Abstract
Objectives
State and federal recommendations for infection control/prevention (IC) in nursing homes (NHs) have become more frequent, but little is known about actual NH policies/practices.
Design and setting
In 2012, we conducted a national survey about the extent to which NHs follow suggested IC practices with regard to three common healthcare-associated pathogens: methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile (C.diff), and extended-spectrum β-lactamase (ESBL) producers, and their prevalence in NHs. We adapted a previously used and validated NH infection control survey, including questions on prevalence, admission and screening policies, contact precaution, decolonization, and cleaning practices.
Results
1,002 surveys were returned. 14.2% of NHs are less likely to accept residents with MRSA, with principal reason being lack of single/cohort rooms. NHs do not routinely perform admission screening (96.4%) because it is not required by regulation (56.2%) and would not change care provision (30.7%). Isolation strategies vary substantially, with gloves being most commonly used. Most NHs (75.1%) do not decolonize MRSA carriers, but some (10.6%) decolonize over 90% of residents. Despite no guidance on how resident rooms on contact precautions should be cleaned, 59.3% of NHs report enhanced cleaning for such rooms.
Conclusions
Overall, NHs tend to follow voluntary infection control guidelines only if doing so does not require substantial financial investment in new/dedicated staff or infrastructure.
Keywords: Nursing home, infection prevention, healthcare-associated infection, multidrug-resistant organism
INTRODUCTION
Nursing home (NH) residents are at a particularly high risk of developing healthcare-associated infections (HAIs) due to frail health, sharing of closed common environments, and frequent hospitalizations.1,2 Among the common causes of these HAIs are multidrug-resistant organisms (MDROs)3,4 such as Methicillin-resistant Staphylococcus aureus (MRSA)5,6 or Extended-spectrum β-lactamase (ESBL).7 Clostridium difficile (C.diff) is also increasingly common,8,9 often due to the overuse of antibiotics.10
The Centers for Medicare and Medicaid Services (CMS) require all Medicare/Medicaid certified NHs to have active infection control (IC) and prevention programs,11 but offer no specific practice standards. The only existing national standards for NHs, published by the Society for Healthcare Epidemiology of America (SHEA) and the Association for Professionals in Infection Control and Epidemiology (APIC),3 are largely adapted from acute care settings.12,13 Its recommendations are broad, allowing for modifications based upon the residents’ clinical situation and facility resources, and defer to guidance developed by a handful of states.14–16
Currently, little is known about actual IC policies and practices that NHs adopt. Existing studies17–19 are based on a small number of NHs and largely pre-date the most recent 2009 CMS infection control requirements for certified NHs, which have mandated more robust IC programs and revisions to internal policies and practices.11
In this study we examined the prevalence of healthcare-associated pathogens and infection control policies and practices in a national sample of NHs. We focused on the extent to which facilities follow existing national guidelines3 with regard to dedicated time spent on infection control duties, admission and screening policies, isolation and contact precautions, decolonization, and room cleaning practices, as they relate to MRSA, ESBL, and C. diff.
METHODS
In 2012 we conducted a national survey of Medicare/Medicaid certified facilities. A random sample of 6,700 US NHs was identified using the CMS Nursing Home Compare website. Surveys were addressed to Directors of Nursing (DONs), asking for the survey to be completed by the person most knowledgeable about infection control and prevention in the facility. Two follow-up mailings were sent to non-respondents 4 and 8 weeks post initial mailing.
We adapted a previously validated NH infection control survey.17 The survey (Appendix 1) was composed of 56 mostly close-ended questions about residents who are colonized or infected, admission policies for such residents, policies for routine screening on admission and for contact precautions, residents’ activity restrictions, decolonization practices, and the environmental cleaning practices for rooms of residents on contact precautions. Respondents were also asked about their title and the amount of time dedicated to infection control. The study was approved by the Institutional Review Board.
Secondary data, 2012 CMS Nursing Home Compare report, the Online Survey, Certification and Reporting (OSCAR), and the LTC focus website,20 were also employed to provide information on NH characteristics and infection control deficiency citations. Rural-Urban Commuting Area Codes (RUCA) file (zip code level) was used to determine NHs’ rural-urban location. The primary and secondary databases were linked at the facility-level, using a unique provider identification number.
To investigate the generalizability and the potential response bias, we compared facility characteristics between responding and non-responding NHs using Wilcoxon rank-sum tests for continuous variables, and chi-square tests for categorical variables.
RESULTS
Completed questionnaires were received from 1,002 NHs for an adjusted response rate of 15.1%.
Nursing Home Characteristics
We did not observe statistically significant differences between responding and non-responding NHs with regard to bed size, percent of Medicare residents, staffing of licensed practical nurses (LPNs), infection control citations, and facility case-mix index (Table 1). However, responding facilities had higher occupancy rates, higher staffing levels of registered nurses (RNs) and certified nursing assistants (CNAs), lower percent of Medicaid residents, and fewer total deficiency citations. Respondents were more likely to be five-star facilities, and less likely to be for-profit and chain-affiliated.
