Abstract
Background
E9802 was a phase 2 multi-institution study conducted to evaluate the safety and effectiveness of vaccinia and fowlpox prostate-specific antigen (PSA) vaccine (step 1) followed by combination with androgen ablation therapy (step 2) in patients with PSA progression without visible metastasis.
Objective
To test the hypothesis that vaccine therapy in this early disease setting will be safe and have a biochemical effect that would support future studies of immunotherapy in patients with minimal disease burden.
Design, setting, and participants
Patients had PSA progression following local therapy were treated with PROSTVAC-V (vaccinia)/TRICOM on cycle 1 followed by PROSTVAC-F (fowlpox)/TRICOM for subsequent cycles in combination with granulocyte-macrophage colony-stimulating factor (step 1). Androgen ablation was added on progression (step 2).
Outcome measurements and statistical analysis
Step 1 primary end points included progression at 6 mo and characterization of change in PSA velocity pretreatment to post-treatment. Step 2 end points included PSA response with combined vaccine and androgen ablation.
Results and limitations
In step 1, 25 of 40 eligible patients (63%) were progression free at 6 mo after registration (90% confidence interval [CI], 48–75). The median pretreatment PSA velocity was 0.13 log(PSA)/mo, in contrast to median postregistration velocity of 0.09 log(PSA)/mo (p = 0.02), which is an increase in median PSA doubling time from 5.3 mo to 7.7 mo. No grade ≥4 treatment-related toxicity was observed. In the 27 patients eligible and treated for step 2, 20 patients achieved a complete response (CR) at 7 mo (CR rate: 74%; 90% CI, 57–87). Although supportive of larger studies in the cooperative group setting, this study is limited by the small number of patients and the absence of a control group as in a phase 3 study.
Conclusions
A viral PSA vaccine can be administered safely in the multi-institutional cooperative group setting to patients with minimal disease volume alone and combined with androgen ablation, supporting the feasibility of future phase 3 studies in this population.
Patient summary
These data support consideration of vaccine therapy earlier in the course of prostate cancer progression with minimal disease burden in clinical practice and future studies of vaccine approaches in earlier stages of disease.
Keywords: Prostate cancer, Pox virus, Vaccine, PSA
1. Introduction
Despite significant recent improvements in the treatment of advanced castration-resistant prostate cancer (CRPC), standard therapy with low risk of toxicity for men with early relapse of prostate cancer (PCa) (defined only by prostate-specific antigen [PSA] progression) remains an unmet need [1–3]. Immune therapy with poxvirus vaccines are particularly attractive options because they can induce potent immune responses by mimicking natural infection, have great flexibility regarding antigen composition, and are easily administered [4,5]. PROSTVAC-V is a highly immunogenic vaccinia virus transduced with a modified full-length PSA with a single amino acid substitution, PSA(L155) as the encoded antigen and three costimulatory molecules— B7.1, ICAM-1, and LFA-3 (TRICOM)—that were found to be synergistic when added to the poxviral system. PROSTVAC-F is a fowlpox virus transduced with TRICOM and PSA(L155). In our prior Eastern Cooperative Oncology Group (ECOG) study, ECOG 7897, we evaluated the optimal sequencing of recombinant vaccinia and fowlpox vaccines without costimulatory molecules TRICOM demonstrating that vaccinia priming followed by fowlpox boost led to the strongest immune response and demonstrated the longest time to PSA progression [6]. PROSTVAC-VF (a single dose of PROSTVAC-V followed by PROSTVAC-F boosts) safety has been demonstrated in phase 1 and phase 2 studies including a randomized phase 2 of PROSTVAC-VF versus empty fowlpox vector (2:1) in 125 asymptomatic or minimally symptomatic men with metastatic CRPC [7]. Although the primary end point of progression-free survival (PFS) was not met, the trial did show a statistically significant improvement in median overall survival, and it led to an ongoing phase 3 study in men with metastatic CRPC (ClinicalTrials.gov identifier NCT01322490).
Although these promising studies address therapy in patients with higher tumor burdens, models of response to therapeutic vaccines suggest that patients with lower tumor burdens (ie, patients without visible metastatic disease) are more likely to benefit from a decrease in tumor growth rate over a longer period of time [8,9]. Prior preclinical and clinical data support the hypothesis that androgen ablation therapy is synergistic with vaccine approaches [10,11]. Building on these prior data, we tested PROSTVAC-VF in men with hormone-sensitive nonmetastatic PCa and PSA recurrence to provide an optimized clinical setting for immune treatment testing for an initial indication of activity and its feasibility in a multi-institutional setting for planning future larger studies.
