Abstract
Aims
The Impella is a percutaneous ventricular assist device. The majority of published data describes the 2.5L and 5.0L devices, and little data is available for the newer 3.8L device. We examined the indications and outcomes from our single-centre “real-world” registry at The Queen Elizabeth Hospital, Birmingham, UK, using all three pump sizes.
Methods and Results
Records from all patients who underwent attempted Impella-assisted procedures at our centre were examined retrospectively. Impella implantation was attempted in 49 patients (mean age 72 ± 13 years; 80% male) and was successful in 48 (98%). 45 patients underwent high-risk percutaneous coronary intervention (PCI), one patient underwent balloon aortic valvuloplasty and 3 patients had Impella as a bridge to cardiac transplantation. The 2.5L and 3.8L devices were used in 36 (75%) and 11 (23%) patients, respectively, while one patient (2%) had the 5L device. Vascular complications occurred in only one patient (2%) and stroke and peri-procedural myocardial infarction occurred in one patient (2%), while in-hospital mortality was 20% (10/49).
Conclusions
In this large real-world registry, we have demonstrated the safety and feasibility of the Impella device for a wide range of indications. This includes the first series of the 3.8L device which provides superior support with no increase in vascular complications.
Electronic supplementary material
The online version of this article (doi:10.1007/s40119-014-0033-8) contains supplementary material, which is available to authorized users.
Keywords: High-risk PCI, Impella 3.8L, Impella device, Outcomes, Percutaneous coronary intervention, Vascular complications
Introduction
Due to an ageing population, percutaneous coronary intervention (PCI) is being increasingly undertaken in patients with multiple comorbidities and complex lesions. As a result, PCI is increasingly performed in patients considered to be at prohibitive risk for coronary artery bypass grafting (CABG) [1, 2]. Many of these patients also have significant left ventricular (LV) impairment, and PCI in this setting is associated with an increased risk of peri-procedural complications [3, 4]. Mechanical-assist devices have been frequently employed to support high-risk PCI in these situations in the hope of reducing this risk.
Such mechanical assistance has been conventionally provided by intra-aortic balloon counterpulsation (IABP), although randomised trials have failed to show benefit of elective IABP both in high-risk PCI [5] and cardiogenic shock complicating acute myocardial infarction [6]. This may be due to the limited support that counter-pulsation provides in augmenting cardiac output and in reducing left ventricular afterload. Percutaneous left ventricular assist devices (LVAD) such as the TandemHeart® (Cardiac Assist Inc., Pittsburg, USA) have been shown to be safe and feasible in this setting, and also provide superior haemodynamic support as compared to IABP [7, 8]. However, the TandemHeart® is complex to use and requires a trans-septal puncture.
The Impella® (Abiomed, Danvers, USA) device is a percutaneous catheter-based impeller-driven LVAD which aspirates blood from the LV cavity expelling the removed blood into the aorta. It has been shown to provide superior cardiac support compared with IABP in both animal [9, 10] and human studies [11], reducing LV end diastolic pressure, wall stress, myocardial oxygen consumption and improving coronary perfusion and cardiac output [12–14].
The Impella device has gained increasing popularity in acute cardiac care, most commonly in high-risk PCI. Large registries like USpella [1] and Europella [15] have demonstrated the feasibility and safety of this device in this setting. More recently, the PROTECT-II trial is the first randomised controlled trial demonstrating the haemodynamic benefit of this device over IABP in elective patients undergoing high-risk PCI and a trend to improved clinical outcomes at 30 days [16]. In addition to high-risk PCI, Impella has also been used successfully in cardiogenic shock [17–20], acute cardiac transplant rejection [21, 22] and refractory heart failure as a bridge to transplantation [23, 24].
Historically, IABP has been the mechanical assist device of choice in the UK, although recent trials from the UK have shown no early benefit of prophylactic IABP in elective high-risk PCI [5], with a recent analysis showing a beneficial effect on 5-year mortality [25]. We therefore, present our experience with the use of the Impella device in a UK quaternary cardiac centre.