Table 1.
Comparison of nursing home characteristics: responding versus non-responding facilities
| Variable | Respondent (N=996) |
Non respondent (N=5704) |
p-value |
|---|---|---|---|
| Facility characteristics | |||
| Number of beds, mean (SD) | 126.4 (61.8) | 123.8 (56.7) | 0.50 |
| Occupancy, %, mean(SD) | 85.6 (11.8) | 84.4 (12.3) | <0.01 |
| For-profit, no. (%) | 604 (60.6) | 4387 (76.9) | <0.01 |
| Chain affiliation, no. (%) | 509 (51.1) | 3353 (58.8) | <0.01 |
| % Medicare patients, mean (SD) | 14.3 (10.9) | 14.6 (10.8) | 0.28 |
| % Medicaid patients, mean (SD) | 59.8 (18.9) | 63.9 (19.0) | <0.01 |
| Located in the rural area, no. (%) | 175 (17.6) | 848 (14.9) | <0.01 |
| Staffing | |||
| RN hours, mean (SD) | 0.73 (0.34) | 0.67 (0.33) | <0.01 |
| LPN hours, mean (SD) | 0.82 (0.33) | 0.82 (0.33) | 0.46 |
| CNA hours, mean (SD) | 2.49 (0.58) | 2.40 (0.56) | <0.01 |
| Quality of the NH | |||
| Total number of deficiency citations, mean (SD) | 9.8 (6.6) | 10.5 (7.3) | 0.04 |
| Any infection control citation, no. (%) | 380 (38.2) | 2196 (38.5) | 0.84 |
| Five-star NHa, no. (%) | 182 (18.3) | 826 (14.6) | <0.01 |
| Resident acuity | |||
| Average RUG -III case mixed index (all admissions)b, mean (SD) | 1.07 (0.09) | 1.07 (0.10) | >0.99 |
RN, registered nurse; LPN, licensed practical or vocational nurse; CNA, certified nursing assistants; NH, nursing home. RUG, Resource Utilization Group. SD, standard deviation.
This indicator shows whether a NH is a five-star facility, an overall measure for NH quality based on the CMS Nursing Home Compare five-star quality rating system that takes into account performance on state health inspections, quality measures and nurse staffing levels. NHs assigned five-stars are considered to have above average quality compared to other facilities in that state.
This index measures the resident acuity of a facility, and is calculated by averaging the scores for all residents admitted to the facility based on the Resource Utilization Group-III classification system used by CMS for Medicare payment adjustment.
Prevalence of Healthcare-Associated Pathogens
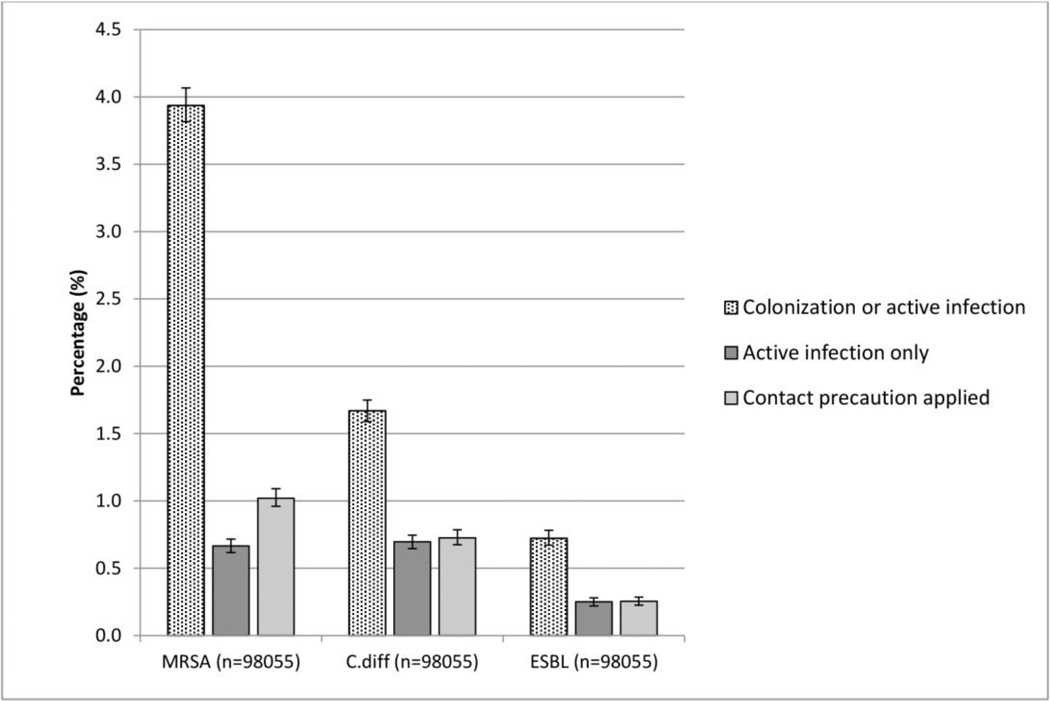
Overall, NHs reported 3.9% of residents as MRSA-positive (95% confidence interval: 3.8%–4.1%). Of these, 16.9% had active infections and were on antibiotic therapy, and 25.9% were on contact precautions. For C.diff, the reported prevalence rate was 1.7%, (95%CI: 1.6%–1.8%); 41.7% were active infections and 43.5% were on contact precautions. Less than 1% of residents were reported harboring ESBL (0.7%; 95% CI: 0.7%–0.8%), among them 34.7% with active infections and 35.3% on contact precautions (Figure 1).
