Abstract
Despite higher rates of prostate cancer–related mortality and later stage of prostate cancer diagnosis, Black/African American men are significantly less likely than non-Hispanic White men to use early detection screening tools, like prostate-specific antigen (PSA) testing for prostate cancer. Lower screening rates may be due, in part, to controversy over the value of prostate cancer screenings as part of routine preventive care for men, but Black men represent a high-risk group for prostate cancer that may still benefit from PSA testing. Exploring the role of social factors that might be associated with PSA testing can increase knowledge of what might promote screening behaviors for prostate cancer and other health conditions for which Black men are at high risk. Using multilevel logistic regression, this study analyzed self-report lifetime use of PSA test for 829 Black men older than 45 years across 381 Philadelphia census tracts. This study included individual demographic and aggregated social capital data from the Public Health Management Corporation’s 2004, 2006, and 2008 waves of the Community Health Database, and sociodemographic characteristics from the 2000 U.S. Census. Each unit increase in community participation was associated with a 3 to 3.5 times greater likelihood of having had a PSA test (odds ratio = 3.35). Findings suggest that structural forms of social capital may play a role in screening behaviors for Black men in Philadelphia. A better understanding of the mechanism underlying the link between social capital and screening behaviors can inform how researchers and interventionists develop tools to promote screening for those who need it.
Keywords: prostate cancer, screening, prostate-specific antigen (PSA), Black/African American men, social capital, community participation, Philadelphia
Introduction
Prostate Cancer and Screening in Black Men
Black/African American men have a greater than 1 in 5 lifetime risk of developing prostate cancer (incidence rate = 248.5/100,000; American Cancer Society, 2013a), the second leading cause of cancer-related death in American men (American Cancer Society, 2014). Compared with non-Hispanic White men, Black men are more than twice as likely to die from prostate cancer (American Cancer Society, 2013a; Brooks, 2013; Ross, Meade, Powe, & Howard, 2009), due to late detection of prostate cancer at advanced disease stage (American Cancer Society, 2013a; Merkin, Stevenson, & Powe, 2002; Myers et al., 1996; Shen et al., 2007; Smith-Bindman et al., 2006). Early detection by screenings, like the prostate-specific antigen (PSA) test, has been an effective way to reduce the likelihood that a man will be diagnosed at late stage, leading to better treatment prognosis thereby reducing the likelihood of cancer-related mortality (American Cancer Society, 2009); however, only about 35% of Black men over the age of 50 will have had a PSA test within a given 12-month period (for those who were not diagnosed with prostate cancer already) compared with 44% among non-Hispanic White men (American Cancer Society, 2009, 2013a). Increasing screening behaviors can help reduce the likelihood that Black men will be screened at a late stage and can reduce the disparity in prostate cancer mortality rates.
Several barriers, actual and perceived, contribute to low screening rates for Black men, like lack of health insurance, lack of knowledge, cost, lack of a sense of urgency, inconvenient doctors’ office hours, and a lack of information about what type of medical professional to seek and where to find one (S. P. Weinrich, Reynolds, Tingen, & Starr, 2000). Another potential barrier may be the lack of recommendation for routine screening from primary care providers (Brooks, 2013) and national policy. In 2012, the United States Preventive Services Task Force (USPSTF) recommended against routine PSA screening for the early detection of prostate cancer due to the high rate of detecting clinically insignificant disease (a “false-positive” result). This over-diagnosis of prostate cancer often leads to unnecessary treatment and treatment-associated side effects including incontinence, erectile dysfunction, bowel control, and death (USPSTF, 2012). Because of uncertainty of the efficacy of routine prostate cancer screening, the Affordable Care Act does not currently include requirements of health insurance coverage for prostate exams. Although the USPSTF no longer recommends routine screening, other organizations such as the American Cancer Society now recommend shared decision making between patient and provider about screening, suggesting that some organizations still see a role for routine screening for certain subgroups of men (Wolf et al., 2010). Physician-led groups, including the American Society of Clinical Oncology (Basch et al., 2012) suggest that the use of PSA screening be based on the patient’s age group and individual risk level, taking into account life expectancy and other concurrent medical conditions. The American Urological Association Foundation (Carter et al., 2013) also differs from USPSTF recommendations, and suggests that men older than 40 years be in discussion with their physicians about PSA screening based on health and family history. Even though routine screening is no longer recommended by the USPSTF, understanding the factors that lead to higher screening rates among Black men is important in understanding how to motivate men to get screening earlier, especially those who are at highest risk for late-stage disease detection. Black men represent a high-risk group for prostate cancer that may still benefit from PSA testing as a preventive screening.
Factors promoting screening for Black men include individual-level demographics like older age, being married, higher education, and higher income (Ross et al., 2009). Focus groups of elderly Black men have consistently reported that family, religious institution and partner are influential on a Black man’s decision to pursue prostate cancer screening (Blocker et al., 2006), reflecting that one’s social environment is important to the decision to use prostate cancer screening. Recognizing the need for increasing preventive screenings among Black men, religious organizations, civic organizations, and community groups serving Black men have worked to promote screenings (Dean & Gilbert, 2013; Holt et al., 2009; Jakes, 2013; Moran, 2013; Simons, 2012; Wilson, 2013). The role of those organizations point to a direct usage of social capital to promote screening; however, social capital itself has not been explored as a contributor to promoting screening (Leader & Michael, 2013).
Social Capital’s Potential Role in Prostate Cancer Screening Behaviors
Although no studies have explicitly attempted to use social capital to explain cancer preventive behavior (Brody et al., 2007), social capital and cancer preventive behaviors are plausibly linked. The concept of social capital grows from the observation that social relationships can create a form of capital or power that can have positive effects on multiple outcomes, including health (Hanifan, 1916; Kawachi, Kennedy, & Glass, 1999; Putnam, 1993, 1995). Social capital may be considered the ecological analogue to individually based social support, and is considered a social determinant of health and health behaviors. Social capital is distinguished from social support because while social support relates to interpersonal relationships among individuals, social capital is about resources embedded within groups, that is, it is a contextual construct.