Methods
The Queen Elizabeth Hospital, Birmingham, UK, is a large quaternary cardiac centre and provides regional cardiac transplantation. We retrospectively analysed our interventional procedural database and identified all patients undergoing Impella implantation since the start of the programme in October 2008 until January 2014 on an intention to treat basis. Clinical and procedural data was procured from electronic patient records, the procedural database and procedure logs in the cardiac catheter laboratory. All patients were included in an intention to treat manner; there were no exclusions.
The 2.5L and 3.8L Impella devices were inserted via the femoral approach. The 5L Impella was inserted via the subclavian artery following surgical exposure and application of a Dacron graft, as previously described [26]. For trans-femoral access, arterial puncture was performed after fluoroscopic localisation of the femoral head, with or without ultrasound guidance and, more recently, with the use of a 4F (French) micropuncture kit (Micropuncture® Introducer Set Silhouette™ Transitionless, Cook Medical Inc., USA) to minimise vascular complications. A femoral angiogram was then performed to ensure adequate vessel calibre (>4 mm) and to assess tortuosity and calcification. Pre-closure was achieved using two sutures (Perclose ProGlide® 6F Suture-Mediated Closure (SMC) System, Abbott Vascular, Illinois, USA) following which a 13F or 14F sheath was inserted for the 2.5L and 3.8L devices, respectively.
A Judkins right or Amplatz catheter was used to cross the aortic valve following which the 0.018″ Impella guide wire was positioned in the aortic apex. The Impella device was then positioned carefully in the LV apex over the 0.018″ wire and set to maximal output. Special manoeuvres were required for insertion of the device in the five patients who had concomitant severe aortic stenosis (AS), as described recently by our group [27].
Outcome data for mortality was obtained from electronic patient records linked to the Office of National Statistics database. Peri-procedural myocardial infarction (PMI) was defined as a total creatinine kinase level greater than three times the upper limit of normal on the morning after the procedure [28, 29]. Data are presented as mean ± standard deviation (SD) for continuous variables and percentages for discrete variables.
The analysis in this article is based on previously conducted data, and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Impella implantation was attempted in a total of 49 patients during the study period: of these, 45 patients underwent high-risk PCI, 3 patients required emergency haemodynamic support as a bridge to cardiac transplantation and one patient with severe AS underwent balloon valvuloplasty (BAV) with Impella support. Implantation of a 2.5L Impella failed in one patient undergoing high-risk PCI due to extreme calcific iliofemoral disease. Implantation was successful in 48 (98%) patients. Of these, the 2.5L Impella device was used in 36 (75%) patients, the 3.8L device in 11 (23%) patients, and the 5L device in one patient (2%).
The baseline clinical characteristics of these patients are shown in Table 1.
Table 1.