Figure 1.
Overall prevalence rate of healthcare-associated pathogens in nursing homes for MRSA; C.diff; ESBL. Error bars indicate 95% confidence intervals. (n=total number of residents).
Resources and Staffing for Infection Control Activities
In most facilities, the individuals most knowledgeable about infection control and prevention practices were the DONs (n=520, 52.5%), followed by dedicated infection preventionists (IPs) (n=245, 24.8%). NHs devoted 10.5 hours per week (median) per 100 residents to infection control and prevention activities, with interquartile range (IQR) of 5.6–18.7 (Table 2). Approximately 6.5% of the NHs reported over 40 hours per week per 100 residents. NHs assigned 18 rooms (median) to each cleaning staff member (IQR, 15–22) (Table 2).
Table 2.
Staffing for infection control activities, screening policies for MRSA on admission to nursing homes, decolonization policies for MRSA-positive residents
| Variable | Distribution |
|---|---|
| Dedicated infection control hours per week per 100 residents, median (IQR) | 10.5 (5.6–18.7) |
| Assigned rooms per cleaning staff member, median (IQR) | 18 (15–22) |
| Any screening policies adopted | 35 (3.6) |
| Site of screening a | |
| Nares | 22 (62.9) |
| Wounds | 18 (51.4) |
| Axilla | 3 (8.6) |
| Groin | 3 (8.6) |
| Throat | 2 (5.7) |
| Reasons for not screening a | 941 (96.4) |
| Not required by regulatory agencies | 529 (56.2) |
| Results would not change care provision | 289 (30.7) |
| MRSA is rare at our facility | 198 (21.0) |
| Screening cost | 165 (17.5) |
| Never considered | 165 (17.5) |
| Impact on staff time | 57 (6.1) |
| Other (reasons provided) | |
| Already performed by hospitals prior to admission | 120 (12.8) |
| Only if indicated by symptoms | 25 (2.7) |
| Not applicable in long-term care settings | 12 (1.3) |
| Not recommended by the facility’s internal policy | 9 (1.0) |
| Not recommended by CDC/other infection control agencies | 5 (0.5) |
| No physician orders | 5 (0.5) |
| Universal /standard precautions applied to all admissions | 5 (0.5) |
| MRSA colonization is so common that results will be mostly positive | 5 (0.5) |
| No screening on employees | 4 (0.4) |
| Positive results require additional tracking and treatment | 2 (0.2) |
| Percentage of MRSA-positive residents being decolonized | |
| None | 615 (75.1) |
| <10 | 66 (8.1) |
| 10–90 | 51 (6.2) |
| >=90 | 87 (10.6) |
MRSA, methicillin- resistant Staphylococcus aureus. CDC, Centers for Disease Control and Prevention. IQR, interquartile range.
Respondents could select more than one option. Data are number (%) of nursing homes unless otherwise indicated.
Policies/Practices for Infection Control and Prevention
In this section, we compare infection control policies and practices reported by the NHs with the currently available recommendations and guidelines (in italics).
