Abstract
Background &Aims
Cephalosporin antibiotics are popular because they have a broad spectrum of activity and are generally well tolerated; cephalosporin-induced liver injury is considered to be rare. We describe a new syndrome associated with a single intravenous dose of cefazolin and the clinical features of cephalosporin-induced liver injury.
Methods
The drug-induced liver injury (DILI) network collected detailed clinical data on 1212 patients with DILI between 2004 and 2012. We analyzed data from 41 patients in whom cephalosporins were implicated as primary agents of liver disease; 33 were formally adjudicated as having cephalosporin-induced DILI.
Results
Nineteen patients developed clinically apparent DILI after a single intravenous dose of cefazolin. All patients developed self-limited liver injury 3–23 days after receiving cefazolin during surgery—often during a minor outpatient procedure. The latency period was 20 days. Clinical features included itching, jaundice, nausea, fever, and rash. We identified 14 more patients with DILI attributed to other cephalosporins (5 first-, 2 second-, 6 third-, and 1 fourth-generation agents). Although latency and injury patterns were similar for cefazolin and other cephalosporins, the other cephalosporins were associated with more severe courses of injury, including 2 deaths from liver failure.
Conclusions
DILI can develop following a single dose of cefazolin. It is characterized by a latency period of 1–3 weeks after exposure, marked cholestasis, and a self-limited moderate to severe clinical course. Other cephalosporins can cause a similar but more severe injury.
Keywords: hepatotoxicity, DILIN, antibiotic, cephalosporin
Introduction
Cephalosporins are some of the most widely used antibiotics, popular because of their excellent tolerance and broad spectrum of activity 1–6. Serious adverse events from cephalosporins are uncommon, and drug-induced liver injury (DILI) is considered to be particularly rare 7–17. Indeed, the majority of the literature on cephalosporin induced liver injury consists of single case reports 8–18. In prospective registries of DILI, cephalosporins are not prominently mentioned, accounting for only 4 of 461 (0.9%) episodes in a Spanish Registry 18, 1 of 77 (1.2%) in a Swedish study 19 and 1 of 96 (1%) in a recent Icelandic survey 20. In contrast, in the ongoing prospective study from the United States known as the Drug-Induced Liver Injury Network (DILIN), cephalosporins were relatively common causes of DILI, and the commonly used, first generation parenteral cephalosporin, cefazolin, was observed to rank highly among implicated single agents. This study describes 19 patients with DILI attributed to cefazolin. These patients had a distinctive clinical phenotype, and cefazolin was frequently overlooked as the putative cause.
Methods
Study Design
The DILIN prospective study is an ongoing, multicenter study of all cases of DILI presenting at 8 geographically diverse medical centers in the US as described 21, 22. In brief, patients with a strong clinical suspicion of liver injury caused by a medication or an herbal agent were enrolled. Patients met minimal pre-established criteria for liver injury including the following: (1) aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level > 5 times the upper limit of normal (ULN) or > 5 times baseline levels if pre-treatment values were known to be abnormal on at least 2 consecutive occasions, or (2) alkaline phosphatase level 2 times the ULN (or baseline level if pre-treatment values were known to be abnormal) on 2 consecutive occasions, or (3) total serum bilirubin level ≥ 2.5 mg/dL accompanied by any elevation in AST, ALT or alkaline phosphatase level, or (4) international normalized ratio (INR) > 1.5 accompanied by any elevations in AST, ALT or alkaline phosphatase level. Patients with acetaminophen toxicity were excluded as were those with bone marrow or liver transplantation. Patients with pre-existing chronic hepatitis C, hepatitis B or nonalcoholic fatty liver disease were eligible; those with autoimmune hepatitis, primary biliary cirrhosis or sclerosing cholangitis were excluded.
Eligible patients had a baseline visit during which extensive clinical history was obtained and laboratory, histologic, and imaging results were documented; pre-established laboratory testing was performed to exclude competing causes of liver injury. All patients were then followed for at least 6 months, and those with evidence of continuing liver injury were asked to return at 12 and 24 months. Chronic liver injury was defined as the presence of liver-related laboratory, radiologic, or histologic abnormalities at least 6 months after onset.
The pattern of liver injury was based upon the ratio (R) of the serum ALT to alkaline phosphatase (both expressed as multiples of the ULN): an R ratio of <2 indicating cholestatic, >5 hepatocellular and 2–5 as mixed cholestatic-hepatocellular injury 22, 23. Severity of injury was scored on a 5 point scale: 1 (mild)=serum enzyme elevations without jaundice (bilirubin <2.5 mg/dL), 2 (moderate)=enzyme elevations and jaundice; 3 (moderate hospitalized)=enzyme elevations, jaundice and hospitalization for liver injury; 4 (severe)=jaundice and signs of liver failure (ascites, encephalopathy, INR ≥1.5) or other organ failure; 5 (fatal)=death or liver transplantation within 6 months of onset.23 The study was approved by the Institutional Review Boards (IRBs) of each participating center and all patients provided written informed consent.
Causality
The diagnosis of DILI and the causal relationship between the event and the implicated agent(s) were evaluated in a formal and standardized fashion by the DILIN Causality Committee as described.22, 23 This process is based on expert opinion and has been shown to have greater reproducibility, and is better differentiation among levels of causality than the commonly used RUCAM.22, 23 Causality was assessed as either definite (>95% likelihood), highly likely (75%–95% likelihood), probable (50%– 74% likelihood), possible (25%–49% likelihood), and unlikely (<25% likelihood). In cases in which several agents were considered possibly implicated, the overall event was adjudicated for the likelihood that it represented DILI and then each agent was given a separate score, but only one agent was permitted to be considered probable, highly likely or definite.
