Abstract
Introduction
In the US, more than 10% of national health expenditures are for prescription drugs. Assessing drug costs in US economic evaluation studies is not consistent, as the true acquisition cost of a drug is not known by decision modelers. Current US practice focuses on identifying one reasonable drug cost and imposing some distributional assumption to assess uncertainty.
Methods
We propose a set of Rules based on current pharmacy practice that account for the heterogeneity of drug product costs. The set of products derived from our Rules, and their associated costs, form an empirical distribution that can be used for more realistic sensitivity analyses, and create transparency in drug cost parameter computation.
The Rules specify an algorithmic process to select clinically equivalent drug products that reduce pill burden, use an appropriate package size, and assume uniform weighting of substitutable products. Three diverse examples show derived empirical distributions and are compared with previously reported cost estimates.
Results
The shapes of the empirical distributions among the three drugs differ dramatically, including multiple modes and different variation. Previously published estimates differed from the means of the empirical distributions. Published ranges for sensitivity analyses did not cover the ranges of the empirical distributions. In one example using lisinopril, the empirical mean cost of substitutable products was $444 (range $23–$953) as compared to a published estimate of $305 (range $51–$523).
Conclusions
Our Rules create a simple and transparent approach to create cost estimates of drug products and assess their variability. The approach is easily modified to include a subset of, or different weighting for, substitutable products. The derived empirical distribution is easily incorporated into one-way or probabilistic sensitivity analyses.
Introduction
In economic evaluations of a health technology or treatment strategy, drug costs are frequently an important element in a decision model. (1, 2) While best practices have been described for estimation and uncertainty for many parameters in decision models, uncertainty around drug costs has been largely unstudied. (3–8) An obvious explanation of this omission is that in many countries, like Canada, Australia and the UK, a single per unit price list is published by a national payer. (6–8) As a result, economic evaluators use this published cost, scaled appropriately, and do not need to account for uncertainty in the cost of medications. (9–11)
In the United States no corollary of this per unit drug cost exists publically. (12, 13) The goal of our work is to improve upon current methodologies for costing medications in the US, by deriving a set of Pharmacy Practice-Based Substitution Rules (Rules) that transparently consider the full set of drug products to be used to fill a given medication order. A medication order is the unit of analysis for an economic evaluator and is a specific quantity of a particular formulation of a drug, taken a specified number of times a day over a specific time period. This full set of products provides a natural way to systematically account for uncertainty in drug costs. In addition to added precision and transparency, this procedure improves uncertainty analysis by more fully covering the real heterogeneity experienced by payers in the US. Finally it adds a uniformity that is lacking in drug cost parameter estimation for US-based decision analyses.
Prior studies reflecting the cost of drugs in the US have defaulted to some function of Average Wholesale Price (AWP) as the base-case estimate. While much has been made of the inadequacy of AWP as a cost metric, a literature review of US-based economic evaluation published in the last five years (identified by querying PubMed for keywords "markov model" "drug cost" "sensitivity" “United States”) found 34 of 36 studies used AWP, some percent discount of AWP or Wholesale Acquisition Cost (WAC, that is generally computed as 75–80% of AWP). (13, 14) It was not clear in these studies how their base-case estimates were derived, that is, precisely which particular drug product was used to estimate their cost. These US-based economic studies did account for uncertainty in drug cost, yet the approaches to quantify uncertainty varied greatly across the studies in our literature review. (10, 15–46) Overall there was a lack of justification for both the estimates and the uncertainty strategy in these papers including their estimated base-case costs, their choice of the minimum and maximum used in one-way sensitivity analyses, and their distributional assumptions used for probabilistic sensitivity analyses. These arbitrary distributions and assumptions potentially bias base-case estimates and the validity of sensitivity analysis findings.
Our derived Rules use the information available in the Truven Health Analytics Red Book, an on-line compendia of all drug products currently available in the United States, to compute a full set of drug products that could be used for any medication order of interest. (44) We selected AWP as our cost metric because our literature review revealed AWP as the de facto cost metric for the US. Although WAC is frequently used in studies, this metric is not universally reported in the Red Book and other drug costing compendia.
We determine which drug products could be selected to fill a medication order, and then scale the cost appropriately. This approach provides a distribution of potential costs for that medication order. We propose to use this empirical distribution as the input for drug cost in US-based decision analyses. The mean or median of the empirical distribution can be used as the base-case estimate, the derived minimum and maximum can be used as the range for a one-way sensitivity analysis, and the empirical distribution can be sampled for use in a probabilistic sensitivity analyses.
The paper proceeds as follows. Rules to determine the set of substitutable products for inclusion in the costing process are described in the Methods section; the findings after implementing the Rules for three drugs are compared to prior published base-case and uncertainty estimates in the Results section; the impact of the Rules and their applicability to future research, as well as ways to adapt the Rules to be the most relevant for particular perspectives, are described in the Discussion section.
Methods
Overview
The key innovation of our method is that it evaluates all drug products available in the US, and determines which products could be used to fill a medication order of interest by encoding expert knowledge of pharmacy practice. A drug product is an entity that is available for sale in the US, distinguished by a unique National Drug Code, and includes a drug of precise amount, formulation, package size, and manufacturer. A medication order is a drug of exact quantity, route of delivery, frequency and length of use.
We apply our Rules to three real-world drug costing scenarios and compare their results to previously published studies. (47–49) For any given medication order, these Rules identify clinically equivalent products, choose drug products that minimize pill-burden as defined later, and consider only drug products with an appropriate package size. We use the Red Book as it includes necessary information to apply our Rules, such as the quantity and unit of delivery for every drug product (strength, route, formulation) package size, manufacturer, generic and unit-dose designation, and AWP. In this paper we focus only on oral formulations of drugs (tablets, capsules, liquids) as these account for the majority of outpatient drug use, though our method can be applied to other drug formulations with slight modification in the calculation.
