Abstract
Introduction
Pyruvate dehydrogenase (PDH) is a key gatekeeper enzyme in aerobic metabolism. The main purpose of this study was to determine if PDH activity is affected by major stress in the form of coronary artery bypass grafting (CABG) which has previously been used as a model of critical illness.
Methods
We conducted a prospective, observational study of patients undergoing CABG at an urban, tertiary care hospital. We included adult patients undergoing CABG with or without concomitant valve surgery. Measurements of PDH activity and quantity and thiamine were obtained prior to surgery, at the completion of surgery, and 6 hours post-surgery.
Results
Fourteen patients were enrolled (age: 67 ± 10 years, 21 % female). Study subjects had a mean 41.7 % (SD: 27.7) reduction in PDH activity after surgery and a mean 32.0% (SD: 31.4) reduction 6 hours after surgery (p < 0.001). Eight patients were thiamine deficient (≤ 7 nmol/L) after surgery compared to none prior to surgery (p = 0.002). Thiamine level was a significantly associated with PDH quantity at all time points (p = 0.01). Post-surgery lactate levels were inversely correlated with post-surgery thiamine levels (r = −0.58 and p = 0.04).
Conclusion
The stress of major surgery causes decreased PDH activity and quantity, and depletion of thiamine levels.
Keywords: thiamine, activity, quantity, lactate, critical illness, surgery, metabolism, major stress
Introduction
Coronary artery bypass grafting (CABG) is a common major surgical procedure performed on more than 245,000 patients in the United States each year.(1) While overall mortality is relatively low, morbidity remains substantial with complications such as atrial fibrillation, post-operative delirium, infections, stroke, prolonged mechanical ventilation, and prolonged length of hospital and intensive care unit stay.(2, 3) During CABG, a shift from aerobic to anaerobic metabolism occurs, causing increased levels of pyruvate and lactate.(4) Elevated lactate post-surgery is correlated with increased mortality and morbidity (including length of mechanical ventilation, length of intensive care unit stay and risk of reoperation).(5–7) Although elevation of lactate after CABG is common, the pathophysiology is not fully understood.
When pyruvate, the end-product of glycolysis, cannot enter the Krebs cycle, lactate is produced. Pyruvate dehydrogenase (PDH) is a key mitochondrial enzyme responsible for the conversion of pyruvate to acetyl-CoA and the enzyme is therefore essential in facilitating aerobic metabolism (see Figure 1). Thiamine, or vitamin B1, is a crucial co-factor for pyruvate dehydrogenase. In the absence of thiamine, the conversion of pyruvate to acetyl-CoA is inhibited and lactate is produced.(8)
Figure 1.
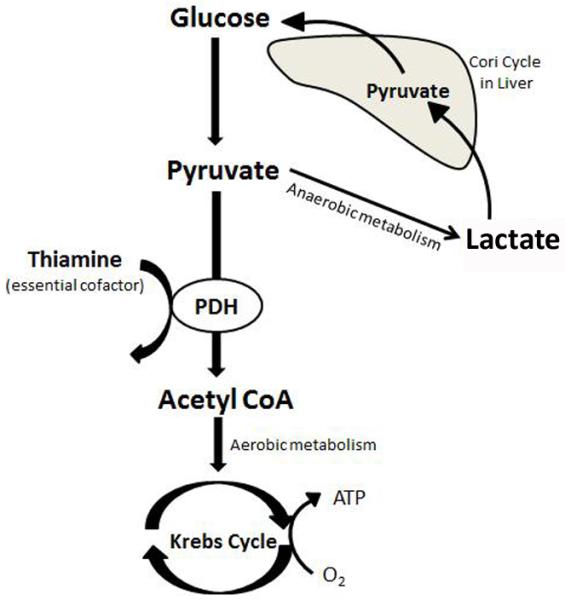
The role of PDH and thiamine in aerobic metabolism. Reprinted/adapted from Andersen et al.(35) with permission from Elsevier.