Public health researchers suggest that social capital may be important to the adoption of health-promoting behaviors, like encouraging preventive screening. Public health researchers have offered the following suggested mechanisms by which social capital may be related to health: (a) diffusion of information about health-promoting behaviors, (b) maintenance of healthy behavioral norms or deterrence of risky behaviors through informal social control, (c) promotion of access to services (d) affective support or other psychosocial pathways that act directly or indirectly, and (e) empowerment to engage political policies that affect community health (Berkman & Kawachi, 2000; Kawachi & Berkman, 2001; Kim, Subramanian, & Kawachi, 2007). Each of these mechanisms offer ways that Black men might be reached with information about screenings, feel social influences that encourage screening, or that allow access to screenings.
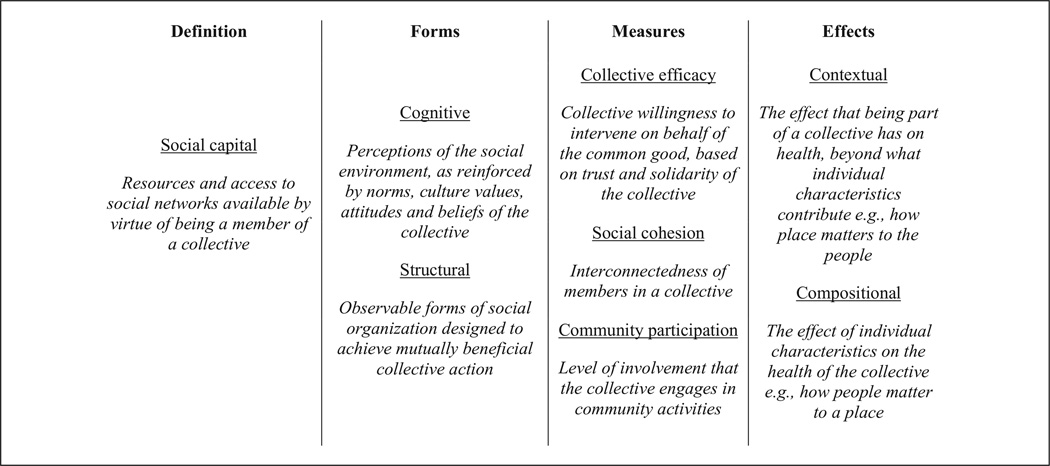
Although social capital is often associated with health outcomes, it has also been associated with health behaviors. A review of social capital’s links to health-related behaviors such as drug and alcohol use, physical activity, diet, and sexual behavior has suggested that social capital may influence behavior through norms and values, communication channels and information diffusion, and psychosocial stress mechanisms (Lindstrom, 2007). For adults, high community trust has been associated with lower alcohol abuse (Lindstrom, 2007), community social capital attributed to religious groups has been associated with few numbers of cigarettes by individual smokers in the community (Brown, Scheffler, Seo, & Reed, 2006), and findings are mixed on whether or not social capital is protective against risky sexual behaviors (Lindstrom, 2007). Furthermore, the influence of geographic area and environment may vary according to the behavior (Lindstrom, 2007). Differences in forms of social capital, measurement of social capital, and the effects of social capital (see Figure 1 for key terms used in this article) make it difficult to extend the role of social capital in these health behaviors to prostate cancer screening, leaving a gap in knowledge on the role of social capital in prostate cancer screening behaviors.
Figure 1.
Key social capital terms use in this article.
Although it is inherently a contextual collective-level construct, it is important to measure both individual-level perceptions and community-level perceptions of social capital, as they represent different characteristics of the group. Tools such as multilevel modeling help determine whether community-level social capital (contextual effect) influences individual health over and above individual perceptions (compositional effect). Contextual effects refer to the influences of the collective that are exerted on the individual (Berkman & Kawachi, 2000), while compositional effects are the influences that the individual contributes to the collective. An example of the distinction between contextual and compositional influences may be that a neighborhood can be low-resource due to having few tangible resources for its residents regardless of the actual income of its residents, which would be contextual, or it can be low-resource because the residents in that neighborhood are all low-income, even if the neighborhood environment has many resources to offer, which is compositional. It is important to measure both contextual and compositional components of social capital, as each has been reported to have different associations with health (Kim, Subramanian, & Kawachi, 2007).
Social capital indicators fall into categories of cognitive social capital, which is the perceptual component, and structural social capital, which is the behavioral/ activity component that are observable (Dasgupta & Serageldin, 1999; De Silva, McKenzie, Harpham, & Huttly, 2005; Kim et al., 2007). Differing health outcomes have been identified based on whether the type of social capital being assessed is structural or cognitive (Kim et al., 2007). Cognitive social capital stems from mental processes and perceptions of one’s social environment, as reinforced by norms, culture, values, attitudes, and beliefs of a collective. Cognitive social capital is often operationalized by asking respondents to rate the degree to which they trust their neighbors, or whether they believe their neighbors would work together toward achieving a common goal. The structural category pertains to forms of social organization and the organization of cooperative networks to achieve “mutually beneficial collective action” (Dasgupta & Serageldin, 1999). Structural social capital is often operationalized as an individual’s or group’s involvement in voluntary associations or civic participation.
Both cognitive and structural forms of social capital have been assessed in survey-based data, using both individual-level and community-level units of analysis. Inclusive of these indicators, measures focus on collective efficacy, social cohesion, and community participation. Collective efficacy refers to the collective willingness of residents to intervene on behalf of the common good, and largely depends on mutual trust and solidarity among residents (Kim et al., 2007; Sampson, Raudenbush, & Earls, 1997). Social cohesion measures the property of how interconnected the group is, while community participation measures how involved the group is in community activities. Each of these can have cognitive or structural representations, but community participation is largely structural since it points to tangible observable events. One study that explicitly examined the use of community participation in the design of a prostate cancer prevention project in Mississippi, attributed the growth of social capital to the successful levels of community participation in the intervention planning (Tataw & Ekúndayò, 2012); however, the role of social capital on the actual prostate screening behaviors was not explicitly explored, leaving a gap in understanding of the role of social capital on screening behaviors.
Study Purpose
The primary aim of this study was to examine the link between social capital and prostate cancer preventive screening behaviors for African American men in Philadelphia, Pennsylvania neighborhoods. The key research question is: are cognitive (perceived social cohesion and perceived collective efficacy) and/or structural (community participation) measures of social capital associated with cancer preventive behaviors within Philadelphia neighborhoods, net of individual-level compositional characteristics (age, socioeconomic position)? Given the supposed diffusion of information mechanisms for social capital, the hypothesis was that African American men older than 45 years residing in neighborhoods with high cohesion, high collective efficacy, or high community participation will be likely to have undergone PSA screening at some point in their lifetime.
