Abstract
Background
Platelet hyperactivity induced by inflammation is a known risk factor for atherosclerosis and thrombosis, but its underlying mechanisms remain poorly understood.
Methods and Results
The signal transducers and activators of transcription 3 (STAT3) was activated in collagen-stimulated platelets. Activated STAT3 served as a protein scaffold to facilitate the catalytic interaction between the kinase Syk and the substrate PLCγ2 to enhance collagen-induced calcium mobilization and platelet activation. The same interaction of STAT3 with Syk and PLCγ2 was also detected in HEK293 cells transfected with cDNAs for Syk and PLCγ2, and stimulated with interleukin-6 (IL-6). Pharmacological inhibition of STAT3 blocked ~50% of collagen- and a collagen-related peptide-, but not TRAP- or ADP-induced aggregation and ~80% of thrombus formation of human platelets on a collagen matrix. This in vitro phenotype was reproduced in mice infused with STAT3 inhibitors and mice with platelet specific STAT3 deficiency. By forming a complex with its soluble receptor, the proinflammatory cytokine IL-6 enhanced the collagen-induced STAT3 activation in human platelets.
Conclusions
These data demonstrate a non-transcriptional activity of STAT3 that facilitates a crosstalk between proinflammatory cytokine and hemostasis/thrombosis signals in platelets. This crosstalk may be responsible for platelet hyperactivity found in conditions of inflammation.
Keywords: STAT3, collagen, GPVI, platelet intracellular signaling, G-quartet oligonucleotide
INTRODUCTION
Signal Transducers and Activators of Transcription 3 (STAT3) is a transcription factor activated by cytokine-induced intracellular signals 1;2. This signal pathway plays a critical role in inflammation and megakaryopoiesis 3. For the latter, thrombopoietin (TPO) binds its receptor (a product of the proto-oncogene c-Mpl) on megakaryocytes to activate the receptor-associated Janus kinase (JAK) 3;4, resulting in the recruitment and tyrosine phosphorylation of STAT3. Activated STAT3 changes conformation, dimerizes through its SH2 domain, and translocates into the nucleus, where it regulates the transcription of multiple genes required for platelet production 2;3.
As the offspring of megakaryocytes, platelets maintain much of the megakaryopoiesis signaling machinery, including JAK and STAT3 5;6. However, platelets are anucleated cells with a limited capacity for protein synthesis. Therefore, it is unclear if platelet STAT3 is merely a “leftover” with no specific function, remains as an active transcription factor, or participates in intracellular signaling independent of its transcriptional activity. It has been reported that the transcription inhibitor actinomycin D blocks the TPO-dependent potentiation of platelet reactivity and that TPO induces the receptor-dependent tyrosine phosphorylation and dimerization of STAT3 in platelets 7;8. Activated STAT3 binds to the regulatory D-loop region of platelet mitochondrial DNA to regulate its transcription 8. This suggests that STAT3 remains active as a transcriptional factor in platelets. However, several lines of evidence also suggest a non-transcriptional role for STAT3 in platelets. First, TPO does not directly activate human platelets 5;6;9, but primes them for activation and aggregation induced by adenosine diphosphate (ADP) 6;10, epinephrine 5;10, and collagen 10;11. This TPO effect is detected within minutes after stimulation, too short to be explained by transcriptional and translational activities in activated platelets. Second, thrombin has been reported to induce STAT3 activation in platelets through a JAK3-dependent pathway 12 that is mediated through a G protein-coupled receptor 13. Consistent with this report, the JAK3 inhibitor WHI-P131 prevents thrombin-induced platelet shape changes, granule secretion, and aggregation 12.
These reports led us to hypothesize that STAT3 has a non-transcriptional activity that regulates agonist-mediated platelet activation. We tested this hypothesis in collagen-stimulated platelets because Spleen Tyrosine Kinase (Syk), which is essential for collagen-mediated signaling in platelets, also participates in cytokine-induced signaling pathways 14. Here, we present supportive data from in vitro experiments on human and mouse platelets and in vivo experiments on mice with megakaryocytes/platelet-specific STAT3 deficiency.
MATERIALS AND METHODS
Materials
Human blood was obtained from healthy donors under a protocol approved by the IRB of Baylor College of Medicine and Puget Sound Blood Center. For platelets aggregation, STAT3 activation and GPVI-mediated signaling, blood was drawn into 10% acid-citrate-dextrose buffer (85 mM sodium citrate, 111 mM glucose and 71 mM citric acid, pH 6.5). The whole blood was centrifuged at 150 × g for 15 min at 24°C to obtain platelet-rich plasma (PRP), which was then centrifuged at 900 × g for 10 min to obtain platelets 15. Platelets were washed with a CGS buffer (13 mM sodium citrate, 30 mM glucose, and 120 mM sodium chloride, pH 7.2) and suspended in Ca2+- and Mg2+-free Tyrode's buffer (138 mM sodium chloride, 5.5 mM glucose, 12 mM sodium bicarbonate, 2.9 mM potassium chloride, and 0.36 mM sodium phosphate dibasic, pH 7.4). To measure thrombus formation under flow conditions, blood was collected in 0.32% sodium citrate (final concentration) and tested directly.
Antibodies against total STAT3, Tyr705-phospho STAT3, Ser727-phospho STAT3, Tyr525/526-phospho Syk, and Tyr1217-phospho PLCγ2, total and phospho-STAT1 and phospho-STAT5 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against total Syk and PLCγ2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescence-conjugated antibodies to integrin αIIbβ3, GP Ibα, CD62P and CD45 were purchased from BD Biosciences (San Jose, CA). Mouse CD41-APC, mouse CD45-PE-Cy7 and mouse TER119-APC-Cy7 antibodies were purchased from eBioscience (San Diego, CA).
A protease inhibitor cocktail (10.4 mM AEBSF, 8 μM Aprotinin, 0.2 mM Leupeptin, 0.4 mM Bestatin, 0.15 mM Pepstatin A, 0.14 mM E-64) was purchased from Sigma-Aldrich (St. Louis, MO). Fura-2/AM was from Molecular Probes (Eugene, OR). Human fibrinogen was from Enzyme Research Laboratories (South Bend, IN). Type 1 collagen and ADP were from Helena Laboratories (Beaumont, TX). The crosslinked collagen-related peptide [CRP, Gly-Lys-Hyp-Gly(Pro-Hyp-Gly)10Lys-Hyp-Gly] was synthesized and crosslinked in the protein core laboratory of Baylor College of Medicine. The thrombin-receptor activating peptide (TRAP) was purchased from Bachem Bioscience (King Of Prussia, PA). Human IL-6 and soluble IL-6 receptor (sIL-6R) were purchased from the R&D Systems (Minneapolis, MN). Recombinant (r) Syk and rSTAT3 were purchased from Promega (Madison, WI) and Abcam (Cambridge, MA), respectively. The Syk inhibitors I (3,4-Methylenedioxy-b-nitrostyrene) and II [3-(1-Methyl-1H-indol-3-yl-methylene)-2-oxo-2,3-dihydro-1H-indole-5-sulfonamide] were purchased from EMD Chemicals (Darmstadt, Germany). Actinomycin D was from Sigma (St. Louis, MO).
STAT3 inhibitors
The STAT3 inhibitor STA21 [(S)-Ochromycinone Deoxytetrangomycin (C19H14O4)] 16 was purchased from Enzo Life Sciences (Plymouth Meeting, PA). A stock solution was made by dissolving STA21 in 30% dimethyl sulfoxide (DMSO) and the working solution was made by diluting the stock solution 300 folds in phosphate buffered saline (PBS) to a final DMSO concentration of 0.1% immediately before use. The vehicle control solution was PBS containing 0.1% DMSO.