Baseline characteristics
| Clinical characteristics | Total | High-risk PCI | BAV | Bridge to transplant |
|---|---|---|---|---|
| N = 49 | N = 45 | N = 1 | N = 3 | |
| Age (years) (mean*) | 72 ± 13 (37–92) | 74 ± 11 (47–92) | 67 | 48 ± 11(37–63) |
| Male | 39 (80%) | 35 (78%) | 1 | 3 (100%) |
| BMI (kg/m2) (meana) | 27 ± 4 (18–40) | 27 ± 4 (20–40) | 25 | 22 ± 3 (18–24) |
| Hypertension | 40 (82%) | 37 (82%) | 0 | 3 (100%) |
| Diabetes | 17 (35%) | 16 (36%) | 0 | 1 (33%) |
| Smoking | 28 (57%) | 26 (58%) | 0 | 2 (66%) |
| Dyslipidemia | 32 (65%) | 31 (69%) | 0 | 1 (33%) |
| Renal function | ||||
| eGFR (ml/min) (meana) | 51 ± 6 (11–117) | 54 ± 27(16–117) | 43 | 15 ± 4 (11–20) |
| eGFR 30–60 | 22 (45%) | 21 (47%) | 1 | 3 (100%) |
| eGFR <30 | 12 (30%) | 9 (14%) | 0 | 3 (100%) |
| PVD | 4 (8%) | 4 (9%) | 0 | 0 |
| CVD | 3 (6%) | 3 (7%) | 0 | 0 |
| Previous MI | 24 (49%) | 22 (49%) | 0 | 2 (67%) |
| Previous CABG | 3 (6%) | 3 (7%) | 0 | 0 |
| Previous PCI | 9 (18%) | 7 (16%) | 0 | 2 (67%) |
| LVEF <35% | 39 (80%) | 36 (80%) | 1 | 3 (100%) |
| LVEF (%) (meana) | 28 ± 14 (10–60) | 28 ± 14 (10–60) | 10 | 15 ± 4 (10–20) |
| Impella characteristics | ||||
| Successful implant | 48 (98%) | 44 (98%) | 1 | 3 (100%) |
| 2.5L Device | 36 (76%) | 34 (77%) | 0 | 2 (67%) |
| 3.8L Device | 11 (23%) | 10 (23%) | 1 | 0 |
| 5.0L Device | 1 (2%) | 0 | 0 | 1 (33%) |
BAV balloon valvuloplasty, BMI body mass index, CABG coronary artery bypass grafting, CVD cerebrovascular disease, ECMO extracorporeal membrane oxygenation, eGFR estimated glomerular filtration rate, LVEF left ventricular ejection fraction, MI myocardial infarction, PCI percutaneous coronary intervention, PVD peripheral vascular disease, SD standard deviation
aMean ± SD (range)
High Risk PCI
45 patients, including five patients with concomitant severe AS, underwent high-risk PCI. Impella was successfully implanted in all but one patient (44/45 [98%]). Of these, 10 patients were given the 13F 3.8L device, while 34 patients were given the 12F 2.5L device. Impella was removed following PCI before the patient left the catheter laboratory in all but two patients and in these patients, successful vascular closure was achieved with pre-closure use of one or two ProGlide devices. PCI was performed via the radial approach in 11 patients (25%) and the femoral approach in 33 (75%). The peri-procedural variables are shown in Table 2.
Table 2.
Procedural characteristics in patients undergoing PCI
| Pre-procedural characteristics | N = 45 |
|---|---|
| Urgency of procedure | |
| Elective PCI (Angina) | 17 (38%) |
| Urgent PCI (NSTEMI) | 28 (62%) |
| Cardiogenic shock (NSTEMI) | 3 (7%) |
| Pulmonary oedema (NSTEMI) | 2 (4%) |
| High-risk PCI features | |
| Unprotected left main stem | 24 (53%) |
| Last remaining vessel | 9 (20%) |
| Multi-vessel | 18 (40%) |
| Severe LV impairment (LVEF <35%) | 36 (80%) |
| Decision for PCI | |
| Refused CABG (MDT) | 28 (62%) |
| Patient preference | 2 (4%) |
| Physician’s decision (no MDT) | 8 (18%) |
| Haemodynamic compromise (no MDT) | 5 (11%) |
| Other | 2 (4%) |
| Logistic Euroscore | 8 ± 3 (1–15) |
| NWQIP PCI risk score | 6 ± 11 (0.4–71) |
| Peri-procedural characteristics | N = 45 |
|---|---|
| Number of lesions treated | 2.0 ± 1.0 |
| Number of stents | 2.7 ± 1.7 (1–8) |
| Rotational atherectomy | 14 (31%) |
| Glycoprotein inhibitor use | 1 (2%) |
| Complete revascularisation | 45 (100%) |
| Impella characteristics | |
| Successful insertion | 44 (98%) |
| 2.5L device | 34 (77%) |
| 3.8L device | 10 (23%) |
| Removal on table | 42 (95%) |
CABG coronary artery bypass grafting, LVEF left ventricular ejection fraction, MDT multi-disciplinary team, NSTEMI non-ST elevation myocardial infarction, NWQIP north west quality improvement programme, PCI percutaneous coronary intervention
The majority of patients (80%) had severe LV impairment (LVEF <35%) with 53% (24/45) of patients undergoing PCI to an unprotected left main stem. Five patients had severe AS with coronary artery disease and PCI was performed in preparation for transcatheter aortic valve replacement (TAVR) or balloon valvuloplasty for clinical stabilisation.