ATP = Adenosine triphosphate; CoA = Coenzyme A; PDH = Pyruvate dehydrogenase
PDH activity in connection with ischemia-reperfusion injury has been extensively studied in in vitro models(9–13) but there is a lack of human studies examining PDH activity in connection with major stress. We hypothesize that critical illness/major stress will decrease the activity of PDH. CABG has previously been used as a model for critical illness and offers an opportunity for measurements before and after the induction of major stress.(14–16) The aim of the current study was therefore to examine the effect of major stress, in the form of CABG, on the activity of the mitochondrial enzyme PDH and furthermore relate this to thiamine and lactate levels.
Materials and Methods
Design
We conducted a prospective, observational, single-center study at an urban, tertiary care center with approximately 400 CABG surgeries each year and a total of 77 intensive care unit beds. The study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center, Boston, Massachusetts. Patients were screened and enrolled over a five week period in October and November 2012. All patients provided informed written consent before enrollment.
Inclusion and exclusion criteria
Inclusion criteria were: 1) CABG with or without concomitant valve surgery and 2) age ≥ 18 years. Exclusion criteria were: 1) Emergency or salvage CABG, 2) revision of a previous CABG, 3) thiamine supplementation within the last 14 days greater than 6 mg per day (i.e., more than a multivitamin), 4) known allergy to thiamine, 5) non-English speaking, 6) inability to provide informed written consent, and 7) research-protected populations (pregnant women, prisoners, and the intellectually disabled).
Procedures
Blood draws were obtained just prior to surgery (pre-surgery), at arrival to the intensive care unit after surgery (post-surgery) and six hours after arrival (6h post-surgery) from already established arterial or venous lines. Blood was collected into 10 mL EDTA (ethylenediaminetetraacetic acid) tubes. Seven mL were used for measurement of PDH activity (see below). The rest was centrifuged at 3500 rpm for 10 minutes; plasma was aliquoted into light blocked cryotubes and frozen at −80°C. At the above mentioned time points we also collected clinical variables such as vital signs and laboratory values including clinically drawn lactate levels. Intra-surgery variables were collected from operative reports and anesthesia records. The EuroSCORE II(17), which is a validated score to predict morbidity and mortality in patients undergoing CABG, was calculated prospectively for all patients. All patients underwent cardiopulmonary bypass which was primed with Lactated Ringers solution (approximately 1 liter), 50 mEq bicarbonate, 12.5 g mannitol, and 1mg/kg tranexamic acid (max. of 100 mg) as per standard procedures at BIDMC.
PDH activity and thiamine levels
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood using density gradient (Ficoll-Paque premium, GE Healthcare Bio-Science Corp, Piscataway, NJ) separation method. We measured pyruvate dehydrogenase (PDH) activity and quantity in PBMCs. Briefly, protein concentration of each sample was determined by BCA (Bicinchoninic acid) method and adjusted to 15mg/mL. Then intact, functional PDH was solubilized from each sample by adding 1 volume of a non-denaturing detergent (lauryl maltoside) to 29 volume of each sample. Finally, the PDH enzyme is immunocaptured within each well by PDH antibodies and then subjected to functional activity and quantitative microplate assays (Abcam, Inc, Cambridge, MA). Absorbance of each well was measured at 37 °C by kinetic program for 20 minutes. PDH activity was expressed as the initial rate of reaction, determined from the slopes of the curves generated. After the activity assay, wells were washed twice with 1x stabilizer and incubated with a secondary detection antibody at room temperature for one hour. Wells were washed two and horseradish peroxidase label was added to each well and incubated for another hour. A final wash was performed and horseradish peroxidase development solution was rapidly added to each empty well. The absorbance of each well was measured at room temperature by kinetic program at 600 nm for 15 minutes. The PDH quantity was expressed as an initial reaction rate determined from the slopes of the curves generated. The PDH activity and quantity is calibrated to the total amount of protein in the sample in order to allow for differences in total protein content. PDH specific activity was calculated as PDH activity/ln(PDH quantity).
Thiamine levels were measured via Liquid Chromatography/Tandem Mass Spectrometry by Quest Diagnostics (Nichols Institute, Chantilly, VA, USA). Absolute thiamine deficiency was determined using previously established standard lab reference range from Quest Diagnostics; specifically, absolute thiamine deficiency was defined as a level ≤ 7 nmol/L. A conservative value of 7 nmol/L was imputed for those measurements where the thiamine level was below the detectable level (7 nmol/L).