Method
Research Design
This study used two main sources of data: the Public Health Management Corporation (PHMC) random-digit dialing survey data (2004, 2006, and 2008) and 2000 U.S. Census Data.Because the study was composed of de-identified secondary data, the study met criteria as institutional review board exempt.
The PHMC data set provided individual-level data on the outcome variable of prostate cancer screening, individual-level demographics for male respondents, and for the social capital variables. To increase power for the multilevel analysis, data were combined from the 2004 (n = 4,415), 2006 (n = 4,193) and 2008 (n = 4,394) Southeastern Pennsylvania Household Health Survey data set administered by the PHMC (Design and Implementation of the Southeastern Pennsylvania Household Survey, Household Health Survey Documentation, PHMC). The survey is of individuals, 18 years of age and older from a probability sample of households in the five major counties of the Greater Philadelphia Metropolitan Area; however, only Black males older than 45 years and who responded to the question about prostate screening were included for analysis of individual-level data. Due to low response rates in some census tracts, PHMC supplies balancing weights to balance out census tracts with a sample that is underrep-resented. Instead of using weights, this study’s approach was to combine data from several waves of the PHMC data set, to boost the sample size in each census tract. Multilevel analysis was performed using the weights available, but this did not change the results of the article, so the use of unweighted variables was preserved.
Summary demographic data on age and race for each of the 381 census tracts in Philadelphia came from the 2000 U.S. Census. Census-tract-level information was chosen as a proxy for neighborhoods in this analysis because the census tract was the smallest area unit of analysis available across all of the variables. Census-tract boundaries are designed to be homogeneous with respect to population characteristics, economic status, and living conditions and are sensitive to physical changes in street layout that may constrain or broaden where tract residents go. Census tracts are more stable boundaries than zip codes, which permits easier statistical comparisons when looking at data from different points in time (Blocker et al., 2006; Krieger et al., 2003). Although Philadelphia residents may not use census tracts to define their neighborhoods, social capital and segregation each depend on neighborhood spatial layout as well as physical boundaries of social interaction, and hence, census tracts may be an appropriate proxy.
Measures
The dependent variable of lifetime prostate screening use was asked in each wave of the PHMC data. At the time of the surveys, prostate exam recommendations were based on level of risk, and not necessarily on a predetermined frequency. Thus, for the prostate tests, the item used was, “About how long has it been since you last had a PSA test or rectal exam for prostate cancer?” which was asked of all males 45 years and older. Due to the nonnormal distribution of responses to this question, which was the primary outcome, responses were recoded so that “0” corresponded to never having a screening test and “1” corresponded to ever having a screening test at some point in the past.
Individual-Level Predictors
The PHMC data set provided the age (continuous), health insurance status, income, education level, and whether the respondent was below the 200% federal poverty level. Health insurance status was coded as yes or no. Education was measured as “graduated from high school” or “not graduated from high school” in alignment with the U.S. Census’ education measure. For ease of interpretation, the midpoint of each of the 19 income categories was used, and was treated as continuous in regression models.
Dummy variables were assigned to account for time in the model, based on whether the individual-level demographic variables were from the 2004, 2006, or 2008 wave of the PHMC data.
Ecological-Level Predictors
Population Demographics
The 2000 U.S. Census provided raw counts of population demographics for each census tracts, which allowed calculation of the percentage of high school graduates and the average age per tract. The number of men who lived above poverty and who have never had a prostate exam represented less than 10% of the entire sample of men, meaning there were too few respondents to include a census-level poverty variable.
Social Capital
The PHMC data included measures of social cohesion, collective efficacy, and community participation. Social cohesion was measured by inclusion of a single summary score of answers to several survey questions. An oblique (promax) rotated principal components factor analysis suggested a three-measure composite score to represent social cohesion (α = .71) addressing feelings of belongingness, interpersonal trust, and neighborliness. All variables were reverse scored so that higher numbers represented high social cohesion. Each question was asked on a 5-point Likert-type agreement scale. Two were on a scale from strongly disagree to strongly agree: “Please tell me if you strongly agree, agree, disagree, or strongly disagree with the following statement: I feel that I belong and am a part of my neighborhood” [feelings of belongingness]. “Most people in my neighborhood can be trusted” [interpersonal trust]. The third asked,
Please rate how likely people in your neighborhood are willing to help their neighbors with routine activities such as picking up their trash cans, or helping to shovel snow. Would you say that most people in your neighborhood are always, often, sometimes, rarely, or never willing to help their neighbors? [neighborliness]
All variables were scored so that higher numbers represented high social cohesion. Collective efficacy was represented by the yes/no (1/0) item, “Have people in your neighborhood ever worked together to improve the neighborhood?” In later waves of the data, the question added the prompt, “For example, through a neighborhood watch, creating a community garden, building a community playground, or participating in a block party.”
Community participation was measured by the continuous item, “How many local groups or organizations in your neighborhood do you currently participate in such as social, political, religious, school-related, or athletic organizations?”
This data were collected at the individual level, but since social capital is inherently a collective-level measure, these were aggregated to have one average value per census tract. Individual-level data from all races were included in the aggregate scores since members from each race within a census tract would be expected to contribute to the overall social capital. Higher numbers represented higher levels of social capital.
Previous research in multilevel modeling (Subramanian, Lochner, & Kawachi, 2003) highlights the importance of distinguishing compositional effects from contextual effects, since individual-level factors may confound the area-level social interactions and the development of social capital. To account for the compositional effects of social capital (i.e., the contribution of social capital as measured by individual-level data) and avoid multicollinearity across levels, group-mean centered variables were included along with the aggregated social capital variables. Conceptually, using the group-mean centered approach is a way to disentangle the contribution of the individual perceptions of social capital from the community-level characteristics of social capital by subtracting the social capital score at the individual-level from the community-level mean score.
Statistical Analysis
Means and standard deviations were calculated for individual-level variables and two-sample t tests were performed to determine whether individuals who had undergone screening were demographically different from those without prior screening, at a significance of p < .05.