The STAT3 inhibitor T40214 is a guanine-rich oligonucleotide (GGGCGGGCGGGCGGGC). It is in a linear structure in low extracellular [K+] (~5 mM), but forms a symmetrical G-quartet structure in high intracellular [K+] (~140 mM) when it is delivered into cells using polyethyleneamine nanobeads (PEI, MW ~25,000, Aldrich Chemical, WI) as a carrier 17. It binds the SH2 domain of STAT3 to inhibit the tyrosine phosphorylation and dimerization of STAT3 18;19. T40214 and a scrambled control oligonucleotide (CGGGGCGGGGCGGGGC) were commercially synthesized (Midland Certified Reagent Co., Midland, TX) and purified by anion exchange high pressure liquid chromatography on Q Sepharose followed by pressure filtration in H2O. Immediately before use, T40214 and the control oligonucleotide (1 mg/ml) were coupled to PEI nanobeads in a oligo/PEI bead ratio of 1 to 2 (w/w) with a coupling efficiency of 60–70%. The coupled beads were extensively washed with PBS before use.
Agonist-induced platelet aggregation
Washed platelets were incubated with STA21 or vehicle control (0.1% DMSO) for 10 min at 37°C and then mixed with purified human fibrinogen (0.2 U/ml). Platelets were re-calcificated immediately before being stimulated with type I fibrillar collagen, crosslinked collagen-related peptide (CRP), thrombin receptor-activating peptide (TRAP), or ADP, and monitored for aggregation for 10 min at 37°C on an optical aggregometer (Bio/Data, Horsham, PA) with constant stirring at 1,200 rpm.
For mouse experiments, C57BL/J6 mice (18–22 weeks old) were infused with STA21 or vehicle control daily for 3 days. They were then anesthetized with 3% isoflurane by inhalation to draw blood from the inferior vena cava (0.38% sodium citrate, final concentration). Blood samples were diluted with an equal volume of Ca2+- and Mg2+-free Tyrode's buffer and centrifuged at 70 × g for 20 min to obtain PRP. Platelet counts were normalized to 2.5 × 108/ml with homogenous plasma before samples were tested for agonist-induced platelet aggregation. The G-quartet STAT3 inhibitor T40214 19 was also tested in the same fashion.
Platelet granule release
A whole blood aggregometer was also used to measure ATP released per manufacturer's instructions (Chrono-log, Havertown, PA). A commercial ELISA was used to measure serotonin released from platelets according to manufacturer's instructions (Immuno-Biological Laboratories, Minneapolis, MN). Briefly, washed platelets before and after treatment with 20 μM of STA21 were stimulated with 5 μg/ml of collagen for 10 min and centrifuged at 900 × g for 10 min. The supernatants from each sample were collected and diluted 50 times in PBS for serotonin measurements.
Flow cytometry
Expression of the platelet adhesion receptors integrin αIIbβ3 and GP Ibα was measured by flow cytometry 20. The surface expression of the activation marker CD62P was measured before and after stimulating platelets with collagen for 20 min at room temperature. The samples were analyzed on an Epics XL-MCL flow cytometer (Beckman Coulter, Miami, FL). The expression of GP 130 and binding of IL-6-sIL-6R complex to platelets was also detected by flow cytometry using a FITC-conjugated mouse anti-human GP 130 (Abcam, Cambridge, MA) and a FITC-conjugated mouse anti-human IL6 antibody (Abcam).
To quantify potential leukocyte contamination, washed platelets and PRP were incubated with V450 anti-human CD45 (BD Biosciences, San Jose, CA) for 20 min at room temperature. Samples were first analyzed for CD45 positivity and then for particle size in the leukocyte gate on the forward scatter. We did not detect leukocytes in PRP and washed platelet preparations (Supplemental Figure 1A).
Immunoblotting and immunoprecipitation
Washed platelets were incubated with either STA21 or one of two Syk inhibitors for 10 min at 37°C before being stimulated with collagen, CRP, or TRAP for 5-10 min at 37°C. They were then solubilized with a hypotonic lysis buffer (10 mM Tris, 1 mM EDTA, 1 mM Na3VO4, 10 mM NaPyroPO4, 10 mM β-glyceroPO4, 10 mM NaF, 1% Igepal CA-630, pH 7.5) in the presence of a protease inhibitor cocktail (Supplemental Materials and Methods). Platelet lysates were centrifuged at 13,000 × g for 15 min at 4°C to remove cellular debris. The supernatant was separated on 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE) and probed for phosphorylated and total STAT isoforms as well as Syk, and PLCγ2 with specific antibodies. The total and phosphorylated STAT3 were also probed in lysates from washed platelets pretreated with interleukin 6 (IL-6, 10 ng/ml) or a complex of IL-6 (10 ng/ml) and an equal molar concentration of soluble IL-6 receptor (sIL-6R) in the presence or absence of collagen for 5-10 min at 37°C.
For immunoprecipitation, washed platelets were incubated with collagen for 5 min at 37°C. Platelet lysates were incubated with antibodies against total and phosphorylated PLCγ2 overnight at 4°C, followed by incubation with protein A sepharose beads (30 μg/ml) for 1 hour at 4°C. Samples were centrifuged at 8,000 × g for 5 min to collect protein A beads, which were washed and boiled in a SDS sample buffer. Bead eluates were separated by 10% SDS-PAGE and immunoblotted for STAT3, Syk and PLCγ2. For negative control, non-immune isotype IgGs were used for immunoblots. In a subset of experiments, platelets were treated with 5 μg/ml of actinomycin D, a global transcription inhibitor, for 2 hrs at 37°C before collagen stimulation and immunoprecipitation in order to determine a role of residual transcriptional activity in the interaction of STAT3 with Syk and PLCγ2.
Transfection and test of HEK293 cells
To further evaluate a role of STAT3 in mediating Syk and PLCγ2 interaction, Human Embryonic Kidney (HEK) 293 cells were co-transfected with human Syk and PLCγ2 cDNAs. These transiently transfected cells were treated with IL-6 for 1 hr in the presence or absence of 20 μM of STA21 and then solubilized by a lysis buffer (Cell Signaling Technology, Danvers, MA) 48 hr after transfection. Cell lysates were either separated by SDS-PAGE and probed for STAT3 phosphorylation or used for immunoprecipitation. For the latter, cell lysates were incubated with a STAT3 antibody overnight at 4°C, followed by incubation with protein A sepharose beads for 1 hr at 4°C. Bead eluates were then separated by SDS-PAGE and probed for Syk, PLCγ2 and STAT3.
Parallel-plate flow chamber assay
Citrated blood was perfused over a glass coverslip coated with type I fibrillar collagen (10 μg/ml coating concentration, overnight at 4°C) at a flow rate of 1 ml/min in a parallel-plate flow chamber. Platelet adhesion and aggregation were monitored in real time with time-lapse image acquisition and the thrombus-covered areas (from a total area of 3 × 105 cm2) were quantified offline using the Element Program (Nikon, Melville, NY).
