present in normal atrium and failing ventricles.47,48 These SK currents track the Ca transient, similar to Ito2, and likewise are expected to fall within the parameter ranges that we tested. Given these limitations, however, our virtual Ito model with three independently adjustable parameters should be viewed as a rough guideline for identifying the key EAD-promoting characteristics of Ito–like currents.
Although the ranges of virtual Ito parameters in this study encompasses the endogenous Ito parameter ranges from multiple species, the caveat is that we injected the virtual Ito only into rabbit ventricular myocytes. Therefore, we cannot exclude the possibility that myocytes from other species, including humans, or myocytes remodeled by heart diseases, would behave differently. However, we believe that the differences would likely be quantitative rather than qualitative given that Zhao et al 3 found that Ito block with 4-AP suppressed H2O2-induced EADs in multiple species exhibiting markedly different action potential properties.
Acknowledgments
We thank Dr. David Christini for invaluable technical assistance with the dynamic clamp, Drs. Alan Garfinkel and Hrayr Karagueuzian for critical review of the manuscript, and Dr. Jeffrey Gornbein for statistical support.
Funding Sources: This work was supported by the NIH grant UCLA CTSI UL1TR000124 (TPN), the American Heart Association National Center Scientist Development Grant (TPN), the Lauren B. Leichtman and Arthur E. Levine Cardiovascular Discovery Fund Investigator Award (TPN), the NIH grant P01 HL078931 (JNW), and the Laubisch and Kawata endowments (JNW).
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Roden DM. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 2.Workman AJ, Marshall GE, Rankin AC, Smith GL, Dempster J. Transient outward K+ current reduction prolongs action potentials and promotes afterdepolarisations: a dynamic-clamp study in human and rabbit cardiac atrial myocytes. J Physiol. 2012;590:4289–4305. doi: 10.1113/jphysiol.2012.235986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Z, Xie Y, Wen H, Xiao D, Allen C, Fefelova N, Dun W, Boyden PA, Qu Z, Xie LH. Role of the transient outward potassium current in the genesis of early afterdepolarizations in cardiac cells. Cardiovasc Res. 2012;95:308–316. doi: 10.1093/cvr/cvs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu Z, Xie LH, Olcese R, Karagueuzian HS, Chen PS, Garfinkel A, Weiss JN. Early afterdepolarizations in cardiac myocytes: beyond reduced repolarization reserve. Cardiovasc Res. 2013;99:6–15. doi: 10.1093/cvr/cvt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen TP, Xie Y, Garfinkel A, Qu Z, Weiss JN. Arrhythmogenic consequences of myofibroblast-myocyte coupling. Cardiovasc Res. 2012;93:242–251. doi: 10.1093/cvr/cvr292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorval AD, Christini DJ, White JA. Real-Time linux dynamic clamp: a fast and flexible way to construct virtual ion channels in living cells. Ann Biomed Eng. 2001;29:897–907. doi: 10.1114/1.1408929. [DOI] [PubMed] [Google Scholar]
- 7.Nabauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- 8.Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 9.Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V, 3rd, Houser SR, Tomaselli GF. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004;286:H602–609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- 10.Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75:473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- 11.Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- 12.Nabauer M, Beuckelmann DJ, Erdmann E. Characteristics of transient outward current in human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:386–394. doi: 10.1161/01.res.73.2.386. [DOI] [PubMed] [Google Scholar]
- 13.Rozanski GJ, Xu Z, Whitney RT, Murakami H, Zucker IH. Electrophysiology of rabbit ventricular myocytes following sustained rapid ventricular pacing. J Mol Cell Cardiol. 1997;29:721–732. doi: 10.1006/jmcc.1996.0314. [DOI] [PubMed] [Google Scholar]