PCI was performed electively in 17 (38%) patients: 11 of these patients were discussed at a heart multi-disciplinary team (MDT) meeting and were thought to be at higher risk for CABG. 28 (62%) patients had a non-ST elevation myocardial infarction (NSTEMI) and underwent in patient revascularisation. These 28 patients—11 elective and 17 urgent—were discussed by the heart MDT and refused CABG due to co-morbidities. Two of the 17 urgent patients expressed a clear preference for PCI over CABG. Five other patients developed ischaemic haemodynamic compromise, three of which had cardiogenic shock and two pulmonary oedema, while awaiting MDT discussion. One patient with severe LV dysfunction due to non-compaction and a single coronary ostium had Impella-assisted PCI to a severely diseased right coronary artery (RCA).
Finally, 18 patients (40%) underwent multi-vessel PCI; 117 stents (mean ± SD 2.7 ± 1.7 lesions) were used to treat 89 lesions (mean ± SD 2.0 ± 1.0 stents) in 45 patients. Rotational atherectomy was carried out in 14 (31%) patients. Complete revascularisation, defined as revascularisation of all intended targets was achieved in all patients.
Impella for Balloon Aortic Valvuloplasty
One patient with severe AS and intractable cardiogenic shock underwent successful bail-out balloon valvuloplasty with Impella (3.8L) support. The patient tolerated the procedure well with a favourable haemodynamic response. However, he had a suspected retroperitoneal haematoma which prompted removal of the device. Shortly afterwards, the patient succumbed to pulmonary oedema and cardiogenic shock.
Impella as a Bridge to Transplant
Three patients had Impella as a bridge to cardiac transplantation. Two of these patients had acute cardiogenic shock following ST-elevated myocardial infarction (STEMI), despite successful primary angioplasty and conventional management including IABP. One patient had a 2.5L Impella implanted via the femoral approach, which allowed sufficient haemodynamic recovery and made the patient suitable for transplantation with an excellent outcome. The other patient had a 5L Impella (21F) inserted via surgical subclavian access, but unfortunately, passed away due to gastro-intestinal bleeding and multiple organ system failure—complications unrelated to Impella.
The third patient with acute decompensated heart (and renal) failure due to dilated cardiomyopathy had a 2.5L Impella implanted femorally but needed early extracorporeal membrane oxygenation (ECMO) due to insufficient haemodynamic response; he eventually underwent successful cardiac and renal transplantation.
Outcomes
The 30-day outcomes are shown in Table 3. In-hospital death occurred in 10 patients (20%). One patient in whom Impella could not be implanted due to vessel tortuosity underwent high-risk PCI but died due to ischaemic cardiogenic shock immediately following the procedure. Two further patients in this group died due to cardiogenic shock, while one died of pre-existing severe sepsis, having also had a stroke with a dense neurodeficit in the setting of significant bilateral carotid artery stenoses. Two patients were given blood transfusions but only as an aid to clinical recovery in the setting of pre-existing anaemia with no evidence of bleeding or a fall in haemoglobin levels. One patient who underwent rotablation and PCI to an unprotected left main stem and left-anterior descending (LAD) artery unfortunately developed coronary perforation and tamponade. Impella provided excellent support while this was managed and the device was removed successfully at the end of the procedure. The patient subsequently died on day 2 post-PCI. Another patient who underwent BAV had a suspected retroperitoneal haematoma which could not be radiologically confirmed, as he passed away as soon as the Impella device was removed. There were no other major vascular complications.