Generation and test of platelet-specific STAT3 null mice
Megakaryocytes/platelet-specific STAT3 deleted mice (pSTAT3Δ/Δ) were generated using C57BL/6-TG(PF4-Cre) mice generated in our laboratory (Supplemental Materials) and crossed with mice carrying STAT3 alleles flanked by loxP sites (STAT3F/F; provided by Dr. Shizuo Akira of Department of Host Defense, Osaka University 21). Colonies were expanded by crossing PF4-Cre-STAT3F/F mice with STAT3F/F mice. These mice were genotyped via PCR by amplifying a fragment of the STAT3 gene flanked by loxP sites (primers: 5’CCTGAAGACCAAGTTCATCGTTGTGA3’ and 5’CACACAAGCCATCAAACTCTGGTCTCC3’) and a fragment that included the PF4 promoter and the cDNA for Cre recombinase. For data verification, a second strain of pSTAT3Δ/Δ mice was also generated using a commercial strain of PF4-Cre mice (Stock #008535, Jackson laboratories, Bar Harbor, ME).
Collagen-induced calcium influx
PRP from STAT3F/F and pSTAT3Δ/Δ mice was incubated with 2 mM fura-2/AM for 1hr at room temperature and then washed. Labeled platelets were suspended in PBS to a final concentration of 1.5 × 108/ml. Calcium (final concentration of 2 mM) was added to the platelet suspension immediately before adding 0.75 μg/ml of collagen. Calcium influx [Ca2+]i was monitored at 340 nm (excitation) and 505 nm (emission) on a PTI QuantaMaster fluorimeter (Photon Technology International, Birmingham, NJ) for 3 min at 37°C with constant stirring.
Statistical Analysis
Quantitative data are presented as mean ± SEM. Repeated measures ANOVA was used to analyze data from experiments where comparisons were made between multiple observations from the sample. Standard ANOVA models were fit to data where all observations included in an analysis were independent. Normal probability plots were used to assess the assumption of normally distributed errors. In repeated measures analyses, Huynh-Feldt adjustments were made for cases where the sphericity assumption came into question. An experiment-wise Type I error rate of 0.05 was maintained in each experiment using the Sidak method to adjust p-values for testing multiple hypotheses.
RESULTS
STAT3 inhibitors on platelet aggregation
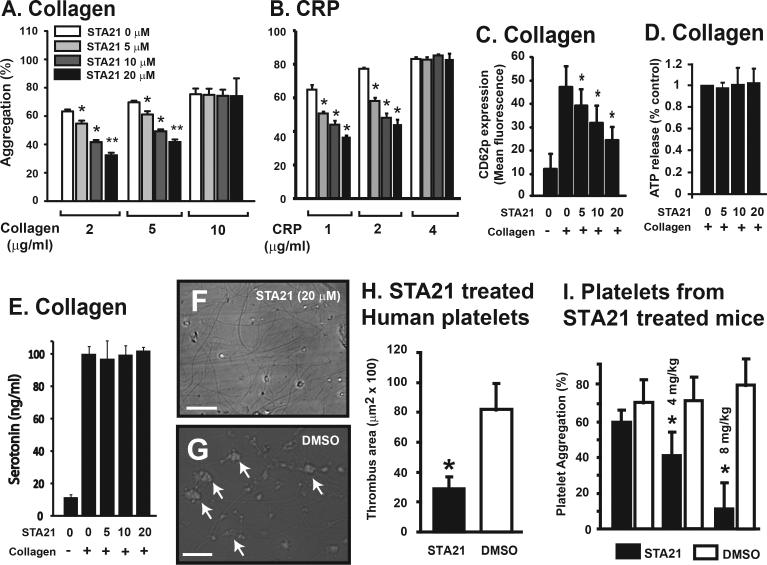
The STAT3 inhibitor STA21 dose-dependently inhibited ~50% of platelet aggregation induced by low, but not maximal doses of collagen and the collagen-related peptide (CRP) (Figure 1A-B). It also partially blocked the collagen-induced expression of the platelet activation marker CD62P (Figure 1C), but not the release of ATP and serotonin (Figure 1D and E). In contrast, STA21 had no effect on ADP and TRAP-induced platelet aggregation and did not alter expression levels of the adhesion receptors integrin αIIbβ3 and GP Ibα (Supplemental Figure 1B & 1C). We then tested the effect of STA21 on platelet thrombus formation by perfusing whole blood over a type I collagen matrix for 1 min at a flow rate of 1 ml/min, which generated a wall shear stress of 62.5 dyn/cm2 (with a measured viscosity of 6 cp). STA21 at 20 μM, which maximally inhibited collagen-induced platelet aggregation, significantly reduced the number of adherent platelets, whereas the DMSO vehicle control did not (614 ± 24 for STA21 vs. 1,542 ± 125 for DMSO in 6 random view fields of 400 × magnification, n = 4). Consistent with reduced platelet adhesion and aggregation, thrombus formation on a collagen matrix under flowing conditions was also reduced ~80% by 20 μM of STA21 (Figure 1G-H).
Figure 1.
Effects of STA21 on platelet aggregation: PRP was incubated with STA21 or DMSO vehicle control (0.1%) for 10 min at 37°C and then induced to aggregate with various concentrations of collagen (A, Univariate Repeated Measures for each collagen dose compared to baseline, n = 9, *p < 0.001) or CRP (B, compared to baseline, n = 3, *p < 0.001). Secretion from α-granule was measured by detecting surface expression of CD62P by flow cytometry after platelets were stimulated with 2 μg/ml of collagen for 10 min (C, Univariate Repeated Measures for each CRP dose compared to baseline, n = 6, *p < 0.001). ATP (D, n = 6) and serotonin (E, n = 3) released from dense granules were measured by whole blood aggregometry and ELISA, respectively, in the supernatant of platelets stimulated with 2 μg/ml of collagen. The data for the panels A-C are also presented as box blots in Supplemental Figure 5. Thrombus formation in vitro was induced by perfusing STA21-treated (20 μm, 10 min at 37°C) blood over immobilized collagen for 1 min at a flow rate of 1 ml/min. Representative images show platelet thrombi in the presence (F) and absence (G) of STA21 (Bar = 200 μm). The areas covered by thrombi were quantified in 6 random images from each experiment (H, n = 3 separate sets of experiments, *p < 0.001). For mouse assay, C57BL/6J mice were daily injected with 4 or 8 mg/kg body weight of STA21 or vehicle control through tail veins for 3 days and collagen-induced platelet aggregation was measured on the third day on an optical aggregomater (I, n = 8, *p < 0.01).
Since human and mouse STAT3 share more than 99% of sequence identity 22, we tested the function of platelets from C57BL/6J mice that had been daily infused with STA21 or its vehicle control for 3 days. Platelets from STA21 infused mice had reduced collagen-induced aggregation (Figure 1I) without detectable reduction in platelet counts (Supplement Figure 1D).