Statistics
Demographic and clinical data were extracted and analyzed. Descriptive statistics including means, medians, 25th to 75th percentiles, frequencies, and percentages were used to summarize the data. A non-parametric test or chi-square (Fisher’s exact test in the case of small sample) was used to compare groups of continuous and categorical variables, respectively. The LOESS regression model was used to fit smooth curves (and 95% confidence intervals of the curves) of liver tests over time. In the case of multiple peaks of the smoothed curves, smoothed curves were fitted for subgroups as indicated by the data. All P values reported are two-sided; a level of 0.05 was considered statistically significant. All data were analyzed with SAS 9.2.
Results
Cefazolin induced DILI
Among 1212 patients with DILI enrolled into a U.S. prospective database between 2004 and 2012, in which 1019 cases were adjudicated, 19 (2%) were attributed to cefazolin (Supplemental Figure 1). This made cefazolin the 6th most common single agent identified in the entire dataset. Analysis of the course and outcome of these 19 patients revealed distinctive, yet highly consistent clinical features. All 19 patients had undergone surgery and had received a single intravenous dose of cefazolin (1–2 gm) in the perioperative period for prophylaxis against infection. The cefazolin used included different commercial preparations (Ancef™, Kefzol™, and several generic forms). Patients included 11 men and 8 women, all of whom were white and one of whom was Hispanic (Table 1).
Table 1.
Clinical and causality characteristics of cefazolin induced liver injury (n = 19)
| Feature | No (%) |
|---|---|
| Age (median-years) | 53 |
| Female | 8 (42%) |
| BMI (median-kg/m2) | 26 |
| Prior history of any drug allergy | 9 (47) |
| Heavy alcohol use* | 12 (63) |
| Diabetes mellitus | 5 (26) |
| Hepatitis C (HCV RNA positive) | 1 (5) |
| Time from administration to symptom onset | |
| < 1 week | 3 (16) |
| 2–4 weeks | 13 (68) |
| > 4 weeks | 3 (16) |
| Time to first laboratory abnormality | |
| < 1 week | 1 |
| 1–2 weeks | 5 |
| 3–4 weeks | 13 |
| Jaundice | 18 (95) |
| Itching | 19 (100) |
| Nausea | 15 (79) |
| Abdominal pain | 5 (26) |
| Rash | 6 (32) |
| Fever | 9 (47) |
| Eosinophilia | 5 (26) |
| Hospitalization | 11(69) |
| Severity | |
| Mild | 1 (5) |
| Moderate | 6 (32) |
| Moderate & hospitalized | 10 (53) |
| Severe | 2 (11) |
| Fatal | 0 |
| Causality | |
| Probable | 1(5) |
| Highly likely | 16 (84) |
| Definite | 2 (11) |
| Persistent abnormal liver tests more than 6 months | 3 (16) |
BMI, body mass index
>2 drinks/day in men and >1 drink/day women
All 19 patients developed itching within 3 to 23 days of receiving cefazolin; all but 1 patient was reported to have jaundice (Table 1/2). Symptoms generally lasted for a few days to several weeks and jaundice resolved in all patients within 31 days of onset. Immuno-allergic features were present in 9 patients (fever 9, rash 6, eosinophilia 5) but were generally mild and transient. Two patients had a previous history of jaundice following surgery, which had been attributed to halothane and methimazole, respectively. Importantly, both had also received cefazolin at the time of surgery. The latency from administration of cefazolin exposure to onset during the second episode in these two patients was 3 and 6 days, representing 2 of the 3 cases with latencies of less than one week (implying that the clinical course is more rapid when there has been prior exposure and sensitization). A narrative description of the clinical course of all patients is available in LiverTox (http://livertox.nih.gov).