Pharmacy Practice-Based Substitution Rules
To define the Rules, we first select clinically equivalent products for a particular medication order, by identifying all products containing the same active ingredient. The list of drug products can range in number from one to potentially thousands, depending on the number of unique drug products that contain the active ingredient. Next we remove drug products that have a different route of delivery than defined in the medication order, as these are not clinically equivalent. For example, intravenous products are eliminated when a medication order specifies an oral route. Even with requiring an identical route, we still allow for variation in drug formulation, as an oral route product can be delivered in various formulations such as tablets, capsules, and liquids. These different forms of an orally administered drug are clinically equivalent and are often used interchangeably based on prescriber and patient preferences. (50)
Once we identify the clinically equivalent drug products, we define the notion of pill-burden. (51, 52) For most medication orders, it is possible to obtain the desired daily dose of a medication using different strength drug products. For a given active ingredient, 20mg of a tablet can be achieved with two, 10mg tablets or one, 20mg tablet. In practice, a provider may have a preference in prescribing one strength over another, a provider may be unaware that an alternative strength exists, a patient may prefer the size of 10mg tablets, or the provider may prefer smaller units of the drug to allow for altering the dose over time. If multiple units of a drug product cannot be used to provide the exact dose prescribed on the medication order, these products are eliminated. For instance, a drug product of 600mg per unit could not be used to fill a medication order for a dose of 750mg.
Yet it is not practical to allow any strength of product to be used. For instance, using ten 10mg tablets to achieve a desired dose of 100mg is not considered practical; we label this as pill burden. As a general rule providers and pharmacists seek to minimize pill burden. To fill a medication order, we consider only drug products with (i) 50% or 100% of the desired strength, (ii) 200% for tablets that can be scored, or (iii) liquids, as these can be reduced by consuming less.
The next step in applying the Rules involves an assessment of how many individual doses of a drug are contained inside the drug product, or what we have labeled package size. Only drug products that have an equal or larger package size to fill the entire medication order are included. Thus drug products with a small package size, say 10 capsules, would not be used to fill a prescription that requires 90 capsules. This definition aligns with current pharmacy practice, as pharmacies generally prefer to use bulk drug products for filling prescriptions. When considering medication orders with a duration that is greater than three months, we consider all drug products that fill at least three months of that medication order. This aligns with current pharmacy practice, as the maximum allowable days supply per filling by most insurance programs is 90-days. If the medication order is 1 tablet a day for a year, we consider all 1-tablet products with package size greater than 90 (instead of 365) and scale the result to reflect one year.
Next we consider whether a product as packaged can be partitioned into a specified number of doses per medication order. Some products denoted as unit dose are dispensed to a patient if and only if the medication order is identical to the number of doses for the drug product. For example, many antibiotics come in blister packs that are designed to be exact dosages. If the medication order does not match identically with a unit dose drug product, then that drug product is not included in the set of substitutable products.
The cost of the medication order depends on how many total units of the drug product are required to fill the medication order. We scale all drug products to the units of the medication order (e.g. milliliters to milligrams, grams to milligrams, teaspoons to milligrams), compute the cost of one dose of the medication using each drug product, and multiply by the frequency per day and the number of days. An empirical distribution of the cost is created and shows the variety and variation of the number of clinically appropriate drug products and their associated costs that satisfy the Rules. For our examples we use 100% of the AWP in 2010 US Dollars as our cost metric.
We demonstrate the costing process for three oral drugs: cephalexin, lisinopril and naproxen. Cephalexin, a general antibiotic, is used to treat infections over a short treatment window (7–10 days). The drug is off-patent and has hundreds of equivalent drug products. Lisinopril is an angiotensin-converting-enzyme inhibitor for the treatment of high-blood pressure. Naproxen is a chronic pain medication available both with a prescription and over the counter. These drugs were selected as they are frequently used, each was in the Top 100 Products by Total Prescriptions in America in 2012, but vary in how long they have been available (first date of introduction 1967–1987), as well as which conditions they treat, and have previously published estimates of base-case and uncertainty of their costs. (53)
Results
We illustrate a detailed summary of the step-by-step application of the Rules for cephalexin. For lisinopril and naproxen we show only the derived empirical distribution resulting from applying the Rules.
A medication order for 500mg cephalexin, twice a day for 10 days is illustrated. Table 1 shows a small sample of 32 drug products of the available 557 drug products with the active ingredient cephalexin extracted from the Red Book. Lines that are printed in bold indicate drug products in this sample that meet the Rules.
Table 1.