- 14.Saegusa N, Garg V, Spitzer KW. Modulation of ventricular transient outward K(+) current by acidosis and its effects on excitation-contraction coupling. Am J Physiol Heart Circ Physiol. 2013;304:H1680–1696. doi: 10.1152/ajpheart.00070.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lan YF, Zhang JC, Gao JL, Wang XP, Fang Z, Fu YC, Chen MY, Lin M, Xue Q, Li Y. Effects of nerve growth factor on the action potential duration and repolarizing currents in a rabbit model of myocardial infarction. J Geriatr Cardiol. 2013;10:39–51. doi: 10.3969/j.issn.1671-5411.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji Y, Opthof T, Kamiya K, Yasui K, Liu W, Lu Z, Kodama I. Pacing-induced heart failure causes a reduction of delayed rectifier potassium currents along with decreases in calcium and transient outward currents in rabbit ventricle. Cardiovasc Res. 2000;48:300–309. doi: 10.1016/s0008-6363(00)00180-2. [DOI] [PubMed] [Google Scholar]
- 17.Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, O’Rourke B, Kass DA, Marban E, Tomaselli GF. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol. 2005;288:H2077–2087. doi: 10.1152/ajpheart.00526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cordeiro JM, Calloe K, Moise NS, Kornreich B, Giannandrea D, Di Diego JM, Olesen SP, Antzelevitch C. Physiological consequences of transient outward K+ current activation during heart failure in the canine left ventricle. J Mol Cell Cardiol. 2012;52:1291–1298. doi: 10.1016/j.yjmcc.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaab S, Nuss HB, Chiamvimonvat N, Orourke B, Pak PH, Kass DA, Marban E, Tomaselli GF. Ionic mechanism of action potential prolongation in ventricular myocytes from dogs with pacing-induced heart failure. Circ Res. 1996;78:262–273. doi: 10.1161/01.res.78.2.262. [DOI] [PubMed] [Google Scholar]
- 20.Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85:1175–1188. doi: 10.1161/01.cir.85.3.1175. [DOI] [PubMed] [Google Scholar]
- 21.Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- 22.Pacioretty LM, Gilmour RF., Jr Developmental changes of action potential configuration and I(to) in canine epicardium. Am J Physiol. 1995;268:H2513–2521. doi: 10.1152/ajpheart.1995.268.6.H2513. [DOI] [PubMed] [Google Scholar]
- 23.Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varro A, Nattel S. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol. 2004;561:735–748. doi: 10.1113/jphysiol.2004.075861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin D, Zhang ZH, Caref EB, Boutjdir M, Jain P, El-Sherif N. Cellular and ionic basis of arrhythmias in postinfarction remodeled ventricular myocardium. Circ Res. 1996;79:461–473. doi: 10.1161/01.res.79.3.461. [DOI] [PubMed] [Google Scholar]
- 25.Benitah JP, Gomez AM, Bailly P, Da Ponte JP, Berson G, Delgado C, Lorente P. Heterogeneity of the early outward current in ventricular cells isolated from normal and hypertrophied rat hearts. J Physiol. 1993;469:111–138. doi: 10.1113/jphysiol.1993.sp019807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Keung EC. Effects of myocardial hypertrophy on transient outward current. Am J Physiol. 1994;266:H1738–1745. doi: 10.1152/ajpheart.1994.266.5.H1738. [DOI] [PubMed] [Google Scholar]
- 27.Xu XP, Best PM. Decreased transient outward K+ current in ventricular myocytes from acromegalic rats. Am J Physiol. 1991;260:H935–942. doi: 10.1152/ajpheart.1991.260.3.H935. [DOI] [PubMed] [Google Scholar]
- 28.Dukes ID, Morad M. The transient K+ current in rat ventricular myocytes: evaluation of its Ca2+ and Na+ dependence. J Physiol. 1991;435:395–420. doi: 10.1113/jphysiol.1991.sp018516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gidh-Jain M, Huang B, Jain P, el-Sherif N. Differential expression of voltage-gated K+ channel genes in left ventricular remodeled myocardium after experimental myocardial infarction. Circ Res. 1996;79:669–675. doi: 10.1161/01.res.79.4.669. [DOI] [PubMed] [Google Scholar]