2. Patients and methods
2.1. Patient eligibility
Eligible patients for step 1 were men aged ≥18 yr with ECOG performance status 0 or 1 who had a histologically confirmed diagnosis of PCa and completed local therapy, now with elevated PSA and no evidence of visible metastatic disease on physical examination, computed tomography or magnetic resonance imaging, or bone scan within 4 wk prior to registration. Patients required evidence of biochemical progression as determined by a reference PSA value (PSA1) followed by two rising PSA values (PSA2, PSA3), each higher than the previous value, obtained at least 4 wk apart. Patients must have had PSA doubling time (PSA DT) <12 mo. The baseline PSA value must have been obtained within 1 wk prior to registration and been >0.4 ng/ml (after prostatectomy) or >1.5 ng/ml (after radiation therapy). Prior neoadjuvant or adjuvant hormonal therapy or chemotherapy was allowed if discontinued ≥1 yr before enrollment, without progression. All patients signed institutional review board–approved consent forms before the study screening began. Patients with biochemical or clinical progression during step 1 were eligible to continue on to androgen blockade in step 2.
2.2. Study design and treatment
This single-arm study was conducted at participating sites in the ECOG. PROSTVAC-V (vaccinia; 2 × 108 pfu) was given subcutaneously on day 1 with subsequent boosts using PROSTVAC-F (1 × 109 pfu) given subcutaneously on day 1 of weeks 5, 9, 13, and 17, and then every 12 wk until biochemical or clinical progression. Starting the day of vaccination and continuing once daily for a total of 4 doses after each vaccination, granulocyte-macrophage colony-stimulating factor (GM-CSF) 100 µg was administered subcutaneously within 5 mm of the vaccination. At progression, eligible patients (without metastatic disease) were offered treatment (step 2) with androgen-deprivation therapy (bicalutamide 50 mg orally every day for 1 mo and luteinizing hormone-releasing hormone agonist for the duration of study participation). PROSTVAC-F and GM-CSF were continued at the same dose every 12 wk until biochemical or clinical progression (defined as in our prior study, with a 50% increase in PSA that is reconfirmed), evidence of metastasis by radiographic imaging, clinical progression, or a maximum of 12 mo. PROSTVAC-V (rV-PSA-TRICOM) and PROSTVAC-F(rF-PSA-TRICOM) were manufactured by Therion Biologics (Cambridge, MA, USA) and provided by the National Cancer Institute (NCI) Cancer Therapeutics Evaluation Program.
2.3. Immunologic monitoring
HLA-A2 typing by flow cytometry and enzyme-linked immunospot (ELISPOT) assays were performed using cryopreserved peripheral blood mononuclear cells. ELISPOT assays for interferon-γ production by T cells exposed to modified PSA (L155) were performed in the ECOG Central Immunology Laboratory with a 7-d in vitro stimulation, as previously described [6]. All conditions were plated in triplicate, and healthy donor assay controls were included on each plate to confirm reagent and assay performance. Because the trial was not designed for HLA-A2+ patients only, the subset tested for peptide-specific responses by ELISPOT was exploratory. Antibody titers against PSA were tested by a standardized enzyme-linked immunosorbent assay developed in the ECOG reference laboratory (University of Pittsburgh Immunologic Monitoring Lab). Results for anti-PSA antibodies were repeated at NCI laboratories with a different methodology used for prior NCI studies, as previously described [12].
2.4. Statistical considerations
The primary end points of step 1 of this study were the proportion of patients free of PSA progression before 6 mo (before the start of androgen ablation) and characterization of change in PSA velocity pretreatment to post-treatment. The primary analysis population included eligible and treated patients. PSA was assessed as complete response (CR), partial response (PR), stable disease, or progression. CR was considered in patients treated with prior radical prostatectomy as a PSA <0.2 ng/ml confirmed by a repeat PSA 1 mo later. In patients treated with radiation therapy only, a PSA <1 ng/ml on three separate occasions taken at least 1 mo apart was considered a complete biochemical response. A PR was considered with a reduction in PSA ≥50% from baseline, confirmed by a repeat PSA 1 mo later. Stable disease was defined as a reduction in PSA <50% from baseline or an increase in PSA not meeting the criteria of progressive disease. PSA progression was defined as an increase in PSA value >50% of baseline (on trial) or nadir PSA, whichever was lower, confirmed by a repeat PSA 2 wk later. The PSA must have risen by at least 5 ng/ml.
A target rate of 60% of progression-free patients at 6 mo was considered worthy of further study based on a prior study that used a similar 6-mo end point in a group of patients less likely to progress than this current proposal with more specified entry criteria [13]. Despite enrollment of patients with less aggressive disease, this prior study demonstrated that 64% were free of biochemical and clinical progression at 6 mo (36% progressed). Because our current study was testing a similar vaccine for patients with more aggressive disease, a true PSA progression-free rate of 60% (observing ≥21 free of progression at 6 mo) would be considered worthy of further study. With 45 patients (41 eligible patients), this design had a 10% probability of declaring the treatment effective if the true progression-free rate was 40% and a 90% probability of declaring the treatment effective if the true progression-free rate was 60%. With a final accrual of 40 eligible patients, the study still had 79% power with one-sided α of 0.07 using the same design (observing ≥21 free of progression at 6 mo). Although fewer than the prespecified sample size (40 eligible patients instead of 41 in the design), this study still observed 25 patients free of progression at 6 mo, exceeding the predefined rule of ≥21. Furthermore, the 80% confidence interval (CI) of the progression-free rate at 6 mo (51%, 73%) excluded the null hypothesis of 40% and included the target rate of 60%.
