Abstract
Objectives
Impaired insight into illness is a prevalent feature of schizophrenia, which negatively influences treatment adherence and clinical outcomes. Little is known about the effects of aging on insight impairment. We aimed to review the available research literature on the effects of aging on insight into illness in schizophrenia, in relation to positive, negative, and cognitive symptoms. Ultimately, we propose a trajectory of insight in schizophrenia across the lifespan.
Method
A systematic Medline® literature search was conducted, searching for English language studies describing the relationship of insight into illness in schizophrenia with aging.
Results
We identified 62 studies. Insight impairment is associated with illness severity, premorbid intellectual function (i.e. IQ), executive function, and memory. Insight impairment improves modestly during midlife, worsening again in late life. It tends to fluctuate with each episode of psychosis, likely in relation to worsening positive symptoms that improve with antipsychotic treatment. The relationship between insight impairment and cognitive dysfunction appears to attenuate with age, while the relationship with lower premorbid intellectual function is preserved. The association between impaired insight and negative symptoms is unclear.
Conclusions
The available literature suggests that the course of insight impairment follows a U-shaped curve, where insight impairment is severe during the first episode of psychosis, modestly improves over midlife, and declines again in late life. Future studies are required to investigate the trajectory of insight into illness and its core domains across the lifespan from prodromal phase to late life.
Keywords: age, aging, insight, schizophrenia, illness awareness, denial
Introduction
Schizophrenia is a chronic brain disorder characterized by impaired insight into illness. Insight into illness is recognized as a multidimensional construct consisting of having awareness of a mental disorder, its symptoms, its implications, and need for treatment (David, 1990; Lincoln et al., 2007). Moderate-to-severe insight impairment occurs in over 50% of patients with schizophrenia (Amador et al., 1994; Schwartz et al., 1997) and is associated with medication non-adherence and poor clinical and functional outcomes (Amador et al., 1994; Schwartz, 1998b; Lysaker et al., 2002; Smith et al., 2002; Buckley et al., 2007; Saravanan et al., 2010; Segarra et al., 2012). Moreover, impaired insight has limited responsiveness to pharmacological and psychological interventions, including cognitive behavioral therapy (CBT) and psychoeducation (Mojtabai et al., 1998; Pijnenborg et al., 2013). Although the significance of insight into illness is evident, it is poorly understood. Recently, insight into illness in schizophrenia has received more attention in the research literature (Mintz et al., 2003; Ouzir et al., 2012). Yet, despite the increasing number of older patients with schizophrenia (Cohen et al., 2000), the effects of aging on insight impairment, and to an even lesser degree, its role in late life, remain largely unexplored.
Insight into illness in schizophrenia appears to fluctuate with illness severity, worsening with an acute psychotic episode and improving with treatment and recovery (Schwartz, 1994; Weiler et al., 2000; Wiffen et al., 2010b; Parellada et al., 2011; Comparelli et al., 2013; Koren et al., 2013). Impaired insight is independently associated with illness severity and cognitive function, namely executive function, premorbid intellectual function (i.e. IQ), memory, and to a lesser degree, attention (Mintz et al., 2003; Aleman et al., 2006; Shad et al., 2006; Orfei et al., 2008; De Hert et al., 2009; Mohamed et al., 2009; Trevisi et al., 2012; Gerretsen et al., 2013b, 2013a; Mingrone et al., 2013). Positive symptoms attenuate and cognitive function appears to decline with age, while the effects of aging on negative symptoms are mixed (Cohen et al., 2000, 2008; Rajji and Mulsant, 2008; Rajji et al., 2013). As such, one's insight into illness may change as a function of the aging process.
The aims of this review are to discuss the following: (1) the multidimensional construct of insight into illness in schizophrenia; (2) the explanatory models of insight into illness and their relation to aging; (3) the effects of aging on the course of schizophrenia, in terms of the interaction among insight into illness and positive, negative, and cognitive symptoms; (4) the effects of episode of psychosis on changes in insight into illness; (5) the influence of phase of illness on insight into illness; (6) a proposed trajectory of insight into illness in schizophrenia across the lifespan; and (7) future directions.
Methods
A systematic Medline® literature search (1949–December 2013) was conducted, searching for English language case reports, studies, or reviews describing the relationship of insight into illness in schizophrenia with aging. The search was performed using the terms “schizophrenia” or “psychotic disorders” and “aging” or “age” (“age of onset” OR “Age factors” OR “Age distribution”), which were cross-referenced with the terms “insight,” “illness awareness,” “denial,” “awareness,” “anosognosia,” and “agnosia.” All MeSH terms were explored, and all subheadings were included. Reference sections were gleaned for relevant articles overlooked by the search strategy.
Results
This search identified 83 publications. All of the titles or abstracts were read by two of the authors (P. G. and E. P.), and 28 relevant papers were selected and reviewed. We identified an additional 57 articles from reviewing cited and citing articles. We retained all studies (n = 62) that reported on insight impairment in schizophrenia spectrum disorders and aging. The data are summarized in Table 1. The majority of cross-sectional studies did not find an association between insight into illness and age; however, results from the few longitudinal studies were mixed.
Table 1. Relationship between age and insight into illness in schizophrenia spectrum disorders.
| Authors, year, journal |
N | Dxa | Mean age (SD), rangeb |
Statusc | Measure of insight into illness |
Cross-sectional association |
Significant (Y/N) |
Longitudinal follow-up |
Longitudinal association |
Significant (Y/N) |
Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gerretsen et al., 2013a, Hum Brain Mapp | 52 | 2 | 41.5 (14.5), 23–77 | U | PANSS G12 | r = 0.12, p = 0.395, n.s. | N | N/A | N/A | – | |
| Gerretsen et al., 2013b, Schizophr Res | 50 | 1 | 65.2 (5.5), 60–79 | U | PANSS G12, BIS | PANSS G12, r = 0.05, p = 0.729, n.s.; BIS, r=−0.23,p = 0.117, n.s. | N | N/A | N/A | – | |
| Mingrone et al., 2013, Compr Psychiatry | 158 | 2 | 40.1 (10.8), ? | O | SUMD items 1–3 | SUMD 1,r=0.08, n.s.; SUMD 2, r=0.03, n.s.; SUMD 3, r=0.01, n.s. | N | N/A | N/A | – | |
| Braw et al., 2012, Eur Psychiatry | 66 | 3 | SZ, 26.8 (8.4), <60; bipolar, 43.9 (12.4), <60 | O | SUMD items and subscales, PANSS G12 | SUMD 1, r=−0.36, p< 0.01; SUMD 2, n.s.; SUMD 3, r=−0.30, p< 0.05; SUMD awareness of anhedonia asociality, r= −0.64, p< 0.001; PANSS G12, r= −0.32, p< 0.01 | Y | N/A | N/A | – | Differences in insight between bipolar and schizophrenia patients disappeared when age was used as a covariate. |
| Schennach et al., 2012, Eur Psychiatry | 399 | 3 | 35.4 (11.1), <65 | H | PANSS G12 | r= −0.01, p = 0.77 | N | Mean 64.7 days (SD = 46.5) | F-test, p = 0.88, n.s. | N | Age was not associated with changes in insight from admission to discharge among those characterized as “improved,” “worsened,” “unchanged,” or “no lack of insight.” |
| Xiang et al., 2012, Compr Psychiatry | 139 | 2 | 33.4 (9.8), <50 | O | ITAQ | r=−0.01, n.s. | N | N/A | N/A | – | |
| Ayesa-Arriola et al., 2011, Early Interv Psychiatry | 164 | 4, 1 | 27.3 (7.8), <60 | B | SUMD items 1–3 | t-test, n.s. | N | N/A | N/A | – | No difference in age between “good” and “poor” (>1) insight groups. |
| Gilleen et al., 2011, Schizophr Bull | 31 | 2 | 38.3 (10.4), 21–62 | B | SAI-E, SUMD item 1 | Weak association between age and SAI informant-rated treatment compliance | Y | N/A | N/A | – | |
| Lysaker et al., 2011, Compr Psychiatry | 65 | 1 | 46.3 (8.9), ? | O | SUMD items 1–3 | n.s. | N | N/A | N/A | – | |
| Parellada et al., 2011, Schizophr Bull | 53 | 4, 1 | 15.43 (1.95), <17 | B | SUMD items and subscales | N/A | – | 2 years | Baseline age and insight at 2 years: SUMD 1, r=−0.49, p< 0.01; SUMD 2, r= −0.33, p< 0.05; SUMD 3, r= −0.46, p<0.01; symp. awareness, r=−0.39, n.s.; symp. attribution, r=−0.17, n.s. | Y | |
| Buchy et al., 2010, Early Interv Psychiatry | 165 | 4, 3 | 22.5 (4.0), 14–30 | B | SUMD item 1 | F= 0.76, p = 0.56, n.s. | N | N/A | N/A | – | Five insight groups did not significantly differ in age. |
| Nakamae et al., 2010, Psychiatry Clin Neurosci | 47 | 2 | 53.0 (13.0), ? | H | SUMD general items | R2 n.s. | N | N/A | N/A | – | Age did not predict insight into illness. |