Table 2.
Detailed clinical features of cefazolin induced DILI
| N | Age | Prior Drug Allergies |
Days from Drug Start to DIL IN Onset |
Nausea | Fever | Rash | Itching | Liver Biopsy Performed at Screen Visit |
At Onset- ALT |
At Onset- AST |
At Onset- ALP |
At Onset- Total Bili |
Peak Values from DILIN Onset to Month 6 Visit- ALT |
Peak Values from DILIN Onset to Month 6 Visit- AST |
Peak Values from DILIN Onset to Month 6 Visit- ALP |
Peak Values from DILIN Onset to Month 6 Visit- Total Bili |
Patient was hospitalized |
DILIN Causality Score |
DILIN Severity Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | Yes | 18 | Yes | Yes | No | Yes | Yes | 558 | 286 | 390 | 6.2 | 558 | 286 | 390 | 6.2 | No | Definite | Moderate |
| 2 | 53 | No | 21 | Yes | Yes | No | Yes | No | 401 | 151 | 432 | 6.5 | 401 | 151 | 521 | 10.6 | Yes | Very likely | Severe |
| 3 | 51 | No | 23 | No | Yes | No | Yes | Yes | 751 | 246 | 170 | 4.7 | 1233 | 472 | 266 | 9.8 | Yes | Very likely | Moderate-H |
| 4 | 67 | Yes | 20 | Yes | No | No | Yes | Yes | 639 | 623 | 1407 | 2.7 | 659 | 654 | 1527 | 18.1 | Yes | Very likely | Moderate-H |
| 5 | 56 | Yes | 20 | No | Yes | No | Yes | No | 614 | 281 | 508 | 2.2 | 614 | 281 | 564 | 2.8 | No | Very likely | Moderate |
| 6 | 45 | Yes | 20 | Yes | No | No | Yes | Yes | 377 | 280 | 689 | 2.9 | 468 | 280 | 781 | 18.8 | Yes | Very likely | Moderate-H |
| 7 | 49 | No | 24 | Yes | No | No | Yes | Yes | 618 | 238 | 328 | 4 | 945 | 414 | 406 | 5.1 | No | Very likely | Moderate |
| 8 | 60 | Yes | 3 | Yes | No | No | Yes | No | 194 | 109 | 309 | 5.7 | 194 | 135 | 385 | 5.7 | Yes | Very likely | Moderate-H |
| 9 | 72 | No | 23 | Yes | No | No | Yes | Yes | 135 | 134 | 354 | 6.4 | 135 | 155 | 441 | 11.1 | Yes | Very likely | Moderate-H |
| 10 | 83 | No | 29 | No | Yes | No | Yes | No | 197 | 159 | 443 | 10.7 | 200 | 159 | 513 | 11.4 | Yes | Very likely | Moderate-H |
| 11 | 57 | Yes | 28 | Yes | Yes | Yes | Yes | No | 303 | 201 | 448 | 1.2 | 378 | 212 | 889 | 1.9 | Yes | Very likely | Mild |
| 12 | 53 | Yes | 15 | Yes | Yes | Yes | Yes | Yes | 170 | 65 | 224 | 4.3 | 170 | 65 | 227 | 7.6 | Yes | Very likely | Moderate-H |
| 13 | 68 | No | 4 | Yes | Yes | Yes | Yes | No | 155 | 76 | 357 | 6.9 | 155 | 82 | 435 | 13 | No | Very likely | Moderate |
| 14 | 59 | No | 26 | Yes | No | No | Yes | Yes | 380 | 136 | 329 | 5.3 | 409 | 191 | 370 | 6.4 | Yes | Very likely | Severe |
| 15 | 45 | No | 21 | Yes | No | No | Yes | Yes | 769 | 143 | 305 | 5.3 | 769 | 143 | 305 | 9.3 | Yes | Probable | Moderate-H |
| 16 | 76 | Yes | 29 | Yes | Yes | Yes | Yes | No | 496 | 194 | 1457 | 7.9 | 496 | 194 | 1457 | 10.9 | No | Very likely | Moderate |