Statistical approach
Descriptive statistics were used to summarize the study population. Data for continuous variables are presented as means with standard deviations (SD) or medians with quartiles depending on normality of the data. Categorical data are presented as counts with frequencies. Percent change in PDH activity, quantity and specific activity was calculated as: (post-surgery level – pre-surgery level) divided by pre-surgery level. All the variables were tested for normal distribution, and logarithmically transformed before statistical analysis if not normally distributed. For comparing changes across the entire study duration, we used repeated measures analysis to evaluate changes in plasma thiamine, and PDH activity, quantity and specific quantity over time. A mixed-model approach with adjustment for age and gender was used to evaluate associations between thiamine levels and PDH activity and quantity. McNemar's test was used to test the difference between prevalence of thiamine deficiency before and after surgery. Pearson's correlation coefficient was obtained between thiamine levels and lactate levels immediately after surgery and between intra surgical variables and post-surgery PDH activity. A p-value < 0.05 was considered statistically significant. All tests of the data were performed in SAS v. 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Twenty-six patients were screened. Twenty-two patients met all inclusion criteria and none of the exclusion criteria and were approached for consent. Of these, a total of 19 patients were consented. Five patients had their surgery cancelled/rescheduled after consent leaving 14 patients for the primary analysis. The mean age was 67 ± 10 years and 21 % were female. Ten (71%) had isolated CABG, four (29%) had concomitant valve surgery and all patients were on cardiopulmonary bypass during surgery. The mean post-operative lactate was 2.8 mmol/L (SD 1.0). No patients had known liver disease, history of malnutrition, current alcohol abuse or other diseases traditionally associated with thiamine deficiency. Additional patient characteristics are shown in Table 1.
Table 1.
| Characteristics | All Patients (n = 14) |
|---|---|
| Demographics | |
| Gender (female) | 3 (21) |
| Age (years) | 67 ± 10 |
| Race (white) | 13 (93) |
| Body mass index (kg/(m2)) | 31 ± 11 |
| Co-morbidities | |
| Congestive heart failure | 2 (14) |
| COPD | 1 (7) |
| Diabetes | 5 (36) |
| Dyslipidemia | 10 (71) |
| Hypertension | 8 (57) |
| Cancer | 3 (21) |
| CABG characteristics | |
| EuroSCORE IIc(%) | 1.9 ± 0.1 |
| Concomitant valve surgery | 4 (29) |
| Length of surgery (minutes) | 346 ± 87 |
| Bypass time (minutes) | 106 ± 37 |
| Cross clamp time (minutes) | 88 ± 38 |
CABG: Coronary artery bypass grafting, COPD: Chronic obstructive pulmonary disease
Continuous variables are expressed as mean ± standard deviation and categorical variables as counts (frequencies)
The EuroSCORE (European System for Cardiac Operative Risk Evaluation) II is a validated score to predict operative mortality. The score incorporates patient related factors (e.g. age and certain co-morbidities), cardiac related factors (e.g. angina class and left ventricular function) and surgery related factors (e.g. urgency and type).
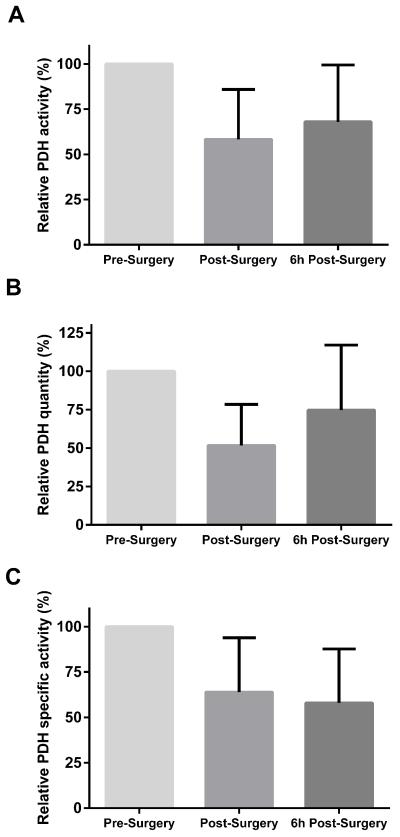
Study subjects had a mean 41.7 % (SD: 27.7) reduction in PDH activity after surgery and a mean 32.0% (SD: 31.4) reduction 6 hours after surgery (p < 0.001, Figure 2A). The amount of PDH protein was also reduced immediately after surgery with a mean loss of 48.3% (SD: 26.7) and six hours after surgery with a mean loss of 25.3% (SD: 42.3, p < 0.001, Figure 2B). Specific PDH activity was reduced 36.1% (SD: 30.0) after surgery and 41.8% (SD: 29.7) 6 hours after surgery (p < 0.001, Figure 2C).