| Wiffen et al., 2010, Schizophr Res | 303 | 1 | Median 30.6, 18–62 | U | SAI-E | F= 2.31, p = 0.08, n.s. | N | 6 months | F = 2.77, p = 0.04 | Y | Age predicted insight improvement. |
| Wiffen et al., 2010, Clin Schizophr Relat Psychoses | 670 | 1 | 42 (14.0), 18–84 | U | PANSS G12 | F= 8.26, p< 0.0001 | Y | N/A | N/A | – | Oldest and youngest groups had less insight than midlife groups. |
| De hert et al., 2009, Eur Psychiatry | 1213 | 1 | 35.5 (11.9), 16–83 | H | PECC | Impaired insight into illness (AMI), r= 0.06, p = 0.042; impaired insight into symptom attribution (ASAMI), r= 0.08, p = 0.004; AMI parameter est. = 0.03, p = 0.263; ASAMI parameter est. = 0.004, p = 0.137 | Y | N/A | N/A | – | |
| Mohamed et al., 2009, Schizophr Bull | 1432 | 2 | 40.5 (11.1), 18–67 | B | ITAQ | r= 0.07, p< 0.01 | Y | N/A | N/A | – | |
| Parellada et al., 2009, Psychol Med | 110 | 4, 3 | 15.5 (?), 9–17 | B | SUMD items and subscales | n.s. | N | N/A | N/A | – | |
| Raffard et al., 2009, Psychiatry Res | 60 | 1 | 33.4 (9.5), 18–56 | O | SUMD items and subscales | SUMD 1, r= 0.00, n.s; SUMD 2, r= −0.14, n.s.; SUMD 3, r= −0.03, n.s.; symptom awareness, r=0.00, n.s.; symptom attribution, r= −0.06, n.s. | N | N/A | N/A | – | |
| Stefanopoulou et al., 2009, Psychiatr Q | 36 | 2 | 34.9 (9.8), 20–52 | H | ITAQ | n.s. | N | N/A | N/A | – | |
| Karow et al., 2008, Eur Arch Psychiatry Clin Neurosci | 59 | 1 | 34.7 (13.0), ? | H | BIS, SUMD, PANSS G12 | r=−0.17 to 0.08, n.s. | N | N/A | N/A | – | |
| Bassitt et al., 2007, Eur Arch Psychiatry Clin Neurosci | 50 | 2 | 31.7 (7.1), 18–50 | O | SUMD symptom awareness + symptom attribution | r= 0.05, p = 0.75, n.s. | N | N/A | N/A | – | |
| Sapara et al., 2007 Schizophr Res | 28 | 2 | 39.0 (10.5), 19–60 | O | SAI-E, BIS | r (controlling for duration of illness) = 0.25–0.23, p>0.20, n.s. | N | N/A | N/A | – | |
| Tirupati et al., 2007, Compr Psychiatry | 183 treated; 143 never treated | 2 | Treated patients, 44.4 (13.6), ?; never-treated patients, 46.9 (16.3), ? | O | PANSS G12 | Treated, r=0.37, p < 0.01; never treated, r= −0.05, n.s. | Y | N/A | N/A | – | |
| Haddock et al., 2006, Br J Psychiatry | 304 | 4, 3 | <22 years, 19.6 (1.6), ?; >21 years, 32.9 (9.9), ? | B | BIS | t-test, n.s. | N | 18 months | F = 3.88, p = 0.023 | Y | No difference in <22-year and >21-year groups in insight at baseline. There was an interaction between insight, age, and treatment at 18 months. |
| Lysaker et al., 2006, J Neuropsychiatry Clin Neurosci | 53 | 1 | 47.5 (9.1), ? | O | SUMD items 1–3 | n.s. | N | N/A | N/A | – | |
| McEvoy et al., 2006, Psychol Med | 251 | 4, 3 | 23.9 (4.7), 16–40 | B | ITAQ | r=0.16, p = 0.016 | Y | N/A | N/A | – | |
| Shad et al., 2006, Psychiatry Res | 14 | 4, 3 (antipsychotic naive) | 26.23 (7.5), ? | H | SUMD | Symptom awareness, β = 0.08; p = 0.78, n.s.; symptom attribution, β = 0.004; p = 0.99, n.s. | N | N/A | N/A | – | |
| Simon et al., 2006, Cogn Neuropsychiatry | 38 | 2 | 24 (7), 16–38 | H | SUMD items and subscales factor analysis | n.s. | N | N/A | N/A | – | |
| Donohoe et al., 2005, J Nerv Ment Dis | 30 | 2 | ? (?), <55 | O | BIS | t-test, n.s. | N | N/A | N/A | – | No difference in age between “poor” and “good” (>9) insight groups |
| Keshevan et al., 2004 Schizophr Res | 535 | 4, 1 | 16–45 | U | PANSS G12 | F = 0.10, p = 0.75, n.s. | N | N/A | N/A | No difference in insight impairment between age groups | |
| Nakano et al., 2004, Psychiatry Res | 37 | 2 | 53 (10), 33–75 | H | SAI-Japanese | SAI-J total, r= −0.21, n.s.; SAI 1 (treatment acceptance), r= −0.40, p< 0.05; SAI 2 (illness awareness), r = −0.11, n.s.; SAI 3 (symptom awareness/attribution), r= −0.17, n.s. | Y | N/A | N/A | – | |
| Shad et al., 2004, Neuroimage | 35 | 4, 1 | Good insight, 25.4 (7.8), N/A; poor insight, 26.1 (6.7), N/A | H | HDRS insight item | t = −0.31, p = 0.75, n.s. | N | N/A | N/A | – | |
| Arduini et al., 2003, Can J Psychiatry | 42 SZ; 22 bipolar | 3 | SZ, 37.4 (12.2), ?; bipolar, 36.7 (11.8), ? | H | SUMD items 1–3 | n.s. | N | N/A | N/A | – | |
| Rossell et al., 2003, Psychol Med | 78 | 2 | 33.7 (8.50), <55 | B | SAI-E | n.s. | N | N/A | N/A | – | |
| Yen et al., 2002, J Nerv Ment Dis | 44 SZ; 33 psychotic bipolar; 32 nonpsychotic bipolar | 3 | SZ, 33.8 (9.9), 19–61; psychotic bipolar, 33.5 (12.4), 16–64; nonpsychotic bipolar, 41.2 (11.1), 21–71 | O | SAI-E total | β =0.02, n.s. | N | N/A | N/A | – | Age did not predict insight into illness. |
| Goldberg et al., 2001, J Nerv Ment Dis | 211 | 3 | 41 (8.6), 21–69 | B | PANSS G12 | β =−0.01, n.s. | N | N/A | N/A | – | |
| Pyne et al., 2001, J Nerv Ment Dis | 177 | 2 | 34.5 (8.7), 18–54 | B | Awareness of mental illness Likert scale | OR = 2.98, p< 0.05 for illness denial if <30 years | Y | N/A | N/A | – | |
| Flashman et al., 2000, A J Psychiatry | 30 | 1 | Aware, 36.4 (14.9), ?; unaware, 33.9 (9.9), ? | B | SUMD | t = 0.55, p = 0.59, n.s. | N | N/A | N/A | – | |
| Laroi et al., 2000, Psychiatry Research | 21 | 1 | 36 (10.2), <60 | B | SUMD items and subscale total | n.s. | N | N/A | N/A | – | |
| Marks et al., 1995, Schizophr Res | 59 | 2 | 42.7 (10.8), ? | O | SAIQ | SAIQ tot, n.s.; worry, n.s.; need for treatment, r = 0.32, p<0.05; illness presence/outcome, r= 0.31, p< 0.05. Neither significant after controlling for length of illness | Y | N/A | N/A | – | |
| Weiler et al., 2000, Schizophr Res | 81 SZ; 14SA | 1 | SZ, 37.3 (8.4); SA, 35.6 (14.5) | H | ITAQ | n.s. | N | N/A | N/A | – | |
| Carroll et al., 1999 Schizophr Res | 110 | 2 | 35.6 (10.9), <64 | B | ITAQ | r=0.05, n.s. | N | N/A | N/A | – | |
| Schwartz et al., 1998, Compr Psychiatry | 66 | 2 | 42.0 (6.7), 21–53 | O | SUMD items 1–3 | R2 n.s. | N | N/A | N/A | – | Age did not predict nsight into illness. |
| Collins et al., 1997, Schizophr Res | 58 | 2 | 34.1 (8.0), ? | O | SAI | r=0.32, p = 0.01; β = 0.19, p = 0.091, n.s. | N | N/A | N/A | – | |
| Dickerson et al., 1997, Psychiatr Serv | 87 | 1 | 39.4 (9.9), <65 | O | PANSS G12 | n.s. | N | N/A | N/A | – | |
| Kim et al., 1997, Compr Psychiat | 63 | 2 | 38.2 (13.3), 20–61 | B | SAI | SAI-J total, r= −0.21, n.s.; SAI 1 (treatment acceptance), r= −0.28, p = 0.060; SAI 2 (illness awareness), r= −0.23, p = 0.113; SAI 3 (symptom awareness/attribution), r= −0.37, p = 0.010 | Y | N/A | N/A | – | |
| Schwartz et al., 1997, Compr Psychiatry | 23 | 2 | 40.1 (8.1), 20–52 | H | Modified total SUMD score | r= 0.07, n.s. | N | N/A | N/A | – | |
| Almeida et al., 1996, Int J Geriatr Psychiatry | 40 | Late paraphrenia | ? (?), >55 years | B | SAI | n.s. | N | N/A | N/A | – | |
| Cuffel et al., 1996, J Nerv Merit Dis | 89 | 2 | 38.9 (7.1), <55 | O | Awareness of illness interview | r= 0.08, n.s. | N | N/A | N/A | – | |
| Fennig et al., 1995, Schizophr Res | 309 | 3 | ? (?), <60, 52.4% under 29 years old. | H | HDRS insight item | n.s. | N | N/A | N/A | – | |
| Macpherson et al., 1996, Br J Psychiatry | 64 | 2 | ? | H | SAI | r= −0.19, p = 0.14 | N | N/A | N/A | – | |
| Aga et al., 1995, Indian J. Psychiatry | 59 | 2 | 35.4 (10.5), 18–55 | H | SAI | t-test, n.s. | N | N/A | N/A | – | No difference in impaired insight between “low” (<66% max score) and “high” insight groups. |
| Cuesta et al., 1995, Am J Psychiatry | 52 | 3 | 30.8 (7.9), 19–45 | H | Lack of insight index (3 items from AMDP) | n.s. | N | N/A | N/A | – | |
| David et al., 1995. Br J Psychiatry | 150 | 3 | 26.4 (6.5), 16–50 | B | PSE | β = 0.12, p = 0.2, n.s. | N | N/A | N/A | – | Age did not predict insight into illness. |
| Kemp and Lambert, 1995, Schizophr Res | 29 | 2 | 28.4 (7.0), 16–45 | H | Modified SUMD symptom awareness and attribution | N/A | – | 3–6 weeks | n.s. | N | Age did not correlate with improvements in insight. |
| Lysaker and Bell, 1995, J Nerv Ment Dis | 44 | 1 | 45 (10), ? | B | PANSS G12 | N/A | – | 26 weeks | n.s. | N | Age did not correlate with changes in insight. |
| Amador et al., 1994, Arch Gen Psychiatry | 221 SZ; 49 SA | 1 | SZ, 34.4 (11.2), ?; SA, 33.6 (12.1), ? | U | SUMD items and subscales | n.s. | N | N/A | N/A | – | |
| Cuesta and Peralta, 1994, Schizophr Bull | 40 | 2 | 27.7 (7.5), 17–46 | H | Lack of insight index (3 items from AMDP) | n.s. | N | N/A | N/A | – | |
| Amador et al., 1993, Am J Psychiatry | 43 | 1 | 31.2 (8.8), ? | H | SUMD items and subscales | n.s. (no statistics provided) | N | N/A | N/A | – | |
| Young et al., 1993, Schizophr Res | 31 | 2 | 38.4 (7.3), 25–53 | B | SUMD symptom awareness | t-test, n.s. | N | N/A | N/A | No difference in age between “low” and “high” insight groups | |
| David et al., 2007b, Br J Psychiatry | 91 | 3 | 31.4 (9.8), <65 | B | PSE | r<0.15, n.s. | N | N/A | N/A | – |
Dx, diagnosis; PANSS, Positive and Negative Syndrome Scale; BIS, Birchwood Insight Scale; SUMD, Scale to Assess Unawareness of Mental Disorder; ITAQ, Insight and Treatment Attitudes Questionnaire; SAI-E, Schedule for the Assessment of Insight—Expanded version; PECC, Psychosis Evaluation Tool for Common Use by Caregivers; HDRS, Hamilton Rating Scale for Depression; SAIQ, Self-Appraisal of Illness Questionnaire; PSE, Present State Examination.