1) Denial of admissions solely on the basis of colonization or infection with MDROs is not appropriate.3,12,21
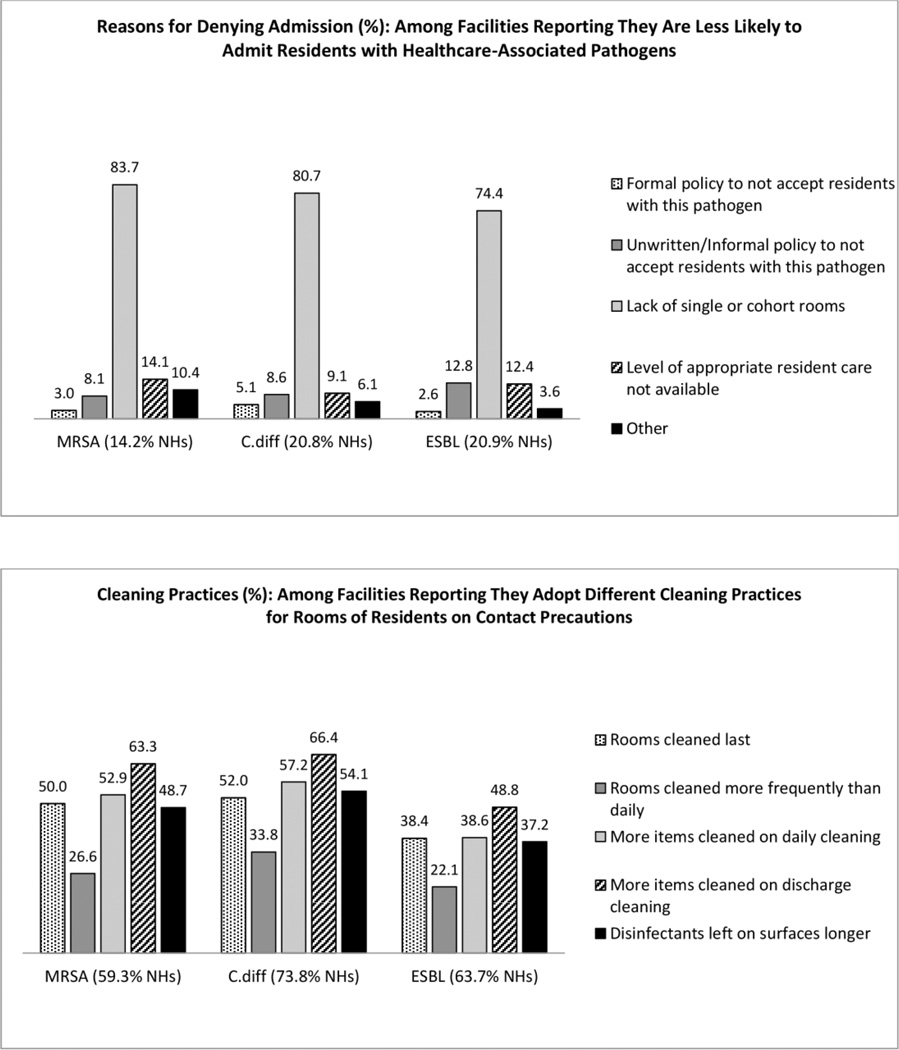
This practice was not very common (14.2%–20.9%) (Figure 2), and was mostly attributed to a lack of single or cohort rooms (74.4%–83.7%). Few NHs (2.6%–5.1%) reported having formal denial policies for MDROs or C.diff, but 8.1% to 12.8% reported informal denial policies.
Figure 2.
Admission denial policies and cleaning practices for residents harboring healthcare-associated pathogens: MRSA; C.diff, and ESBL.
2) There are currently no recommendations for routine screening for MRSA on NH admission
Very few (3.6%) NHs performed routine screening for MRSA on admission (Table 2). When screening occurred, nares and wounds were the two most common sites. More than half of NHs stated that they do not screen because it is not required by regulatory agencies. Thirty percent reported that screening would not affect care provision and 12.8% stated that screening was done in hospitals prior to NH transfer. They also identified cost (17.5%) and limited staff resources (6.1%) as reasons for not screening.
3) Isolation precautions and restrictions on activities for residents harboring MRSA
Apply contact precautions for residents with draining wounds, including single room, gloves and gowns for all resident contact and upon room entry, and dedicated care equipment.12,13,21
Use mask in addition to contact precautions when near residents with respiratory symptoms or performing splash-generating procedures,12,13,21 but not routinely to prevent transmission from patient to health care workers.12
Limit the movement or transport of residents with draining wounds from the room for essential purposes only.21Allow colonized or infected residents whose site of colonization or infection can be appropriately contained, and who can observe good hand hygiene practices, to enter common areas and participate in group activities.12,21
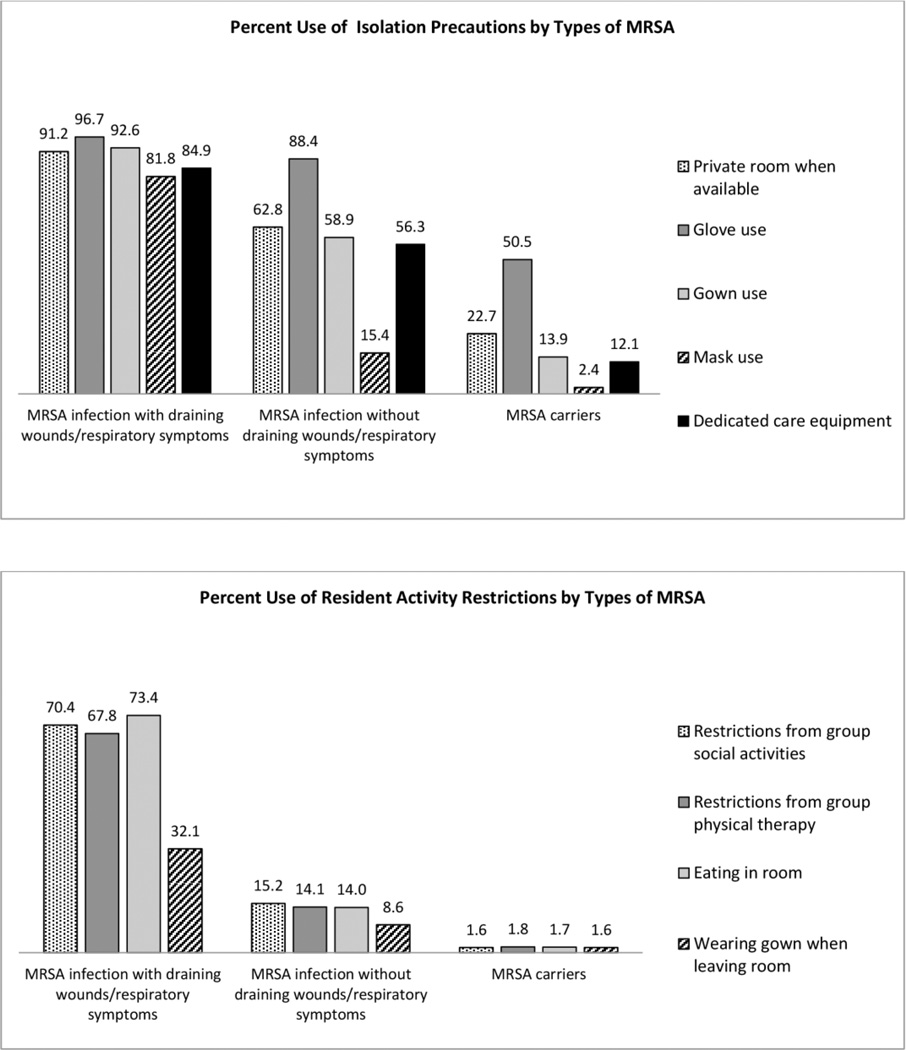