Step 1 data were assessed as of May 2013 and step 2 data as of April 2014. The pretreatment and postregistration PSA velocities were calculated using the three PSA values required for study entry and PSA measurements obtained every 4 wk for the first 6 mo of treatment by a piecewise linear model, with an additional analysis using the values obtained in the first 3 mo following baseline. To be considered evaluable for PSA velocity, patients must have completed at least 3 mo of treatment. The Wilcoxon signed rank test was used to test the difference between pretreatment and post-treatment PSA velocities, and effect by GM-CSF the changes in anti-PSA antibodies from baseline to 12 wk or 24 wk. The Kaplan-Meier method was used to characterize PFS and overall survival. Exact binomial CIs were used to describe PSA progression-free rate and PSA response. All p values are two sided.
3. Results
3.1. Characteristics of the patients
The study was activated in ECOG institutions on February 3, 2006, and terminated on December 11, 2007, after reaching its accrual goal of 50 patients. Table 1 shows the demographics and disease characteristics of the eligible patients at study entry. Patients with biochemical or clinical progression during initial therapy (step 1) were eligible to continue on with vaccine and androgen blockade in step 2. In step 1, 10 patients were ineligible (Table 2), and 40 patients were included in the main analysis. A total of 31 patients were registered for step 2. Among these, three were ineligible because criteria for progression were not met, and one patient never started assigned therapy. Therefore, 27 eligible and treated patients were included in the step 2 analysis. For step 1, the median duration of treatment among eligible patients was 9.9 mo with an interquartile range (IQR) of 4.3–15.4 mo. Overall, 75% were off treatment due to progressive disease. For step 2, the median duration of treatment among eligible and treated patients was 8.6 mo with an IQR of 7.5–10.3 mo.
Table 1.
Patient characteristics
| Characteristics (n = 40) | Result |
|---|---|
| Age, yr | |
| Median | 62.5 |
| IQR | 56–66.5 |
| Race, n (%) | |
| White | 36 (92) |
| Black | 3 (8) |
| Unknown | 1 |
| ECOG PS, n (%) | |
| 0 | 39 (97.5) |
| 1 | 1 (2.5) |
| Prior treatment, n (%) | |
| Single-agent cytotoxic systemic chemotherapy | 1 (3) |
| Multiagent cytotoxic systemic chemotherapy | 3 (8) |
| Hormonal therapy | 13 (33) |
| Radiation therapy | 32 (80) |
| Surgery | 39 (98) |
| Other | 6 (15) |
| PSA DT, mo* | |
| Median | 4.3. |
| IQR | 3.3–7.1 |
| Serum testosterone level, ng/dl | |
| Median | 379 |
| IQR | 277–455 |
ECOG PS = Eastern Cooperative Oncology Group performance status; IQR = interquartile range; PSA = prostate-specific antigen; PSA DT = prostate-specific antigen doubling time.
Submitted by the sites based on the formula provided in the protocol using two PSA measurements prior to study entry, therefore different from the pretreatment PSA DT assessed by multiple PSA measurements prior to the start of therapy using a piecewise linear model in the text.
Table 2.
Reasons for ineligibility
| Case | Reason |
|---|---|
| 98002 | No rising PSA |
| 98004 | PSA DT >365 d; baseline lab evaluation >4 wk prior to registration |
| 98011 | Testosterone level <150 ng/dl at baseline; no prior radiation or surgery; prior hormonal therapy within a year of registration |
| 98013 | No rising PSA |
| 98015 | Testosterone level <150 ng/dl at baseline |
| 98017 | Baseline testosterone level obtained after registration |
| 98028 | Prior hormonal therapy within a year of registration |
| 98029 | Baseline PSA obtained >1 wk prior to registration |
| 98039 | No rising PSA; baseline PSA obtained >1 wk prior to registration |
| 98046 | Baseline testosterone level obtained after registration |
PSA = prostate-specific antigen; PSA DT = prostate-specific doubling time.