1 = schizophrenia (SZ), schizoaffective (SA), and/or schizophreniform; 2 = schizophrenia only; 3 = unspecified/mixed psychoses; and 4 = first-episode psychosis.
?, indicates data not specified.
Hospitalized (H), out-patient (O), both (B), or unspecified (U).
Insight into illness in schizophrenia: a multidimensional construct
Insight into illness in schizophrenia has evolved from the dichotomous notion of being “present” or “not present” to a multidimensional construct that exists on a continuum (David, 1990). There are several different definitions of insight into illness, and although not exactly alike, they generally share their acknowledgement of four core domains: (1) awareness of having a severe brain disorder; (2) awareness and appropriate attribution of symptoms to mental illness; (3) acceptance of the need for treatment, most commonly with an antipsychotic medication; and (4) awareness of social, occupational, legal, or other negative consequences attributable to the mental disorder (David, 1990; Orfei et al., 2008). Intriguingly, awareness in one domain does not ensure awareness in another domain (Bota et al., 2006).
A number of instruments are available for the quantitative measurement of insight into illness and its domains, including single-item measures that provide a global assessment of insight into illness and multiple-item measures that can be divided into clinician-rated (McEvoy et al., 1989; Amador and Strauss, 1990; David, 1990; Medalia and Thysen, 2008) and self-report scales (Markova and Berrios, 1992; Selten et al., 1993; Birchwood et al., 1994; Hayashi et al., 1999; Marks et al., 2000; Beck et al., 2004).
Explanatory models of impaired insight in schizophrenia
There are generally four explanatory models of impaired insight into illness in schizophrenia: (1) psychological; (2) cognitive or neuropsychological; (3) clinical/psychopathological; and (4) neuroanatomical. A fifth, (5) lack of information/education, may also play an influential role in illness awareness deficits in patients with schizophrenia.
According to the (1) psychological model, impaired insight into illness, or illness denial, serves as a defense mechanism that acts as a coping strategy for the emotional consequences of acknowledging a severe mental illness (McGlashan and Carpenter, 1976; Moore et al., 1999). Although results are mixed, some research has shown that greater insight into illness is associated with hopelessness, lower self-esteem, depression, and suicidal ideation, along with other negative effects (Carroll et al., 1999, 2004; Cunningham Owens et al., 2001; Drake et al., 2004; Cooke et al., 2007a, 2007b). This model of illness denial appears to be applicable across the lifespan with arguably greater influence in the early phases of schizophrenia, where there exists the strongest risk for depression and suicide in relation to developing insight into illness (Melle and Barrett, 2012).
The (2) cognitive/neuropsychological model suggests that an underlying cognitive dysfunction mediates impaired insight. The greatest evidence in support of this theory is the association between insight impairment and executive dysfunction, especially set-shifting, as measured by the Wisconsin Card Sorting Test or Trail Making Test B, and lower premorbid intellectual function (Young et al., 1993; Lysaker and Bell, 1994; Keshavan et al., 2004; Aleman et al., 2006; Shad et al., 2006; Nair et al., 2014). Impaired insight, according to this model, is thought to result from mental rigidity, faulty error monitoring, and the inability to entertain alternative hypotheses for the etiology of delusional experiences. Memory and attention deficits have also been associated with impaired insight (Voruganti et al., 1997; Keshavan et al., 2004; Nair et al., 2014). The effects of aging on cognition will be discussed later (see Section on Aging, cognitive function, and insight into illness).
The related construct of “cognitive insight” proposes that one's degree of self-certainty and self-reflectiveness dictate one's capacity for illness awareness (Beck et al., 2004). Studies on the whole suggest that cognitive insight explains at most a modest portion of the variance of insight into illness (Pedrelli et al., 2004; Warman et al., 2007; Uchida et al., 2009b; Greenberger and Serper, 2010; Nair et al., 2014). To understand this, individuals with schizophrenia may have significant rigidity about their illness beliefs, but relatively preserved mental flexibility and self-reflectiveness in other domains.
The (3) clinical/psychopathological model considers impaired insight to be a product of the severity of one's clinical psychopathology, in particular positive symptoms (Cuesta and Peralta, 1994; Collins et al., 1997; Cuesta et al., 2006). More frequent, intense hallucinations, greater delusional severity, and duration of untreated psychosis lead to greater insight impairment (Sevy et al., 2004; Parellada et al., 2011) (see Section on Aging, Positive Symptoms, and Insight into Illness).
The (4) neuroanatomical model posits that structural alterations underlie insight impairment. Anosognosia or impaired illness awareness can occur with right hemisphere brain lesions secondary to stroke, traumatic brain injury, and dementia (Orfei et al., 2008). To explain the role of the right hemisphere in illness awareness, the cerebral hemispheres are thought to serve distinct functions when confronted with discrepant cognitive or sensory stimuli (Ramachandran et al., 2007). Impaired illness awareness in these contexts is thought to arise from interhemispheric imbalance and serves as a model for understanding impaired insight in other neuropsychiatric disorders, such as schizophrenia (Ramachandran, 1995; Ramachandran et al., 2007; Shad et al., 2007).
Volume-based analyses using a region of interest approach support the neuroanatomical model of impaired insight by reporting reduced right hemisphere volume in the right frontal lobe, including the orbitofrontal cortex, dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortices (Flashman et al., 2001; Shad et al., 2004, 2006), and the right parietal lobe (Shad et al., 2007). However, voxel-based morphometry studies using a whole brain approach have produced mixed results (Ha et al., 2004; Bassitt et al., 2007; Cooke et al., 2008; Morgan et al., 2010; Berge et al., 2011). A more recent study, exploring hemisphere asymmetry, found relatively less right hemisphere volume in the DLPFC, and parietal and anteroinferior temporal lobe in relation to impaired insight (Gerretsen et al., 2013a). In a study of first-episode patients, cortical thickness, but not voxel-based morphometry, was associated with regional thinning in the left middle frontal and inferior temporal gyri (Buchy et al., 2011). Other studies have associated impaired insight with the following: reduced brain volume, particularly within the frontal and parietal regions (Flashman et al., 2000; Laroi et al., 2000; Sapara et al., 2007; Parellada et al., 2011); reduced white matter volume (Gerretsen et al., 2013a); white matter deficits as measured with diffusion tensor imaging (Antonius et al., 2011); and right posterior insula structural alterations (Palaniyappan et al., 2011). Age-related cerebral atrophy may enhance the relationship between structural changes and impaired insight, particularly in late life, where there may be greater insight impairment than in midlife (Wiffen et al., 2010b). Studies are required to test this hypothesis.
A final explanation for impaired insight in schizophrenia is the notion that (5) lack of information/education contributes to unawareness of illness. According to this model, patients often do not have the knowledge base required to score high on assessments of insight into illness. Upon gaining information about schizophrenia, insight into illness may improve in a subset of patients (Chan et al., 2009; Aho-Mustonen et al., 2011; Pijnenborg et al., 2013).
None of these models seem to explain impaired insight in its entirety; at the same time, they are not mutually exclusive. All models may coexist and contribute to a fuller understanding of the complex construct of insight into illness. For example, the functional aspects of illness denial as a defense mechanism may involve the same brain structures that are damaged in anosognosia secondary to a cerebral lesion.
The interaction among aging, symptoms, and insight into illness in schizophrenia
Aging, positive symptoms, and insight into illness
Impaired insight into illness is consistently associated with severity of positive symptoms in a number of cross-sectional studies (David et al., 1992; Amador et al., 1993; Young et al., 1993; Collins et al., 1997; Schwartz, 1998a; Chan et al., 2012), longitudinal studies (Weiler et al., 2000; Saeedi et al., 2007) and a meta-analytic review (Mintz et al., 2003). However, some studies have failed to find a relationship between positive symptoms and insight into illness (Cuesta and Peralta, 1994; Smith et al., 1998; Carroll et al., 1999; Braw et al., 2012).
The research literature suggests that positive symptoms improve with age in schizophrenia (McGlashan and Fenton, 1993; Davidson et al., 1995; Cohen et al., 2000, 2008; Harrison et al., 2001; Jeste et al., 2003; Bankole et al., 2008; Shepherd et al., 2012). In a sample 40 to 85 years, there was a trend towards improvement of positive symptoms in older patients with schizophrenia (Jeste et al., 2003). The decline in positive symptoms is proposed to represent “burnout” (Jeste et al., 2003), resulting from age-related degeneration of the dopaminergic system (Schultz et al., 1997), countering the hyperdopaminergic state hypothesized to cause psychosis (Howes and Kapur, 2009). Dopaminergic neuronal and receptor loss may manifest in a reduction of positive symptom severity. Age-related decreases in striatal D2 receptors (Volkow et al., 1996; Kaasinen et al., 2000) likely contribute to the increased antipsychotic drug sensitivity of older adults (Jeste et al., 2003; Uchida et al., 2009a; Leon et al., 2010) and the need for lower doses to achieve remission of positive symptoms. The observation of age-related improvement in positive symptoms is used to argue that schizophrenia does not follow a debilitating course, as in Alzheimer's disease or other dementias, with clinical deterioration usually limited to the first 5 years after onset (Lieberman, 1999; Levine et al., 2011). There exists, however, heterogeneity among different illness course trajectories, with a minority of patients deteriorating over time (Harding, 1988; Olesen and Mortensen, 2002; Rabinowitz et al., 2007).
Overall, there appears to be an improvement of insight into illness with age, at least during midlife, in conjunction with the attenuation of positive symptoms that may be related, in part, to dopaminergic system “burnout.” It is important to note that longitudinal data on the effects of aging on insight into illness and its relationship to symptomatology are, for the most part, limited to samples less than 65 years.
Aging, negative symptoms, and insight into illness
The relationship among aging, insight into illness, and negative symptoms is unclear, seemingly as a result of the inconsistent association between negative symptoms and aging, and similarly, negative symptoms and impaired insight.
Positive symptoms have traditionally characterized the early course of schizophrenia with negative symptoms emerging later in life, dominating the clinical picture with age (Pfohl and Winokur, 1982; McGlashan and Johannessen, 1996). However, explorations of the literature on the effects of age on negative symptoms suggest that findings are mixed. In earlier reports, negative symptom severity was observed to increase with age (Soni and Mallik, 1993; Davidson et al., 1995; Gur et al., 1996). Conversely, subsequent and more recent studies have indicated that negative symptoms stabilize or attenuate with age (Schultz et al., 1997; Jeste et al., 2003; Cohen et al., 2013). One review reported that negative symptoms were prevalent in 50–90% of first-episode patients, with only 20–40% of patients experiencing persistent negative symptoms that remained stable in long-term follow-up studies (Makinen et al., 2008).