| 17 | 31 | No | 6 | No | No | No | Yes | Yes | 168 | 47 | 340 | 5 | 168 | 76 | 397 | 8.7 | No | Very likely | Moderate |
| 18 | 19 | Yes | 24 | Yes | No | Yes | Yes | Yes | 515 | 189 | 360 | 13.5 | 515 | 189 | 360 | 14.2 | Yes | Very likely | Moderate-H |
| 19 | 47 | No | 12 | Yes | No | Yes | Yes | Yes | 293 | 359 | 1332 | 14.9 | 390 | 415 | 1489 | 14.9 | Yes | Definite | Moderate-H |
Definite - Greater than 95%; Very likely - 75–95%; Probable 50–75%
H = hospitalized
DILIN onset has been defined previously as the time at which the patient met study entry criteria, which is based on liver test abnormalities (see Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf 2009;32:55–68).
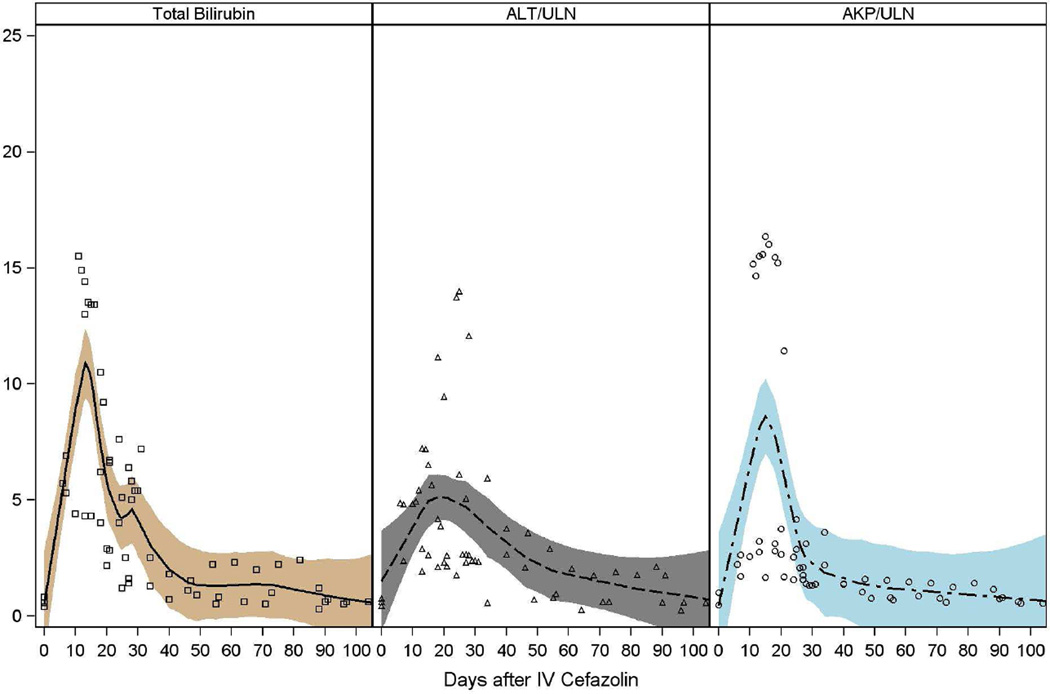
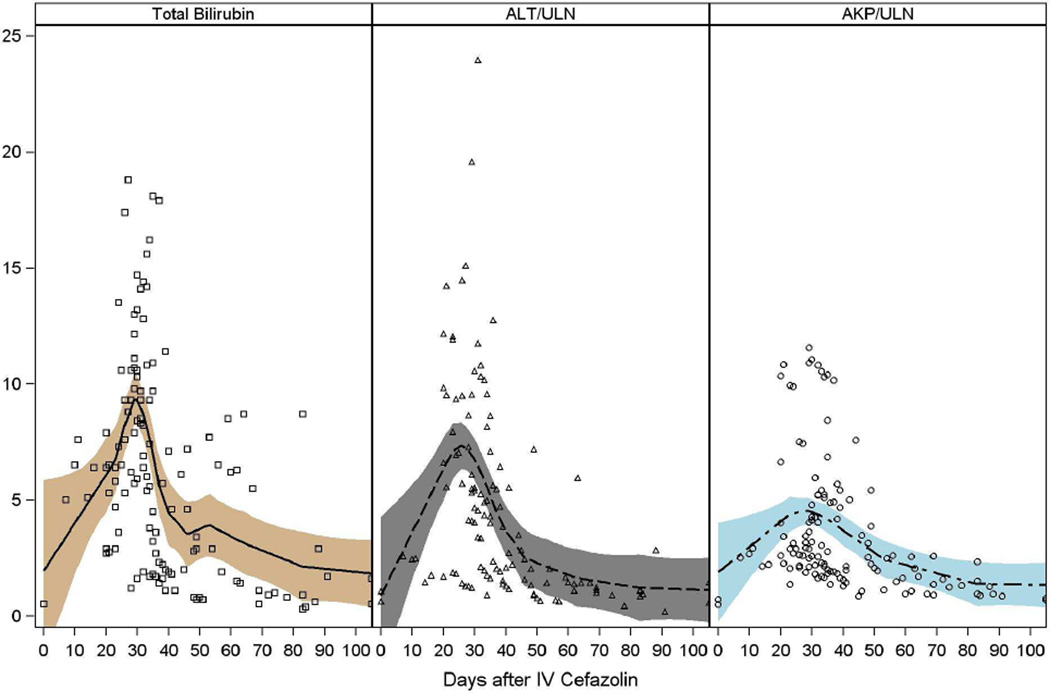
Laboratory documentation of liver injury was first obtained 6 to 31 days after exposure and 2 to 23 days after initial symptoms (Tables 2/3, Figure 1, Supplemental Table 2). Initial abnormalities included elevations in total serum bilirubin and typically a mixed or cholestatic pattern of serum enzyme elevations. Total serum bilirubin rose to greater than 2.5 mg/dL in 18 patients (95%) and to above 10 mg/dL in 7 (37%). Liver test curves revealed two peaks in bilirubin elevation, with each patient fitting into only one of the two peaks occurring either before or after 25 days. The separate LOESS curves for these groups revealed that 7 had a peak in bilirubin level within 15 days (Figure 1A) and 12 with a peak within 30 days (Figure 1B).
Table 3.
Laboratory features of cefazolin induced liver injury
| Feature | Median | Range |
|---|---|---|
| Time from administration to laboratory abnormalities (days) | 21 | 6–29 |
| ALT (U/L), initial | 380 | 135–769 |
| AST (U/L), initial | 189 | 47–623 |
| Alkaline phosphatase (U/L), initial | 360 | 170–1457 |
| Bilirubin (mg/dL), initial | 5.3 | 1.2 – 14.9 |
| INR, initial | 1.0 | 0.8–1.2 |
| R value, initial | 1.8 | 0.4 – 8.8 |
| ALT (U/L), peak | 409 | 135–1233 |
| AST (U/L), peak | 191 | 65–654 |
| Alkaline phosphatase (U/L), peak | 435 | 227–1527 |
| Bilirubin (mg/dL), peak | 9.8 | 1.9–18.8 |
| INR, peak | 1.1 | 1.0–2.0 |
| Eosinophilia (no, % and count/µL) | 5 (26%) | 533 – 3400 |
R value = ratio of the serum ALT to alkaline phosphatase (both expressed as multiples of the ULN)
Figure 1. Liver test abnormalities.
The liver test abnormalities in 19 patients with cefazolin induced DILI are depicted. In (A) are shown patients falling into the early peak abnormality group, and in (B), the later peak group. Values on the X axis include bilirubin (mg/dL) or fold elevations over the upper limit of normal (ULN) in international units.
The severity of the liver injury was scored as severe (INR rising transiently to ≥ 1.5) in 2 patients, moderate (either 2+/3+) in 16, and mild (anicteric) in 1 (Tables 2/3). No patient died or underwent liver transplantation, and none developed clinical evidence of ascites, hepatic encephalopathy or variceal hemorrhage. In follow up, 3 patients had liver test abnormalities when evaluated 6 months after DILI onset, but serum bilirubin levels were normal and abnormalities were minimal (Supplemental Table 2) and eventually resolved in all patients.