Figure 2.
Relative PDH activity (A), quantity (B) and specific activity (C) before and after the surgery. The graphs represent mean with the error bar indicating one standard deviation. There was a significant change in all three measurements over time (p < 0.001). .
PDH = Pyruvate dehydrogenase
There was no correlation between post-surgery PDH activity and surgery time, cross-clamp time or bypass time. We also did not find a correlation between post-surgery PDH activity and estimated blood loss during the surgery.
Thiamine levels were lower after surgery (7 nmol/L [quartiles: 7 – 11]) as compared to pre-surgery levels (13 nmol/L [quartiles: 9 – 16]) and remained low six hours after surgery (8 nmol/L [quartiles: 7 – 10], p < 0.001, Figure 3). The decrease in thiamine remained statistical significant when adjusting for total sample protein indication that the decrease cannot be explained by hemodilution. Eight patients were thiamine deficient (≤ 7 nmol/L) after surgery compared to none of the patients before (p = 0.001). Thiamine level was associated with PDH quantity at all time points after adjustment for age and sex (p = 0.01) and we found a trend towards an association between thiamine level and PDH activity (p = 0.09). Immediate post-surgery lactate levels were inversely correlated with post-surgery thiamine levels (r = −0.58, p = 0.04).
Figure 3.
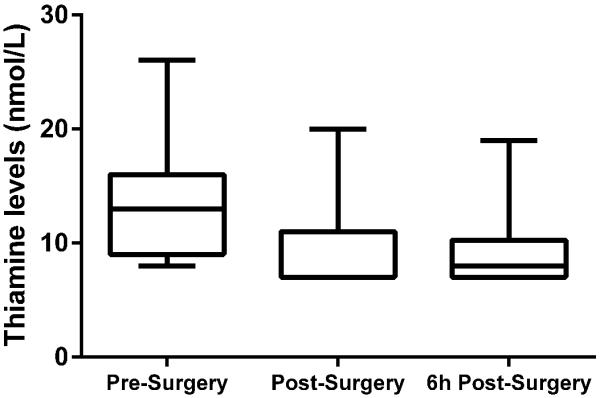
Thiamine levels before and after the surgery. The box plots represent minimum, 1st quartile, median, 3rd quartile and maximum. There was a significant change in thiamine over time (p < 0.001).
Discussion
We found that PDH activity and quantity decreases in connection with profound stress in the form of CABG. More specifically we found a more than 40% reduction in PDH activity immediately after surgery as compared to before surgery and activity was still decreased six hours after surgery. We furthermore found that CABG depletes thiamine levels and more than half the patients became thiamine deficient after their surgery.
PDH activity has been studied in different in vitro models of ischemic-reperfusion injury. Kobayashi and Neely examined isolated rats hearts and induced ten minutes of ischemia followed by reperfusion.(9) While PDH activity was not markedly affected immediately after the ischemic period, the activity decreased by more than half within two minutes of reperfusion. Also using isolated rats hearts Patel and Olson showed that PDH activity decreases with induced ischemia and found that more profound ischemia causes a greater decrease in PDH activity.(10) Using indirect methods Lewandowski et al. likewise found signs of decreased PDH activity in connection with ischemia.(11) Similar findings have been established using isolated human cardiomyocytes (12, 13) as well as in animal cardiac arrest and brain ischemia models of ischemia-reperfusion injury.(18−22) The results in the current study are in accordance with these findings showing that major stress, whether it being experimental induced ischemic-reperfusion injury or clinically in the form of CABG, causes a decrease in the activity of PDH.