The findings of studies reporting on the relationship between impaired insight and negative symptoms are also mixed. Impaired insight and negative symptoms are modestly associated in a number of cross-sectional studies (Nakano et al., 2004; De Hert et al., 2009; Braw et al., 2012; Chan et al., 2012) and longitudinal studies (Kemp and Lambert, 1995; Saeedi et al., 2007). Similarly, the results of a meta-analysis found a modest but significant relationship between impaired insight and negative symptoms (Mintz et al., 2003). Conversely, other cross-sectional (Amador et al., 1994; Collins et al., 1997; Schwartz, 1998a) and longitudinal studies (Smith et al., 1998; Carroll et al., 1999) have failed to find a relationship between impaired insight and negative symptoms.
Aging, cognitive function, and insight into illness
Impaired insight is negatively correlated with cognitive function in cross-sectional (Young et al., 1993; Drake and Lewis, 2003; Goodman et al., 2005) and longitudinal studies (Lysaker and Bell, 1994). Other studies, however, have failed to find a relationship between cognitive function and insight into illness (Cuesta and Peralta, 1994; David et al., 1995; Kemp and David, 1996; Collins et al., 1997; Aleman et al., 2002; Cuesta et al., 2006). In two comprehensive meta-analyses, global cognitive function, premorbid intellectual function (i.e. IQ), executive function, and memory were the cognitive domains associated with insight into illness (Aleman et al., 2006; Nair et al., 2014).
The normal aging process is associated with cognitive decline, particularly with attention and executive function, while the relationship with premorbid intellectual function is preserved (Hedden and Gabrieli, 2004; Dickinson and Hiscock, 2010). Reports on the course of cognitive function are mixed in schizophrenia and may depend on the sample studied. Individuals with schizophrenia tend to score 1 to 3 standard deviations below normal healthy controls on tests of cognition (Saykin et al., 1991; Wilk et al., 2004; Frangou, 2010; Rajji et al., 2013) and have lower premorbid intellectual function as assessed with measures of IQ prior to the onset of the first episode of psychosis (Woodberry et al., 2008). Some studies report that cognitive function progressively declines (Bilder et al., 1992; Sheitman et al., 2000), while other studies suggest that cognitive function remains stable, especially among community-dwelling patients (Gold et al., 1999; Heaton et al., 2001; Kurtz, 2005; Keefe et al., 2006; Bonner-Jackson et al., 2010). In studies of community-dwelling patients, age-related cognitive decline occurs at a similar rate to healthy controls (Jeste et al., 2003; Rajji et al., 2013). However, as a result of having less cognitive reserve, individuals with schizophrenia may experience “premature aging” (Rajji et al., 2013). By comparison, the rate of cognitive decline is accelerated in institutionalized patients and in those with more severe psychopathology (Kurtz, 2005; Rajji and Mulsant, 2008). This suggests that these patients may have less “cognitive reserve” than community-dwelling patients or the possibility of a neurodegenerative process (Cohen et al., 2008).
Recent preliminary research suggests that aging in late life (greater than 60 years) attenuates the relationship between insight impairment and measures of cognitive dysfunction seen in younger samples, while its relationship with premorbid intellectual function is preserved (Gerretsen et al., 2013b). In contrast to the modest improvement in insight into illness observed in younger adults, a cross-sectional study that measured insight at different points across the lifespan found the greatest insight impairment in the >56 years group (Wiffen et al., 2010a). The reason for this decline may be related to the effects of “premature aging”; however, this study did not assess cognition.
The effects of medications in late life, particularly high doses of antipsychotics and the use of drugs with anticholinergic properties, may contribute to cognitive impairment and possibly insight impairment (Leon et al., 2010; Gerretsen and Pollock, 2013). The effects of drugs on insight impairment remain to be studied.
Insight into illness and episode of psychosis
To understand the relationship between aging and insight into illness, it is important to distinguish it from its relation to an episode of psychosis. At any point across the lifespan, a patient with schizophrenia can experience a new psychotic episode or an exacerbation of preexisting psychotic symptoms, during which insight may fluctuate considerably. During a psychotic episode, insight impairment tends to worsen in conjunction with worsening psychosis and then improves with hospitalization and initiation of antipsychotic treatment (David et al., 1995; Kemp and Lambert, 1995; Smith et al., 1998; Weiler et al., 2000; Parellada et al., 2011; Schennach et al., 2012; Koren et al., 2013). With recovery, insight into illness appears to either remain stable or continue to improve. The greatest degree of insight improvement may occur during the acute phase of treatment and may be influenced by multiple factors, including psychosocial stressors, medication adherence, IQ, age, duration of untreated psychosis, severity, and phase of illness (Buckley et al., 2007; Wiffen et al., 2010b; Parellada et al., 2011; Quee et al., 2011).
Insight into illness and phase of illness
We are not aware of any cohort studies that have measured insight impairment longitudinally over the course of schizophrenia from the prodromal phase or first episode of psychosis to late life. However, the progression of insight impairment at each phase of illness (i.e. prodromal, first episode of psychosis, and chronic, including late life) may be characteristically different, providing some clarity regarding the effects of aging. A caveat to this approach is the likelihood that some, although few, patients may experience their first episode of psychosis in mid-to-late life. The following section discusses the course of insight into illness during the different phases of schizophrenia.
Prodromal phase of schizophrenia
Little is known about the relationship between impaired insight into illness and the prodromal phase of schizophrenia. Prior to the onset of the first episode of psychosis, patients with schizophrenia experience subsyndromal symptoms (e.g. ideas of reference, persecutory ideation, and perceptual disturbances) for which they have greater capacity for mental flexibility, discernment, and insight than during subsequent phases of psychosis. We are aware of just one study that has explored insight into illness during the prodrome of schizophrenia, which consisted of a retrospective analysis of 24 participants diagnosed with schizophrenia within a 2-year period (Bota et al., 2006). The investigators reported that insight into illness is usually maintained during the initial perceptual disturbance phase yet dissipates later in the prodrome as the experiences become delusional. As would be expected, the average age of these subjects at the time of first diagnosis was mid-20s (25 ± 9.5 years); however, no age differences were reported between the “insight” and “no insight” groups.
First-episode schizophrenia
Longitudinal studies tracking insight into illness in patients with first-episode psychosis report that insight into illness improves at follow-up visits (Fennig et al., 1996; Mintz et al., 2004; Sim et al., 2006; Saeedi et al., 2007; Parellada et al., 2009; Saravanan et al., 2010; Segarra et al., 2012; Capdevielle et al., 2013). A 5-year longitudinal study reported continuous improvement in insight into illness measurements at 6months, 1 year, and 5 years (Johnson et al., 2012). In another longitudinal study, insight into illness improved at 1-year follow-up yet failed to further improve during the subsequent year (Parellada et al., 2011), suggesting greatest improvement during the acute phase of treatment and hospitalization.
Midlife chronic phase of schizophrenia
During the chronic phase in midlife, individuals with schizophrenia appear to have greater insight into illness than during the first episode of psychosis. Studies that have compared multiple-episode versus first-episode patients have reported more awareness of having a mental illness in the multiple-episode group (Thompson et al., 2001; Schennach et al., 2012; Koren et al., 2013). Explanations for this difference included the following: acceptance of illness by chronic patients (psychological model); greater psychological defensiveness by the first-episode patients (psychological model); lack of education or knowledge about schizophrenia during the first episode of psychosis (psychoeducation model); and a longer time undergoing treatment (clinical/psychopathological model). In one study, although the multi-episode group had greater insight into illness at the time of admission, first-episode patients had significantly greater improvement in insight during the acute phase of treatment and at the time of discharge (Schennach et al., 2012), which suggests that first-episode patients (and possibly younger patients) may have a greater capacity for developing insight into illness than those in later phases of schizophrenia. This idea is supported by another study, in which younger patients experienced greater changes in insight into illness during a psychotic episode (Chen et al., 2001).
A number of cross-sectional studies whose samples consisted primarily of subjects during the chronic phase of schizophrenia have reported a modest-to-moderate association between age and insight into illness (Mohamed et al., 2009; Braw et al., 2012), while others have failed to find such a relationship (Amador et al., 1993; David et al., 1995; Gerretsen et al., 2013b). These mixed findings among cross-sectional studies are confounded within this phase of illness by heterogeneous sampling, possibly including first-episode and late-life patients, schizoaffective disorder patients, and combining hospitalized (institutionalized) and community-dwelling patients.
Late-life schizophrenia
There exists a paucity of literature that tracks insight into illness in late life. Of the aforementioned studies, few included subjects greater than 60 years, thus failing to adequately assess the effects of aging on insight into illness in late life. One cross-sectional study included patients up to 84 years and separated subjects into four age groups (Wiffen et al., 2010a). The greatest insight impairment was found in the >56 years group, followed by the 18–28 years group. Insight into illness was significantly better in the two intermediate groups. These findings, although preliminary, suggest that insight impairment is severe with the onset of psychosis, modestly improves over midlife, and worsens again in late life.
The decline in insight into illness in late life may be a function of the premature aging observed in schizophrenia. Although age-related cognitive decline occurs at a similar rate between patients with schizophrenia and healthy controls, functional impairment tends to occur earlier because of lower cognitive reserve (Rajji and Mulsant, 2008; Rajji et al., 2013).
Proposed trajectory of insight into illness
Based on the literature outlined in the previous sections, we propose that the course of insight impairment follows a U-shape trajectory (Figure 1). During adolescence and early adulthood, when prodromal and first episode of psychosis phases typically occur, insight impairment progresses with the emergence of psychosis (Mintz et al., 2004; Bota et al., 2006; Saeedi et al., 2007; Parellada et al., 2009, 2011). During hospitalization and treatment with antipsychotic medication, insight into illness tends to improve during the recovery period (Weiler et al., 2000; Johnson et al., 2012; Schennach et al., 2012; Segarra et al., 2012).
Figure 1.
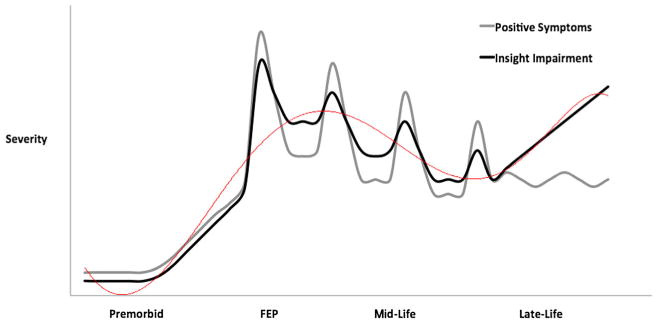
Theoretical trajectory of insight impairment and positive symptom severity across the course of schizophrenia (red line—insight impairment trend line).
With the exception of episodic worsening during recurrent psychotic episodes, there is a modest improvement in insight into illness throughout midlife, during the chronic phase of schizophrenia (Thompson et al., 2001; Schennach et al., 2012; Koren et al., 2013). Possible explanations for this improvement include the following: a longer duration of treatment; amelioration of psychotic symptoms; acceptance of one's condition; acquisition of knowledge about the illness; and improved coping techniques (Koren et al., 2013).