Sample of Potential Clinically Substitutable Drug Products for 500mg Cephalexin Twice a Day for 10 Days
| Row | Product Name | Manufacturer | Strength | Route | Form | Package Size |
Generic | Unit Dose | AWP Package Price ($) |
AWP Unit Price ($) |
Cost of Medication Order ($) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CEPHALEXIN | A | 250 mg | ORAL | CAP | 28 | Y | N | 6.59 | 0.24 | 9.41 |
| 2 | CEPHALEXIN | J | 250 mg | ORAL | CAP | 500 | Y | N | 140.91 | 0.28 | 11.27 |
| 3 | CEPHALEXIN | K | 250 mg/5 ml | ORAL | PDR | 200 ml | Y | N | 31.5 | 0.16 | 31.50 |
| 4 | CEPHALEXIN | N | 125 mg/5 ml | ORAL | PDR | 200 ml | Y | N | 15.75 | 0.08 | 31.50 |
| 5 | CEPHALEXIN | N | 250 mg/5 ml | ORAL | PDR | 200 ml | Y | N | 31.5 | 0.16 | 31.50 |
| 6 | CEPHALEXIN | J | 500 mg | ORAL | CAP | 40 | Y | N | 32.36 | 0.81 | 32.36 |
| 7 | CEPHALEXIN | J | 500 mg | ORAL | CAP | 20 | Y | N | 19.18 | 0.96 | 38.36 |
| 8 | BIO-CEF | E | 250 mg/5 ml | ORAL | PDR | 100 ml | Y | N | 20.34 | 0.20 | 40.68 |
| 9 | CEPHALEXIN | J | 500 mg | ORAL | CAP | 60 | Y | N | 62.16 | 1.04 | 41.44 |
| 10 | BIO-CEF | E | 125 mg/5 ml | ORAL | PDR | 100 ml | Y | N | 10.49 | 0.10 | 41.96 |
| 11 | BIO-CEF | E | 500 mg | ORAL | CAP | 100 | Y | N | 162.02 | 1.62 | 64.81 |
| 12 | CEPHALEXIN | I | 250 mg | ORAL | CAP | 30 | Y | N | 48.95 | 1.63 | 65.27 |
| 13 | CEPHALEXIN | I | 250 mg | ORAL | CAP | 40 | Y | N | 65.27 | 1.63 | 65.27 |
| 14 | CEPHALEXIN | I | 250 mg | ORAL | CAP | 28 | Y | N | 45.69 | 1.63 | 65.27 |
| 15 | CEPHALEXIN | D | 250 mg | ORAL | CAP | 28 | Y | N | 57.17 | 2.04 | 81.67 |
| 16 | CEPHALEXIN | M | 500 mg | ORAL | CAP | 120 | Y | N | 340 | 2.83 | 113.33 |
| 17 | KEFLEX | H | 250 mg | ORAL | CAP | 30 | N | N | 86.22 | 2.87 | 114.96 |
| 18 | KEFLEX | H | 250 mg | ORAL | CAP | 20 | N | Y | 57.56 | 2.88 | 115.12 |
| 19 | CEPHALEXIN | I | 500 mg | ORAL | CAP | 28 | Y | N | 80.76 | 2.88 | 115.37 |
| 20 | CEPHALEXIN | I | 500 mg | ORAL | CAP | 40 | Y | N | 115.38 | 2.88 | 115.38 |
| 21 | CEPHALEXIN | I | 500 mg | ORAL | CAP | 8 | Y | N | 23.08 | 2.89 | 115.40 |
| 22 | CEPHALEXIN | F | 250 mg | ORAL | CAP | 20 | Y | N | 57.8 | 2.89 | 115.60 |
| 23 | KEFLEX | H | 500 mg | ORAL | CAP | 40 | N | N | 115.85 | 2.90 | 115.85 |
| 24 | CEPHALEXIN | C | 500 mg | ORAL | CAP | 28 | Y | N | 92.06 | 3.29 | 131.51 |
| 25 | CEPHALEXIN | C | 500 mg | ORAL | CAP | 14 | Y | N | 46.03 | 3.29 | 131.51 |
| 26 | KEFLEX | L | 250 mg | ORAL | CAP | 100 | N | N | 329.92 | 3.30 | 131.97 |
| 27 | CEPHALEXIN | F | 250 mg | ORAL | CAP | 28 | Y | N | 92.44 | 3.30 | 132.06 |
| 28 | CEPHALEXIN | B | 500 mg | ORAL | CAP | 10 | Y | N | 35.43 | 3.54 | 141.72 |
| 29 | CEPHALEXIN | G | 500 mg | ORAL | CAP | 20 | Y | N | 72.2 | 3.61 | 144.40 |
| 30 | KEFLEX | J | 500 mg | ORAL | CAP | 40 | N | N | 150.99 | 3.77 | 150.99 |
| 31 | CEPHALEXIN | G | 250 mg | ORAL | CAP | 20 | Y | N | 75.6 | 3.78 | 151.20 |
| 32 | CEPHALEXIN | I | 250 mg/5 ml | ORAL | PDR | 20 ml | Y | N | 63 | 3.15 | 630.00 |
Note: Bold indicates drug product meets Pharmacy Practice-Based Substitution Rules. Form abbreviations: CAP-Capsules PDR-Powder for Liquid Suspension. All Costs are listed in 2010 US Dollars.
The name of the drug products varies from Cephalexin, Bio-Cef, or Keflex, with the name of the manufacturer de-identified. The strength of the drug products varies from 125mg to 750mg over all 557 drug products. All routes are oral, but the formulation varies showing capsules, tablets, and powders for liquid suspension. The column labeled Package Size illustrates the wide variation of the number of tablets or capsules in a package from 28 to 500, or 5ml to 200ml for the formulations used in liquid suspensions.
The column labeled AWP Package Price is the cost of the package that reflects the strength, package size, generic and unit dose features used in creating the empirical distribution. The AWP unit price is the Package Price divided by the number of units.
The Cost of Medication Order is the only column not taken directly from the Red Book, and is computed by determining the number of units needed to fill the medication order multiplied by the AWP Unit Price. For example, the drug product in row 2 of Table 1 requires 40 units (2 capsules, twice a day, for 10 days), multiplying 40 by $0.28 equals $11.27. The Unit Price for a liquid is the price per single milliliter, thus the total number of milliliters required to reach the strength of the medication order are used.
We apply the Rules to the 557 drug products that have the active ingredient cephalexin. The iterative elimination of drug products is described in the top section of Table 2. In this example, all drug products are administered orally and thus are clinically equivalent.
Table 2.