3.2. Prostate-specific antigen biomarker assessment
Table 3 shows the PSA progression-free rate at 6 mo following vaccine therapy in step 1, as the planned primary end point. A total of 25 patients treated with vaccine alone were free of progression at 6 mo after registration, and the progression-free rate at 6 mo was 63% (90% CI, 48–75), exceeding the target rate of 60%, signifying that the treatment is worthy of further study. All the 25 patients deemed progression free at 6 mo had a 24-wk PSA assessment within 10 d of the prespecified schedule except for three patients. These three patients had 24-wk PSA assessments 3–5 wk away from the prespecified schedule, but all had progression-free status documented at 7 and 8 mo after registration. These three patients did not have progression until 9, 12, and 30 mo, respectively, after registration.
Table 3.
Prostate-specific antigen progression-free rate at 6 mo (eligible patients)
| Progression status | n | % |
|---|---|---|
| Progression free at 6 mo | 25 | 63 |
| Progression within 6 mo | 11 | 28 |
| Other* | 4 | 10 |
| Total | 40 | – |
Cases 98007 and 98030 started nonprotocol therapy prior to 6 mo; case 98041 experienced progression after 6 mo, but the last disease assessment documenting progression-free was prior to 6 mo. Case 98043 received step 2 treatment at 5 mo without meeting the criteria of progressive disease in step 1.
Although not included in the planned prospective analysis for the primary end point, Table 4 is included for completeness to show PSA progression–free rates including the ineligible patients. Table 5 shows PSA velocities before and after treatment with vaccine alone. The median pretreatment PSA velocity was 0.13 log (PSA)/mo (IQR: 0.08–0.17), in contrast to median postregistration (6 mo) velocity of 0.09 log (PSA)/mo (IQR: 0.06– 0.12), which represents an improvement of PSA DT from 5.3 mo to 7.7 mo (p = 0.02 by Wilcoxon signed rank test). Table 6 shows data collected to test for a potential early direct effect of GM-CSF on PSA based on prior data demonstrating a decrease over the 2 wk following GM-CSF administration in this population in a sawtooth pattern [14]. PSA values were obtained on days 4 and 15 of the study to test the hypothesis that PSA will decrease over the course of 2 wk following GM-CSF administration. The median day 4 PSA was, in fact, less than the day 15 PSA, 4.5 ng/ml and 4.9 ng/ml, respectively (p = 0.003), which contrasts to the hypothesis that GM-CSF alone, independent of vaccine, could directly decrease PSA by day 15.
Table 4.
Prostate-specific antigen progression-free rate at 6 mo*
| Progression status | n | % |
|---|---|---|
| Progression free at 6 mo | 29 | 58 |
| Progression within 6 mo | 13 | 26 |
| Other† | 8 | 16 |
| Total | 50 | – |
All patients including 10 ineligible patients.
Cases 98007, 98015 (ineligible), and 98030 started nonprotocol therapy prior to 6 mo; cases 98011 (ineligible) and 98041 experienced progression after 6 mo, but the last disease assessment documenting progression free was prior to 6 mo. Case 98043 received step 2 treatment at 5 mo without meeting the criteria of progressive disease in step 1. Cases 98004 (ineligible) and 98028 (ineligible) had <6 mo of follow-up.
Table 5.
Prostate-specific antigen slopes before and after vaccine treatment (step 1)
| (n = 31)* | Median (log PSA/mo) |
IQR (log PSA/mo) |
p value** |
|---|---|---|---|
| 6-mo PSA data | |||
| Pretreatment slope | 0.13 | (0.08–0.17) | – |
| During treatment slope | 0.09 | (0.06–0.12) | – |
| Difference between pretreatment and during treatment | −0.04 | (−0.08 to 0.01) | 0.02 |
| 3-mo PSA data | |||
| Pretreatment slope | 0.13 | (0.08–0.15) | – |
| During treatment slope | 0.08 | (0.04–0.15) | – |
| Difference between pretreatment and during treatment | −0.03 | (−0.08 to 0.04) | 0.10 |
IQR = interquartile range; PSA = prostate-specific antigen.
Among 30 patients (one patient was excluded because his 6-mo PSA was measured >3 mo away from the 6-mo time point) included in the PSA velocity analysis, the baseline PSA median was 2.0 (IQR: 1.5–5.4) and the 6-mo PSA median was 4.2 (IQR: 2.4–9.6).
Wilcoxon signed rank test.
Table 6.
Day 4 and day 15 prostate-specific antigen measurements (step 1)
| (n = 22) | Median, ng/ml | IQR, ng/ml | p value* |
|---|---|---|---|
| Day 4 PSA | 4.5 | 1.4–9.3 | – |
| Day 15 PSA | 4.9 | 1.6–9.6 | – |
| Difference between day 4 and day 15 | 0.45 | 0.1–1.1 | 0.003 |
IQR = interquartile range; PSA = prostate-specific antigen.
Wilcoxon signed rank test.