The trajectory of insight into illness into late life remains unclear, although limited evidence suggests that it may decline during this phase. This may be due to a longer duration of illness (Wiffen et al., 2010b); however, if this were the case, one would expect a decline in midlife rather than the observed improvement in insight into illness. We propose that worsening insight impairment in late life may, in part, be explained by the cognitive decline associated with premature aging (Figure 2). Although cognitive decline in patients with schizophrenia appears to progress at a similar rate in comparison with healthy individuals, patients with schizophrenia have less cognitive reserve and accompanying neuroanatomical alterations (e.g. cerebral atrophy and decreased neuroreceptor density) that may lead to premature aging (Rajji et al., 2013). Insight impairment in late life may also be influenced by the sample studied, as the severity of psychopathology and illness trajectory are worse in institutionalized versus community-dwelling patients with schizophrenia (Rajji and Mulsant, 2008). Longitudinal studies that include participants with late-life schizophrenia are required to test this hypothesis.
Figure 2.
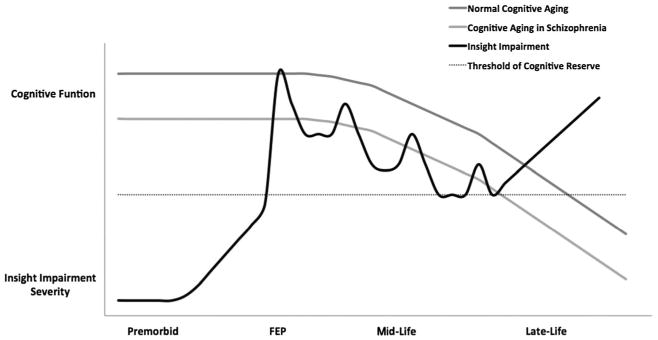
Theoretical trajectory of insight impairment in relation to cognition across the course of schizophrenia.
Conclusion and future directions
Impaired insight into illness is a common feature of schizophrenia, which negatively influences treatment adherence and clinical outcomes. Our systematic review of the literature suggests that insight impairment is associated with illness severity, duration of untreated psychosis, lower premorbid intellectual function (i.e. IQ), executive dysfunction, and memory deficits. The course of insight impairment appears to follow a U-shaped curve (Figure 1), where insight impairment increases with the first episode of psychosis, decreases over midlife, and increases again in late life. Insight impairment also tends to fluctuate with each episode of psychosis, likely in relation to worsening positive symptoms that improve with antipsychotic treatment. Preliminary evidence suggests that the relationship between impaired insight and measures of cognitive dysfunction appears to attenuate with age, while the relationship with premorbid intellectual function is preserved. The association between impaired insight and negative symptoms is unclear.
This review has a number of limitations: First, many relevant articles may have been omitted from our Medline® search. For most studies, although they may comment on the relationship between aging and insight impairment, the effects of aging were not their primary aim. Second, lack of longitudinal studies that track insight impairment over a significant period of time renders it challenging to make generalizations about the effects of aging. Third, the negligible representation of older adults with schizophrenia in the insight literature serves as a barrier to truly understanding the effects of aging on insight into illness in late life.
Insight into illness is recognized as a multidimensional construct that usually consists of awareness of having a mental disorder, its symptoms, its implications, and need for treatment; however, little-to-nothing is known about the effects of aging on these core domains, which may follow different courses over the lifespan. We suggest a longitudinal study to track insight into illness and its core domains in relation to other clinical features (e.g. positive, negative, and cognitive symptoms; treatment adherence; and functional outcomes) from early to late life in patients with schizophrenia. Naturally, the impracticality of such a study poses difficulties. As such, a more pragmatic approach would be to utilize a cross-sectional methodology, including samples from across the lifespan from prodromal phase to late life. A greater understanding of the course and correlates of insight at each phase of illness across the lifespan may help us better understand a complex phenomenon with significant implications for treatment adherence and treatment outcomes. The ultimate clinical application of gaining insight into illness is developing the capacity to consent to treatment. Future research may demonstrate that different strategies at different phases of illness may yield better clinical results. For example, psychoeducation and CBT for psychosis may be of greater benefit in younger patients to improve insight into illness, while neurostimulation and cognitive remediation strategies may be indicated for those in later life.
Key points.
Impaired insight into illness appears to follow a U-shaped trajectory, where insight impairment is severe during the first episode, modestly improves over mid-life, and declines again in late-life.
Insight impairment fluctuates during each episode of psychosis, likely in accordance with positive symptoms.
The association between insight impairment and cognitive dysfunction attenuates with age, while the relationship with premorbid intellectual function is preserved.
Future studies are required to investigate the trajectory of insight into illness and its core domains across the lifespan from prodromal phase to late life.
Footnotes
Names of the institutions at which the research was conducted: Centre for Addiction and Health and University of Toronto.
Conflict of interest: None declared.
References
- Aga VM, Agarwal AK, Gupta SC. The relationship of insight to psychopathology in schizophrenia: a cross-sectional study. Indian J Psychiatry. 1995;37:129–135. [PMC free article] [PubMed] [Google Scholar]
- Aho-Mustonen K, Tiihonen J, Repo-Tiihonen E, et al. Group psychoeducation for long-term offender patients with schizophrenia: an exploratory randomised controlled trial. Crim Behav Ment Health CBMH. 2011;21:163–176. doi: 10.1002/cbm.788. [DOI] [PubMed] [Google Scholar]
- Aleman A, de Haan EH, Kahn RS. Insight and neurocognitive function in schizophrenia. J Neuropsychiatry Clin Neurosci. 2002;14:241–242. doi: 10.1176/jnp.14.2.241. [DOI] [PubMed] [Google Scholar]
- Aleman A, Agrawal N, Morgan KD, David AS. Insight in psychosis and neuro-psychological function: meta-analysis. Br J psychiatry J Ment Sci. 2006;189:204–212. doi: 10.1192/bjp.189.3.204. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Levy R, Howard RJ, David AS. Insight and paranoid disorders in late life (late paraphenia) Int J Geriatr Psychiatry. 1996;11:653–658. [Google Scholar]
- Amador X, Strauss D. The scale to assess unawareness of mental disorder (SUMD) Columbia University and. New York State Psychiatric Institute; New York: 1990. [Google Scholar]
- Amador XF, Strauss DH, Yale SA, et al. Assessment of insight in psychosis. Am J Psychiatry. 1993;150:873–879. doi: 10.1176/ajp.150.6.873. [DOI] [PubMed] [Google Scholar]
- Amador XF, Flaum M, Andreasen NC, et al. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry. 1994;51:826–836. doi: 10.1001/archpsyc.1994.03950100074007. [DOI] [PubMed] [Google Scholar]
- Antonius D, Prudent V, Rebani Y, et al. White matter integrity and lack of insight in schizophrenia and schizoaffective disorder. Schizophr Res. 2011;128:76–82. doi: 10.1016/j.schres.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduini L, Kalyvoka A, Stratta P, et al. Insight and neuropsychological function in patients with schizophrenia and bipolar disorder with psychotic features. Can J Psychiatry Rev Canad Psychiatr. 2003;48:338–341. doi: 10.1177/070674370304800510. [DOI] [PubMed] [Google Scholar]
- Ayesa-Arriola R, Rodriguez-Sanchez JM, Morelli C, et al. Insight dimensions in first-episode psychosis patients: clinical, cognitive, pre-morbid and socio-demographic correlates. Early Interv Psychiatry. 2011;5:140–149. doi: 10.1111/j.1751-7893.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- Bankole A, Cohen CI, Vahia I, et al. Symptomatic remission in a multiracial urban population of older adults with schizophrenia. Am J Geriatr Psychiatry. 2008;16:966–973. doi: 10.1097/JGP.0b013e31818af801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassitt DP, Neto MR, de Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257:58–62. doi: 10.1007/s00406-006-0685-z. [DOI] [PubMed] [Google Scholar]
- Beck AT, Baruch E, Balter JM, et al. A new instrument for measuring insight: the Beck Cognitive Insight Scale. Schizophr Res. 2004;68:319–329. doi: 10.1016/S0920-9964(03)00189-0. [DOI] [PubMed] [Google Scholar]
- Berge D, Carmona S, Rovira M, et al. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123:431–439. doi: 10.1111/j.1600-0447.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Lipschutz-Broch L, Reiter G, et al. Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull. 1992;18:437–448. doi: 10.1093/schbul/18.3.437. [DOI] [PubMed] [Google Scholar]
- Birchwood M, Smith J, Drury V, et al. A self-report Insight Scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89:62–67. doi: 10.1111/j.1600-0447.1994.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Grossman LS, Harrow M, Rosen C. Neurocognition in schizophrenia: a 20-year multi-follow-up of the course of processing speed and stored knowledge. Compr Psychiatry. 2010;51:471–479. doi: 10.1016/j.comppsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota RG, Munro JS, Ricci WF, Bota DA. The dynamics of insight in the prodrome of schizophrenia. CNS Spectr. 2006;11:355–362. doi: 10.1017/s1092852900014486. [DOI] [PubMed] [Google Scholar]
- Braw Y, Sitman R, Sela T, et al. Comparison of insight among schizophrenia and bipolar disorder patients in remission of affective and positive symptoms: analysis and critique. Eur Psychiatry. 2012;27:612–618. doi: 10.1016/j.eurpsy.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Buchy L, Bodnar M, Malla A, Joober R, Lepage M. A 12-month outcome study of insight and symptom change in first-episode psychosis. Early Interv Psychiatry. 2010;4:79–88. doi: 10.1111/j.1751-7893.2010.00166.x. [DOI] [PubMed] [Google Scholar]
- Buchy L, Ad-Dab'bagh Y, Malla A, et al. Cortical thickness is associated with poor insight in first-episode psychosis. J Psychiatr Res. 2011;45:781–787. doi: 10.1016/j.jpsychires.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Wirshing DA, Bhushan P, et al. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 2007;21:129–141. doi: 10.2165/00023210-200721020-00004. [DOI] [PubMed] [Google Scholar]
- Capdevielle D, Norton J, Jaussent I, et al. A multi-dimensional approach to insight and its evolution in first-episode psychosis: a 1-year outcome naturalistic study. Psychiatry Res. 2013;210:835–841. doi: 10.1016/j.psychres.2013.09.020. [DOI] [PubMed] [Google Scholar]