Results From Applying Pharmacy Practice-Based Substitution Rules to Three Medication Orders
| Cephalexin 500mg Twice a Day for 10 Days |
Lisinopril 20mg Once a Day for One year |
Naproxen 500mg Twice a Day for 3 Months |
||||
|---|---|---|---|---|---|---|
| Empirical Distribution |
Previous Research |
Empirical Distribution |
Previous Research |
Empirical Distribution |
Previous Research |
|
|
Iterative Costing Process |
||||||
| Total Active Ingredient Matches | 557 | 632 | 31 | |||
| Clinically Equivalent | −0 | −0 | −0 | |||
| Minimize Pill Burden | −31 | −332 | −0 | |||
| Reasonable Package Size | −217 | −189 | −0 | |||
| Unit Dose | −2 |
−2 |
−0 |
|||
| Total Substitutable Sample Size | 307 | 109 | 31 | |||
|
Costs |
||||||
| Base-case | - | $97.56 | - | $304.63 | - | $157.61 |
| Mean | $106.37 | - | $443.71 | - | $242.02 | - |
| Median | $113.33 | - | $388.69 | - | $254.16 | - |
| Min | $11.27 | $13.77 | $23.00 | $50.99 | $154.64 | $118.21 |
| Max | $259.40 | $97.56 | $952.98 | $522.97 | $359.10 | $197.01 |
| SD | 60.07 | - | 222.23 | - | 43.56 | - |
| Coefficient of Variation | 0.56 | - | 0.50 | - | 0.18 | - |
Previous research base-case estimates and uncertainty ranges are from previously published estimates of identical medication orders scaled to 2010 dollars; Cephalexin (Hayes and Williamson 2001) Lisinopril (Rosen et al. 2005) Naproxen (Wielage et al. 2013).
Of the 557 drug products, 526 are either of 250mg or 500mg strength, 50% or 100% of the dose in the medication order, and minimize pill burden. The 10-day length of the medication order requires a package size of at least 20, 500mg strength units or 40, 250mg strength units, eliminating 217 products. Two drug products are eliminated as they are unit dose and their package size is not equal to the medication order.
After the application of the Rules, 307 drug products remain. The cost of the medication order varies across the drug products with some notable systematic features. For instance the drug products in lines 6 and 7 of Table 1 are made by the same manufacturer (J) but differ only in package size. The price per 500 milligram is $0.15 greater in the smaller package size ($0.81 vs. $0.96), a relationship that is expected. Drug products in lines 13 and 14 made by manufacturer (I) also only differ by package size but have identical prices per unit ($1.63).
The bottom section of Table 2 illustrates key statistics. The range of the costs is from $11.27 to $259.40 with a mean of $106.37, median of $113.33 and a standard deviation of $60.07. The full empirical distribution of costs derived from our Rules is displayed in Figure 1a, and indicates the relative number of drug products at different costs. This is contrasted with the cost estimate and sensitivity analysis from a previously published paper by Hayes & Williamson (2001). Their base-case estimate of $97.56 (2010 US dollars) is defined as the maximum value, as the authors compared costs between a certain generic (the minimum) and non-generic drug product (the maximum). The difference in costs between a certain generic and non-generic drug product was used in a one-way sensitivity analysis as shown as dashed lines in the empirical distribution reflecting our Rules in Figure 1a. Hayes & Williamson (2001) assumed a uniform distribution between the minimum and maximum for probabilistic sensitivity analysis as shown in Figure 1b.
Figure 1.
Empirical distribution of costs of medication order for 500mg cephalexin twice a day for 10 days. Figure 1a uses Pharmacy Practice-Based Substitution Rules with the dashed vertical lines representing the one-way sensitivity analysis range used in Hayes and Williamson (2001) scaled to 2010 Dollars. Figure 1b displays the strategy specified by Hayes & Williamson (2001) for probabilistic sensitivity analysis which used a uniform distribution between $13.77 and $97.56, representing a generic and non-generic cost estimate respectively.
The costs of 135 of 307 (44%) substitutable drug products are included in the range used for uncertainty analysis by Hayes & Williamson (2001). One hundred seventy of the selected drug products (55%) are greater than the study’s base-case estimate of $97.56.
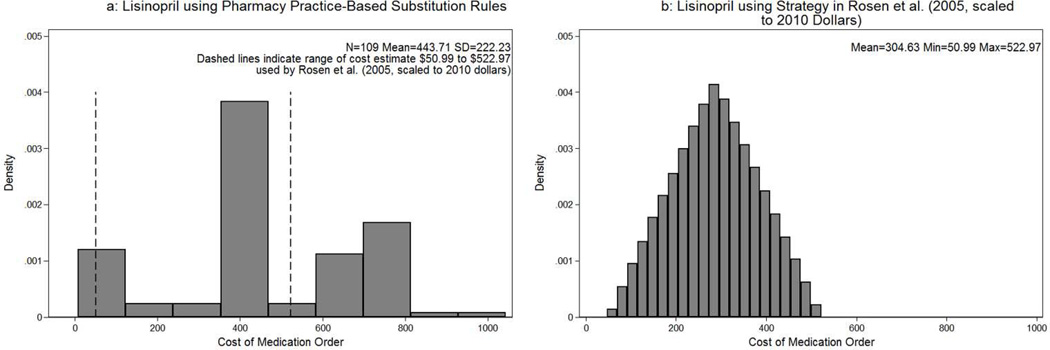
Next we illustrate the empirical distribution of 20mg lisinopril once a day for one year that is shown in Figure 2a and in Table 2. Of 632 drug products with the active ingredient lisinopril, there are 109 drug products that meet the Rules. The distribution is bi-modal with a mode just under $400 and another just over $700, indicating a large spread in overall costs (standard deviation of $222.23). Eighteen generic products (16% of selected drug products overall) have a cost of less than $200, while the majority of other generics and most non-generics were at least $400. Figure 2b reflects the published uncertainty analysis of Rosen et al. (2005) that used a base-case value at $304.63 and assumed a triangular distribution between the minimum and the maximum values for probabilistic sensitivity analysis. This previously published range includes costs of 62 (57%) substitutable drug products, omitting a substantial number of higher cost drug products.
Figure 2.