Table 3.
Outcomes
| Outcomes | Total (N = 49) | High-risk PCI (N = 45) | BAV (N = 1) | Transplant (N = 3) |
|---|---|---|---|---|
| 30-day mortality | 10 (20%) | 8 (18%) | 1 (100%) | 1 (33%) |
| Blood transfusion | 3 (6%) | 2 (5%) | 0 | 1 (33%) |
| Vascular complications | 1 (2%) | 0 | 1 (100%) | 0 |
| Stroke | 1 (2%) | 1 (2%) | 0 | 0 |
| PMI | 1 (2%) | 1 (2%) | 1 (100%) | 0 |
| Hospital stay (days) | 5 ± 6 (1–22) | 5 ± 6 (1–22) | – | – |
BAV balloon aortic valvuloplasty, PCI percutaneous coronary intervention, PMI peri-procedural myocardial infarction
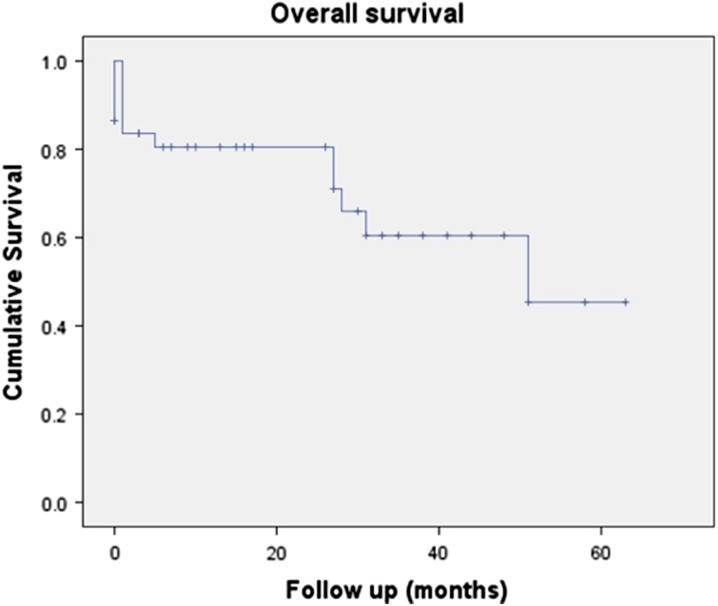
Within the group of patients studied, 30-day survival was 80% (39/49), while overall survival after a median follow-up of 29 months (range 1–71 months) was 65% (32/49), as shown in Fig. 1.
Fig. 1.
Kaplan–Meier Curve showing survival over a median follow-up period of 29 months (range 1–71)
Discussion
We have reported the first real-world experience of the Impella percutaneous left ventricular assist device including the 3.8L device across a range of indications. We have demonstrated its safety, feasibility and efficacy in high-risk PCI, to support BAV and as a bridge to transplantation in acute decompensated heart failure.
The BCIS-1 investigators reported no reduction in 30-day major adverse cardiac events (MACE) with the use of IABP prophylactically in elective high-risk PCI [5]. However, on longer follow-up of 5 years, there was a statistically significant reduction in all––cause mortality in the IABP group [25], although the mechanism of this benefit is unclear. While the role of IABP in the elective setting remains controversial, current guidelines [30] and recent trials [6] have also questioned the efficacy of IABP in cardiogenic shock complicating acute myocardial infarction.