For MRSA-infected residents with draining wounds 76.9% of NHs report using full contact precautions (private rooms, glove and gown use, dedicated equipment); another 21.4% implemented less than complete contact precautions, primarily due to lack of dedicated equipment (62.2%) and private rooms (32.7%). More than half reported using precautions for infections not involving draining wounds. While contact precautions for MRSA carriers were less frequent, 50.5% reported using gloves when caring for these residents. Masks were used in 81.8% of NHs when near infected residents with respiratory symptoms, and 15.4% used masks regardless of respiratory symptoms or for asymptomatic carriers (2.4%) (Figure 3). Similar patterns were observed Vis-a-Vis activity restrictions (Figure 3). Over two thirds of NHs reported using restrictions on activities of infected residents with uncontained wounds; fewer (14.0%–15.2%) reported use for those without draining wounds. Restrictions for MRSA carriers were rarely adopted (<2%).
Figure 3.
Isolation precaution and activity restriction policies for residents harboring MRSA.
4) Decolonization for MRSA-positive residents is not recommended routinely but should be used as a component of intensified MRSA control program for a limited period of time on a case-by-case basis12,21
Most NHs (75.1%) reported they do not decolonize residents harboring MRSA, and another 8.1% reported decolonizing <10% (Table 2). However, 10.6% reported they decolonize at least 90% of such residents.
5) Prioritize room cleaning of residents on contact precautions;12,21 however, no guidance exists as to how such rooms should be cleaned
For environmental cleaning, approximately two thirds of NHs reported adopting different cleaning practices for rooms of residents on contact precautions (Figure 2). For example, on discharge more items were cleaned in rooms previously occupied by residents on contact precautions for MRSA (63.3%), C.diff (66.4%), and ESBL (48.8%). In addition, 37.2%–54.1% of NHs reported leaving disinfectants on surfaces longer in these rooms. The least common approach was to increase the frequency of cleaning (26.6% for MRSA; 33.8% for C.diff, and 22.1% for ESBL).
DISCUSSION
In the last two decades, an increasing number of state and regional guidelines or recommendations have been issued for NH surveillance and infection control activities. More recently CMS has issued national requirements for NHs to implement more robust infection prevention and control programs. Yet, little is known about the NHs’ response to these largely voluntary infection control approaches. This national study is one of the first to shed light on NHs’ practices in this regard.