Empirical distribution of costs of medication order for 20mg lisinopril once a day for 1 year. Figure 2a uses Pharmacy Practice-Based Substitution Rules with the dashed vertical lines representing the one-way sensitivity analysis range used in Rosen et al. (2005) scaled to 2010 Dollars. Figure 2b displays the strategy specified by Rosen et al. (2005) for probabilistic sensitivity analysis which used a triangular distribution between $50.99 and $522.97.
Finally we illustrate the results of the costing procedure for naproxen using a medication order of 500mg capsules twice a day for 3 months. The complete results are shown in Table 2 and Figures 3a and 3b. All 31 drug products with an active ingredient naproxen meet the Rules. The previously reported base-case estimate of $157.61 is at the lower end of the empirical distribution.(42) The sensitivity analysis in the published work, Wielage et al. (2013), considered a range for one-way analysis of $118.21 to $197.01. A normal distribution where six standard deviations covered the range, was used for probabilistic sensitivity analysis. This range would only include the costs of 4, or 13%, of the substitutable products.
Figure 3.
Empirical distribution of costs of medication order for 500mg naproxen once a day for 3 months. Figure 3a uses Pharmacy Practice-Based Substitution Rules with the dashed vertical lines representing the one-way sensitivity analysis range used in Wielage et al. (2013) scaled to 2010 Dollars. Figure 3b displays the strategy specified by Wielage et al. (2013) for probabilistic sensitivity analysis which used a normal distribution with 6 standard deviations between $118.21 and $197.01.
A visual comparison of Figures 1, 2 and 3 shows the differences in distributions between our method and the previous studies for probabilistic sensitivity analysis.
Discussion
Our work describes a practical solution to an unconsidered problem when performing US-based economic evaluations. This is a unique issue to the United States where no single national payer publishes a price for given units of all particular drugs. Since this cost does not publically exist, and given the fragmented nature of reimbursement in the United States where multiple payers are known to face varying levels of price discrimination, our method offers a more transparent parameter estimate for both the base-case cost and its uncertainty. (54)
We defined our Pharmacy Practice-Based Substitution Rules as an approach to consider all reasonable products that could be used to fill the medication order and computed the empirical distribution of the costs. Our work highlights the careful consideration that should be given to drug costs, as there tends to be real and substantial differences in costs of identical products that can directly affect the results of cost effectiveness/decision analysis studies depending on the ratio of drug costs relative to non-drug costs.(55) In this work, for simplicity, we only present our Rules and examples for oral drug formulations, but our Rules can be adapted to all drug formulations with minor adjustments.
The empirical distributions derived with the Rules illustrate the substantial heterogeneity in costs for a particular medication order. The shapes of the empirical cost distributions of the three illustrative drugs were shown on the same horizontal cost scale for each drug example to illustrate the wide variation in shapes and costs of medication orders. We have shown that the cost of a given medication order varies substantially depending upon which drug product is used. This heterogeneity is inconsistent between drugs and classes of drugs, and is not explained by a separate examination of generics or non-generics.
It is clear that a single distributional assumption to reflect different cost distributions across drugs and drug classes does not exist. While the uniform distribution is chosen most frequently in the literature, other distributions such as triangular, normal and gamma are used. Our examples illustrate that these distributional choices are clearly not valid across all drugs. By considering all possible drug products, our Rules allow for a transparent method that better follows actual practice and provides a practical guide for applied studies in the US. Sampling from these empirical distributions for probabilistic sensitivity analyses and their range for one-way analyses provide better inputs to decision analyses.
The differences in drug cost for clinically equivalent products appear to be a function of differing package sizes and individual manufacturer differences. A detailed review of all records found many inconsistencies. The cost per unit by manufacturer varied for most drug products. Some drug products produced by the same manufacturer cost more for a smaller package size as one might expect, while for other manufactures, the cost per unit is less for a smaller package size. These inconsistencies highlight the need to account for the full set of products that could be used to fill a medication order. One single estimate does not capture what occurs in a real world setting where a heterogeneous drug product set is available.
Using our method could have substantial impact on the results of US-based economic evaluations, not only in drug studies, but also in most health technology studies, as these decision analyses often have a drug component. The extent to which our procedure will effect the overall results relies on two key factors: (i) how different our distribution is from what was used previously and (ii) the relative amount of drug costs to non-drug costs in the study. In some instances the derived distribution by the Rules may be tighter than what was previously estimated, suggesting a smaller range of ICERs seen in sensitivity analysis. Our three examples, however, showed far wider ranges of drug costs as compared to previous estimates, suggesting that the ranges of ICERs computed in sensitivity analysis will increase. For studies where a decision maker chooses one treatment over another that was close to a particular willingness to pay threshold, the overall decision or treatment strategy recommended could change if our method were adopted.
Our method is transparent and requires three main inputs: the cost metric, the frame of possible drug products, and the weighting of the drug products selected for inclusion. We chose AWP as our cost metric. We chose the entire Red Book as our frame, and we assumed equal weighting of the drug products selected in the empirical distribution.
The choice of the appropriate cost metric is beyond the scope of this study. Our literature review revealed that AWP is the most widely used cost metric. AWP and other publically available metrics such as WAC are flawed, as they rely on drug manufacturer self-reported data to compute an estimate of the acquisition cost. Both AWP and WAC have been shown to be an overestimate of the true acquisition cost. (14) The goal of our work is not to present a perfect solution to drug costing in the US, but to use the information currently available to provide a more realistic and data driven assessment of uncertainty. If better or more relevant cost metrics are available for certain payers, these can be transparently substituted in our approach with new empirical distributions generated.
With regard to the choice of the frame for reflecting drug products, we could have restricted the sample to include only generics or exclude certain drug products. The Rules can be altered to only include those drug products that yield exact matches of desired tablet strength. Given expert guidance, choices as to the inclusion of certain drug products or the weighting of these drugs to reflect actual market share could be incorporated.