We furthermore found a decrease in the quantity of PDH protein after surgery. It should be noted that this decrease cannot be explained by hemodilution or blood loss since the PDH measurements are calibrated to the total amount of protein. We cannot determine the mechanism from the current study but the decrease could be related to the decrease in thiamine levels. Thiamine deficiency has been shown to negatively affect PDH mRNA levels in in vitro experiments suggesting that thiamine is important in the regulation of PDH production.(23) Furthermore, the binding of thiamine to PDH might serve as a protection against intracellular proteolysis and breakdown, which has been shown for other thiamine dependent mitochondrial enzymes.(24)
Our group has previously reported that CABG depletes thiamine (25) and thiamine deficiency has been described in the critical ill population.(26) A prominent feature in some cases of thiamine deficiency is severe lactic acidosis which has been described to rapidly resolve with the administration of intravenous thiamine.(27) Our group has previously found a significant inverse relationship between thiamine levels and lactate levels in critical ill patients with diabetic ketoacidosis (28) and sepsis (29). The current study found a similar inverse relationship between thiamine levels and lactate suggesting that thiamine might play an important role in lactate metabolism during major surgery and critical illness.
CABG causes a major stress response to the human body including a systemic inflammatory response (30, 31), and has therefore been used as a prospective “human model” of critical illness in a number of studies.(14−16) The association between lactate levels and outcome has been established in CABG (5−7) and a wide range of critical illnesses including sepsis (32), post-cardiac arrest(33) and trauma (34). Meanwhile, the exact pathophysiology of lactate production in these patients remains unclear and is likely multifactorial.(35) The current finding suggests a defect in PDH during CABG that may be translatable to a broader population of critical ill patients but further studies are needed to clarify this.
The current study has certain limitations. First, the number of participants was small, making extensive statistical analyses difficult to perform and we may have had inadequate statistical power for some comparisons. The size of the study also limits our ability to analyze the association between PDH measurements and patient centered outcomes. Secondly, we did not measure lactate levels as part of our protocol but utilized clinical drawn lactate levels. That stated lactate levels in this population were drawn immediately post-operatively in 100% of patients and in 64% of patients six hours posto-peratively.
In conclusion, we found a significant decrease in PDH activity/quantity and a depletion of thiamine levels in patients undergoing CABG. Whether there is a causal relationship between depletions of thiamine, decreased PDH activity and quantity and increased lactate production cannot be determined from the current study. Further research is warranted to clarify the complex pathophysiology of anaerobic metabolism and lactate production in connection with major surgery and critical illness.
Acknowledgments
Sources of Funding Dr. Donnino is supported by NHLBI (1K02HL107447-01A1).
List of abbreviations
- PDH
pyruvate dehydrogenase
- CABG
coronary artery bypass grafting
- SD
standard deviation
- PMBC
peripheral blood mononuclear cell
Footnotes
Conflicts of interests On behalf of all authors, the corresponding author states that there is no conflict of interest.
Authors' contributions LWA and MD were responsible for study conception and design. LWA and TP enrolled patients. XL performed PDH measurements. LWA, XL and TG performed statistical analyses. All authors took part in critical revision of the manuscript, interpreted the data, and provided intellectual content. All authors were responsible for drafting or revising the manuscript. All authors read and approved the final submission and agree to be accountable for all aspects of the work.