Table 7 shows PSA levels at 7 mo of combined vaccine and androgen ablation therapy in step 2. As shown, 74% achieved a PSA ≤0.1 ng/ml with 48% having a totally undetectable PSA. Median 7-mo PSA was 0.1 ng/ml with an IQR of 0–0.3. Overall, 20 patients achieved a CR at 7 mo (CR rate: 74%; 90% CI, 57–87), and seven patients experienced a PR at 7 mo, as defined by protocol criteria. As for the best PSA response, 21 patients achieved a CR (CR rate: 78%; 90% CI, 61–90), and 6 patients experienced a PR during step 2.
Table 7.
7-mo prostate-specific antigen on step 2
| PSA level, ng/ml |
n | % |
|---|---|---|
| 0 | 13 | 48 |
| 0.1 | 7 | 26 |
| 0.2–1.0 | 3 | 11 |
| 1.1–5.0 | 2 | 7 |
| >5.0 | 2 | 7 |
| Total | 27 | 100 |
| Median | 0.1 | |
| IQR | 0–0.3 | |
IQR = interquartile range; PSA = prostate-specific antigen.
3.3. Overall and progression-free survival
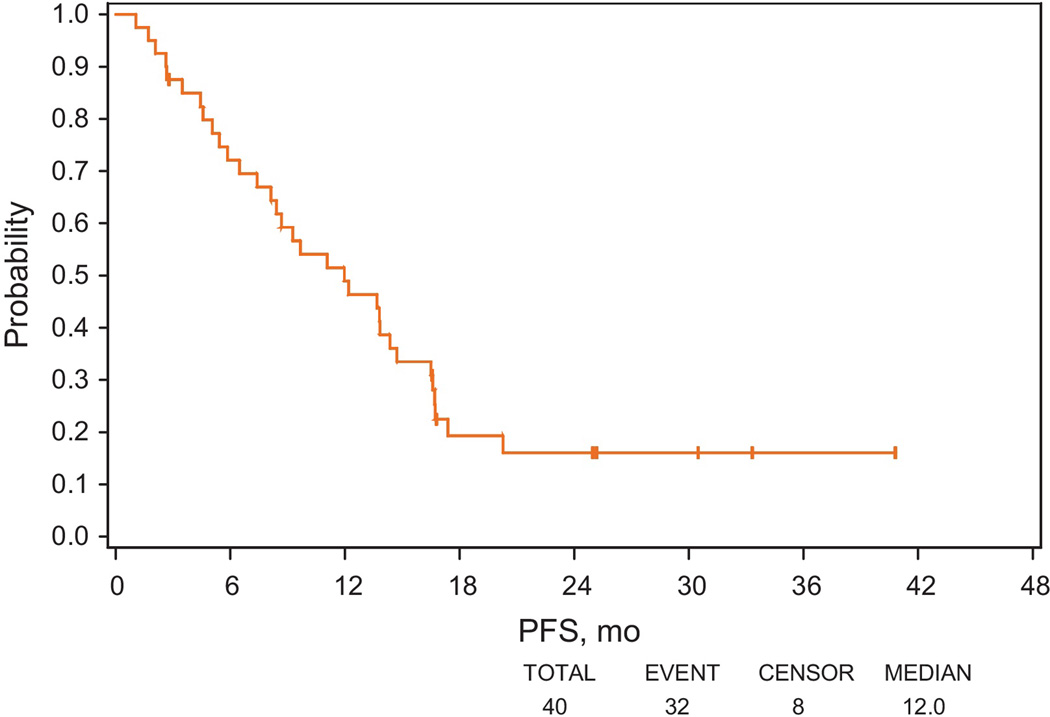
PFS was defined as the time from registration in step 1 to PSA progression, clinical progression, or death, whichever occurred first, as shown in Figure 1. Median PFS was 12.0 mo (95% CI, 7.4–14.7). Survival time was defined as the time from study entry until death or date last known alive. At the time of analysis, only 3 patients died, and 37 patients were alive among eligible patients. Median follow-up among patients still alive was 58.8 mo, and median survival has not been reached. Among 25 patients considered progression free at 6 mo, 19 experienced PSA progression; 6 remained progression free as of the analysis. Of the 19 patients with PSA progression, the earliest PSA progression occurred at 7.4 mo, and 14 patients had PSA progression after 10 mo from registration.
Fig. 1.
Progression-free survival (PFS) in step 1 (including both prostate-specific antigen [PSA] and clinical progression as events). PFS was defined as the time from registration to PSA progression, clinical progression, or death.
PFS = progression-free survival.
3.4. Toxicity
Information about symptoms and toxicities was collected during treatment. All patients who received protocol therapy, regardless of eligibility, were evaluated for toxicities. During step 1 therapy, no grade ≥4 toxicities were observed; all toxicities were mild and moderate, except for three cases of grade 3 adverse events of fever, joint effusion, and muscle pain (Table 8). Injection site reaction, muscle pain, and fatigue were the most frequently occurring toxicities. Similarly, during step 2 no grade ≥3 toxicities were observed, with all toxicities either mild or moderate. Hot flashes and injection site reaction were the most frequently occurring toxicities.