Consistent with the current lack of recommendations for MDRO screening, the vast majority of NHs reported no routine screening activities. Aside from the absence of regulatory mandates, many NHs identified lack of actionable response to screening (positive results would not change care provision) and lack of resources (impact on staffing, cost of screening) as reasons for not screening. Some NHs also reported relying on hospital screening to detect colonization on admission. Nine states have enacted laws requiring active surveillance cultures at hospital admissions.22
In absence of routine screening, it is not surprising that MDRO prevalence reported by the survey respondents (e.g. 3.9% for MRSA) is considerably lower than prevalence based on screening cultures (24%–58%).17,23,24A recent study using the Minimum Data Set (MDS) to assess prevalence, reported results similar to ours.25 Unlike surveillance testing, both the MDS and surveys rely on staff knowledge about residents’ disease status,26 and thus are unlikely to reflect true carriage burden or risk of transmission.
Infection control programs in NHs are supposed to be spearheaded by IPs,3 but this practice is far from being universally adopted. Dedicated IPs were found in only one quarter of NHs and full-time IPs were rarely available. Although empirical data justifying a fulltime IP in NHs are still lacking, a ratio of 1 IP/250 beds has been suggested as optimal.3,27 By this standard, the NHs in our study are nearly 40% below the expected IP staff levels,3,27 and 60% below that for small rural hospitals.28
The disparity in infection control resources between hospitals and NHs is worrisome, especially in an era when patient transfers between the two care settings are more and more frequent, potentially contributing to the spread of healthcare-associated pathogens.29 A recent study found that an outbreak in one NH influences MRSA prevalence in multiple hospitals with which that NH shares patients.30
Current guidelines do not recommend denying NH admission solely based on colonization or infection with MDROs,3,12,21 and indeed our results suggest that formal NH policies do not support such practice. However, a substantive number of NHs report they are less likely to admit such patients. Shortage of single rooms and inability to provide appropriate level of care are cited as reasons for denying admission, as previously reported in other studies.18,31
Following concerns about delivering care that is consistent with a “home-like” environment,32 most NHs impose only limited restriction on activities of residents whose site of colonization or infection can be contained. This practice is consistent with a prior study showing that compared with universal glove use for all residents, contact isolation precautions did not decrease the frequency of MRSA acquisition but did result in 40% higher costs and 21% lower rates of hand hygiene.33 Importantly, our study found that gloves were more commonly used than any other approach, indicating that NHs may be more aware and supportive of this barrier precaution, particularly when compared to more expensive approaches such as the use of single rooms.
Due to concerns with resistance to decolonizing agents and risks of re-colonization, current guidelines for NHs do not recommend routine decolonization, except in conditions of increased transmission.12,21 We found that most NHs did not decolonize MRSA carriers. A prior study suggested several factors that might temper NHs’ decision to decolonize – such as time and cost, lack of support from physicians, need for a dedicated decolonization team, and risks of re-colonization due to frequent patient transfers to and from hospitals.34
Environmental contamination also plays a key role in the transmission of MDROs and C.diff. CDC guidelines recommend that NHs prioritize room cleaning of residents on contact precautions and focus on frequently touched items12; however, there are no specific instruction about cleaning practices. Despite this lack of clarity, we found that many NHs adopt a variety of enhanced cleaning practices for rooms of residents on contact precautions. These practices may be particularly important in NHs as they do not disrupt social and care activities, but directly intervene on the path of person-to-person transmission.
When infection control practice recommendations are available, most NHs appear to follow them. Recommendations for change in practice, which do not require substantial resource investment, e.g. the use of gloves, masks, room cleaning, have substantially better chances of being adopted. However, when additional dedicated staff (e.g. IPs) or single rooms specifically designated for infected patients may be indicated, NHs are reluctant or unable to comply. While regulatory mandates may be more effective than recommendations in assuring compliance, to be successful such tactics must be accompanied by adequate financial and educational supports, given the already financially constrained circumstances of most facilities.
Perhaps a meaningful starting point is to promote working partnerships between NHs and hospitals sharing significant numbers of patients with a potential for high infection transmission risk. Care transitions between hospitals and NHs are often fragmented, poorly informed, and rarely coordinated.35 If MRSA screening results were accurately and promptly shared with NHs during transfers, NHs may be able to more effectively use this information to treat or contain transmissions, potentially reducing re-hospitalizations of infected residents. Today however, the lack of information and communication between hospital and NH staff, have been cited as barriers to effective infection control/prevention in NHs.36
The principal study limitation is the low response rate, which may limit the generalizability of our findings. It is interesting to note that this response rate might also indicate lack of interest or perceived importance of this topic in NHs. A prior study has shown that only 59% of NH staff perceived MRSA to be a risk to residents’ safety.36 Lack of managerial interest in and emphasis on residents’ safety culture have also been identified as barriers to infection control/prevention in NHs.34 Overall, our survey’s response is similar to an earlier national survey of C.diff prevalence and control practices in US hospitals (response rate, 12.5%), suggesting that expecting higher response rates may not be realistic.37
Our study suggests that NHs are quite compliant in following infection control guidelines, as long as such recommendations do not require substantial financial investments. Additional research to better understand which infection control practices are effective, while maintaining a “home-like” environment for all residents, is critical to assure increased NH compliance.