References
- 1.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW. Coronary revascularization trends in the United States, 2001–2008. JAMA. 2011;305(17):1769–76. doi: 10.1001/jama.2011.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silber JH, Rosenbaum PR, Schwartz JS, Ross RN, Williams SV. Evaluation of the complication rate as a measure of quality of care in coronary artery bypass graft surgery. JAMA. 1995;274(4):317–23. [PubMed] [Google Scholar]
- 4.Mandak J, Pojar M, Cibicek N, Lonsky V, Palicka V, Kakrdova D, Nedvidkova J, Kubicek J, Zivny P. Impact of cardiopulmonary bypass on peripheral tissue metabolism and microvascular blood flow. Perfusion. 2008;23(6):339–46. doi: 10.1177/0267659109105359. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay AJ, Xu M, Sessler DI, Blackstone EH, Bashour CA. Lactate Clearance Time and Concentration Linked to Morbidity and Death in Cardiac Surgical Patients. Ann Thorac Surg. 2012 doi: 10.1016/j.athoracsur.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Toraman F, Evrenkaya S, Yuce M, Aksoy N, Karabulut H, Bozkulak Y, Alhan C. Lactic acidosis after cardiac surgery is associated with adverse outcome. Heart Surg Forum. 2004;7(2):E155–9. doi: 10.1532/HSF98.20041002. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar LA, Almeida JP, Fukushima JT, Rhodes A, Vincent JL, Osawa EA, Galas FR. High lactate levels are predictors of major complications after cardiac surgery. J Thorac Cardiovasc Surg. 2013 doi: 10.1016/j.jtcvs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Naito E, Ito M, Yokota I, Saijo T, Matsuda J, Kuroda Y. Thiamine-responsive lactic acidaemia: role of pyruvate dehydrogenase complex. Eur J Pediatr. 1998;157(8):648–52. doi: 10.1007/s004310050903. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi K, Neely JR. Effects of ischemia and reperfusion on pyruvate dehydrogenase activity in isolated rat hearts. J Mol Cell Cardiol. 1983;15(6):359–67. doi: 10.1016/0022-2828(83)90320-6. [DOI] [PubMed] [Google Scholar]
- 10.Patel TB, Olson MS. Regulation of pyruvate dehydrogenase complex in ischemic rat heart. Am J Physiol. 1984;246(6 Pt 2):H858–64. doi: 10.1152/ajpheart.1984.246.6.H858. [DOI] [PubMed] [Google Scholar]
- 11.Lewandowski ED, Johnston DL. Reduced substrate oxidation in postischemic myocardium: 13C and 31P NMR analyses. Am J Physiol. 1990;258(5 Pt 2):H1357–65. doi: 10.1152/ajpheart.1990.258.5.H1357. [DOI] [PubMed] [Google Scholar]
- 12.Rao V, Merante F, Weisel RD, Shirai T, Ikonomidis JS, Cohen G, Tumiati LC, Shiono N, Li RK, Mickle DA, Robinson BH. Insulin stimulates pyruvate dehydrogenase and protects human ventricular cardiomyocytes from simulated ischemia. J Thorac Cardiovasc Surg. 1998;116(3):485–94. doi: 10.1016/S0022-5223(98)70015-7. [DOI] [PubMed] [Google Scholar]
- 13.Merante F, Mickle DA, Weisel RD, Li RK, Tumiati LC, Rao V, Williams WG, Robinson BH. Myocardial aerobic metabolism is impaired in a cell culture model of cyanotic heart disease. Am J Physiol. 1998;275(5 Pt 2):H1673–81. doi: 10.1152/ajpheart.1998.275.5.H1673. [DOI] [PubMed] [Google Scholar]
- 14.Spratt DI, Kramer RS, Morton JR, Lucas FL, Becker K, Longcope C. Characterization of a prospective human model for study of the reproductive hormone responses to major illness. Am J Physiol Endocrinol Metab. 2008;295(1):E63–9. doi: 10.1152/ajpendo.00472.2007. [DOI] [PubMed] [Google Scholar]
- 15.Debono M, Sheppard L, Irving S, Jackson P, Butterworth J, Brookes ZL, Newell-Price J, Ross JJ, Ross RJ. Assessing adrenal status in patients before and immediately after coronary artery bypass graft surgery. Eur J Endocrinol. 2011;164(3):413–9. doi: 10.1530/EJE-10-0996. [DOI] [PubMed] [Google Scholar]
- 16.Spratt DI, Frohnauer M, Cyr-Alves H, Kramer RS, Lucas FL, Morton JR, Cox DF, Becker K, Devlin JT. Physiological effects of nonthyroidal illness syndrome in patients after cardiac surgery. Am J Physiol Endocrinol Metab. 2007;293(1):E310–5. doi: 10.1152/ajpendo.00687.2006. [DOI] [PubMed] [Google Scholar]
- 17.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 18.Bogaert YE, Sheu KF, Hof PR, Brown AM, Blass JP, Rosenthal RE, Fiskum G. Neuronal subclass-selective loss of pyruvate dehydrogenase immunoreactivity following canine cardiac arrest and resuscitation. Exp Neurol. 2000;161(1):115–26. doi: 10.1006/exnr.1999.7250. [DOI] [PubMed] [Google Scholar]