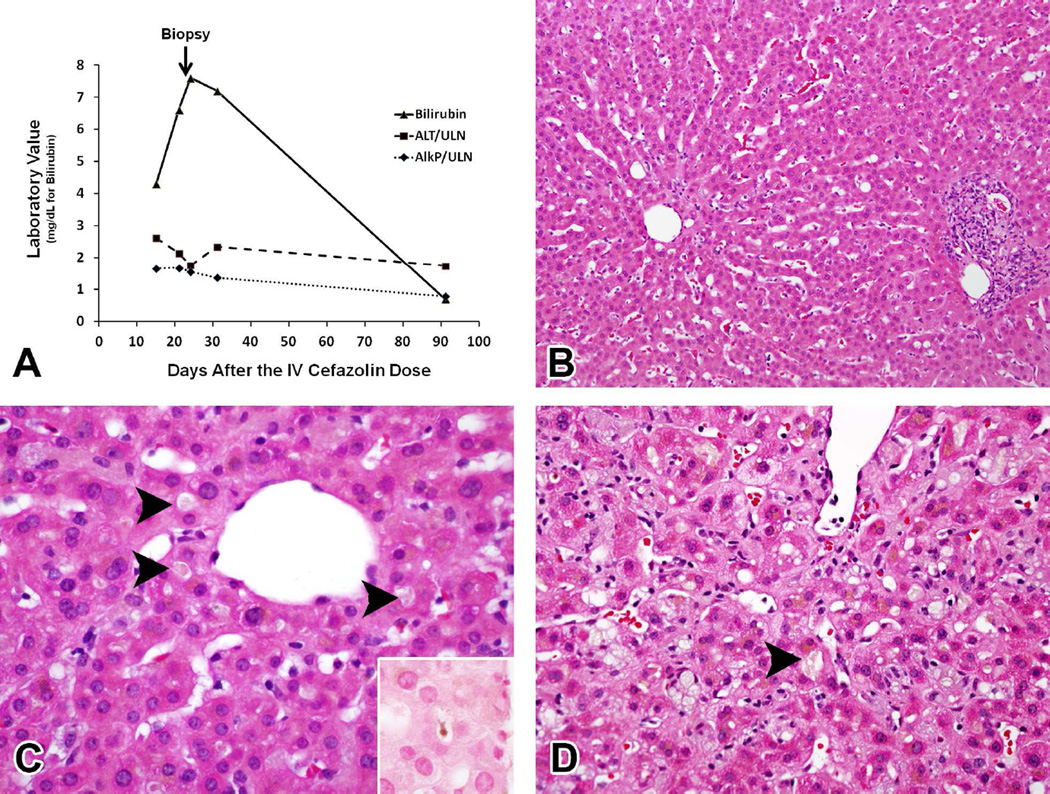
Liver biopsies were performed in 10 patients, 6 of which were available for central review [DEK] (the mean time between onset and biopsy was 30 days). Five patients had a cholestatic pattern of injury (3 had typical cholestatic hepatitis, see Figure 2; one had acute cholestasis and one had chronic cholestasis), and one had a mild acute hepatitis injury pattern. Among the cholestatic injury patients, four had moderate to marked zone 3 cholestasis with varying degrees of parenchymal inflammation while the chronic cholestatic patient had duct injury and pseudoxanthomatous changes without bile accumulation. The sole acute hepatitis case had mild parenchymal injury and inflammation without cholestasis. Finally, 2 of the 6 biopsies had infiltrates of eosinophils.
Figure 2. Cefazolin induced DILI - histology.
In (A) is shown the liver test profile of a patient with typical cholestasis from cefazolin induced DILI. Values on the X-axis include bilirubin (mg/dL) or fold elevations over the upper limit of normal (ULN) in international units (IU). In (B-C) are shown photomicrographs of the liver biopsy specimen taken 30 days after exposure to cefazolin in a representative patient. In (B), the image depicts cholestatic hepatitis with mild portal and lobular inflammation (H&E, 200x). In (C), high magnification of zone 3 revealed prominent canalicular cholestasis (arrowheads), which was demonstrated more clearly on the iron stain (inset) (H&E, 600x). In (D), is depicted prominent canalicular cholestasis (arrowhead) in a different patient with cefazolin induced DILI (H&E, 400x) in a different patient.
At the time of initial clinical evaluation, cefazolin was considered to be a potential responsible agent in only 10 of 19 patients, and was often not initially a suspected agent; other drugs initially considered as possibly causative included tramadol, propofol, sevoflurane, azithromycin, clindamycin, oxcarbazepine, ketorolac, chaparall, and oxycodone/acetaminophen in one case each. In some instances, the patient was unaware of having received cefazolin and the information was found only after detailed review of medical records. In the final adjudication by the DILIN Causality Committee for the 19 cases here in which cefazolin was the primary implicated agent, scores were highly likely in all except 3 patients, two of which were considered definite and one probable.
Liver Injury due to other cephalosporins
During the study period, 14 other patients had DILI attributed to non-cefazolin cephalosporins, including both oral and intravenous formulations and agents from all four “generations” of this class of antibiotics (Table 4, Supplemental Figure 1, Supplemental Table 1). One of these cases was scored as definite, 8 as highly likely and 5 as probable. None was administered as a single intravenous injection. All were given for a limited time (2 to 14 days) and clinical symptoms began 1 to 4 weeks (range 4 to 29 days) later. Symptoms included jaundice (79%), itching (64%), nausea (79%), and fever (79%). Immuno-allergic features (fever, rash, eosinophilia) occurred in 36%. Laboratory test abnormalities typically reflected a cholestatic or a mixed cholestatic/hepatocellular injury pattern, similar to those in cefazolin cases (Figure 1). In six of the subjects, liver biopsies were available for central review [DEK]. Five had a cholestatic injury pattern and one had mild acute hepatocellular injury. Finally, 3 of the 6 biopsies had abnormal eosinophilia.
Table 4.