Table 8.
Treatment-related toxicities
| Toxicity type | (n = 50) Grade |
||
|---|---|---|---|
| 1, | 2, | 3, | |
| n | n | n | |
| Allergic reaction | 1 | 1 | – |
| Hemoglobin | 4 | – | – |
| Leukocytes | 1 | – | – |
| Lymphopenia | 1 | – | – |
| Fatigue | 18 | 2 | – |
| Fever without neutropenia | 12 | 2 | 1 |
| Rigors/chills | 7 | – | – |
| Sweating | 2 | – | – |
| Weight loss | 1 | – | – |
| Flushing | 1 | – | – |
| Injection site reaction | 23 | 5 | – |
| Erythema multiforme | – | 1 | – |
| Hand-foot reaction | 1 | – | – |
| Hot flashes | 3 | – | – |
| Anorexia | 3 | – | – |
| Diarrhea without prior colostomy | 1 | – | – |
| Flatulence | 1 | – | – |
| Nausea | 2 | – | – |
| Edema limb | 1 | – | – |
| Lymphatics: other | 1 | – | – |
| Alkaline phosphatase | 2 | – | – |
| ALT, SGPT | 2 | – | – |
| AST, SGOT | 2 | – | – |
| Bilirubin | 3 | – | – |
| Creatinine | 1 | – | – |
| Hyperglycemia | 6 | – | – |
| Hypernatremia | 1 | – | – |
| Joint effusion | – | – | 1 |
| Dizziness | 2 | – | – |
| Abdomen, pain | 1 | – | – |
| Bone, pain | 1 | – | – |
| Head/headache | 1 | – | – |
| Joint, pain | 3 | – | – |
| Muscle, pain | 18 | 3 | 1 |
| Pain: other | 9 | – | – |
| Renal/GU: other | 1 | – | – |
| Worst degree | 30 | 12 | 2 |
ALT = alanine aminotransferase; AST = aspartate aminotransferase; GU = genitourinary; SGOT = serum glutamic-oxaloacetic transaminase; SGPT = serum glutamic-pyruvic transaminase.
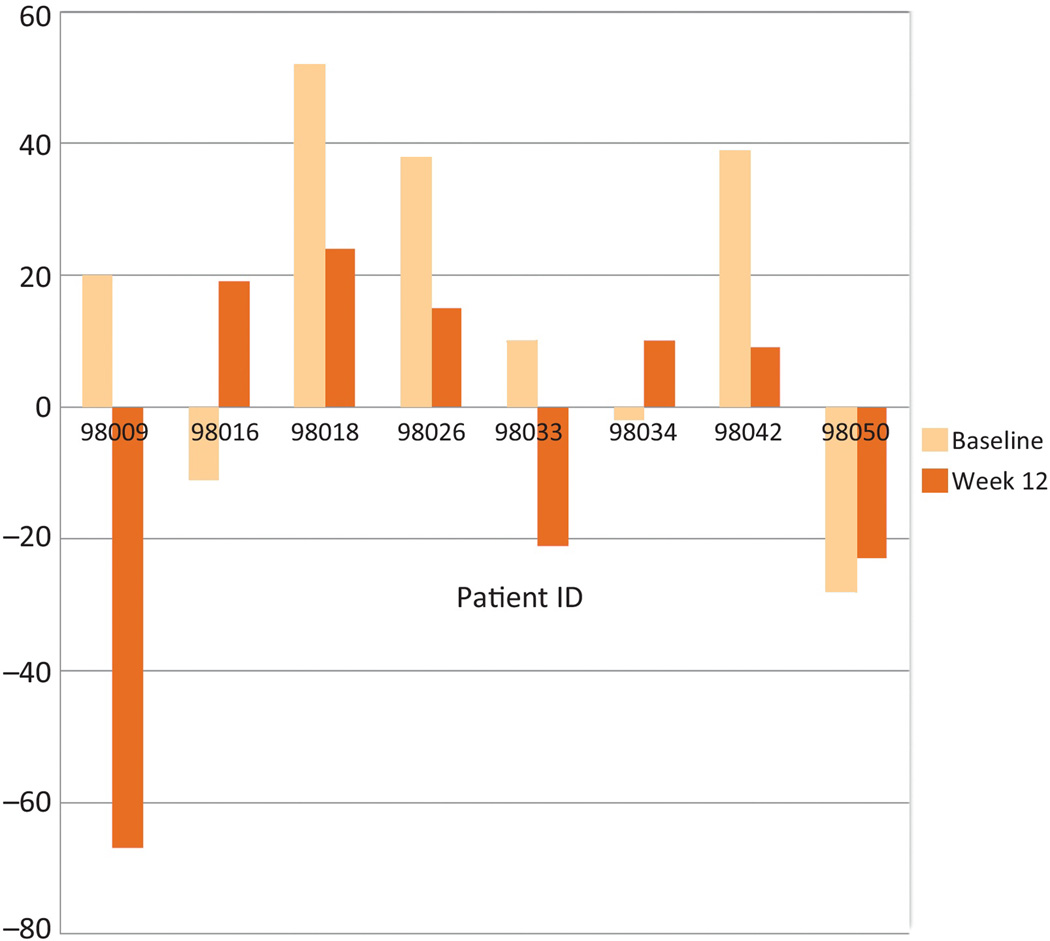
3.5. Immune effect
In this small cohort of patients, we did not find a clear and significant T-cell or PSA antibody immune effect. The interferon (IFN)-γ ELISPOT assay was utilized in eight HLA-A2+ patients at baseline and at week 12. Overall, as shown in Figure 2, no significant change was detected in this small sample set (p = 0.2). Anti-PSA antibody titers were evaluated by enzyme-linked immunosorbent assay (ELISA) by two different methodologies. Initial assessment at the ECOG Central Immunology Laboratory, testing a higher serum concentration, demonstrated an increase overall at 12 wk (n = 21; p = 0.03) and 24 wk (n = 19; p = 0.02). Repeat assay within NCI laboratories, at previously standardized lower concentrations, demonstrated no significant increase that was subsequently confirmed by Western analysis. Specifically, three patients who were positive on initial ELISA showed no significant increase in anti-PSA at both 1:50 and 1:250 dilution by ELISA. Western analysis of PSA and bovine serum albumin (control) at 1:50 was conducted in these three patients. Two patients were negative at all three time points. One patient was weakly positive at baseline (7.1 by densitometer), at 12 wk (12.3), and at 24 wk (7.9).
Fig. 2.
Effect of vaccination on interferon-γ production by enzyme-linked immunospot assay. Eight HLA-A2+ patients were assessed at baseline and at week 12. The y-axis represents net mean spots per 105 cells after 7 d in vitro stimulation with 10 µg/ml prostate-specific antigen peptide (50 µg total) and interleukin (IL)-2 plus IL-7. An increase from baseline activity is shown in patients 98016, 98034, and 98050.
4. Discussion