These enhancements also apply to different payers perspectives. A certain payer, such as a health system, may have the ability to influence which drug products are used. The empirical distribution could only consider drug products that maximize revenue, or minimize cost, to that payer. Additionally if the true distribution or market share of a given drug were known by the payer, the actual distribution of products and corresponding cost would be used in lieu of the equal weight assumption.
Our approach defines the Rules to derive an empirical distribution for medication orders costs and include that distribution in a probabilistic sensitivity analysis simulation where multiple iterations reflect the full range of costs used in drug costing along with the other estimates in the model. The base-case estimate can be defined using either the mean or median of the cost distribution depending on the level of skew.
When a researcher uses a parameter estimate and subsequent sensitivity analysis on drug product cost, they are seeking to control for two simultaneous phenomena. One phenomenon is the uncertainty about the extent to which the cost is correctly measured by the chosen metric for the reported payer’s perspective. The second phenomenon is the uncertainty about which drug product is used to fill a certain medication order. Our Rules have examined and offered a solution to the latter.
Our method can be used with any cost metric to improve estimates of all kinds of drug costs and provide an assessment of the variation of cost estimates from the perspective chosen. Our solution of creating an empirical distribution creates a more realistic distribution than has previously been available for US based drug cost estimates.
Acknowledgments
Financial support for this study was provided in part by grants from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources 1UL1RR025011 (Rosenberg) and the National Institute of Mental Health T32 MH18029 (Levy). The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Contributor Information
Joseph F Levy, University of Wisconsin-Madison Department of Population Health Sciences.
Patrick D Meek, Albany College of Pharmacy and Health Sciences Department of Pharmacy.
Marjorie A Rosenberg, University of Wisconsin-Madison Department of Actuarial Science, Risk Management and Insurance and Department of Biostatistics and Medical Informatics.
References
- 1.Hay JW, Smeeding J, Carroll NV, Drummond M, Garrison LP, Mansley EC, et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR Drug Cost Task Force report--Part I. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2010;13(1):3–7. doi: 10.1111/j.1524-4733.2009.00663.x. [DOI] [PubMed] [Google Scholar]
- 2.Garrison LP, Jr, Mansley EC, Abbott TA, 3rd, Bresnahan BW, Hay JW, Smeeding J. Good research practices for measuring drug costs in cost-effectiveness analyses: a societal perspective: the ISPOR Drug Cost Task Force report--Part II. Value Health. 2010;13(1):8–13. doi: 10.1111/j.1524-4733.2009.00660.x. [DOI] [PubMed] [Google Scholar]
- 3.Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford university press; 2006. [Google Scholar]
- 4.Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM modeling good research practices task force-6. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15(6):835–842. doi: 10.1016/j.jval.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health economics. 2005;14(4):339–347. doi: 10.1002/hec.985. [DOI] [PubMed] [Google Scholar]
- 6.Lewis G, Peake M, Aultman R, Gyldmark M, Morlotti L, Creeden J, et al. Cost-Effectiveness of Erlotinib versus Docetaxel for Second-Line Treatment of Advanced Non-Small-Cell Lung Cancer in the United Kingdom. Journal of International Medical Research. 2010;38(1):9–21. doi: 10.1177/147323001003800102. [DOI] [PubMed] [Google Scholar]
- 7.Hiligsmann M, Reginster JY. Potential cost-effectiveness of denosumab for the treatment of postmenopausal osteoporotic women. Bone. 2010;47(1):34–40. doi: 10.1016/j.bone.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Lidgren M, Jonsson B, Rehnberg C, Willking N, Bergh J. Cost-effectiveness of HER2 testing and 1-year adjuvant trastuzumab therapy for early breast cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19(3):487–495. doi: 10.1093/annonc/mdm488. [DOI] [PubMed] [Google Scholar]
- 9.Lathia N, Isogai PK, De Angelis C, Smith TJ, Cheung M, Mittmann N, et al. Cost-effectiveness of filgrastim and pegfilgrastim as primary prophylaxis against febrile neutropenia in lymphoma patients. Journal of the National Cancer Institute. 2013;105(15):1078–1085. doi: 10.1093/jnci/djt182. [DOI] [PubMed] [Google Scholar]
- 10.Liew D, De Abreu Lourenço R, Adena M, Chim L, Aylward P. Cost-effectiveness of 12-month treatment with ticagrelor compared with clopidogrel in the management of acute coronary syndromes. Clin Ther. 2013;35(8):1110–1117. doi: 10.1016/j.clinthera.2013.06.015. e9. [DOI] [PubMed] [Google Scholar]
- 11.Dorian P, Kongnakorn T, Phatak H, Rublee DA, Kuznik A, Lanitis T, et al. Cost-effectiveness of apixaban vs. current standard of care for stroke prevention in patients with atrial fibrillation. European heart journal. 2014 doi: 10.1093/eurheartj/ehu006. ehu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gencarelli DM. Average wholesale price for prescription drugs: is there a more appropriate pricing mechanism? NHPF issue brief / National Health Policy Forum, George Washington University. 2002;2002(775):1–19. [PubMed] [Google Scholar]
- 13.Willke RJ. Beyond AWP. Way Beyond. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2010;13(1):1. doi: 10.1111/j.1524-4733.2009.00673.x. [DOI] [PubMed] [Google Scholar]