DILI caused by cephalosporins
| Drug | No Cases |
Generation | Route | Duration of treatment (range, days) |
Latency (range, days) |
Initial ALT (U/L) (mean ± SD and median) |
Initial Alk P (U/L) (mean ± SD and median) |
Initial Bilirubin (mg/dL) (mean ± SD and median) |
Severe (n, %) |
|---|---|---|---|---|---|---|---|---|---|
| Cefazolin | 19 | 1st | IV | 1 | 6–29 | 407 ± 211 380 | 536 ± 399 360 | 6.1 ± 3.6 5.3 | 2/19 (11%) |
| Cephalexin | 3 | 1st | Oral | 2–14 | 11–20 | 378 ± 365 316 | 313 ± 179 397 | 2.3 ± 3.4 0.4 | 1/3 (33%) * fatal |
| Cefadroxil | 2 | 1st | Oral | 14 | 27 | 486 ± 381 486 | 234 ± 79 234 | 19.6 ± 20.0 19.6 | 0 (0%) |
| Cefuroxime | 1 | 2nd | IV | 11 | 56 | 268 | 651 | 8.4 | 0 (0%) |
| Cefaclor | 1 | 2nd | Oral | 2 | 6 | 64 | 104 | 4.1 | 0 (0%) |
| Ceftriaxone | 4 | 3rd | IV | 3–13 | 4–29 | 292 ± 181 227 | 362 ± 191 412 | 1.8 ± 1.9 1.1 | 1/4(25%) |
| Cefdinir | 1 | 3rd | Oral | 9 | 12 | 232 | 409 | 4.7 | 0 (0%) |
| Cefotaxime | 1 | 3rd | IV | 4 | 1 | 23 | 127 | 2.3 | 1/1 (100%)* fatal |
| Cefipime | 1 | 4 | IV | 7 | 20 | 205 | 62 | 11.3 | 0(0%) |
Severe = proportion scored as severe or fatal
Cholestatic = proportion with jaundice, itching and cholestatic or mixed pattern of serum enzyme elevations (R value <5) Typical signature - The signature of cefazolin hepatotoxicity is defined as symptomatic liver injury with jaundice and pruritus, cholestatic or mixed aminotransferase elevations, and an onset of 1 to 4 weeks after exposure.
Denotes 1 fatal case
In contrast to patients with cefazolin-induced DILI, patients with DILI due to the other cephalosporins had a more severe course. Two patients died, 1 other patient was considered to have severe liver injury, 9 moderate and 2 mild. The majority (64%) of patients required hospitalization and the mortality rate was 14%. One patient developed Stevens Johnson Syndrome (i.e., severe skin injury, multi-organ failure) and died of liver and multisystem organ failure. One death was in a patient with underlying cirrhosis due to alpha-1-antitrypsin disease, who developed a severe immuno-allergic syndrome, including a rash (without desquamation) and eosinophilia and died of liver and multisystem organ failure. In follow up, 3 patients had liver test abnormalities 6 months after DILI onset, but serum bilirubin levels were normal and the enzyme elevations were mild (Supplemental Table 2).
Discussion
While the cephalosporins have been thought to be a rare cause of idiosyncratic DILI, this has not been the experience in an ongoing study from the US. Among 1019 patients with DILI collected between 2004 and 2012 and undergoing careful causality assessment, 19 were attributed to cefazolin and 14 more to other cephalosporins. This made cefazolin the 6th most commonly identified specific agent responsible for DILI, and likewise, the cephalosporins, the 6th most common drug class to cause DILI. For cefazolin, the clinical presentation or “signature” was cholestatic or mixed hepatocellular-cholestatic injury arising 1 to 3 weeks after a single intravenous injection of the antibiotic given at the time of surgery, often unknown to the patient. Jaundice (95%) and pruritus (100%) were the most prominent signs or symptoms. The course was usually self-limited. Notably, because of confusion about the specific diagnosis, patients underwent substantial diagnostic testing (including multiple CT scans, MRI’s, ERCPs, liver biopsies, and others), which was often unnecessary and/or led to severe complications.
The findings reported here have several important clinical implications. First, cefazolin was often overlooked as a cause of DILI. In our experience, many patients were not aware that they had received cefazolin during outpatient procedures, and further, clinicians and often even DILIN investigators often did not initially consider or recognize cefazolin as a cause of DILI. For example, by definition, DILIN enrolls only patients recruited because of a high suspicion of DILI; even given this scenario, cefazolin was implicated only in retrospect after careful review of the medical record in 53% of patients. For these reasons, we speculate that cefazolin is and has been underappreciated as a cause of DILI. The appearance of jaundice and pruritus 1 to 3 weeks after minor surgery should lead to a search of operative records and medications that might have been given during surgery. These results also imply that the merits of routine use of cefazolin at the time of uncomplicated surgery should be carefully reconsidered 1, 24, 25.
Reports of delayed DILI after a single intravenous dose of medication are rare, but have been reported sporadically 26. A search of the FDA Adverse Event Reporting system (http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/) failed to reveal a report of DILI after a single intravenous dose of medication. Notwithstanding, the most common drugs associated with a single “dose” of medications include the halogenated anesthetics which are given during anesthesia, including halothane, isoflurane, enflurane, desflurane, and sevoflurane. While they are not given intravenously, the inhaled route is likely similar from a pharmagologic delivery standpoint. Although infrequently currently used, halothane’s pattern of liver injury is assumed to be typical for the other halogenated anesthetics; it is well described to cause an acute and often fulminant DILI picture, in a primary hepatocellular pattern, occurring shortly after exposure 27,28. Since the halogenated anesthetics are often given during surgeries in which cefazolin may also be given prophylactically, it is important to consider each of these agents as potential causes of DILI in patients having orthopedic type surgeries for which cefazolin is used prophylactically to prevent infection. Based on the data from our study, several points are noteworthy. First, the timing of injury for the halogenated anesthetics is typically more acute than what we have reported here for cefazolin, the former occurring within hours or days of exposure 29. Additionally, the injury pattern for the halogenated anesthetics is typically hepatocellular, rather than mixed or cholestatic as is typical for cefazolin (Table 2). Finally, in our cohort, a distinct minority of patients (2 sevoflurane, 1 desflurane) received halogenated anesthetics, making it highly unlikely that these agents confounded causality assessment in our patients.