In this phase 2 trial of PROSTVAC-VF in men with biochemical recurrence, PROSTVAC-VF was well tolerated, and 63% of eligible patients were free of PSA, radiographic, and clinical progression at 6 mo of vaccine therapy alone, meeting the trial end point considered worthy of further study. Activity of the vaccine was also assessed by estimating the rate of rise of PSA before and after PROSTVAC-VF therapy, with the PSA DT increasing from 5.3 mo to 7.7 mo. Although the benefit modulating the rate of PSA rise has not been validated as an end point that will ultimately lead to improvement in metastasis-free or overall survival, it suggests possible biologic activity of the agent. Additional limitations recognized include the possibility that alteration of PSA DT occurred by chance in the absence of treatment effect, differences in timing, and schedule of PSA assessments or that this vaccine altered PSA secretion or elimination without an effect on the natural history of the disease. Despite these potential limitations, this study supports the feasibility of this approach in patients with low-volume disease within the cooperative group or national multi-institutional setting, contributing to the design of larger and more definitive studies of vaccine therapies in earlier stages of disease [8,15–17].
In step 2 of the study, the combination of vaccine and androgen ablation therapy also demonstrated biochemical activity as defined by a PSA at 7 mo of therapy. As shown in Table 7, 74% had a significant PSA nadir, defined by the study as ≤0.1 ng/ml. These data are only hypothesis generating, but interesting given a biologic rationale for the synergy of vaccine therapy with androgen ablation in a population of patients with aggressive disease characterized by a median PSA DT of 4.3 mo at study entry [11]. Historically, in a study by Hussain et al of 1345 patients with castration-sensitive PCa and more aggressive disease as characterized by visible new metastasis treated with androgen ablation therapy, 48% of patients achieved a PSA ≤0.2 ng/ml by 7 mo of therapy [18,19]. In a recent retrospective analysis of 294 men with less aggressive disease characterized by a median pretreatment PSA of only 2.1 treated with androgen ablation therapy following radical prostatectomy, 76% had an undetectable nadir and only 8% a nadir >0.2 ng/ml [20]. Given such contradictory benchmarks, a phase 3 prospective study would be needed with an appropriately matched control group, and our data support the feasibility of such a larger pox viral vaccine study in the national cooperative group setting. A clear and significant T-cell or PSA antibody immune effect was not demonstrated in this small cohort of patients. Assessment of PSA-specific CD8+ T cells via IFN-γ ELISPOT in only eight patients was not significant overall, as shown in Figure 1, in contrast to prior studies of ELISPOT in this population [6]. Initial analysis of PSA antibody in a small sample demonstrated an increase, but further analysis and Western confirmation demonstrated no significant increase. These data support a low likelihood of a significant biologic effect to alter PSA antibody but attest to the need to further study and validate a standard approach to anti-PSA assessment for future studies. Further assessment of these potential biomarkers may be warranted following the results of the ongoing NCI study (ClinicalTrials.gov identifier NCT01322490) with overall survival as the primary end point. Taken together, these biomarker studies highlight some of the limitations and pitfalls of immune assessments in clinical trials of vaccine therapies including the clear demonstration of differences in PSA antibody results depending on methodology.