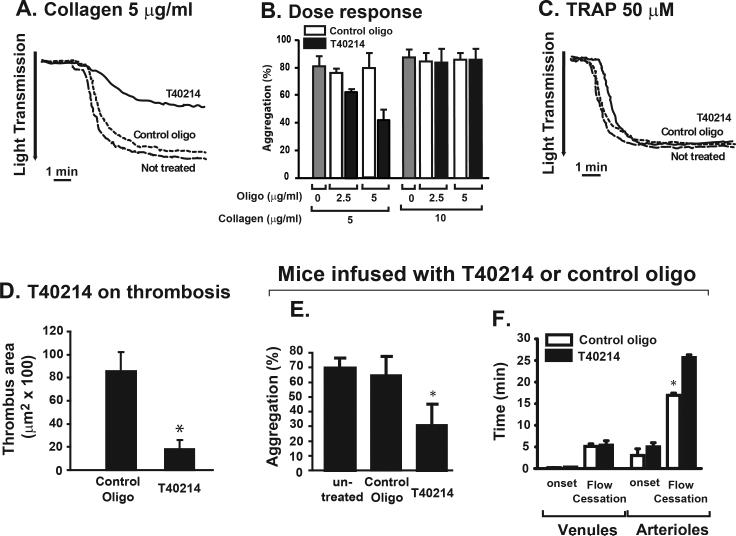
To validate data generated with STA21, we also tested the oligonucleotide G-quartet T40214, a different class of STAT3 inhibitor that specifically blocked phosphorylation-dependent STAT3 dimerization 17;18. Compared to those treated with a scrambled control oligonucleotide, T40214 significantly reduced the aggregation of human platelets induced by 5 μg/ml, but not 10 μg/ml of collagen (Figure 2A & 2B). Platelet aggregation induced by 50 μM of TRAP was not affected (Figure 2C). Platelets pretreated with T40214 also formed significantly smaller thrombi on collagen matrix under a wall shear stress of 62.5 dyn/cm2 as compared to those treated with a scrambled control oligonucleotide (Figure 2D). Platelets from C57BL/6J mice infused with T40214 had reduced collagen-induced aggregation (Figure 2E). The ex vivo thrombus formation in the cremaster arterioles after photochemical-induced vascular injury was also significantly delayed in T40214-infused mice (Figure 2F).
Figure 2.
Effects of T40214 on human and mouse platelets: PRP was incubated with the PEI-coupled T40214 or the scrambled control oligonucleotide for 1 hrs at 37°C and then induced to aggregate with 5 μg/ml or 10 μg/ml of collagen at two doses of T40214 or control oligonucleotide (A & B). Platelet aggregation was also induced by 50 μM of TRAP in the presence of either T40214 or the control scrambled oligonucleotide (C). Citrated blood treated with T40214 or the control oligonucleotide was perfused over immobilized collagen for 1 min at a flow rate of 1 ml/min and areas covered by platelet thrombi were measured (D, n = 6, *p < 0.01). Male C57BL/J6 mice (n = 8) were infused with 1 mg/kg of PEI-coupled T40214 or the control oligonucleotide daily for 3 days. PRP was then tested for platelet aggregation induced by 0.75 μg/ml collagen (E, n = 5, *p < 0.01). Thrombus formation after photochemical injury was examined by the onset and time to closure of venules and arterioles of cremaster muscles of mice infused with T40214 or the control oligonucleotide (F, n = 8, *p < 0.01).
Collagen-induced platelet aggregation in pSTAT3Δ/Δ mice
Because STAT3 is ubiquitously expressed, a STAT3 inhibitor infused systemically could interfere with platelet aggregation through its effects on endothelial cells and leukocytes. We therefore generated megakaryocyte/platelet-specific STAT3 deletant mice (pSTAT3Δ/Δ) by crossing the C57BL/6-Tg(CXCL4-cre) (PF4-Cre) mice with STAT3F/F mice. The selectivity and robustness of Cre expression in the PF4-Cre line were verified by crossing with reporter lines that express β-galactosidase or eYFP only in cells that experience Cre recombinase activity (Supplemental Figure 2). The platelet-specific deletion of STAT3 was verified by immunoblotting platelet lysates with a STAT3 antibody (Supplemental Figure 3A). pSTAT3Δ/Δ mice were fertile without detectable physical abnormalities and their hematological parameters were comparable to those of STAT3F/F littermates (Table 1).
Table 1.
Hematological measurements of pSTAT3Δ/Δ and STAT3F/F mice*
| Measurements | Unit | pSTAT3Δ/Δ | STAT3F/F | p value |
|---|---|---|---|---|
| Platelet counts | 103/mm3 | 613.26 ± 25.2 | 663.63 ± 16.2 | 0.08 |
| Mean platelet vol. | FL | 5.14 ± 0.08 | 5.11 ± 0.03 | 0.67 |
| White cell count | 103/mm3 | 2.51 ± 0.35 | 2.19 ± 0.13 | 0.39 |
| Red cell count | 106/mm3 | 7.08 ± 0.15 | 7.36 ± 0.12 | 0.13 |
| Hemoglobin | g/dl | 11.63 ± 0.22 | 11.20 ± 0.19 | 0.18 |
| Hematocrit | % | 33.11 ± 0.73 | 33.30 ± 0.63 | 0.84 |
Data are expressed as mean ± SEM and analyzed with two way ANOVA test (n = 27 mice/group).
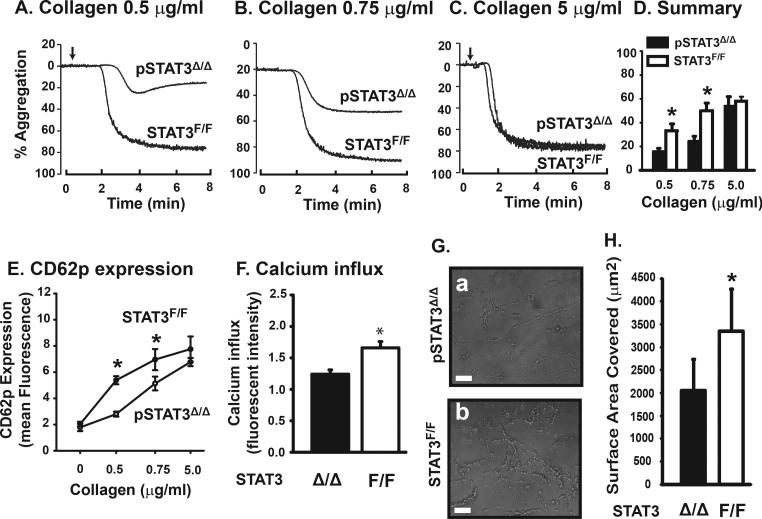
Platelets from these pSTAT3Δ/Δ mice had reduced aggregation, CD62P expression, and calcium influx induced by 0.5 and 0.75 μg/ml, but not 5 μg/ml of collagen (Figure 3A-F). The rate of thrombus formation was also reduced when blood from pSTAT3Δ/Δ mice was perfused over a collagen matrix under arterial shear stress (Figure 3G & H). In contrast, TRAP- and ADP-induced platelet aggregation was minimally affected (Supplemental Figure 3B & C).
Figure 3.
Platelet function of STAT3Δ/Δ mice: Platelets from pSTAT3Δ/Δ and STAT3F/F littermates were induced to aggregate by 0.5, 0.75, or 5 μg/ml of collagen (A-C) and data from 32 mice/group were quantified (D, *p < 0.01). CD62p expression was measured after platelets were stimulated with 0.5, 0.75 or 5 μg/ml of collagen. The comparison was made between WT and STAT3 KO platelets at each collagen level with a two-way ANOVA (E, n = 12, *p < 0.001). No interaction between litter and genotype was found at any of these collagen doses. Calcium influx was detected in platelets stimulated with 0.75 μg/ml of collagen (F, *p < 0.003). Blood was perfused over immobilized collagen for 10 min at a flow rate of 1 ml/min to measure thrombus formation (G, representative images, bar = 50 μm), which was quantified by measuring surface areas covered by platelet thrombi (H, n = 10, *p < 0.001).