Multilevel modeling software, MLwIN 2.11 (Rasbash, Charlton, Browne, Healy, & Cameron, 2009) generated two-level variance component models with random intercepts. Level 2 was census tracts and Level 1 was individuals. The models were estimated with a dichotomous outcome using the binomial logit function, and were based on a first-order marginal quasi-likelihood approximation of the second-order Taylor linearization procedure, and estimated under iterative generalized least squares assumptions.
After examining bivariate associations between each predictor and the outcome using logistic regression in an iterative generalized least squares model, the model was built. The baseline model (null model) contained no predictor variables. Subsequent models separately included variables for: survey timing, individual-level covariates of age, insurance status, income, poverty, and high school completion, census tract analogs to the individual-level covariates of age, high school completion, and separate contextual and compositional social capital variables measuring social cohesion, collective efficacy, and community participation. The constant had both fixed (relationship between individual-level factors and screening rates across all census tracts, assuming the effects of the individual factors is the same in every census tract) and random (variation in screening rates between census tracts that is not attributable to individual-level differences) components while the remaining predictors were entered as fixed effects.
To get log odds for each parameter, the coefficient estimates, standard errors, and 95% confidence intervals were exponentiated and assessed at a significance at p < .05. The Level-1 log odds represented the model’s fixed effects, that is, the differences between individuals within each tract. The Level-2 logs odds represented the model’s random effects, that is, the differences between census tracts and across individuals.
Results
Of the entire PHMC sample, 829 respondents were Black males older than 45 years who responded to the question about the use of a prostate screening exam. Of the total 829 respondents, approximately 78.8% had a prostate screening exam at some point in the past (Table 1). Those who had prostate screening were significantly older by an average of 6 years (62 years), were more likely to have health insurance, have higher income, and were less likely to be in poverty; education level was not associated (p = .443). The mean social cohesion score for all 829 Black male respondents to the screening question was 9.43 (SD = 2.06) on a scale of 3 to 13, 79.5% of individuals reported that their neighborhood demonstrated collective efficacy, and participated in less than one community event (range = 0–10). Individuals who ever had a prostate exam reported perceptions of significantly higher social cohesion in their neighborhoods (on a combined scale of 3 to 13; p < .003), were significantly more likely to report collective efficacy in their neighborhoods (p < .007), and participated in more community efforts (p < .02).
Table 1.
Demographic Characteristics of Respondents.
| Total respondents for prostate exam (N = 829) |
Never had prostate exam (n = 176) |
Ever had prostate exam (n = 653) |
p value | |
|---|---|---|---|---|
| Age in years, M (SD) | 60 (11) | 56 (10) | 62 (11) | <.001 |
| High school graduates, n (%) | 640 (77.6) | 132 (75.4) | 508 (78.2) | .443 |
| Have health insurance, n (%) | 750 (90.5) | 145 (82.4) | 605 (92.7) | <.001 |
| Mean income (US$) category, midpoint (range) |
27,300 (14,000–54,500) | 23,000 (10,400–37,200) | 27,300 (14,000–54,500) | <.001 |
| Less than 200% poverty level, n (%) | 385 (46.4) | 106 (60.2) | 279 (42.7) | <.001 |
| Social cohesion score, M (SD)a | 9.43 (2.05) | 9 (2.23) | 9.56 (1.99) | <.003 |
| Collective efficacy = yes, n (%)b | 628 (79.5) | 121 (72) | 507 (81.5) | <.007 |
| Community participation, M (SD)c | 0.84 (1.2) | 0.64 (1.07) | 0.89 (1.35) | <.02 |
n = 696.
n = 790.
n = 817.
Accordingly, in bivariable associations (Table 2, bivariate), older age (odds ratio [OR] = 1.06 [1.04–1.08], p < .0001), having health insurance (OR = 2.70 [1.66–4.39], p < .0001), and having higher income (OR = 1.08 [1.04–1.12], p < .0001) were significantly associated with a likelihood of having had prostate screening. Being in poverty reduced the likelihood of having a prostate screening by nearly 50% (OR = 0.49 [0.35–0.69], p < .0001). Additionally, compositional social cohesion (OR = 1.12 [1.02–1.22], p = .01) and compositional collective efficacy (OR = 1.58 [1.06–2.36], p = .02) were associated with increased likelihood for cancer screening in bivariate analysis. At the census-tract level, older age (OR = 1.04 [1.00–1.07], p = .03), having a higher percentage of high school graduates (OR = 1.02 [1.01–1.03], p = .003), and increases in community participation (OR = 2.63 [1.34–5.15], p = .005) were all associated with prostate screening at p < .05. Compositional community participation (OR = 1.15 [0.98–1.34], p = .08) and contextual (neighborhood-level) social cohesion (OR = 1.30 [0.98–1.72], p = .07) were marginally significant at p < .1.
Table 2.
Multilevel Model for Log Odds (95% Confidence Interval) of Lifetime Prostate Exam.