This study has a number of strengths. First, data were collected in a standardized and prospective fashion; this allowed for collection of important clinical data as well as those focused on outcome. An important part of this data collection was the regular review of all medical records by trained personnel. Additionally, the causality process used was robust, and has been shown to be superior to conventional instruments such as RUCAM 23. The assignment of causality to cefazolin was strengthened by finding a typical clinical signature and the absence of competing diagnoses or exposures to agents that cause cholestatic, self-limited, injury. Other commonly implicated drugs were generally considered to lack a hepatotoxicity signature (propofol, tramadol, oxycodone), or cause a very different pattern of hepatic injury (acetaminophen, sevoflurane, oxcarbamazepine, and azithromycin). This further suggests that the cases included in this study are bona fide cases of cephalosporin induced DILI. In this context, we were surprised to find that, heretofore, cephalosporin-induced DILI has rarely been reported. In fact, there appear to be no more than a total of 10 previously reported cephalosporin-induced DILI cases 8–17.
Cephalosporin-induced liver injury appears to resemble that associated with amoxicillin/clavulanic acid 30. As with amoxicillin/clavulanate-induced liver injury, clinical symptoms arose 1 to 3 weeks after initial exposure and well after the antibiotic was stopped. Immuno-allergic features were often present but usually transient and mild, with full blown “drug reaction with eosinophilia and systemic symptoms” (DRESS) syndrome being uncommon 31. More rapid recurrence upon re-exposure was also common. Furthermore, the injury signature was usually cholestatic with jaundice and itching, features also typical of amoxicillin/clavulanic acid-induced liver injury. We speculate that, because cephalosporins share the beta lactam structure of penicillins and clavulanic acid, a common mechanism of injury may be an immuno-allergic reaction to a structural component of the beta lactam 4-ring molecule.
It was notable that 2 patients receiving non-cefazolin cephalosporins died, and an additional patient had severe injury. However, in each of the fatal cases, patients had a complicated clinical course, with severe hypersensitivity reaction on top of an underlying liver disease. Therefore, we urge caution in concluding that non-cefazolin cephalosporin-induced DILI may be severe or fatal. Since cephalosporins are commonly used in clinical practice, it is likely that the overall mortality rate associated with cephalosporin use is low, but not nil, and it may be more likely in patients with underlying disorders.
In conclusion, cephalosporins appear to be a relatively common cause of antibiotic associated liver injury. The latency period is typically 1 to 3 weeks after exposure, and patients may not become symptomatic until after the antibiotic is stopped – this is particularly true in the unique clinical syndrome in which a single infusion of cefazolin leads to DILI. The course of cephalosporin-induced DILI is usually one of a mild-to-moderate, self-limited cholestatic injury, particularly when associated with a single cefazolin infusion. However, severe and even fatal, DILI may occur in a small number of patients, and some cases may be followed by evidence of chronic injury.
Supplementary Material
Acknowledgements
We thank Huiman Barnhart and Thomas Phillips from the Duke Clinical Research Institute for help with statistical analysis.
Funding
The DILIN Network is structured as a U01 cooperative agreement with Funds provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under grants: 2U01-DK065176-06 (Duke), 2U01- DK065201-06 (UNC), 2U01-DK065184-06 (Michigan), 2U01-DK065211-06 (Indiana), 5U01DK065193-04 (UConn), 5U01-DK065238-08 (UCSF/CPMC), 1U01-DK083023-01 (UTSW), 1U01-DK083027-01 (TJH/UPenn), 1U01- DK082992-01 (Mayo), 1U01-DK083020-01 (USC). Additional funding is provided by CTSA grants: UL1 RR025761 (Indiana), UL1 RR025747 (UNC), UL1 RR024134 (UPenn), UL1 RR024986 (Michigan), UL1 RR024982 (UTSW), UL1 RR024150 (Mayo) and in part by the Intramural Research Program of The NIH, National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s declaration of conflicts of interests
The authors certify that we have no financial arrangements (e.g., consultancies, stock ownership, equity interests, patent-licensing arrangements, research support, honoraria, etc.) with a company whose product figures prominently in this manuscript or with a company making a competing product.
Author contributions
Saleh Alqahtani - salqaht1@jhmi.edu: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis.
Marwan Ghabril - mghabril@iu.edu: analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Jiezhun Gu - jiezhun.gu@duke.edu: data collection and statistical analysis; critical revision of the manuscript for important intellectual content
David Kleiner - kleinerd@mail.nih.gov: data collection; analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Jay Hoofnagle - HOOFNAGLEJ@extra.NIDDK.NIH.GOV: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis.