Collagen induced STAT3 phosphorylation in human platelets
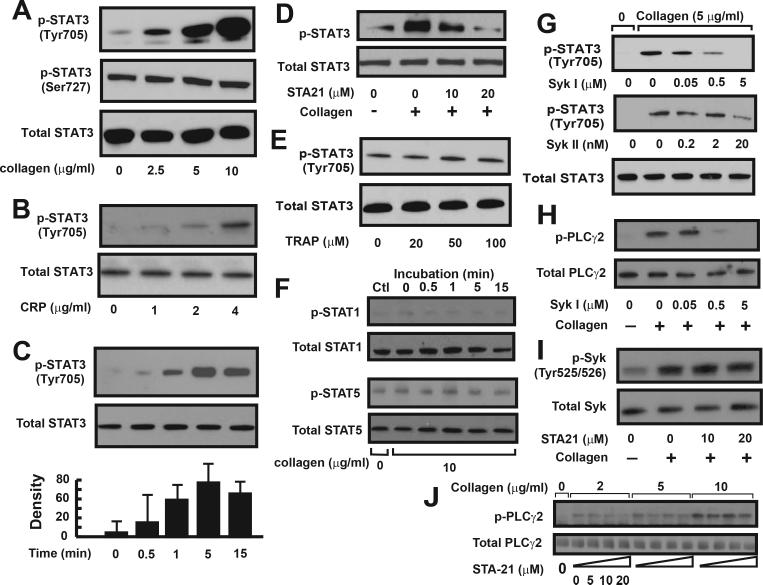
Figures 1–3 show that blocking STAT3 moderately, but specifically inhibits collagen-induced platelet aggregation and thrombus formation. We next assessed whether platelet STAT3 was activated (phosphorylated) after collagen stimulation. Tyr705 and Ser727 of STAT3 are known to be differentially phosphorylated in response to cytokine stimulation in nucleated cells 23;24. We found that collagen and CRP stimulation dose-dependently increased STAT3 phosphorylation at Tyr705, but not Ser727 (Figure 4A-B). This collagen-induced STAT3 tyrosine phosphorylation was detectable 0.5 min after collagen exposure, but reached the maximal level after 5-10 min (Figure 4C). This collagen-induced STAT3 phosphorylation was dose-dependently blocked by STA21 (Figure 4D). In contrast, TRAP had no effect on the level of STAT3 tyrosine phosphorylation (Figure 4E). Collagen stimulation also did not change the levels of tyrosine phosphorylation of STAT1 and STAT5, the other two major STAT proteins in platelets (Figure 4F).
Figure 4.
STAT3 phosphorylation in human platelets: Washed platelets were incubated with various concentrations of collagen (A) or CRP (B) for 10 min at 37°C. Platelet lysates were probed with antibodies against Tyr705 phosphorylated, Ser727 phosphorylated, and total STAT3. Aliquots of platelets were collected and probed for STAT3 phosphorylation over a 15 min after platelets were stimulated with 5 μg/ml of collagen. The optical density of immunoreactive bands of STAT3 phosphorylation was recorded (C). STAT3 phosphorylation induced by 5 μg/ml of collagen was measured in the presence of STA21 (D). STAT3 phosphorylation was also determined in TRAP-treated platelets (E). STAT1 and STAT5 phosphorylation was probed in collagen-stimulated platelet lysates (F). Human washed platelets were first treated with one of two Syk inhibitors for 10 min and then stimulated with 5 μg/ml of collagen. Platelet lysates were probed for the phosphorylation of STAT3 (G) and PLCγ2 (H). STA21-treated platelets were stimulated with collagen and probed for Syk phosphorylation (I). Phosphorylated and total PLCγ2 was probed in platelets treated with various doses of collagen in the presence of increasing doses of STA21 (J). Panel figures represent 3–7 separate experiments.
Syk inhibitors on tyrosine phosphorylation of STAT3
We have shown that collagen specifically induced tyrosine phosphorylation of STAT3, but not STAT1 and STAT5 in human platelets. Previous studies have identified prominent roles for Syk and PLCγ2 in collagen-induced calcium mobilization 25. To decipher the relationship between STAT3 and these two signal molecules, we evaluated STAT3 phosphorylation in the presence of Syk inhibitors 26;27. Two Syk inhibitors dose-dependently reduced collagen-induced STAT3 phosphorylation (Figure 4G). As expected, this Syk inhibitor was also effective in blocking PLCγ2 phosphorylation induced by collagen (Figure 4H). In contrast, STA21 did not inhibit Syk phosphorylation (Figure 4I), but dose-dependently blocked PLCγ2 phosphorylation in platelets treated by 2 and 5 μg/ml, but not 10 μg/ml of collagen (Figure 4J). Together, these data suggest that STAT3 acts downstream of Syk, but upstream of PLCγ2.
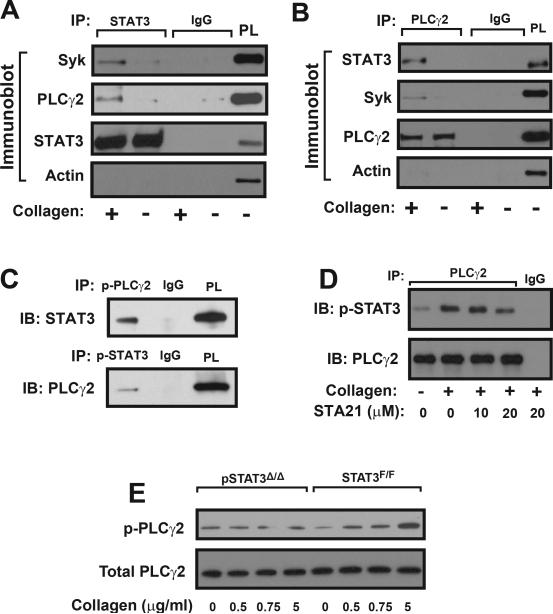
Interaction of STAT3 with Syk and PLCγ2
This spatial relationship among STAT3, Syk, and PLCγ2 was further examined by co-immunoprecipitation experiments. A STAT3 antibody immunoprecipitated Syk and PLCγ2 (Figure 5A) and a PLCγ2 antibody immunoprecipitated Syk and STAT3 (Figure 5B) in lysates of collagen-stimulated platelets. Both antibodies did not immunoprecipitate actin, suggesting the association of STAT3 with Syk and PLCγ2 was not mediated through actin cytoskeleton. The pretreatment of platelets with the transcription inhibitor actinomycin D did not alter this association (Supplemental Figure 4C). Furthermore, co-immunoprecipitation was also achieved by antibodies specifically against phosphorylated PLCγ2 and STAT3 (Figure 5C). The STAT3 inhibitor STA21 reduced the amount of phosphorylated STAT3 immunoprecipitated by a PLCγ2 antibody (Figure 5D). Consistent with data from human platelets, the collagen-induced phosphorylation of PLCγ2 was significantly reduced in platelets from pSTAT3Δ/Δ mice (Figure 5E).
Figure 5.
Co-immunoprecipitation: Washed human platelets were stimulated with 5 μg/ml of collagen and platelet lysates were incubated with a STAT3 antibody followed by immunoprecipitation (IP) with protein A sepharose beads. Precipitated proteins were probed with antibodies to STAT3, Syk, PLCγ2, and actin (A, isotype IgG as control and STAT3 as loading control, platelet lysate [PL] as positive control). The same technique was used to immunoprecipitate PLCγ2 and probe for PLCγ2, Syk, STAT3, and actin (B). STAT3 and PLCγ2 were also immunoprecipitated with antibodies specifically against phosphorylated PLCγ2 and phosphorylated STAT3, respectively (C). STAT3 was co-immunoprecipitated with a PLCγ2 antibody from lysates of platelets stimulated with 5 μg/ml of collagen in the presence of increasing doses of STA21 (D). PLCγ2 phosphorylation was measured in platelets from pSTAT3Δ/Δ and STAT3F/F mice stimulated with increasing doses of collagen (E). Panels in the figure represent of 3–6 separate experiments.