| Bivariate | p | Model I (−null model) |
p | Model II (+time) |
p | Model III (+individual) |
p | Model IV (+compositional) |
p | Model V (+census tract) |
p | Model VI (+social capital) |
p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Individual level (Level 1) | ||||||||||||||
| Constant | 3.71*** (3.14, 4.38) |
<.0001 | 3.71*** (3.14, 4.38) |
<.0001 | 3.58*** (2.73, 4.68) |
<.0001 | 0.05** (0.01, 0.28) |
.0006 | 0.04* (0.01, 0.28) |
.001 | 0.02* (0, 0.25) |
.001 | 0.06 (0, 2.82) |
.15 |
| Year 2004 | 0.95 (0.66, 1.35) |
.78 | 1.00 (0.67, 1.49) |
1.00 | 1.07 (0.67, 1.70) |
.77 | 0.87 (0.52, 1.44) |
.58 | 0.88 (0.53, 1.47) |
.35 | 0.88 (0.53, 1.48) |
.64 | ||
| Year 2006 | 1.13 (0.78, 1.63) |
.51 | 1.13 (0.75, 1.70) |
.55 | 1.03 (0.64, 1.65) |
.89 | 0.81 (0.48, 1.36) |
.42 | 0.82 (0.49, 1.39) |
.37 | 0.81 (0.48, 1.39) |
.45 | ||
| Age continuous (N = 829) |
1.06*** (1.04, 1.08) |
<.0001 | 1.06*** (1.04, 1.08) |
<.0001 | 1.07*** (1.04, 1.09) |
<.0001 | 1.06*** (1.04, 1.09) |
<.0001 | 1.06*** (1.04, 1.09) |
<.0001 | ||||
| Health insurance (N = 829) |
2.70*** (1.66, 4.39) |
<.0001 | 1.96* (1.11, 3.46) |
.02 | 2.00* (1.07, 3.73) |
.03 | 2.03* (1.09, 3.79) |
.03 | 2.00* (1.06, 3.77) |
.03 | ||||
| Income categorical (n = 703) |
1.08*** (1.04, 1.12) |
<.0001 | 1.05 (0.98, 1.12) |
.14 | 1.04 (0.96, 1.12) |
.32 | 1.03 (0.96, 1.11) |
.31 | 1.04 (0.97, 1.12) |
.29 | ||||
| Below 200% poverty (N = 829) |
0 49*** (0.35, 0.69) |
<.0001 | 0.65 (0.32, 1.33) |
.24 | 0.62 (0.28, 1.37) |
.24 | 0.60 (0.28, 1.33) |
.44 | 0.61 (0.27, 1.36) |
.23 | ||||
| High school graduate (n = 825) |
1.17 (0.79, 1.72) |
.44 | 1.18 (0.71, 1.97) |
.51 | 1.40 (0.79, 2.47) |
.25 | 1.33 (0.75, 2.37) |
.25 | 1.24 (0.69, 2.24) |
.48 | ||||
| Compositional social cohesion (n = 696) |
1.12* (1.02, 1.22) |
.01 | 1.07 (0.95, 1.20) |
.25 | 1.07 (0.96, 1.20) |
.3 | 1.08 (0.96, 1.21) |
.19 | ||||||
| Compositional collective efficacy (n = 790) |
1.58* (1.06, 2.36) |
.02 | 1.00 (0.58, 1.75) |
1.00 | 1.02 (0.59, 1.78) |
.31 | 1.06 (0.61, 1.85) |
.84 | ||||||
| Compositional community participation (n = 817) |
1.15† (0.98, 1.34) |
.08 | 1.20† (0.98, 1.47) |
.08 | 1.20† (0.98, 1.47) |
.08 | 1.20† (0.96, 1.49) |
.10 | ||||||
| Neighborhood level (Level 2) | ||||||||||||||
| Median age (n = 381) | 1.04* (1.00, 1.07) |
.03 | 1.00 (0.95, 1.05) |
.32 | 0.99 (0.94, 1.04) |
.66 | ||||||||
| Percent high school graduates (n = 381) |
1.02** (1.01, 1.03) |
.003 | 1.01 (0.99, 1.03) |
.31 | 1.01 (0.98, 1.03) |
.46 | ||||||||
| Social cohesion (n = 362)a |
1.30† (0.98, 1.72) |
.07 | 0.91 (0.56, 1.45) |
.68 | ||||||||||
| Collective efficacy (n = 376)a |
1.00 (1.00, 1.00) |
.58 | 1.00 (0.99, 1.00) |
.02 | ||||||||||
| Community participation (n = 374)a |
2.63** (1.34, 5.15) |
.005 | 3.35* (1.24, 9.06) |
.02 | ||||||||||
| Random effect (Level-2 variance) |
— | 1.00 (1.00, 1.00) |
1.00 (0.94, 1.07) |
1.01 (0.98, 1.08) |
1.00 (1.00, 1.00) |
1.00 (1.00, 1.00) |
1.00 (1.00, 1.00) |
Represents the number of census tracts used to create aggregate measure. All other Level-I variables used Census data across all populated tracts,
p ≤ .10.
p < .05.
p < .01.
p < .001.
When accounting for time and the other individual-level demographics, age (OR = 1.06 [1.04–1.08], p < .0001) and having health insurance (OR = 1.96 [1.11–3.46], p = .02) were both significant predictors of prostate screening (Model III). Compositional social cohesion and collective efficacy variables were no longer significant when controlling for other individual-level demographics (Model IV), as is the case with the census-level demographics (Model V). But compositional community participation remained marginally significant. The effect size for community participation grew larger with the addition of individual- and area-level control variables included in the model, leading to a final OR = 3.35 [1.24–9.06], p = .02 in the final model (Model VI).
Discussion
Both area- and individual-level factors contributed to the likelihood of a Black male in Philadelphia getting prostate screening. A Black man living in a community where community participation is higher by even one unit is 3 to 3.5 times more likely to have had prostate screening than one with less community participation. Since this study used single-item measures of social capital, these estimates may be downwardly biased, as reported in other studies of social capital and health (Kim, et al., 2007), which may mean that the actual effect estimates of social capital are even larger than this study identifies. Having health insurance and younger age were positively associated with cancer screening, which is consistent with what other studies have reported (American Cancer Society, 2013b; Hoffman-Goetz, Breen, & Meissner, 1998; Potosky, Breen, Graubard, & Parsons, 1998). Health insurance had the strongest positive association with prostate cancer screening, which implicates that health care coverage may help reduce disparities in screening. Both health insurance and age are structural factors that might influence screening because health insurance status points to whether or not the man has access to screening, to some extent, and age is a determinant of whether or not a physician will recommend screening. In contrast to contextual community participation as a structural measure of social capital, social cohesion and collective efficacy, as measures of cognitive social capital in this survey, were not significant predictors in the final model, at the individual or community level. Altogether, this may suggest that structural factors, including structural forms of social capital like community participation, have the greatest influence on screening behaviors.
The presence of community-level participatory activities was statistically associated with an individual undergoing prostate cancer screening, while an individual’s level of participation in community events was not statistically associated. At the individual level, participating in community events was not significant at p < .05 but there may be evidence of a weak association, with a p value of .08. This may suggest that an individual’s involvement in community activities may not be as important to prostate cancer screening as the overall strength of the community. This would support this study’s findings at the community level, where the degree of community participation as a measure of structural social capital, had a strong association with prostate cancer screening. Although one might expect that the compositional individual-level measures of community participation would have the strongest association with an individual behavior like prostate screening, contextual neighborhood-level effects had the strongest associations, over and above the effect of the individual age and health insurance which would have the most direct effect on screening behavior. Instead, this study suggests that individual perceptions of community participation were only marginally related to prostate screening rates, which may represent a precursory support of Lynch et al.’s (2001) finding that individual-level community participation is not linked to prostate cancer mortality rates. Still, even Lynch’s findings suggested marginal significance and if there was a downward bias in estimates due to using single-item measures of social capital, then both his study and the current study may suggest that individual-level community participation is a component in men’s screening behaviors and subsequent cancer-related mortality.