- Carroll A, Fattah S, Clyde Z, et al. Correlates of insight and insight change in schizophrenia. Schizophr Res. 1999;35:247–253. doi: 10.1016/s0920-9964(98)00142-x. [DOI] [PubMed] [Google Scholar]
- Carroll A, Pantelis C, Harvey C. Insight and hopelessness in forensic patients with schizophrenia. Aust N Z J Psychiatry. 2004;38:169–173. doi: 10.1080/j.1440-1614.2004.01315.x. [DOI] [PubMed] [Google Scholar]
- Chan SW, Yip B, Tso S, Cheng BS, Tam W. Evaluation of a psychoeducation program for Chinese clients with schizophrenia and their family caregivers. Patient Educ Couns. 2009;75:67–76. doi: 10.1016/j.pec.2008.08.028. [DOI] [PubMed] [Google Scholar]
- Chan SK, Chan KK, Lam MM, et al. Clinical and cognitive correlates of insight in first-episode schizophrenia. Schizophr Res. 2012;135:40–45. doi: 10.1016/j.schres.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Chen EY, Kwok CL, Chen RY, Kwong PP. Insight changes in acute psychotic episodes: a prospective study of Hong Kong Chinese patients. J Nerv Ment Dis. 2001;189:24–30. doi: 10.1097/00005053-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Cohen CI, Cohen GD, Blank K, et al. Schizophrenia and older adults. An overview: directions for research and policy. Am J Geriatr Psychiatry. 2000;8:19–28. doi: 10.1097/00019442-200002000-00003. [DOI] [PubMed] [Google Scholar]
- Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59:232–234. doi: 10.1176/ps.2008.59.3.232. [DOI] [PubMed] [Google Scholar]
- Cohen CI, Natarajan N, Araujo M, Solanki D. Prevalence of negative symptoms and associated factors in older adults with schizophrenia spectrum disorder. Am J Geriatr Psychiatry. 2013;21:100–107. doi: 10.1016/j.jagp.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Collins AA, Remington GJ, Coulter K, Birkett K. Insight, neurocognitive function and symptom clusters in chronic schizophrenia. Schizophr Res. 1997;27:37–44. doi: 10.1016/S0920-9964(97)00075-3. [DOI] [PubMed] [Google Scholar]
- Comparelli A, Savoja V, De Carolis A, et al. Relationships between psychopathological variables and insight in psychosis risk syndrome and first-episode and multiepisode schizophrenia. J Nerv Ment Dis. 2013;201:229–233. doi: 10.1097/NMD.0b013e3182834315. [DOI] [PubMed] [Google Scholar]
- Cooke M, Peters E, Fannon D, et al. Insight, distress and coping styles in schizophrenia. Schizophr Res. 2007a;94:12–22. doi: 10.1016/j.schres.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MA, Peters ER, Greenwood KE, et al. Insight in psychosis: influence of cognitive ability and self-esteem. Br J psychiatry J Mental Sci. 2007b;191:234–237. doi: 10.1192/bjp.bp.106.024653. [DOI] [PubMed] [Google Scholar]
- Cooke MA, Fannon D, Kuipers E, et al. Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res. 2008;103:40–51. doi: 10.1016/j.schres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V. Lack of insight in schizophrenia. Schizophr Bull. 1994;20:359–366. doi: 10.1093/schbul/20.2.359. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, Caro F, de Leon J. Is poor insight in psychotic disorders associated with poor performance on the Wisconsin Card Sorting Test? Am J Psychiatry. 1995;152:1380–1382. doi: 10.1176/ajp.152.9.1380. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, Zarzuela A, Zandio M. Insight dimensions and cognitive function in psychosis: a longitudinal study. BMC Psychiatry. 2006;6:26. doi: 10.1186/1471-244X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffel BJ, Alford J, Fischer EP, Owen RR. Awareness of illness in schizophrenia and outpatient treatment adherence. J Nerv Ment Dis. 1996;184:653–659. doi: 10.1097/00005053-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Cunningham Owens DG, Carroll A, Fattah S, et al. A randomized, controlled trial of a brief interventional package for schizophrenic out-patients. Acta Psychiatr Scand. 2001;103:362–369. doi: 10.1034/j.1600-0447.2001.00132.x. [DOI] [PubMed] [Google Scholar]
- David AS. Insight and psychosis. Br J psychiatry J Mental Sci. 1990;156:798–808. doi: 10.1192/bjp.156.6.798. [DOI] [PubMed] [Google Scholar]
- David A, Buchanan A, Reed A, Almeida O. The assessment of insight in psychosis. Br J psychiatry J Mental Sci. 1992;161:599–602. doi: 10.1192/bjp.161.5.599. [DOI] [PubMed] [Google Scholar]
- David A, van Os J, Jones P, et al. Insight and psychotic illness. Cross-sectional and longitudinal associations. Br J psychiatry J Mental Sci. 1995;167:621–628. doi: 10.1192/bjp.167.5.621. [DOI] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Powchik P, et al. Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am J Psychiatry. 1995;152:197–207. doi: 10.1176/ajp.152.2.197. [DOI] [PubMed] [Google Scholar]
- De Hert MA, Simon V, Vidovic D, et al. Evaluation of the association between insight and symptoms in a large sample of patients with schizophrenia. Eur Psychiatry. 2009;24:507–512. doi: 10.1016/j.eurpsy.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Ringel N, Parente F. Lack of insight among outpatients with schizophrenia. Psychiatric services (Washington, DC) 1997;48:195–199. doi: 10.1176/ps.48.2.195. [DOI] [PubMed] [Google Scholar]
- Dickinson MD, Hiscock M. Age-related IQ decline is reduced markedly after adjustment for the Flynn effect. J Clin Exp Neuropsychol. 2010;32:865–870. doi: 10.1080/13803391003596413. [DOI] [PubMed] [Google Scholar]
- Donohoe G, Corvin A, Robertson IH. Are the cognitive deficits associated with impaired insight in schizophrenia specific to executive task performance? J Nerv Ment Dis. 2005;193:803–808. doi: 10.1097/01.nmd.0000190587.01950.72. [DOI] [PubMed] [Google Scholar]
- Drake RJ, Lewis SW. Insight and neurocognition in schizophrenia. Schizophr Res. 2003;62:165–173. doi: 10.1016/s0920-9964(02)00382-1. [DOI] [PubMed] [Google Scholar]
- Drake RJ, Pickles A, Bentall RP, et al. The evolution of insight, paranoia and depression during early schizophrenia. Psychol Med. 2004;34:285–292. doi: 10.1017/s0033291703008821. [DOI] [PubMed] [Google Scholar]
- Fennig S, Everett E, Bromet EJ, et al. Insight in first-admission psychotic patients. Schizophr Res. 1996;22:257–263. doi: 10.1016/s0920-9964(96)00077-1. [DOI] [PubMed] [Google Scholar]
- Flashman LA, McAllister TW, Andreasen NC, Saykin AJ. Smaller brain size associated with unawareness of illness in patients with schizophrenia. Am J Psychiatry. 2000;157:1167–1169. doi: 10.1176/appi.ajp.157.7.1167. [DOI] [PubMed] [Google Scholar]
- Flashman LA, McAllister TW, Johnson SC, et al. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci. 2001;13:255–257. doi: 10.1176/jnp.13.2.255. [DOI] [PubMed] [Google Scholar]
- Frangou S. Cognitive function in early onset schizophrenia: a selective review. Front Hum Neurosci. 2010;3:79. doi: 10.3389/neuro.09.079.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Pollock BG. Cognitive risks of anticholinergics in the elderly. Aging Health. 2013;9:156–166. [Google Scholar]
- Gerretsen P, Chakravarty MM, Mamo D, et al. Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum Brain Mapp. 2013a;34:1035–1043. doi: 10.1002/hbm.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerretsen P, Mulsant BH, Liu AY, et al. Insight into illness in late-life schizophrenia: a function of illness severity and premorbid intellectual function. Schizophr Res. 2013b;150:217–222. doi: 10.1016/j.schres.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Gilleen J, Greenwood K, David AS. Domains of awareness in schizophrenia. Schizophr Bull. 2011;37:61–72. doi: 10.1093/schbul/sbq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S, Arndt S, Nopoulos P, O'Leary DS, Andreasen NC. Longitudinal study of cognitive function in first-episode and recent-onset schizophrenia. Am J Psychiatry. 1999;156:1342–1348. doi: 10.1176/ajp.156.9.1342. [DOI] [PubMed] [Google Scholar]
- Goldberg RW, Green-Paden LD, Lehman AF, Gold JM. Correlates of insight in serious mental illness. J Nerv Ment Dis. 2001;189:137–145. doi: 10.1097/00005053-200103000-00001. [DOI] [PubMed] [Google Scholar]
- Goodman C, Knoll G, Isakov V, Silver H. Insight into illness in schizophrenia. Compr Psychiatry. 2005;46:284–290. doi: 10.1016/j.comppsych.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Greenberger C, Serper MR. Examination of clinical and cognitive insight in acute schizophrenia patients. J Nerv Ment Dis. 2010;198:465–469. doi: 10.1097/NMD.0b013e3181e4f35d. [DOI] [PubMed] [Google Scholar]
- Gur RE, Petty RG, Turetsky BI, Gur RC. Schizophrenia throughout life: sex differences in severity and profile of symptoms. Schizophr Res. 1996;21:1–12. doi: 10.1016/0920-9964(96)00023-0. [DOI] [PubMed] [Google Scholar]
- Ha TH, Youn T, Ha KS, et al. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132:251–260. doi: 10.1016/j.pscychresns.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Haddock G, Lewis S, Bentall R, et al. Influence of age on outcome of psychological treatments in first-episode psychosis. Br J psychiatry J Mental Sci. 2006;188:250–254. doi: 10.1192/bjp.188.3.250. [DOI] [PubMed] [Google Scholar]
- Harding CM. Course types in schizophrenia: an analysis of European and American studies. Schizophr Bull. 1988;14:633–643. doi: 10.1093/schbul/14.4.633. [DOI] [PubMed] [Google Scholar]
- Harrison G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J psychiatry J Mental Sci. 2001;178:506–517. doi: 10.1192/bjp.178.6.506. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Yamashina M, Igarashi Y. Awareness of being a patient and its relevance to insight into illness in patients with schizophrenia. Compr Psychiatry. 1999;40:377–385. doi: 10.1016/s0010-440x(99)90144-x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, et al. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeste DV, Twamley EW, Eyler Zorrilla LT, et al. Aging and outcome in schizophrenia. Acta Psychiatr Scand. 2003;107:336–343. doi: 10.1034/j.1600-0447.2003.01434.x. [DOI] [PubMed] [Google Scholar]
- Johnson S, Sathyaseelan M, Charles H, Jeyaseelan V, Jacob KS. Insight, psychopathology, explanatory models and outcome of schizophrenia in India: a prospective 5-year cohort study. BMC Psychiatry. 2012;12:159. doi: 10.1186/1471-244X-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Vilkman H, Hietala J, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Karow A, Pajonk FG, Reimer J, et al. The dilemma of insight into illness in schizophrenia: self- and expert-rated insight and quality of life. Eur Arch Psychiatry Clin Neurosci. 2008;258:152–159. doi: 10.1007/s00406-007-0768-5. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Poe M, Walker TM, Kang JW, Harvey PD. The Schizophrenia Cognition Rating Scale: an interview-based assessment and its relationship to cognition, real-world functioning, and functional capacity. Am J Psychiatry. 2006;163:426–432. doi: 10.1176/appi.ajp.163.3.426. [DOI] [PubMed] [Google Scholar]