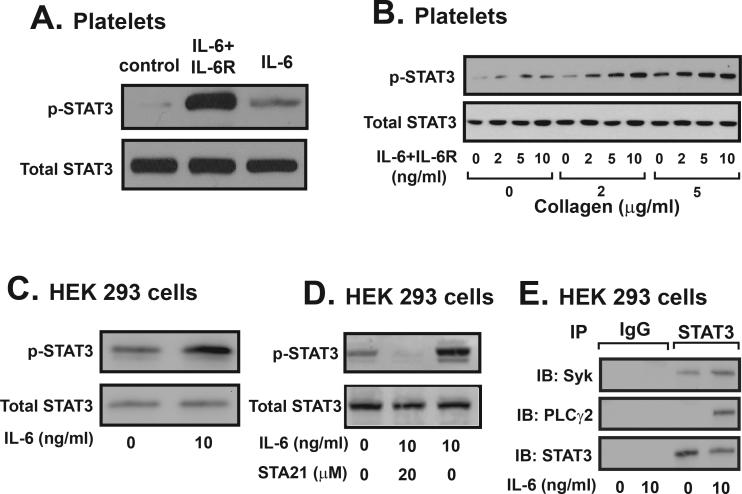
IL-6 on collagen-induced STAT3 phosphorylation
Together, these data suggest that STAT3 interacted with Syk and PLCγ2 to enhance collagen-induced platelet activation and aggregation. A critical implication of this tri-molecule interaction could be to facilitate a crosstalk between collagen-induced and inflammatory cytokine-induced signal pathways. This potential crosstalk was examined in human platelets and HEK293 cells. We found that human platelet expressed the common signal transducer of the IL-6 cytokine family gp130 (Supplemental Figure 4A) and a complex of IL-6 and soluble IL-6 receptor could be detected on the surface of platelets after 10 min incubation with this complex (Supplemental Figure 4B). The IL-6 and soluble IL-6 receptor complex, but not IL-6 alone induced STAT3 phosphorylation (Figure 6A), which was dose-dependently enhanced by collagen in human platelets (Figure 6B). In contrast to platelets, IL-6 induced STAT3 phosphorylation in HEK293 cells transiently transfected with human Syk and PLCγ2 cDNAs (Figure 6C). STA21 at 20 μM blocked this IL-6 induced STAT3 phosphorylation in HEK293 cells (Figure 6D). Consistent with results from platelet experiments, a STAT3 antibody immunoprecipitated Syk and PLCγ2 from lysates of IL-6 stimulated HEK293 cells (Figure 6E).
Figure 6.
Effect of IL-6 on platelet and HEK293 cells. STAT3 phosphorylation was measured in platelets stimulated with IL-6/sIL-6R or IL-6 in the absence (A) and presence (B) of increasing concentrations of collagen. HEK293 cells transiently expressing human Syk and PLCγ2 were stimulated with 10 ng/ml of IL-6 for 1 hr at 37°C and then lysed. Cell lysates were probed for total and phosphorylated STAT3 (C). These cells were also stimulated with IL-6 in the presence of STA21 and probed for STAT3 phosphorylation (D). Resting and IL-6 stimulated HEK293 cells were lysed and incubated with a STAT3 antibody followed by immunoprecipitation by protein A coupled beads (E). Immunoprecipitated proteins were probed for Syk, PLCγ2, and STAT3. Non-immune isotype IgG was used as negative control. The figure represent of 3–4 separate experiments.
DISCUSSION
We have presented several lines of experimental evidence that STAT3 regulates collagen-induced platelet activation and aggregation. First, two STAT3 specific inhibitors of different classes; STA21 (a small molecule) and T40214 (a protein binding oligonucleotide G-quartet) dose-dependently blocked platelet aggregation induced by low doses of collagen- and CRP-, but not TRAP- or ADP. They also inhibited thrombus formation on a collagen substrate under arterial flow, and reduced CD62P expression (Figure 1 & 2). Second, platelets from mice deficient in platelet STAT3 (pSTAT3Δ/Δ) aggregated poorly and had a low level of CD62P expression and calcium influx in response to stimulation by low doses of collagen (Figure 3), but reacted normally to ADP and TRAP (Supplemental Figure 3). These pSTAT3Δ/Δ platelets also formed smaller thrombi on a collagen matrix under arterial flow (Figure 3). There are two known receptors for collagen on platelets: the integrin α2β1 and GP VI/Fc receptor γ chain 28;29. Our data suggest that STAT3 is primarily involved in GP VI–mediated signaling because STA21 blocked platelet activation induced by not only collagen, but also CRP, which specifically targets GPVI 30. STAT3 phosphorylation was also blocked by inhibiting Syk (Figure 4), a critical tyrosine kinase in the GP VI-mediated signaling pathway. The finding is supported by a report that the Syk inhibitor piceatannol blocked STAT3 phosphorylation in lymphocytic cells 31. Through these experiments, we have also made three novel observations.
First, STAT3 enhanced a collagen-induced intracellular signal that resulted in platelet activation, calcium mobilization and aggregation. This activity is independent of transcriptional activity because i) the effects of STAT3 on platelet activation and aggregation occurred as early as 0.5 min after collagen stimulation and reached the plateau 5-10 min after collagen stimulation, a time period insufficient for significant transcriptional activities, especially in anucleated platelets, ii) STAT3 directly interacted with Syk and PLCγ2, likely forming a tri-molecule complex as suggested by co-immunoprecipitation experiments (Figure 5), and iii) the transcription inhibitor actinomycin D did not affect the interaction of STAT3 with Syk and PLCγ2 in collagen-stimulated platelets (Supplemental Figure 4).
Second, STAT3 may act as a protein scaffold in regulating collagen signals. As previously reported, crosslinking GPVI induces the Src kinase-dependent phosphorylation of the immunoreceptor tyrosine-based activation motif, which then recruits and activates Syk 25. Syk activation leads to the phosphorylation of the linker for activation of T cells (LAT) and the adapter Src SH2 domain-containing leukocyte protein of 76 kDa (SLP-76), both are required for tyrosine phosphorylation of PLCγ2 32;33. However, there are likely additional proteins linking Syk to PLCγ2 activation because: i) tyrosine phosphorylation of PLCγ2 was reduced, but not eliminated in CRP-stimulated LAT−/− platelets 32 and ii) SLP-76 is tyrosine phosphorylated in collagen signaling downstream of Syk, but Syk and SLP-76 association could not be detected 34. We found that blocking Syk reduced STAT3 phosphorylation, but not vice versa (Figure 4) and STA21 reduced the amount of STAT3 immunuprecipitated by a PLCγ2 antibody (Figure 5D). Collagen-induced PLCγ2 phosphorylation was reduced in platelets from pSTAT3Δ/Δ mice (Figure 5E). Similarly, a STAT3 antibody immunoprecipitated Syk and PLCγ2 in HEK293 cells that transiently expressed the two signal molecules and were stimulated with IL-6 (Figure 6). These data suggest that an activated (dimerized) STAT3 may enhance or accelerate the catalytic interaction between Syk and PLCγ2 by bringing the substrate to the proximity of its kinase (a dimerized STAT3 has two SH2 binding sites). In this regard, STAT3 functions as a signal enhancer, not a facilitator. This notion is consistent with the finding that effects of STAT3 were detected only at low concentrations of collagen or CRP. The notion that STAT3 functions as a protein scaffold is also supported by a report that STAT3 couples phosphatidylinositol 3-kinase with the type1 interferon receptor in nucleated cells 35.