This discrepancy between an individual’s actual level of participation in community events and the overall participatory activities in the community underscores how the community context may influence individual health behaviors for Black men in Philadelphia. It is possible that having more community events, regardless of whether or not an individual participates in them, facilitates greater diffusion of health messages that would promote screening behaviors. Or it may be that communities that have more events are likely to have events focused on promoting health screenings. Black men themselves have identified elements of social capital as crucial to encouraging prostate cancer screening. In a qualitative study, Black men identified community settings as an important place for prostate cancer screening to be promoted, and emphasized the need for trust-building and a sustained community presence if screening interventions are to be effective (Allen, Kennedy, Wilson-Glover, & Gilligan, 2007). Given the individual-level barriers to prostate cancer screening that Black men cite, like lack of knowledge and issues of cost and access (S. P. Weinrich et al., 2000), areas with high levels of community participation may be addressing these issues directly through community activities. For instance, churches or community health fairs may be getting information out about prostate cancer, and men living in areas near where these activities are placed may increase the likelihood that they benefit from diffusion of information mechanisms facilitating dissemination of information about prostate cancer screening and how to access it. Encouraging participatory communities through churches, civic organizations, and mutual benefit associations may be a strategy for preparing Black men to receive messages about prostate cancer screening. Predominantly, Black civic organizations, fraternities, and religious groups have already been active in carrying the banner of preventive screening for Black men (Holt et al., 2009; Jakes, 2013; Moran, 2013; Simons, 2012; Wilson, 2013). These groups gained credibility as messengers in the Black community as they gained strength and solidarity through unification against persistent forms of institutional racism, and represent a form of social capital that has been leveraged to address health disparities throughout history (Dean & Gilbert, 2013). Of all of these groups, the most empirical evidence exists for church-based efforts, through which studies have been effective for increasing prostate cancer screening among African American men (Boehm et al., 1995; Holt et al., 2009; Tingen, Weinrich, Heydt, Boyd, & Weinrich, 1998; S. Weinrich et al., 1998; Wilkinson, List, Sinner, Dai, & Chodak, 2003). The Black church and other community groups have been considered prime spaces for the cultivation of social capital in the Black community (Baodong, Wright-Austin, & Orey, 2009; Dean & Gilbert, 2013; Putnam, 1995). This study may provide empirical evidence that these are the effective ways to get Black men to cancer screening, and suggest that other cancer screening be promoted at community events.
Community events provide a context for residents to physically join together to bond and interact and may be a means of pooling social resources across communities. That is, community events in one community may be sponsored by members outside of the community. It is possible then, that when it comes to cancer preventive screening for Black men, it is not the immediate neighborhood that has an effect on individual behavior, but the cumulative effect across neighborhoods. This would also explain why social cohesion and collective efficacy were not related to the individual screening behavior, because they are largely properties of what’s happening within the immediate neighborhood, based on how the questions were framed in this survey. Because of the requisite joining together implied, residents living in areas of high community participation may have increased contact with one another. Increased contact may offer more opportunities to be exposed to the diffusion of information mechanism. Thus, they may be more likely to have health messages reinforced through social networks for sharing messages about health-promoting and preventive behaviors. Depending on where the diffusion of information mechanism operates, our findings may suggest culturally relevant strategies for increasing adoption of cancer preventive screening by Black/African Americans (American Cancer Society, 2013b; Hoffman-Goetz et al., 1998; Potosky et al., 1998).
Limitations
All of the data were cross-sectional, making it difficult to determine whether social capital causes residents to seek out cancer preventive screening, or whether or not those who are likely to pursue preventive screening seek out neighborhoods that are high in social capital. The difficulty in establishing a causal relationship between social capital and screening behavior emphasizes the need to conduct social capital examinations with primary data collection designed to explicitly explore the role of social capital on screening behaviors.
The response rate to the PHMC questions was less than 30%, but this is a typical and acceptable rate for community random-digit dialing surveys. PHMC’s response rates fall within the range of response rates of other well-used and respected community surveys that use random-digit dialing (California Health Interview Survey, 2009; Centers for Disease Control and Prevention, 2009; Lee, Brown, Grant, Belin, & Brick, 2009)
There were limited measures of social capital and it was only measured based on neighborhood, and not on kinship networks or other social organizing structures. There were no purely ecological measures and no primary source representations of social capital (e.g., voting block representation, number of community events). Nevertheless, this study makes a contribution to the literature by using three indicators of social capital when studies predominantly use only one indicator of social capital (Kim et al., 2007). Using multiple indicators accounting for multiple dimensions of the social capital construct was a strength of this analysis, leading to a more refined view of which specific social capital mechanisms may be at work.
The measurements for self-rated cancer preventive behaviors as single self-reported items may not be the most valid way to measure these behaviors. In the case of prostate cancer screening, patients may confuse colonoscopy with rectal exams and may not be aware for the name of the PSA test. Self-report lacks medical records or other supporting documentation from a health professional, for validation.
The PHMC questionnaire no longer gathers data on cancer diagnosis (it did in previous waves, but not after 2002) so this study could not distinguish those who already had cancer and for whom these are diagnostic (not early detection) actions. Thus, there could be reverse causation since having prostate cancer would increase the likelihood of having a prostate exam to monitor disease progression or remission and those with prostate cancer may be more likely to seek out community resources for testing or support; however, the results from this study still support that regardless of whether or not it is for prevention or follow-up, having an active community plays a role in this health behavior.