- Kemp RA, Lambert TJ. Insight in schizophrenia and its relationship to psychopathology. Schizophr Res. 1995;18:21–28. doi: 10.1016/0920-9964(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Kemp R, David A. Psychological predictors of insight and compliance in psychotic patients. Br J psychiatry J Mental Sci. 1996;169:444–450. doi: 10.1192/bjp.169.4.444. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Rabinowitz J, DeSmedt G, Harvey PD, Schooler N. Correlates of insight in first episode psychosis. Schizophr Res. 2004;70:187–194. doi: 10.1016/j.schres.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kim Y, Sakamoto K, Kamo T, Sakamura Y, Miyaoka H. Insight and clinical correlates in schizophrenia. Compr Psychiatry. 1997;38:117–123. doi: 10.1016/s0010-440x(97)90091-2. [DOI] [PubMed] [Google Scholar]
- Koren D, Viksman P, Giuliano AJ, Seidman LJ. The nature and evolution of insight in schizophrenia: a multi-informant longitudinal study of first-episode versus chronic patients. Schizophr Res. 2013;151:245–251. doi: 10.1016/j.schres.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res. 2005;74:15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Laroi F, Fannemel M, Ronneberg U, et al. Unawareness of illness in chronic schizophrenia and its relationship to structural brain measures and neuropsychological tests. Psychiatry Res. 2000;100:49–58. doi: 10.1016/s0925-4927(00)00063-9. [DOI] [PubMed] [Google Scholar]
- Leon C, Gerretsen P, Uchida H, et al. Sensitivity to antipsychotic drugs in older adults. Curr Psychiatry Rep. 2010;12:28–33. doi: 10.1007/s11920-009-0080-3. [DOI] [PubMed] [Google Scholar]
- Levine SZ, Lurie I, Kohn R, Levav I. Trajectories of the course of schizophrenia: from progressive deterioration to amelioration over three decades. Schizophr Res. 2011;126:184–191. doi: 10.1016/j.schres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Lullmann E, Rief W. Correlates and long-term consequences of poor insight in patients with schizophrenia. A systematic review. Schizophr Bull. 2007;33:1324–1342. doi: 10.1093/schbul/sbm002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker P, Bell M. Insight and cognitive impairment in schizophrenia. Performance on repeated administrations of the Wisconsin Card Sorting Test. J Nerv Ment Dis. 1994;182:656–660. doi: 10.1097/00005053-199411000-00010. [DOI] [PubMed] [Google Scholar]
- Lysaker P, Bell M. Work rehabilitation and improvements in insight in schizophrenia. J Nerv Ment Dis. 1995;183:103–106. doi: 10.1097/00005053-199502000-00007. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Bryson GJ, Bell MD. Insight and work performance in schizophrenia. J Nerv Ment Dis. 2002;190:142–146. doi: 10.1097/00005053-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Whitney KA, Davis LW. Awareness of illness in schizophrenia: associations with multiple assessments of executive function. J Neuropsychiatry Clin Neurosci. 2006;18:516–520. doi: 10.1176/jnp.2006.18.4.516. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Dimaggio G, Buck KD, et al. Poor insight in schizophrenia: links between different forms of metacognition with awareness of symptoms, treatment need, and consequences of illness. Compr Psychiatry. 2011;52:253–260. doi: 10.1016/j.comppsych.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Macpherson R, Jerrom B, Hughes A. Relationship between insight, educational background and cognition in schizophrenia. Br J psychiatry J Mental Sci. 1996;168:718–722. doi: 10.1192/bjp.168.6.718. [DOI] [PubMed] [Google Scholar]
- Makinen J, Miettunen J, Isohanni M, Koponen H. Negative symptoms in schizophrenia: a review. Nord J Psychiatry. 2008;62:334–341. doi: 10.1080/08039480801959307. [DOI] [PubMed] [Google Scholar]
- Markova IS, Berrios GE. The assessment of insight in clinical psychiatry: a new scale. Acta Psychiatr Scand. 1992;86:159–164. doi: 10.1111/j.1600-0447.1992.tb03245.x. [DOI] [PubMed] [Google Scholar]
- Marks KA, Fastenau PS, Lysaker PH, Bond GR. Self-Appraisal of Illness Questionnaire (SAIQ): relationship to researcher-rated insight and neuropsychological function in schizophrenia. Schizophr Res. 2000;45:203–211. doi: 10.1016/s0920-9964(99)00208-x. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Apperson LJ, Appelbaum PS, et al. Insight in schizophrenia. Its relationship to acute psychopathology. J Nerv Ment Dis. 1989;177:43–47. doi: 10.1097/00005053-198901000-00007. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Johnson J, Perkins D, et al. Insight in first-episode psychosis. Psychol Med. 2006;36:1385–1393. doi: 10.1017/S0033291706007793. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Carpenter WT., Jr Postpsychotic depression in schizophrenia. Arch Gen Psychiatry. 1976;33:231–239. doi: 10.1001/archpsyc.1976.01770020065011. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Fenton WS. Subtype progression and pathophysiologic deterioration in early schizophrenia. Schizophr Bull. 1993;19:71–84. doi: 10.1093/schbul/19.1.71. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Johannessen JO. Early detection and intervention with schizophrenia: rationale. Schizophr Bull. 1996;22:201–222. doi: 10.1093/schbul/22.2.201. [DOI] [PubMed] [Google Scholar]
- Medalia A, Thysen J. Insight into neurocognitive dysfunction in schizophrenia. Schizophr Bull. 2008;34:1221–1230. doi: 10.1093/schbul/sbm144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melle I, Barrett EA. Insight and suicidal behavior in first-episode schizophrenia. Expert Rev Neurother. 2012;12:353–359. doi: 10.1586/ern.11.191. [DOI] [PubMed] [Google Scholar]
- Mingrone C, Rocca P, Castagna F, et al. Insight in stable schizophrenia: relations with psychopathology and cognition. Compr Psychiatry. 2013;54:484–492. doi: 10.1016/j.comppsych.2012.12.014. [DOI] [PubMed] [Google Scholar]
- Mintz AR, Dobson KS, Romney DM. Insight in schizophrenia: a meta-analysis. Schizophr Res. 2003;61:75–88. doi: 10.1016/s0920-9964(02)00316-x. [DOI] [PubMed] [Google Scholar]
- Mintz AR, Addington J, Addington D. Insight in early psychosis: a 1-year follow-up. Schizophr Res. 2004;67:213–217. doi: 10.1016/S0920-9964(03)00047-1. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Rosenheck R, McEvoy J, et al. Cross-sectional and longitudinal relationships between insight and attitudes toward medication and clinical outcomes in chronic schizophrenia. Schizophr Bull. 2009;35:336–346. doi: 10.1093/schbul/sbn067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, Nicholson RA, Carpenter BN. Role of psychosocial treatments in management of schizophrenia: a meta-analytic review of controlled outcome studies. Schizophr Bull. 1998;24:569–587. doi: 10.1093/oxfordjournals.schbul.a033350. [DOI] [PubMed] [Google Scholar]
- Moore O, Cassidy E, Carr A, O'Callaghan E. Unawareness of illness and its relationship with depression and self-deception in schizophrenia. Eur Psychiatry. 1999;14:264–269. doi: 10.1016/s0924-9338(99)00172-8. [DOI] [PubMed] [Google Scholar]
- Morgan KD, Dazzan P, Morgan C, et al. Insight, grey matter and cognitive function in first-onset psychosis. Br J psychiatry J Mental Sci. 2010;197:141–148. doi: 10.1192/bjp.bp.109.070888. [DOI] [PubMed] [Google Scholar]
- Nair A, Palmer EC, Aleman A, David AS. Relationship between cognition, clinical and cognitive insight in psychotic disorders: a review and meta-analysis. Schizophr Res. 2014;152:191–200. doi: 10.1016/j.schres.2013.11.033. [DOI] [PubMed] [Google Scholar]
- Nakamae T, Kitabayashi Y, Okamura A, et al. Insight and quality of life in long-term hospitalized Japanese patients with chronic schizophrenia. Psychiatry Clin Neurosci. 2010;64:372–376. doi: 10.1111/j.1440-1819.2010.02100.x. [DOI] [PubMed] [Google Scholar]
- Nakano H, Terao T, Iwata N, Hasako R, Nakamura J. Symptomatological and cognitive predictors of insight in chronic schizophrenia. Psychiatry Res. 2004;127:65–72. doi: 10.1016/j.psychres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Olesen AV, Mortensen PB. Readmission risk in schizophrenia: selection explains previous findings of a progressive course of disorder. Psychol Med. 2002;32:1301–1307. doi: 10.1017/s0033291702005548. [DOI] [PubMed] [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14:203–222. doi: 10.1177/1073858407309995. [DOI] [PubMed] [Google Scholar]
- Ouzir M, Azorin JM, Adida M, Boussaoud D, Battas O. Insight in schizophrenia: from conceptualization to neuroscience. Psychiatry Clin Neurosci. 2012;66:167–179. doi: 10.1111/j.1440-1819.2012.02325.x. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L, Mallikarjun P, Joseph V, Liddle PF. Appreciating symptoms and deficits in schizophrenia: right posterior insula and poor insight. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:523–527. doi: 10.1016/j.pnpbp.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Parellada M, Fraguas D, Bombin I, et al. Insight correlates in child- and adolescent-onset first episodes of psychosis: results from the CAFEPS study. Psychol Med. 2009;39:1433–1445. doi: 10.1017/S0033291708004868. [DOI] [PubMed] [Google Scholar]
- Parellada M, Boada L, Fraguas D, et al. Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr Bull. 2011;37:38–51. doi: 10.1093/schbul/sbq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrelli P, McQuaid JR, Granholm E, et al. Measuring cognitive insight in middle-aged and older patients with psychotic disorders. Schizophr Res. 2004;71:297–305. doi: 10.1016/j.schres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Winokur G. The evolution of symptoms in institutionalized hebephrenic/catatonic schizophrenics. Br J psychiatry J Mental Sci. 1982;141:567–572. doi: 10.1192/bjp.141.6.567. [DOI] [PubMed] [Google Scholar]