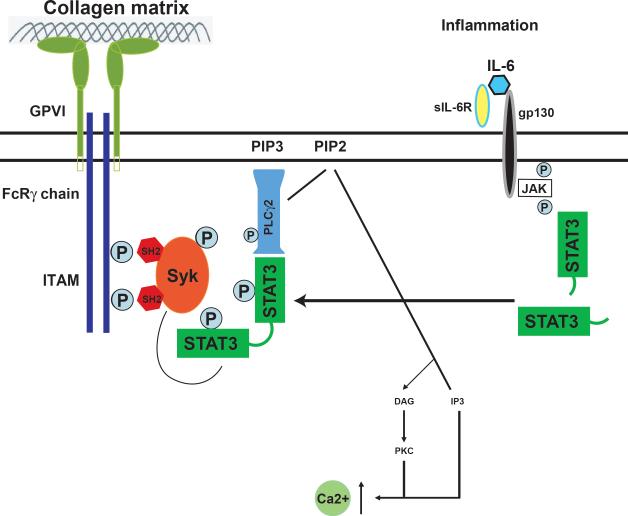
Third, the IL-6•sIL-6R complex, but not IL-6, activated STAT3 and enhanced collagen-induced STAT3 phosphorylation in platelets (Figure 6A & B), whereas IL-6 alone was sufficient to activate STAT3 in HEK293 cells (Figure 6C). These data suggest that, unlike HEK293 cells, platelets express the common signal transducer of the IL-6 cytokine family gp130 (Supplemental Figure 4A), but not IL-6 receptor. As a result, IL-6 acts on platelets only when it forms a complex with soluble IL-6R, which can be released through inflammation-mediated shedding. An IL-6•sIL-6R complex then primes platelets for collagen-induced activation by facilitating or accelerating the catalytic interaction between Syk and PLCγ2. In a similar fashion, IL-15 induces Syk and STAT3 phosphorylation in mouse mast cells 36. Figure 7 schematically illustrates a potential mechanism for IL-6 to enhance collagen-mediated signaling in platelets.
Figure 7.
A schematic crosstalk between IL-6 and collagen signal pathways: Data from this study support a model of crosstalk between collagen-induced and cytokine-mediated STAT3 signals in platelets. This crosstalk may be active during inflammation where the secretion of the proinflammatory cytokine IL-6 and the membrane shedding of IL-6 receptor (gp80) result in the formation of soluble IL-6•sIL-6R complex that binds to gp130 on platelets to activate STAT3. The activated STAT3 serves as a protein scaffold to bring the kinase Syk to the vicinity of the substrate PLCγ2 to enhance or accelerate PLCγ2 phosphorylation in response to collagen stimulation. Activated PLCγ2 could then hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) to produce inositol 1,4,5-triphosphate (IP3) to mobilize calcium.
In summary, we have shown that STAT3 can serve as a protein scaffold to facilitate the catalytic process of activating PLCγ2 by Syk. This novel non-transcriptional activity of STAT3 enhances collagen-induced signaling in platelets, potentially making platelets hyperactive in conditions of inflammation by linking proinflammatory cytokine signals to hemostasis/thrombosis signals. The clinical implications of data presented here remains to be explored, but one could speculate that platelet hyperactivity, which has been widely reported in patients with coronary artery diseases, could be caused by this crosstalk. Furthermore, because hyperactive or activated platelets are insensitive to aspirin, targeting STAT3 could potentially improve aspirin efficacy by reducing inflammation-induced platelet hyperactivity with minimal impacts on the physiologically critical process of hemostasis.
Supplementary Material
Short Commentary.
In conditions of inflammation, platelets often deviate from their normal path of activation to become hyperactive in response to various agonists that are in solution or affixed to subendothelial matrix. This platelet hyperactivity is a known risk factor for atherosclerosis and thrombosis, but its underlying mechanisms remain poorly understood. Using specific inhibitors and transgenic mice, we have shown that the signal transducers and activators of transcription 3 (STAT3) is activated in collagen-stimulated human and mouse platelets. Activated STAT3 serves as a protein scaffold to facilitate the catalytic interaction between the kinase Syk and the substrate PLCγ2, leading to enhanced collagen-induced calcium mobilization and platelet activation/aggregation. These data demonstrate a non-transcriptional activity of STAT3 that facilitates a crosstalk between the proinflammatory cytokine interleukin 6 and hemostasis/thrombosis signals in platelets. This crosstalk could provide a mechanism for platelets to become hyperactive in diverse clinical conditions that share a common pathology of inflammation. Blocking STAT3 and its intracellular signal pathway could potentially reduce and prevent arterial thrombosis. For example, inhibitors to JAK2 (the upstream of STAT3) are being clinically tested for myeloproliferative disorders, which are associated with the high incidence of thrombosis. These inhibitors could potentially improve clinical outcomes of myeloproliferative disorders by not only blocking aberrant proliferation of blood cells, but also preventing thrombosis. Hyperactive platelets are also known to be insensitive to aspirin. Targeting STAT3 could therefore improve aspirin efficacy by reducing inflammation-induced platelet hyperactivity with a minimal impact on the physiologically critical process of hemostasis.
Acknowledgments
Funding Sources: This work was supported by NIH grants HL71895, CA104035, and HL081613, and a Merit Review Grant from the Department of Veterans Affairs.