Conclusion
Neighborhood social capital may make a contribution to promoting individual prostate cancer preventive screening for Black men in Philadelphia. Additionally, health insurance and age were important factors related to cancer screening. Increasing access to health insurance may be especially crucial for increasing screening among Black men who are at high risk for prostate cancer. As the first study to explore the relationship between social capital and cancer preventive screening using multilevel modeling, further investigation could help elucidate the mechanisms through which social capital and cancer screening operate. These findings also underscore a potential mechanism for motivating Black men toward better health behaviors. The results of this study can be useful for public health practitioners to understand the potential health-promoting role of social capital for Black men as a means to address health disparities, and highlights that future research on Black men and their health behaviors should include social capital measures and consider social capital’s influences. Given that this article found differences in cancer screening behavior based on the type of social capital highlights that future investigations of social capital should include multiple indicators to best understand which dimension of social capital is relevant to the behavior and to the group being studied. This understanding could inform effective strategies for promoting screening for Black men for prostate cancer and for other diseases for which they are at high risk.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health and National Cancer Institute (Grant No. 1F31CA136236).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Allen JD, Kennedy M, Wilson-Glover A, Gilligan TD. African-American men’s perceptions about prostate cancer: Implications for designing educational interventions. Social Science & Medicine. 2007;64:2189–2200. doi: 10.1016/j.socscimed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer facts & figures for African Americans 2009–2010. Atlanta, GA: Author; 2009. [Google Scholar]
- American Cancer Society. Cancer facts & figures for African Americans 2013–2014. Atlanta, GA: Author; 2013a. [Google Scholar]
- American Cancer Society. Cancer prevention and early detection facts & figures 2013. Atlanta, GA: Author; 2013b. [Google Scholar]
- American Cancer Society. What are the key statistics about prostate cancer? 2014 Retrieved from http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics.
- Baodong L, Wright-Austin S, Orey B. Church attendance, social capital, and Black voting participation. Social Science Quarterly. 2009;90:576–592. [Google Scholar]
- Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, Parnes H, Nam RK. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology provisional clinical opinion. Journal of Clinical Oncology. 2012;30:3020–3025. doi: 10.1200/JCO.2012.43.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Kawachi I. Social epidemiology. Vol. 1. New York, NY: Oxford University Press; 2000. [Google Scholar]
- Blocker DE, Romocki LS, Thomas KB, Jones BL, Jackson EJ, Reid L, Campbell MK. Knowledge, beliefs, and barriers associated with prostate cancer prevention and screening behaviors among African-American men. Journal of the National Medical Association. 2006;98:1286–1295. [PMC free article] [PubMed] [Google Scholar]
- Boehm S, Coleman-Burns P, Schlenk EA, Funnell MM, Parzuchowski J, Powell IJ. Prostate cancer in African American men: Increasing knowledge and self-efficacy. Journal of Community Health Nursing. 1995;12:161–169. doi: 10.1207/s15327655jchn1203_4. [DOI] [PubMed] [Google Scholar]
- Brody JG, Kavanaugh-Lynch MHE, Olopade O, Shinagawa SM, Steingraber S, Williams DR. Neighborhood context and breast cancer: Identifying gaps in breast cancer research. Report of California Breast Cancer Program Special Research Initiatives. 2007 Retrieved from http://www.cbcrp.org/sri/reports/identifyinggaps/GAPS_full.pdf.
- Brooks DD. Why are black men negatively affected by prostate cancer more than white men? 2013 Retrieved from http://www.cancer.org/cancer/news/expertvoices/post/2013/09/24/why-are-black-men-negatively-affected-by-prostate-cancer-more-than-white-men.aspx.
- Brown TT, Scheffler RM, Seo S, Reed M. The empirical relationship between community social capital and the demand for cigarettes. Health Economics. 2006;15:1159–1172. doi: 10.1002/hec.1119. [DOI] [PubMed] [Google Scholar]
- California Health Interview Survey. Response rates. In: Cervantes IF, Norman GJ, Brick MJ, Edwards S, editors. CHIS 2007 methodology series. Vol. 4. Los Angeles: UCLA Center for Health Policy Research; 2009. [Google Scholar]
- Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, Zietman AL. Early detection of prostate cancer: AUA guideline. Journal of Urology. 2013;190:419–426. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2008 Behavioral Risk Factor Surveillance System (BRFSS). Summary data quality report. Atlanta, GA: U.S. Department of Health and Human Services; 2009. Retrieved from ftp://ftp.cdc.gov/pub/data/brfss/2008_Summary_Data_Quality_Report.pdf. [Google Scholar]
- Dasgupta P, Serageldin I. Social capital: A multifaceted perspective. Washington, DC: World Bank Publications; 1999. [Google Scholar]
- De Silva MJ, McKenzie K, Harpham T, Huttly SR. Social capital and mental illness: A systematic review. Journal of Epidemiology & Community Health. 2005;59:619–627. doi: 10.1136/jech.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean L, Gilbert K. Social capital, social policy, and health disparities: A legacy of political advocacy in African-American communities. In: Kawachi I, Takoa S, Subramanian SV, editors. Global perspectives on social capital and health. New York, NY: Springer; 2013. pp. 307–322. [Google Scholar]
- Hanifan LJ. The rural school community center. Annals of the American Academy of Political and Social Science. 1916;67:130–138. [Google Scholar]
- Hoffman-Goetz L, Breen NL, Meissner H. The impact of social class on the use of cancer screening within three racial/ethnic groups in the United States. Ethnicity & Disease. 1998;8:43–51. [PubMed] [Google Scholar]
- Holt CL, Wynn TA, Litaker MS, Southward P, Jeames S, Schulz E. A comparison of a spiritually based and non-spiritually based educational intervention for informed decision making for prostate cancer screening among church-attending African-American men. Urologic Nursing. 2009;29:249–258. [PMC free article] [PubMed] [Google Scholar]
- Jakes TD. Manpower Men’s Ministry. 2013 Retrieved from http://67.227.231.30/about/bishop-t-d-jakes/
- Kawachi I, Berkman LF. Social ties and mental health. Journal of Urban Health. 2001;78:458–467. doi: 10.1093/jurban/78.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Kennedy BP, Glass R. Social capital and self-rated health: A contextual analysis. American Journal of Public Health. 1999;89:1187–1193. doi: 10.2105/ajph.89.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Subramanian SV, Kawachi I. Social capital and physical health. In: Kawachi I, Subramanian SV, Kim D, editors. Social capital and health. Vol. 1. New York, NY: Springer; 2007. pp. 139–190. [Google Scholar]
- Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The public health disparities geocoding project (US) Journal of Epidemiology and Community Health. 2003;57:186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader AE, Michael YL. The association between neighborhood social capital and cancer screening. American Journal of Health Behavior. 2013;37:683–692. doi: 10.5993/AJHB.37.5.12. [DOI] [PubMed] [Google Scholar]
- Lee E, Brown ER, Grant D, Belin TR, Brick JM. Exploring nonresponse bias in a health survey using neighborhood characteristics. American Journal of Public Health. 2009;99:1811–1817. doi: 10.2105/AJPH.2008.154161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom AM. Social capital and health-related behaviors. In: Kawachi I, Subramanian SV, Kim D, editors. Social capital and health. Vol. 1. New York, NY: Springer; 2007. pp. 215–238. [Google Scholar]
- Lynch J, Smith GD, Hillemeier M, Shaw M, Raghunathan T, Kaplan G. Income inequality, the psychosocial environment, and health: Comparisons of wealthy nations. Lancet. 2001;358:194–200. doi: 10.1016/S0140-6736(01)05407-1. [DOI] [PubMed] [Google Scholar]
- Merkin SS, Stevenson L, Powe N. Geographic socioeconomic status, race, and advanced-stage breast cancer in New York City. American Journal of Public Health. 2002;92:64–70. doi: 10.2105/ajph.92.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D. Kappa Alpha Psi hosts free health screenings for men’s needs at the 1st annual men’s health fair. 2013 Retrieved from http://www.mlive.com/news/saginaw/index.ssf/2013/06/free_health_screenings_hone_in.html.