Supplementary Material
ACKNOWLEGEMENTS
Financial support. This project was support by National Institute of Nursing Research (R01 NR010727).
REFERENCES
- 1.Strausbaugh LJ, Sukumar SR, Joseph CL. Infectious disease outbreaks in nursing homes: an unappreciated hazard for frail elderly persons. Clin Infect Dis. 2003;36:870–876. doi: 10.1086/368197. [DOI] [PubMed] [Google Scholar]
- 2.Richards CL., Jr. Infection control in long-term care facilities. J Am Med Dir Assoc. 2007;8:S18–S25. doi: 10.1016/j.jamda.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Smith PW, Bennett G, Bradley S, et al. SHEA/APIC guideline: infection prevention and control in the long-term care facility, July 2008. Infect Control Hosp Epidemiol. 2008;29:785–814. doi: 10.1086/592416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards CL, Jr, Steele L. Antimicrobial-resistant bacteria in long-term care facilities: infection control considerations. J Am Med Dir Assoc. 2003;4:S110–S114. doi: 10.1097/01.JAM.0000066025.56717.01. [DOI] [PubMed] [Google Scholar]
- 5.Bradley SF. Staphylococcus aureus infections and antibiotic resistance in older adults. Clin Infect Dis. 2002;34:211–216. doi: 10.1086/338150. [DOI] [PubMed] [Google Scholar]
- 6.Raghavendran K, Mylotte JM, Scannapieco FA. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: the contribution of dental biofilms and periodontal inflammation. Periodontol 2000. 2007;44:164–177. doi: 10.1111/j.1600-0757.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rooney PJ, O'Leary MC, Loughrey AC, et al. Nursing homes as a reservoir of extended-spectrum beta-lactamase (ESBL)-producing ciprofloxacin-resistant Escherichia coli. J Antimicrob Chemother. 2009;64:635–641. doi: 10.1093/jac/dkp220. [DOI] [PubMed] [Google Scholar]
- 8.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawar D, Tsay R, Nelson DS, et al. Burden of Clostridium difficile infection in long-term care facilities in Monroe County, New York. Infect Control Hosp Epidemiol. 2012;33:1107–1112. doi: 10.1086/668031. [DOI] [PubMed] [Google Scholar]
- 10.Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011;53:42–48. doi: 10.1093/cid/cir301. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Deparment of Health and Human Serivces. [Accessed June 2, 2014];Revisions to Appendix PP -"Interpretive Guidelines for Long-Term Care Facilities, Tag F-441". 2009 https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/r51soma.pdf.
- 12.Siegel JD, Rhinehart E, Jackson M, Chiarello L Healthcare Infection Control Practices Advisory Committee. Management of Multidrug-Resistant Organisms in Healthcare Settings. [Accessed June 12, 2014];2006 doi: 10.1016/j.ajic.2007.10.006. http://www.cdc.gov/hicpac/mdro/mdro_toc.html. [DOI] [PubMed]
- 13.Siegel JD, Rhinehart E, Jackson M, Chiarello L Healthcare Infection Control Practices Advisory Committee. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. [Accessed June 12, 2014];2007 doi: 10.1016/j.ajic.2007.10.007. http://www.cdc.gov/hicpac/2007IP/2007isolationPrecautions.html. [DOI] [PMC free article] [PubMed]
- 14.Iowa Department of Public Health. [Accessed December 9, 2014];Report of the Iowa Antibiotic Resistance Task Force: a public health guide. (3rd edition). 2011 http://www.idph.state.ia.us/adper/common/pdf/cade/antibioticreport.pdf.
- 15.California Department of Health Services. [Accessed June 12, 2014];Guideline prevention and control of antibiotic resistant microorganisms California long-term care facilities. 1996 http://www.cdph.ca.gov/pubsforms/Guidelines/Documents/armgdepp1999.pdf.
- 16.Maryland Department of Health and Mental Hygiene. [Accessed June 12, 2014];Guidelines for control of management of Methicillin-resistant Staphylococcus aureus in long term care facilities. 2001 http://phpa.dhmh.maryland.gov/IDEHASharedDocuments/guidelines/mrsa-ltcf-guide.pdf.