- 14.Curtiss FR, Lettrich P, Fairman KA. What is the price benchmark to replace average wholesale price (AWP)? Journal of Managed Care Pharmacy. 2010;16:492–501. doi: 10.18553/jmcp.2010.16.7.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ayvaci MU, Shi J, Alagoz O, Lubner SJ. Cost-effectiveness of adjuvant FOLFOX and 5FU/LV chemotherapy for patients with stage II colon cancer. Medical decision making : an international journal of the Society for Medical Decision Making. 2013;33(4):521–532. doi: 10.1177/0272989X12470755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brogan A, Mauskopf J, Talbird SE, Smets E. US cost effectiveness of darunavir/ritonavir 600/100mg bid in treatment-experienced, HIV-infected adults with evidence of protease inhibitor resistance included in the TITAN trial. PharmacoEconomics. 2010;28(1):129–146. doi: 10.2165/11587490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Casciano R, Chulikavit M, Perrin A, Liu Z, Wang X, Garrison LP. Cost-effectiveness of everolimus vs sunitinib in treating patients with advanced, progressive pancreatic neuroendocrine tumors in the United States. Journal of medical economics. 2012;15(Suppl 1):55–64. doi: 10.3111/13696998.2012.720319. [DOI] [PubMed] [Google Scholar]
- 18.Coleman CI, Straznitskas AD, Sobieraj DM, Kluger J, Anglade MW. Cost-effectiveness of clopidogrel plus aspirin for stroke prevention in patients with atrial fibrillation in whom warfarin is unsuitable. The American journal of cardiology. 2012;109(7):1020–1025. doi: 10.1016/j.amjcard.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Gidwani R, Khan ZM, Fenaux P, Beach CL, Pashos CL. A cost-effectiveness analysis of using azacitidine vs. decitabine in treating patients with myelodysplastic syndromes. Journal of medical economics. 2012;15(1):145–154. doi: 10.3111/13696998.2011.631067. [DOI] [PubMed] [Google Scholar]
- 20.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2011;14(6):836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. Journal of viral hepatitis. 2013;20(12):847–857. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 22.Harrington AR, Armstrong EP, Nolan PE, Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke; a journal of cerebral circulation. 2013;44(6):1676–1681. doi: 10.1161/STROKEAHA.111.000402. [DOI] [PubMed] [Google Scholar]
- 23.Kim AS, Nguyen-Huynh M, Johnston SC. A cost-utility analysis of mechanical thrombectomy as an adjunct to intravenous tissue-type plasminogen activator for acute large-vessel ischemic stroke. Stroke a journal of cerebral circulation. 2011;42(7):2013–2018. doi: 10.1161/STROKEAHA.110.606889. [DOI] [PubMed] [Google Scholar]
- 24.Kymes SM, Pusic I, Lambert DL, Gregory M, Carson KR, DiPersio JF. Economic Evaluation of a Plerixafor for Stem Cell Mobilization. The American journal of managed care. 2012;18(1):33. [PMC free article] [PubMed] [Google Scholar]
- 25.Laskin BL, Goebel J, Starke JR, Schauer DP, Eckman MH. Cost-effectiveness of latent tuberculosis screening before steroid therapy for idiopathic nephrotic syndrome in children. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(1):22–32. doi: 10.1053/j.ajkd.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazenby GB, Unal ER, Andrews AL, Simpson K. A Cost-Effectiveness Analysis of Anal Cancer Screening in HIV-Positive Women. Journal of lower genital tract disease. 2012;16(3):275–280. doi: 10.1097/LGT.0b013e31823cde2f. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Mullin R, Blazawski J, Coleman CI. Cost-effectiveness of apixaban compared with warfarin for stroke prevention in atrial fibrillation. PloS one. 2012;7(10):e47473. doi: 10.1371/journal.pone.0047473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauskopf J, Brogan A, Martin S, Smets E. Cost effectiveness of darunavir/ritonavir in highly treatmentexperienced, HIV-1-infected adults in the USA. PharmacoEconomics. 2010;28(1):83–105. doi: 10.2165/11587470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Mauskopf J, Brogan AJ, Talbird SE, Martin S. Cost-effectiveness of combination therapy with etravirine in treatment-experienced adults with HIV-1 infection. AIDS. 2012;26(3):355–364. doi: 10.1097/QAD.0b013e32834e87e6. [DOI] [PubMed] [Google Scholar]
- 30.Miller SM, Goldstein JL, Gerson LB. Cost-effectiveness model of endoscopic biopsy for eosinophilic esophagitis in patients with refractory GERD. The American journal of gastroenterology. 2011;106(8):1439–1445. doi: 10.1038/ajg.2011.94. [DOI] [PubMed] [Google Scholar]
- 31.Newman MJ, Jones LT, Kraft JM, Lee AR, Lech GM, Farrell NM, et al. Cost-effectiveness of fulvestrant-250 mg versus 500 mg in postmenopausal women with estrogen receptor-positive metastatic breast cancer and disease progression after antiestrogen therapy. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2012;18(4):394–401. doi: 10.1177/1078155212438253. [DOI] [PubMed] [Google Scholar]
- 32.Nuijten M, Andress DL, Marx SE, Sterz R. Chronic kidney disease Markov model comparing paricalcitol to calcitriol for secondary hyperparathyroidism: a US perspective. Current medical research and opinion. 2009;25(5):1221–1234. doi: 10.1185/03007990902844097. [DOI] [PubMed] [Google Scholar]