- Myers RE, Wolf TA, McKee L, McGrory G, Burgh DY, Nelson G, Nelson GA. Factors associated with intention to undergo annual prostate cancer screening among African American men in Philadelphia. Cancer. 1996;78:471–479. doi: 10.1002/(SICI)1097-0142(19960801)78:3<471::AID-CNCR14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Potosky AL, Breen N, Graubard BI, Parsons PE. The association between health care coverage and the use of cancer screening tests. Results from the 1992 National Health Interview Survey. Medical Care. 1998;36:257–270. doi: 10.1097/00005650-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Putnam RD. Making democracy work. Civic traditions in modern Italy. Princeton, NJ: Princeton University Press; 1993. [Google Scholar]
- Putnam RD. Bowling alone: America’s declining social capital. Journal of Democracy. 1995;6:65–78. [Google Scholar]
- Rasbash J, Charlton C, Browne WJ, Healy M, Cameron B. MLwIN, (Version 2.1) Bristol, England: Centre for Multilevel Modelling, University of Bristol; 2009. [Google Scholar]
- Ross LE, Meade SA, Powe BD, Howard DL. Prostate-specific antigen test use and digital rectal examinations among African-American men, 2002–2006. Journal of National Black Nurses’ Association. 2009;20:52–58. [PubMed] [Google Scholar]
- Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: A multilevel study of collective efficacy. Science. 1997;277:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- Shen Y, Dong W, Esteva FJ, Kau S, Theriault RL, Bevers TB. Are there racial differences in breast cancer treatments and clinical outcomes for women treated at MD Anderson cancer center? Breast Cancer Research and Treatment. 2007;102:347–365. doi: 10.1007/s10549-006-9337-2. [DOI] [PubMed] [Google Scholar]
- Simons V. Walking away from the abyss. In the know: Confronting prostate cancer. 2012 Retrieved from http://www.theprostatenet.org/12-0702_Prostate_Net_July_Newsletter_Final.pdf.
- Smith-Bindman R, Miglioretti DL, Lurie N, Abraham L, Barbash RB, Strzelczyk J, Kerlikowske K. Does utilization of screening mammography explain racial and ethnic differences in breast cancer? Annals of Internal Medicine. 2006;144:541–553. doi: 10.7326/0003-4819-144-8-200604180-00004. [DOI] [PubMed] [Google Scholar]
- Subramanian SV, Lochner KA, Kawachi I. Neighborhood differences in social capital: A compositional artifact or a contextual construct? Health & Place. 2003;9(1):33–44. doi: 10.1016/s1353-8292(02)00028-x. [DOI] [PubMed] [Google Scholar]
- Tataw DB, Ekúndayò OT. Horizontal participatory planning in prostate cancer prevention: An analysis of the Mississippi prostate cancer project. Journal of Human Behavior in the Social Environment. 2012;22:733–754. [Google Scholar]
- Tingen MS, Weinrich S, Heydt DD, Boyd MD, Weinrich MC. Perceived benefits: A predictor of participation in prostate cancer screening. Cancer Nursing. 1998;21:349–357. doi: 10.1097/00002820-199810000-00006. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Screening for prostate cancer. 2012 doi: 10.1001/archinternmed.2012.135. Retrieved from http://www.uspreventive-servicestaskforce.org/prostatecancerscreening.htm. [DOI] [PMC free article] [PubMed]
- Weinrich S, Holdford M, Boyd M, Creanga D, Cover K, Johnson A, Weinrich M. Prostate cancer education in African American churches. Public Health Nursing. 1998;15(3):188–195. doi: 10.1111/j.1525-1446.1998.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Weinrich SP, Reynolds WA, Jr, Tingen MS, Starr CR. Barriers to prostate cancer screening. Cancer Nursing. 2000;23:117–121. doi: 10.1097/00002820-200004000-00007. [DOI] [PubMed] [Google Scholar]
- Wilkinson S, List M, Sinner M, Dai L, Chodak G. Educating African-American men about prostate cancer: Impact on awareness and knowledge. Urology. 2003;61(2):308–313. doi: 10.1016/s0090-4295(02)02144-1. [DOI] [PubMed] [Google Scholar]
- Wilson J. Free health screenings for men offered May 14–16. The Chattanoogan. 2013 May 8; Retrieved from http://www.chattanoogan.com/2013/5/8/250725/Free-Health-Screenings-For-Men-Offered.aspx.
- Wolf AMD, Wender RC, Etzioni RB, Thompson IM, D’Amico AV, Volk RJ, Smith RA. American Cancer Society guideline for the early detection of prostate cancer: Update 2010. CA: A Cancer Journal for Clinicians. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]