- 19.Bogaert YE, Rosenthal RE, Fiskum G. Postischemic inhibition of cerebral cortex pyruvate dehydrogenase. Free Radic Biol Med. 1994;16(6):811–20. doi: 10.1016/0891-5849(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 20.Fukuchi T, Katayama Y, Kamiya T, McKee A, Kashiwagi F, Terashi A. The effect of duration of cerebral ischemia on brain pyruvate dehydrogenase activity, energy metabolites, and blood flow during reperfusion in gerbil brain. Brain Res. 1998;792(1):59–65. doi: 10.1016/s0006-8993(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 21.Zaidan E, Sims NR. Selective reductions in the activity of the pyruvate dehydrogenase complex in mitochondria isolated from brain subregions following forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13(1):98–104. doi: 10.1038/jcbfm.1993.12. [DOI] [PubMed] [Google Scholar]
- 22.Zaidan E, Sims NR. Reduced activity of the pyruvate dehydrogenase complex but not cytochrome c oxidase is associated with neuronal loss in the striatum following short-term forebrain ischemia. Brain Res. 1997;772(1–2):23–8. doi: 10.1016/s0006-8993(97)00833-0. [DOI] [PubMed] [Google Scholar]
- 23.Pekovich SR, Martin PR, Singleton CK. Thiamine deficiency decreases steady-state transketolase and pyruvate dehydrogenase but not alpha-ketoglutarate dehydrogenase mRNA levels in three human cell types. J Nutr. 1998;128(4):683–7. doi: 10.1093/jn/128.4.683. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Wynn RM, Machius M, Chuang JL, Karthikeyan S, Tomchick DR, Chuang DT. Cross-talk between thiamin diphosphate binding and phosphorylation loop conformation in human branched-chain alpha-keto acid decarboxylase/dehydrogenase. J Biol Chem. 2004;279(31):32968–78. doi: 10.1074/jbc.M403611200. [DOI] [PubMed] [Google Scholar]
- 25.Donnino MW, Cocchi MN, Smithline H, Carney E, Chou PP, Salciccioli J. Coronary artery bypass graft surgery depletes plasma thiamine levels. Nutrition. 2010;26(1):133–6. doi: 10.1016/j.nut.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruickshank AM, Telfer AB, Shenkin A. Thiamine deficiency in the critically ill. Intensive Care Med. 1988;14(4):384–7. doi: 10.1007/BF00262893. [DOI] [PubMed] [Google Scholar]
- 27.Thomas L, Fay D, Moraillon X, Delubac G, Berthet P, Demingeon G. Lactic acidosis treated with thiamine. 3 cases. Presse Med. 1985;14(36):1871–5. [PubMed] [Google Scholar]
- 28.Moskowitz A, Graver A, Giberson T, Berg K, Liu X, Uber A, Gautam S, Donnino MW. The relationship between lactate and thiamine levels in patients with diabetic ketoacidosis. J Crit Care. 2014;29(1):182, e5–8. doi: 10.1016/j.jcrc.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donnino MW, Carney E, Cocchi MN, Barbash I, Chase M, Joyce N, Chou PP, Ngo L. Thiamine deficiency in critically ill patients with sepsis. J Crit Care. 2010;25(4):576–81. doi: 10.1016/j.jcrc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Hess PJ., Jr. Systemic inflammatory response to coronary artery bypass graft surgery. Am J Health Syst Pharm. 2005;62(18 Suppl 4):S6–9. doi: 10.2146/ajhp050302. [DOI] [PubMed] [Google Scholar]
- 31.Kapoor MC, Ramachandran TR. Inflammatory response to cardiac surgery and strategies to overcome it. Ann Card Anaesth. 2004;7(2):113–28. [PubMed] [Google Scholar]
- 32.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–8. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Donnino MW, Andersen LW, Giberson T, Gaieski DF, Abella BS, Peberdy MA, Rittenberger JC, Callaway CW, Ornato J, Clore J, Grossestreuer A, Salciccioli J, Cocchi MN, National Post-Arrest Research C Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med. 2014;42(8):1804–11. doi: 10.1097/CCM.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan LJ, Kellum JA. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury. Crit Care Med. 2004;32(5):1120–4. doi: 10.1097/01.ccm.0000125517.28517.74. [DOI] [PubMed] [Google Scholar]
- 35.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127–40. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]