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenlund AC, Morales MO, Viviano BL, Yan H, Krolewski J, Schreiber RD. Stat recruitment by tyrosine-phosphorylated cytokine receptors: an ordered reversible affinity-driven process. Immunity. 1995;2:677–687. doi: 10.1016/1074-7613(95)90012-8. [DOI] [PubMed] [Google Scholar]
- 2.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat. Rev. Immunol. 2006;6:602–612. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 3.Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89:483–492. [PubMed] [Google Scholar]
- 4.Kaushansky K. Thrombopoietin: the primary regulator of platelet production. Blood. 1995;86:419–431. [PubMed] [Google Scholar]
- 5.Miyakawa Y, Oda A, Druker BJ, Miyazaki H, Handa M, Ohashi H, Ikeda Y. Thrombopoietin induces tyrosine phosphorylation of Stat3 and Stat5 in human blood platelets. Blood. 1996;87:439–446. [PubMed] [Google Scholar]
- 6.Ezumi Y, Takayama H, Okuma M. Thrombopoietin, c-Mpl ligand, induces tyrosine phosphorylation of Tyk2, JAK2, and STAT3, and enhances agonists-induced aggregation in platelets in vitro. FEBS Lett. 1995;374:48–52. doi: 10.1016/0014-5793(95)01072-m. [DOI] [PubMed] [Google Scholar]
- 7.Majka M, Ratajczak J, Villaire G, Kubiczek K, Marquez LA, Janowska-Wieczorek A, Ratajczak MZ. Thrombopoietin, but not cytokines binding to gp130 protein-coupled receptors, activates MAPKp42/44, AKT, and STAT proteins in normal human CD34+ cells, magakaryocytes, and platelets. Exp. Hemato. 2002;30:751–760. doi: 10.1016/s0301-472x(02)00810-x. [DOI] [PubMed] [Google Scholar]
- 8.Vassilev AO, Lorenz DR, Tibbles HE, Uckun FM. Role of the leukemia-associated transcription factor STAT3 in platelet physiology. Leuk. Lymphoma. 2002;43:1461–1467. doi: 10.1080/1042819022386716. [DOI] [PubMed] [Google Scholar]
- 9.Fontenay-Roupie M, Huret G, Loza JP, Adda R, Melle J, Maclouf J, Dreyfus F, Levy-Toledano S. Thrombopoietin activates human platelets and induces tyrosine phosphorylation of p80/85 cortatin. Thromb. Haemost. 1998;79:195–201. [PubMed] [Google Scholar]
- 10.Oda A, Miyakawa Y, Druker BJ, Ozaki K, Yabusaki K, Shirasawa Y, Handa M, Kato T, Miyazaki H, Shimosaka A, Ikeda Y. Thrombopoietin primes human platelet aggregation induced by shear stress and by multiple agonists. Blood. 1996;87:4664–4670. [PubMed] [Google Scholar]
- 11.Pasquet JM, Gross BS, Gratacap MP, Quek L, Pasquet S, Payrastre B, van WG, Mountford JC, Watson SP. Thrombopoietin potentiates collagen receptor signaling in platelets through a phosphatidylinositol 3-kinase-depedent pathway. Blood. 2000;95:3429–3434. [PubMed] [Google Scholar]
- 12.Tibbles HE, Vassilev A, Wendorf H, Schonhoff D, Zhu D, Lorenz D, Waurzyniak B, Liu XP, Uckun FM. Role of a JAK3-dependent biochemical signaling pathway in platelet activation and aggregation. J Biol Chem. 2001;276:17815–17822. doi: 10.1074/jbc.M011405200. [DOI] [PubMed] [Google Scholar]
- 13.Yamaoka K, Saharinen P, Pesu M, Holt VE, III, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks). Genome Biol. 2004;5:253. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uckun FM, Qazi S, Ma H, Tuel-Ahlgren L, Ozer Z. STAT3 is a substrate of Syk tyrosine kinase in B-lineage leukemia/lymphoma cells exposed to oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2902–2907. doi: 10.1073/pnas.0909086107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardo A, Bergeron AL, Sun CW, Guchhait P, Cruz MA, Lopez JA, Dong JF. Von Willebrand factor present in fibrillar collagen enhances platelet adhesion to collagen and collagen-induced platelet aggregation. J. Thromb. Haemost. 2004;2:660–669. doi: 10.1111/j.1538-7836.2004.00661.x. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Wang R, Wang S, Lin J. A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4700–4705. doi: 10.1073/pnas.0409894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing N, Zhu Q, Yuan P, Li Y, Mao L, Tweardy DJ. Targeting signal transducer and activator of transcription 3 with G-quartet oligonucleatides: a potential novel therapy for head and neck cancer. Mol. Cancer Ther. 2006;5:279–286. doi: 10.1158/1535-7163.MCT-05-0302. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q, Jing N. Computational study on mechanism of G-quartet oligonucleotide T40214 selectively targeting Stat3. J. Comput. Aided Mol. Des. 2007;21:641–648. doi: 10.1007/s10822-007-9147-6. [DOI] [PubMed] [Google Scholar]
- 19.Jing N, Li Y, Xu X, Sha W, Li P, Feng L, Tweardy DJ. Targeting Stat3 with G-quartet oligodeoxynucleotides in human cancer cells. DNA Cell Biol. 2003;22:685–696. doi: 10.1089/104454903770946665. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JN, Bergeron AL, Yu Q, Sun C, McBride L, Bray PF, Dong JF. Duration of exposure to high fluid shear stress is critical in shear-induced platelet activation-aggregation. Thromb. Haemost. 2003:90672–678. doi: 10.1160/TH03-03-0145. [DOI] [PubMed] [Google Scholar]
- 21.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3801–3804. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietra LD, Bressan A, Pezzotti AR, Serlupi-Crescenzi O. Highly conserved amino-acid sequence between murine STAT3 and a revised human STAT3 sequence. Gene. 1998;213:119–124. doi: 10.1016/s0378-1119(98)00185-1. [DOI] [PubMed] [Google Scholar]
- 23.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 24.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by rapamycin target mTOR. Curr. Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 25.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J. Thromb. Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang WY, Wu YC, Wu CC. Prevention of platelet glycoprotein IIb/IIIa activation by 3,4-methylenedioxy-beta-nitrostyrene, a novel tyrosine kinase inhibitror. Mol. Pharmacol. 2006;70:1380–1389. doi: 10.1124/mol.106.023986. [DOI] [PubMed] [Google Scholar]
- 27.Lai JY, Cox PJ, Patel R, Sadiq S, Aldous DJ, Thurairatnam S, Smith K, Wheeler D, Jagpal S, Parveen S, Fenton G, Harrison TK, McCarthy C, Bamborough P. Potent small molecule inhibitors of spleen tyrosine kinase (Syk). Bioorg. Med. Chem. Lett. 2003;13:3111–3114. doi: 10.1016/s0960-894x(03)00658-9. [DOI] [PubMed] [Google Scholar]
- 28.Barnes MJ, Knight CG, Farndale RW. The collagen-platelet interaction. Curr. Opin. Hematol. 1998;5:314–320. doi: 10.1097/00062752-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Nieswandt B, Watson SP. Platelet-collagen interaction: is GPVI the central receptor? Blood. 2003;102:449–461. doi: 10.1182/blood-2002-12-3882. [DOI] [PubMed] [Google Scholar]
- 30.Asselin J, Gibbins JM, Achison M, Lee YH, Morton LF, Farndale RW, Barnes MJ, Watson SP. A collagen-like peptide stimulates tyrosine phosphorylation of syk and phospholipase C gamma2 in platelets independent of the integrin alpha2beta1. Blood. 1997;89:1235–1242. [PubMed] [Google Scholar]
- 31.Su L, David M. Distinct mechanisms of STAT phosphorylation via the interferon-alpha/beta receptor. Selective inhibition of STAT3 and STAT5 by piceatannol. J. Biol. Chem. 2000;275:12661–12666. doi: 10.1074/jbc.275.17.12661. [DOI] [PubMed] [Google Scholar]
- 32.Pasquet JM, Gross B, Quek L, Asazuma N, Zhang W, Sommers CL, Schweighoffer E, Tybulewicz V, Judd B, Lee JR, Koretzky G, Love PE, Samelson LE, Watson SP. LAT is required for tyrosine phosphorylation of phospholipase c gamma2 and platelet activation by the collagen receptor GPVI. Mol. Cell Biol. 1999;19:8326–8334. doi: 10.1128/mcb.19.12.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross BS, Melford SK, Watson SP. Evidence that phospholipase C-gamma2 interacts with SLP-76, Syk, Lyn, LAT and the Fc receptor gamma-chain after stimulation of the collagen receptor glycoprotein VI in human platelets. Eur. J. Biochem. 1999;263:612–623. doi: 10.1046/j.1432-1327.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 34.Gross BS, Lee JR, Clements JL, Turner M, Tybulewicz VL, Findell PR, Koretzky GA, Watson SP. Tyrosine phosphorylaion of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets. J. Biol. Chem. 1999;274:5963–6971. doi: 10.1074/jbc.274.9.5963. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer LM, Mullersman JE, Pfeffer SR, Murti A, Shi W, Yang CH. STAT3 as an adaptor to couple phosphatidylinositol 3-kinase to the IFNAR-1 chain of the type 1 interferon receptor. Science. 1997;276:1418–1420. doi: 10.1126/science.276.5317.1418. [DOI] [PubMed] [Google Scholar]
- 36.Bulanova E, Budagian V, Orinska Z, Krause H, Paus R, Bulfone-Paus S. Mast cells express novel functional IL-15 receptor alpha isoforms. J. Immunol. 2003;170:5045–5055. doi: 10.4049/jimmunol.170.10.5045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.