- 30.Takimoto K, Li D, Hershman KM, Li P, Jackson EK, Levitan ES. Decreased expression of Kv4.2 and novel Kv4.3 K+ channel subunit mRNAs in ventricles of renovascular hypertensive rats. Circ Res. 1997;81:533–539. doi: 10.1161/01.res.81.4.533. [DOI] [PubMed] [Google Scholar]
- 31.Arechiga-Figueroa IA, Rodriguez-Martinez M, Albarado A, Torres-Jacome J, Sanchez-Chapula JA. Multiple effects of 4-aminopyridine on feline and rabbit sinoatrial node myocytes and multicellular preparations. Pflugers Arch. 2010;459:345–355. doi: 10.1007/s00424-009-0734-3. [DOI] [PubMed] [Google Scholar]
- 32.Ridley JM, Milnes JT, Zhang YH, Witchel HJ, Hancox JC. Inhibition of HERG K+ current and prolongation of the guinea-pig ventricular action potential by 4-aminopyridine. J Physiol. 2003;549:667–672. doi: 10.1113/jphysiol.2003.043976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nabauer M, Kaab S. Potassium channel down-regulation in heart failure. Cardiovasc Res. 1998;37:324–334. doi: 10.1016/s0008-6363(97)00274-5. [DOI] [PubMed] [Google Scholar]
- 34.Tomaselli GF, Zipes DP. What causes sudden death in heart failure? Circ Res. 2004;95:754–763. doi: 10.1161/01.RES.0000145047.14691.db. [DOI] [PubMed] [Google Scholar]
- 35.Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- 36.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, Nguyen-Tran VT, Gu Y, Ikeda Y, Chu PH, Ross J, Giles WR, Chien KR. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107:801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 37.Delpon E, Cordeiro JM, Nunez L, Thomsen PE, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Hofman-Bang J, Burashnikov E, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calloe K, Cordeiro JM, Di Diego JM, Hansen RS, Grunnet M, Olesen SP, Antzelevitch C. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res. 2009;81:686–694. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukas A, Antzelevitch C. Phase 2 reentry as a mechanism of initiation of circus movement reentry in canine epicardium exposed to simulated ischemia. Cardiovasc Res. 1996;32:593–603. [PubMed] [Google Scholar]
- 40.Wettwer E, Amos G, Gath J, Zerkowski HR, Reidemeister JC, Ravens U. Transient outward current in human and rat ventricular myocytes. Cardiovasc Res. 1993;27:1662–1669. doi: 10.1093/cvr/27.9.1662. [DOI] [PubMed] [Google Scholar]
- 41.Wagner S, Ruff HM, Weber SL, Bellmann S, Sowa T, Schulte T, Anderson ME, Grandi E, Bers DM, Backs J, Belardinelli L, Maier LS. Reactive oxygen species-activated Ca/Calmodulin Kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran DX, Sato D, Yochelis A, Weiss JN, Garfinkel A, Qu Z. Bifurcation and chaos in a model of cardiac early afterdepolarizations. Phys Rev Lett. 2009;102:258103. doi: 10.1103/PhysRevLett.102.258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi BR, Burton F, Salama G. Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol. 2002;543:615–631. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li GR, Feng J, Wang Z, Fermini B, Nattel S. Comparative mechanisms of 4-aminopyridine-resistant Ito in human and rabbit atrial myocytes. Am J Physiol. 1995;269:H463–472. doi: 10.1152/ajpheart.1995.269.2.H463. [DOI] [PubMed] [Google Scholar]
- 46.Zygmunt AC, Gibbons WR. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991;68:424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]
- 47.Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T, Chen PS. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res. 2011;108:971–979. doi: 10.1161/CIRCRESAHA.110.238386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuteja D, Rafizadeh S, Timofeyev V, Wang S, Zhang Z, Li N, Mateo RK, Singapuri A, Young JN, Knowlton AA, Chiamvimonvat N. Cardiac small conductance Ca2+-activated K+ channel subunits form heteromultimers via the coiled-coil domains in the C termini of the channels. Circ Res. 2010;107:851–859. doi: 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]