5. Conclusions
PROSTVAC-V in cycle 1 followed by PROSTVAC-F boosts (designated PROSTVAC-VF) in combination with GM-CSF in noncastrated patients with rising PSA after definitive local therapy was administered safely in the multi-institutional cooperative group setting to patients with minimal disease volume. It supports advancing further studies of vaccine approaches in patients with minimal disease burden.
Take-home message.
These data support consideration of vaccine therapy earlier in the course of prostate cancer progression with minimal disease burden in clinical practice and future studies of vaccine approaches in earlier stages of disease.
Acknowledgments
Financial disclosures: Robert S. DiPaola certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Noah M. Hahn receives remuneration from Millennium and Binomics, honoraria from Medivation, and plays a consulting/advisory role for Sanofi. Michael A. Carducci is a consultant for Astellas, Medivation, Amgen, and Sanofi. Edmund C. Lattime is an inventor of a patent for the use of a recombinant vaccinia virus encoding GM-CSF, licensed to Jennerex Corp (JX-594), currently in clinical trial. Philip M. Arlen is chief executive officer and chief medical officer of Precision Biologics.
Funding/Support and role of the sponsor: This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD, and Mitchell D. Schnall, MD, PhD, group cochairs) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA80775, CA49883, CA16116, CA21076, and from the National Cancer Institute (NCI), National Institutes of Health, and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Robert S. DiPaola had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: DiPaola, Stein, Carducci, Lattime, Gulley, Arlen, Wilding.
Acquisition of data: DiPaola, Chen, Bubley, Stein, Hahn, Butterfield.
Analysis and interpretation of data: DiPaola, Chen, Stein, Hahn, Carducci, Lattime, Gulley, Butterfield.
Drafting of the manuscript: DiPaola, Chen, Bubley, Stein, Hahn, Carducci, Gulley, Butterfield, Wilding.
Critical revision of the manuscript for important intellectual content: None.
Statistical analysis: Chen.
Obtaining funding: None.
Administrative, technical, or material support: DiPaola, Hahn.
Supervision: None.
Other (specify): Provision of study materials or patients: DiPaola, Bubley, Hahn.
Only the first step of the study (step 1: vaccine alone) was presented at the Genitourinary American Society of Clinical Oncology Annual Meeting 2009; step 2 data (combined androgen ablation and vaccine) has not been presented.
References
- 1.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holzbeierlein JM, McLaughlin MD, Thrasher JB. Complications of androgen deprivation therapy for prostate cancer. Curr Opin Urol. 2004;14:177–183. doi: 10.1097/00042307-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bruce JY, Lang JM, McNeel DG, Liu G. Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol. 2012;10:716–722. [PubMed] [Google Scholar]
- 4.Arlen PM, Kaufman HL, DiPaola RS. Pox viral vaccine approaches. Semin Oncol. 2005;32:549–555. doi: 10.1053/j.seminoncol.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci U S A. 1996;93:11349–11353. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 7.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010;15:969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 10.Hurwitz AA, Yanover P, Markowitz M, Allison JP, Kwon ED. Prostate cancer: advances in immunotherapy. BioDrugs. 2003;17:131–138. doi: 10.2165/00063030-200317020-00005. [DOI] [PubMed] [Google Scholar]
- 11.Mercader M, Bodner BK, Moser MT, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 13.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 14.Small EJ, Reese DM, Um B, Whisenant S, Dixon SC, Figg WD. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]
- 15.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 16.Stein WD, Gulley JL, Schlom J, et al. Tumor regression and growth rates determined in five intramural NCI prostate cancer trials: the growth rate constant as an indicator of therapeutic efficacy. Clin Cancer Res. 2011;17:907–917. doi: 10.1158/1078-0432.CCR-10-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulley JL, Madan RA, Schlom J. Impact of tumour volume on the potential efficacy of therapeutic vaccines. Curr Oncol. 2011;18:e150–e157. doi: 10.3747/co.v18i3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 19.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368:1314–1325. doi: 10.1056/NEJMoa1212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keto CJ, Aronson WJ, Terris MK, et al. Detectable prostate-specific antigen nadir during androgen-deprivation therapy predicts adverse prostate cancer-specific outcomes: results from the SEARCH database. Eur Urol. 2014;65:620–627. doi: 10.1016/j.eururo.2012.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]