- 33.Pan F, Goh JW, Cutter G, Su W, Pleimes D, Wang C. Long-term cost-effectiveness model of interferon beta-1b in the early treatment of multiple sclerosis in the United States. Clin Ther. 2012;34(9):1966–1976. doi: 10.1016/j.clinthera.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 34.Pan F, Peng S, Fleurence R, Linnehan JE, Knopf K, Kim E. Economic analysis of decitabine versus best supportive care in the treatment of intermediate- and high-risk myelodysplastic syndromes from a US payer perspective. Clin Ther. 2010;32(14):2444–2456. doi: 10.1016/j.clinthera.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Park H, Rascati KL, Keith MS, Hodgkins P, Smyth M, Goldsmith D, et al. Cost-effectiveness of lanthanum carbonate versus sevelamer hydrochloride for the treatment of hyperphosphatemia in patients with end-stage renal disease: a US payer perspective. Value Health. 2011;14(8):1002–1009. doi: 10.1016/j.jval.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 36.Patel JJ, Mendes MA, Bounthavong M, Christopher ML, Boggie D, Morreale AP. Cost-utility analysis of bevacizumab versus ranibizumab in neovascular age-related macular degeneration using a Markov model. Journal of evaluation in clinical practice. 2012;18(2):247–255. doi: 10.1111/j.1365-2753.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 37.Sikirica V, Erder MH, Xie J, Macaulay D, Diener M, Hodgkins P, et al. Cost Effectiveness of Guanfacine Extended Release as an Adjunctive Therapy to a Stimulant Compared with Stimulant Monotherapy for the Treatment of Attention-Deficit Hyperactivity Disorder in Children and Adolescents. PharmacoEconomics. 2012;30(8):e1–e15. doi: 10.2165/11632920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith KJ, Baik SH, Reynolds CF, III, Rollman BL, Zhang Y. Cost-effectiveness of Medicare drug plans in schizophrenia and bipolar disorder. The American journal of managed care. 2013;19(2):e55. [PMC free article] [PubMed] [Google Scholar]
- 39.Snedecor SJ, Carter JA, Kaura S, Botteman MF. Cost-effectiveness of denosumab versus zoledronic acid in the management of skeletal metastases secondary to breast cancer. Clin Ther. 2012;34(6):1334–1349. doi: 10.1016/j.clinthera.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Snedecor SJ, Carter JA, Kaura S, Botteman MF. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a cost-effectiveness analysis. Journal of medical economics. 2013;16(1):19–29. doi: 10.3111/13696998.2012.719054. [DOI] [PubMed] [Google Scholar]
- 41.Stopeck A, Rader M, Henry D, Danese M, Halperin M, Cong Z, et al. Cost-effectiveness of denosumab vs zoledronic acid for prevention of skeletal-related events in patients with solid tumors and bone metastases in the United States. Journal of medical economics. 2012;15(4):712–723. doi: 10.3111/13696998.2012.675380. [DOI] [PubMed] [Google Scholar]
- 42.Wielage RC, Bansal M, Andrews JS, Wohlreich MM, Klein RW, Happich M. The Cost-Effectiveness of Duloxetine in Chronic Low Back Pain: A US Private Payer Perspective. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2013;16(2):334–344. doi: 10.1016/j.jval.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Wong YN, Meropol NJ, Speier W, Sargent D, Goldberg RM, Beck JR. Cost implications of new treatments for advanced colorectal cancer. Cancer. 2009;115(10):2081–2091. doi: 10.1002/cncr.24246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodward TC, Tafesse E, Quon P, Lazarus A. Cost effectiveness of adjunctive quetiapine fumarate extended-release tablets with mood stabilizers in the maintenance treatment of bipolar I disorder. PharmacoEconomics. 2010;28(9):751–764. doi: 10.2165/11538350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Xie J, Diener M, Sorg R, Wu EQ, Namjoshi M. Cost-effectiveness of denosumab compared with zoledronic acid in patients with breast cancer and bone metastases. Clinical breast cancer. 2012;12(4):247–258. doi: 10.1016/j.clbc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Xie J, Namjoshi M, Wu EQ, Parikh K, Diener M, Yu AP, et al. Economic evaluation of denosumab compared with zoledronic acid in hormone-refractory prostate cancer patients with bone metastases. Journal of Managed Care Pharmacy. 2011;17(8):621. doi: 10.18553/jmcp.2011.17.8.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosen AB, Hamel MB, Weinstein MC, Cutler DM, Fendrick AM, Vijan S. Cost-effectiveness of full Medicare coverage of angiotensin-converting enzyme inhibitors for beneficiaries with diabetes. Annals of internal medicine. 2005;143(2):89–99. doi: 10.7326/0003-4819-143-2-200507190-00007. [DOI] [PubMed] [Google Scholar]
- 48.Wielage RC, Bansal M, Andrews JS, Klein RW, Happich M. Cost-utility analysis of duloxetine in osteoarthritis: a US private payer perspective. Applied health economics and health policy. 2013;11(3):219–236. doi: 10.1007/s40258-013-0031-3. [DOI] [PubMed] [Google Scholar]
- 49.Hayes CS, Williamson H., Jr Management of Group A beta-hemolytic streptococcal pharyngitis. American family physician. 2001;63(8):1557–1564. [PubMed] [Google Scholar]
- 50.Center for Drug E, Research DoDM, Services CfDE, Research OoGD. Orange book approved drug products with therapeutic equivalence evaluations. Rockville, Md.: U.S. Dept. of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Office of Pharmaceutical Science, Office of Generic Drugs; 1985. Available from: http://purl.access.gpo.gov/GPO/LPS1445. [Google Scholar]
- 51.Collier R. Reducing the “pill burden”. Canadian Medical Association Journal. 2012;184(2):E117–E118. doi: 10.1503/cmaj.109-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hauber AB, Han S, Yang J-C, Gantz I, Tunceli K, Gonzalez JM, et al. Effect of pill burden on dosing preferences, willingness to pay, and likely adherence among patients with type 2 diabetes. Patient preference and adherence. 2013;7:937. doi: 10.2147/PPA.S43465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pharmacy Times. [Accessed December 20, 2013];Top 200 Drugs of 2012. 2013 Avaialble from: http://www.pharmacytimes.com/publications/issue/2013/July2013/Top-200-Drugs-of-2012.
- 54.Zodet MW, Hill SC, Zuvekas SH. Evaluating an Alternative Data Source for Editing MEPS Drug Prices. 2012 [Google Scholar]
- 55.Ofman JJ, Gralnek IM, Udani J, Fennerty MB, Fass R. The cost-effectiveness of the omeprazole test in patients with noncardiac chest pain. The American journal of medicine. 1999;107(3):219–227. doi: 10.1016/s0002-9343(99)00219-3. [DOI] [PubMed] [Google Scholar]