- Pijnenborg GH, van Donkersgoed RJ, David AS, Aleman A. Changes in insight during treatment for psychotic disorders: a meta-analysis. Schizophr Res. 2013;144:109–117. doi: 10.1016/j.schres.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Pyne JM, Bean D, Sullivan G. Characteristics of patients with schizophrenia who do not believe they are mentally ill. J Nerv Ment Dis. 2001;189:146–153. doi: 10.1097/00005053-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Quee PJ, van der Meer L, Bruggeman R, et al. Insight in psychosis: relationship with neurocognition, social cognition and clinical symptoms depends on phase of illness. Schizophr Bull. 2011;37:29–37. doi: 10.1093/schbul/sbq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz J, Levine SZ, Haim R, Hafner H. The course of schizophrenia: progressive deterioration, amelioration or both? Schizophr Res. 2007;91:254–258. doi: 10.1016/j.schres.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Raffard S, Bayard S, Gely-Nargeot MC, et al. Insight and executive functioning in schizophrenia: a multidimensional approach. Psychiatry Res. 2009;167:239–250. doi: 10.1016/j.psychres.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Mulsant BH. Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophr Res. 2008;102:122–140. doi: 10.1016/j.schres.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Rajji TK, Voineskos AN, Butters MA, et al. Cognitive performance of individuals with schizophrenia across seven decades: a study using the MATRICS consensus cognitive battery. Am J Geriatr Psychiatry. 2013;21:108–118. doi: 10.1016/j.jagp.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS. Anosognosia in parietal lobe syndrome. Conscious Cogn. 1995;4:22–51. doi: 10.1006/ccog.1995.1002. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, McGeoch PD, Williams L. Can vestibular caloric stimulation be used to treat Dejerine-Roussy Syndrome? Med Hypotheses. 2007;69:486–488. doi: 10.1016/j.mehy.2006.12.036. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Coakes J, Shapleske J, Woodruff PW, David AS. Insight: its relationship with cognitive function, brain volume and symptoms in schizophrenia. Psychol Med. 2003;33:111–119. doi: 10.1017/s0033291702006803. [DOI] [PubMed] [Google Scholar]
- Saeedi H, Addington J, Addington D. The association of insight with psychotic symptoms, depression, and cognition in early psychosis: a 3-year follow-up. Schizophr Res. 2007;89:123–128. doi: 10.1016/j.schres.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Sapara A, Cooke M, Fannon D, et al. Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res. 2007;89:22–34. doi: 10.1016/j.schres.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Saravanan B, Jacob KS, Johnson S, et al. Outcome of first-episode schizophrenia in India: longitudinal study of effect of insight and psychopathology. Br J psychiatry J Mental Sci. 2010;196:454–459. doi: 10.1192/bjp.bp.109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Schennach R, Meyer S, Seemuller F, et al. Insight in schizophrenia-course and predictors during the acute treatment phase of patients suffering from a schizophrenia spectrum disorder. Eur Psychiatry. 2012;27:625–633. doi: 10.1016/j.eurpsy.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Schultz SK, Miller DD, Oliver SE, et al. The life course of schizophrenia: age and symptom dimensions. Schizophr Res. 1997;23:15–23. doi: 10.1016/S0920-9964(96)00087-4. [DOI] [PubMed] [Google Scholar]
- Schwartz CE. Insight in psychosis: state or trait? Am J Psychiatry. 1994;151:788–789. doi: 10.1176/ajp.151.5.aj1515788. [DOI] [PubMed] [Google Scholar]
- Schwartz RC. Insight and illness in chronic schizophrenia. Compr Psychiatry. 1998a;39:249–254. doi: 10.1016/s0010-440x(98)90031-1. [DOI] [PubMed] [Google Scholar]
- Schwartz RC. The relationship between insight, illness and treatment outcome in schizophrenia. Psychiatr Q. 1998b;69:1–22. doi: 10.1023/a:1022141322657. [DOI] [PubMed] [Google Scholar]
- Schwartz RC, Cohen BN, Grubaugh A. Does insight affect long-term impatient treatment outcome in chronic schizophrenia? Compr Psychiatry. 1997;38:283–288. doi: 10.1016/s0010-440x(97)90061-4. [DOI] [PubMed] [Google Scholar]
- Segarra R, Ojeda N, Pena J, et al. Longitudinal changes of insight in first episode psychosis and its relation to clinical symptoms, treatment adherence and global functioning: one-year follow-up from the Eiffel study. Eur Psychiatry. 2012;27:43–49. doi: 10.1016/j.eurpsy.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Selten JP, Sijben NE, van den Bosch RJ, Omloo-Visser J, Warmerdam H. The subjective experience of negative symptoms: a self-rating scale. Compr Psychiatry. 1993;34:192–197. doi: 10.1016/0010-440x(93)90047-8. [DOI] [PubMed] [Google Scholar]
- Sevy S, Nathanson K, Visweswaraiah H, Amador X. The relationship between insight and symptoms in schizophrenia. Compr Psychiatry. 2004;45:16–19. doi: 10.1016/j.comppsych.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Shad MU, Muddasani S, Prasad K, Sweeney JA, Keshavan MS. Insight and pre-frontal cortex in first-episode Schizophrenia. Neuroimage. 2004;22:1315–1320. doi: 10.1016/j.neuroimage.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Shad MU, Tamminga CA, Cullum M, Haas GL, Keshavan MS. Insight and frontal cortical function in schizophrenia: a review. Schizophr Res. 2006;86:54–70. doi: 10.1016/j.schres.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A. Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry. 2007;19:437–446. doi: 10.1080/09540260701486324. [DOI] [PubMed] [Google Scholar]
- Sheitman BB, Murray MG, Snyder JA, et al. IQ scores of treatment-resistant schizophrenia patients before and after the onset of the illness. Schizophr Res. 2000;46:203–207. doi: 10.1016/s0920-9964(00)00034-7. [DOI] [PubMed] [Google Scholar]
- Shepherd S, Depp CA, Harris G, et al. Perspectives on schizophrenia over the lifespan: a qualitative study. Schizophr Bull. 2012;38:295–303. doi: 10.1093/schbul/sbq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim K, Chan YH, Chua TH, et al. Physical comorbidity, insight, quality of life and global functioning in first episode schizophrenia: a 24-month, longitudinal outcome study. Schizophr Res. 2006;88:82–89. doi: 10.1016/j.schres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Simon AE, Berger GE, Giacomini V, Ferrero F, Mohr S. Insight, symptoms and executive functions in schizophrenia. Cogn Neuropsychiatry. 2006;11:437–451. doi: 10.1080/13546800444000308. [DOI] [PubMed] [Google Scholar]
- Smith TE, Hull JW, Huppert JD, Silverstein SM. Recovery from psychosis in schizophrenia and schizoaffective disorder: symptoms and neurocognitive rate-limiters for the development of social behavior skills. Schizophr Res. 2002;55:229–237. doi: 10.1016/s0920-9964(01)00231-6. [DOI] [PubMed] [Google Scholar]
- Smith TE, Hull JW, Santos L. The relationship between symptoms and insight in schizophrenia: a longitudinal perspective. Schizophr Res. 1998;33:63–67. doi: 10.1016/s0920-9964(98)00063-2. [DOI] [PubMed] [Google Scholar]
- Soni SD, Mallik A. The elderly chronic schizophrenic inpatient: A study of psychiatric morbidity in ‘elderly graduates’. Int J Geriatr Psychiatry. 1993;8:665–673. [Google Scholar]
- Stefanopoulou E, Lafuente AR, Saez Fonseca JA, Huxley A. Insight, global functioning and psychopathology amongst in-patient clients with schizophrenia. Psychiatry Q. 2009;80:155–165. doi: 10.1007/s11126-009-9103-9. [DOI] [PubMed] [Google Scholar]
- Thompson KN, McGorry PD, Harrigan SM. Reduced awareness of illness in first-episode psychosis. Compr Psychiatry. 2001;42:498–503. doi: 10.1053/comp.2001.27900. [DOI] [PubMed] [Google Scholar]
- Tirupati S, Padmavati R, Thara R, McCreadie RG. Insight and psychopathology in never-treated schizophrenia. Compr Psychiatry. 2007;48:264–268. doi: 10.1016/j.comppsych.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Trevisi M, Talamo A, Bandinelli PL, et al. Insight and awareness as related to psychopathology and cognition. Psychopathology. 2012;45:235–243. doi: 10.1159/000329998. [DOI] [PubMed] [Google Scholar]
- Uchida H, Kapur S, Mulsant BH, et al. Sensitivity of older patients to antipsychotic motor side effects: a PET study examining potential mechanisms. Am J Geriatr Psychiatry. 2009a;17:255–263. doi: 10.1097/JGP.0b013e318198776d. [DOI] [PubMed] [Google Scholar]
- Uchida T, Matsumoto K, Kikuchi A, et al. Psychometric properties of the Japanese version of the Beck Cognitive Insight Scale: relation of cognitive insight to clinical insight. Psychiatry Clin Neurosci. 2009b;63:291–297. doi: 10.1111/j.1440-1819.2009.01946.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, et al. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18 F-N-methylspiroperidol. Psychiatry Res. 1996;67:11–16. doi: 10.1016/0925-4927(96)02809-0. [DOI] [PubMed] [Google Scholar]
- Voruganti LN, Heslegrave RJ, Awad AG. Neurocognitive correlates of positive and negative syndromes in schizophrenia. Can J Psychiatry. 1997;42:1066–1071. doi: 10.1177/070674379704201008. [DOI] [PubMed] [Google Scholar]
- Warman DM, Lysaker PH, Martin JM. Cognitive insight and psychotic disorder: the impact of active delusions. Schizophr Res. 2007;90:325–333. doi: 10.1016/j.schres.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Weiler MA, Fleisher MH, McArthur-Campbell D. Insight and symptom change in schizophrenia and other disorders. Schizophr Res. 2000;45:29–36. doi: 10.1016/s0920-9964(99)00215-7. [DOI] [PubMed] [Google Scholar]
- Wiffen BD, Rabinowitz J, Fleischhacker WW, David AS. Insight: demographic differences and associations with one-year outcome in schizophrenia and schizoaffective disorder. Clin Schizophr Relat Psychoses. 2010a;4:169–175. doi: 10.3371/CSRP.4.3.3. [DOI] [PubMed] [Google Scholar]
- Wiffen BD, Rabinowitz J, Lex A, David AS. Correlates, change and ‘state or trait’ properties of insight in schizophrenia. Schizophr Res. 2010b;122:94–103. doi: 10.1016/j.schres.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Wilk CM, Gold JM, Humber K, et al. Brief cognitive assessment in schizophrenia: normative data for the Repeatable Battery for the Assessment of Neuropsychological Status. Schizophr Res. 2004;70:175–186. doi: 10.1016/j.schres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Xiang YT, Wang Y, Wang CY, et al. Association of insight with sociodemographic and clinical factors, quality of life, and cognition in Chinese patients with schizophrenia. Compr Psychiatry. 2012;53:140–144. doi: 10.1016/j.comppsych.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Yen CF, Chen CS, Yeh ML, et al. Comparison of insight in patients with schizophrenia and bipolar disorder in remission. J Nerv Ment Dis. 2002;190:847–849. doi: 10.1097/00005053-200212000-00008. [DOI] [PubMed] [Google Scholar]
- Young DA, Davila R, Scher H. Unawareness of illness and neuropsychological performance in chronic schizophrenia. Schizophr Res. 1993;10:117–124. doi: 10.1016/0920-9964(93)90046-l. [DOI] [PubMed] [Google Scholar]