Don Rockey - rockey@musc.edu: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content, statistical analysis
References
- 1.Bohnen JM, Solomkin JS, Dellinger EP, et al. Guidelines for clinical care: anti-infective agents for intra-abdominal infection. A Surgical Infection Society policy statement. Arch Surg. 1992;127:83–89. doi: 10.1001/archsurg.1992.01420010097015. discussion 89. [DOI] [PubMed] [Google Scholar]
- 2.Donowitz GR. Third generation cephalosporins. Infect Dis Clin North Am. 1989;3:595–612. [PubMed] [Google Scholar]
- 3.Klein NC, Cunha BA. Third-generation cephalosporins. Med Clin North Am. 1995;79:705–719. doi: 10.1016/s0025-7125(16)30034-7. [DOI] [PubMed] [Google Scholar]
- 4.Klein NC, Cunha BA. The selection and use of cephalosporins: a review. Adv Ther. 1995;12:83–101. [PubMed] [Google Scholar]
- 5.Molavi A. Cephalosporins: rationale for clinical use. Am Fam Physician. 1991;43:937–948. [PubMed] [Google Scholar]
- 6.Schaad UB, Suter S, Gianella-Borradori A, et al. A comparison of ceftriaxone and cefuroxime for the treatment of bacterial meningitis in children. N Engl J Med. 1990;322:141–147. doi: 10.1056/NEJM199001183220301. [DOI] [PubMed] [Google Scholar]
- 7.Pacik PT. Augmentation mammaplasty: postoperative cephalosporin-induced hepatitis. Plast Reconstr Surg. 2007;119:1136–1137. doi: 10.1097/01.prs.0000253456.58964.6e. [DOI] [PubMed] [Google Scholar]
- 8.Ammann R, Neftel K, Hardmeier T, et al. Cephalosporin-induced cholestatic jaundice. Lancet. 1982;2:336–337. doi: 10.1016/s0140-6736(82)90311-7. [DOI] [PubMed] [Google Scholar]
- 9.Bilici A, Karaduman M, Cankir Z. A rare case of hepatitis associated with cefprozil therapy. Scand J Infect Dis. 2007;39:190–192. doi: 10.1080/00365540600823235. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Ahmad J. Cefdinir-induced hepatotoxicity: potential hazards of inappropriate antibiotic use. J Gen Intern Med. 2008;23:1914–1916. doi: 10.1007/s11606-008-0758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggleston SM, Belandres MM. Jaundice associated with cephalosporin therapy. Drug Intell Clin Pharm. 1985;19:553–555. doi: 10.1177/106002808501900710. [DOI] [PubMed] [Google Scholar]
- 12.Fung-Herrera CG, Mulvaney WP. Cephalexin nephrotoxicity. Reversible nonoliguric acute renal failure and hepatotoxicity associated with cephalexin therapy. JAMA. 1974;229:318–319. doi: 10.1001/jama.229.3.318. [DOI] [PubMed] [Google Scholar]
- 13.Kaur I, Singh J. Cholestatic hepatitis with intravenous ceftriaxone. Indian J Pharmacol. 2011;43:474–475. doi: 10.4103/0253-7613.83133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Longo F, Hastier P, Buckley MJ, et al. Acute hepatitis, autoimmune hemolytic anemia, and erythroblastocytopenia induced by ceftriaxone. Am J Gastroenterol. 1998;93:836–837. doi: 10.1111/j.1572-0241.1998.239_a.x. [DOI] [PubMed] [Google Scholar]
- 15.Peker E, Cagan E, Dogan M. Ceftriaxone-induced toxic hepatitis. World J Gastroenterol. 2009;15:2669–2671. doi: 10.3748/wjg.15.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skoog SM, Smyrk TC, Talwalkar JA. Cephalexin-induced cholestatic hepatitis. J Clin Gastroenterol. 2004;38:833. doi: 10.1097/01.mcg.0000139074.40365.04. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz B, Ekiz F, Coban S, et al. Cefixime-induced hepatotoxicity. Turk J Gastroenterol. 2011;22:445. doi: 10.4318/tjg.2011.0297. [DOI] [PubMed] [Google Scholar]
- 18.Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 19.De Valle MB, Av Klinteberg V, Alem N, et al. Drug-induced liver injury in a Swedish University hospital out-patient hepatology clinic. Aliment Pharmacol Ther. 2006;24:1187–1195. doi: 10.1111/j.1365-2036.2006.03117.x. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsson ES, Bergmann OM, Bjornsson HK, et al. Incidence, Presentation, and Outcomes in Patients With Drug-Induced Liver Injury in the General Population of Iceland. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Chalasani N, Fontana RJ, Bonkovsky HL, et al. Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–1934. doi: 10.1053/j.gastro.2008.09.011. 1934 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fontana RJ, Watkins PB, Bonkovsky HL, et al. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockey DC, Seeff LB, Rochon J, et al. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 2010;51:2117–2126. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: national initiatives to improve outcomes for patients having surgery. Clin Infect Dis. 2006;43:322–330. doi: 10.1086/505220. [DOI] [PubMed] [Google Scholar]
- 25.Classen DC, Evans RS, Pestotnik SL, et al. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326:281–286. doi: 10.1056/NEJM199201303260501. [DOI] [PubMed] [Google Scholar]
- 26.Veit O, Beck B, Steuerwald M, et al. First case of ivermectin-induced severe hepatitis. Trans R Soc Trop Med Hyg. 2006;100:795–797. doi: 10.1016/j.trstmh.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Bjornsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–38. doi: 10.1016/j.dld.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 29.Turillazzi E, D'Errico S, Neri M, et al. A fatal case of fulminant hepatic necrosis following sevoflurane anesthesia. Toxicol Pathol. 2007;35:840–845. doi: 10.1080/01926230701584148. [DOI] [PubMed] [Google Scholar]
- 30.Lucena MI, Andrade RJ, Fernandez MC, et al. Determinants of the clinical expression of amoxicillin-clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44:850–856. doi: 10.1002/hep.21324. [DOI] [PubMed] [Google Scholar]
- 31.Walsh SA, Creamer D. Drug reaction with eosinophilia and systemic symptoms (DRESS): a clinical update and review of current thinking. Clin Exp Dermatol. 2011;36:6–11. doi: 10.1111/j.1365-2230.2010.03967.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.