- 17.Murphy CR, Eells SJ, Quan V, et al. Methicillin-resistant Staphylococcus aureus burden in nursing homes associated with environmental contamination of common areas. J Am Geriatr Soc. 2012;60:1012–1018. doi: 10.1111/j.1532-5415.2012.03978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreman T, Hu J, Pottinger J, Herwaldt LA. Survey of long-term-care facilities in Iowa for policies and practices regarding residents with methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococci. Infect Control Hosp Epidemiol. 2005;26:811–815. doi: 10.1086/502498. [DOI] [PubMed] [Google Scholar]
- 19.Mody L, Langa KM, Saint S, Bradley SF. Preventing infections in nursing homes: a survey of infection control practices in southeast Michigan. Am J Infect Control. 2005;33:489–492. doi: 10.1016/j.ajic.2005.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Accessed July 6, 2014];Shaping Long Term Care in America Project at Brown University funded in part by the National Institute on Aging (1P01AG027296) 2010 http://ltcfocus.org/ [Google Scholar]
- 21.Aureden K, Burdsall D, Harris M, Rosenbaum P. Guide to the elimination of Methicillin-Resistant Staphylococcus aureus (MRSA) in the Long-Term Care Facility. Washington, DC: Associations for Professionals in Infection Control and Epidemiology; 2009. [Accessed June 12, 2014]. http://www.apic.org/Resource_/EliminationGuideForm/08b12595-9f92-4a64-ad41-4afdd0088224/File/APIC-MRSA-in-Long-Term-Care.pdf. [Google Scholar]
- 22.Association for Professionals in Infection Control and Epidemiology. [Accessed December 21, 2014];MRSA Laws. http://www.apic.org/Resource_/TinyMceFileManager/Advocacy-PDFs/Static_map_-_MRSA_revised_4-21-11.gif. [Google Scholar]
- 23.Furuno JP, Hebden JN, Standiford HC, et al. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am J Infect Control. 2008;36:468–471. doi: 10.1016/j.ajic.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mody L, Kauffman CA, Donabedian S, Zervos M, Bradley SF. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin Infect Dis. 2008;46:1368–1373. doi: 10.1086/586751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahvecioglu D, Ramiah K, McMaughan D, et al. Multidrug-resistant organism infections in US nursing homes: a national study of prevalence, onset, and transmission across care settings, October 1, 2010–December 31, 2011. Infect Control Hosp Epidemiol. 2014;35(Suppl 3):S48–S55. doi: 10.1086/677835. [DOI] [PubMed] [Google Scholar]
- 26. [Accessed November 12, 2014];Centers for Medicare & Medicaid Services. MDS 3.0 RAI Manual. 2014 http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/MDS30RAIManual.html.
- 27.Morrison J. Development of a resource model for infection prevention and control programs in acute, long term, and home care settings: conference proceedings of the Infection Prevention and Control Alliance. Am J Infect Control. 2004;32:2–6. doi: 10.1016/j.ajic.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson KB, Murphy CL, Samore MH, et al. Assessing the status of infection control programs in small rural hospitals in the western United States. Am J Infect Control. 2004;32:255–261. doi: 10.1016/j.ajic.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Huang SS, Avery TR, Song Y, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol. 2010;31:1160–1169. doi: 10.1086/656747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee BY, Bartsch SM, Wong KF, et al. The importance of nursing homes in the spread of methicillin-resistant Staphylococcus aureus (MRSA) among hospitals. Med Care. 2013;51:205–215. doi: 10.1097/MLR.0b013e3182836dc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds C, Kim D, Kaplan SH, et al. Are nursing homes less likely to admit methicillin-resistant Staphylococcus aureus carriers? Am J Infect Control. 2014;42:63–65. doi: 10.1016/j.ajic.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mody L, Bradley SF, Huang SS. Keeping the "home" in nursing home: implications for infection prevention. JAMA Intern Med. 2013;173:853–854. doi: 10.1001/jamainternmed.2013.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trick WE, Weinstein RA, DeMarais PL, et al. Comparison of routine glove use and contact-isolation precautions to prevent transmission of multidrug-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 2004;52:2003–2009. doi: 10.1111/j.1532-5415.2004.52555.x. [DOI] [PubMed] [Google Scholar]
- 34.McClean P, Tunney M, Parsons C, Gilpin D, Baldwin N, Hughes C. Infection control and meticillin-resistant Staphylococcus aureus decolonization: the perspective of nursing home staff. J Hosp Infect. 2012;81:264–269. doi: 10.1016/j.jhin.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Naylor MD, Kurtzman ET, Pauly MV. Transitions of elders between long-term care and hospitals. Policy Polit Nurs Pract. 2009;10:187–194. doi: 10.1177/1527154409355710. [DOI] [PubMed] [Google Scholar]
- 36.Wolf R, Lewis D, Cochran R, Richards C. Nursing staff perceptions of methicillin-resistant Staphylococcus aureus and infection control in a long-term care facility. J Am Med Dir Assoc. 2008;9:342–346. doi: 10.1016/j.jamda.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis WR, Schlosser J, Jarvis AA, Chinn RY. National point prevalence of Clostridium difficile in US health care facility inpatients, 2008. Am J Infect Control. 2009;37:263–270. doi: 10.1016/j.ajic.2009.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.