The Impella ventricular assist device (VAD) system, however, has been shown to provide superior haemodynamic support than IABP [16, 17]. The randomised PROTECT-II study [16] demonstrated that in the setting of high-risk elective PCI, the Impella device not only provided superior haemodynamic support than IABP, but also resulted in a statistically significant 22% reduction in major adverse events at 90 days largely driven by a reduction in repeat revascularisation. Impella facilitated more aggressive and complete revascularisation with a higher use of rotational atherectomy (14% vs. 9%). Similarly, we have also shown a high proportion of rotational atherectomy (31%) in our high-risk PCI cohort.
At present, there is no agreed definition of what constitutes a high-risk PCI, although large registries such as Europella [15] and USpella [1] have included patients undergoing PCI to unprotected left main stem, PCI to the last remaining vessel and multivessel PCI in the context of impaired left ventricular function. Patients not suitable for CABG due to high risk are increasingly undergoing PCI which has been shown to be a safe and feasible strategy in these patients [1]. 62% of our patients were discussed by the MDT and were deemed to be at too high risk for CABG due to co-morbidities.
Several risk scoring models such as the Mayo PCI risk score have been developed to anticipate the in-hospital major event rate in PCI. We have used the North West Quality Improvement Programme (NWQIP) scoring system, which had been standardised to a UK population and validated against the established international risk models [31]. Population-based risk models, however, may not capture all the high-risk characteristics of an individual patient, and the definition of high-risk PCI and the need for mechanical LV support remains the discretion of the operator. Indeed our mean NWQIP predicted major event rate was 6 ± 11% (range 0.4–71), which is comparable to the mean Mayo PCI score in the PROTECT II trial (8.8 ± 3.4% in the Impella group) [16], although our in-hospital mortality was significantly higher at 20%.
The overall 30-day major arrhythmic events (MAE) rate in the USpella registry was 8% with 4% in-hospital mortality [1]. In the Europella registry, 30-day mortality was 5.5% with a 30-day MAE rate of 12.3% (including major vascular complications) [15]. Our in-hospital mortality and MAE of 20% is substantially higher than both these registries. This may either be a reflection of smaller patient numbers in this study, or the higher-risk profile of the population, as discussed earlier. Indeed in the smaller cohort of the Protect I trial (N = 20), the 30-day MAE rate was 20% [32], while in the larger PROTECT II trial (N = 225) it was 7% [16].
Interestingly, we report only one major vascular complication in our cohort as compared to 4% in USpella and 5.5% in the Europella registry. While this could be a reflection of smaller patient numbers, it also suggests increasing experience with larger arterial access in the era of percutaneous valvular interventions (TAVI) and the use of newer techniques such as micropuncture and pre-closure with percutaneous suture devices. This is important in a cohort of patients who can ill-afford any further haemodynamic setbacks.
In this cohort, 52% had PCI to unprotected left main stem, 40% underwent multi-vessel PCI and 20% had PCI to the last remaining vessel—essentially comparable to the USpella [1] and Europella [15] registries. However, our patients were older (mean age 75 vs. 70 years in the USpella and Europella registries) and had a greater incidence of severe LV impairment (80% vs. 69%). Although the distribution of elective and urgent PCI was comparable to the USpella registry (the Europella registry only included elective cases), we have used Impella in 3 patients with cardiogenic shock, 2 with ischaemic pulmonary oedema and, importantly, in 5 patients with coexisting severe AS; patients with these conditions were not included in the larger registries. Overall, our patients were a substantially higher-risk cohort when compared to these registries. This reflects a growing confidence in the use of Impella and its expanding indications in acute cardiac care.
Impella in Aortic Stenosis
Severe AS is reported to be a contraindication to Impella use due to theoretical concerns of either reducing effective valve orifice or inducing aortic incompetence. However, with increasing experience there have been anecdotal reports [33, 34] and a recent series [35] describing the use of Impella in this setting. The use of Impella in our patients with severe AS is an example of the expanding indication for this device. The methods used for implanting the device in these patients have been described by our group in a previous report [27]. One patient with severe AS and LV dysfunction underwent emergency BAV with Impella support for intractable cardiogenic shock. The rapid deterioration and death that followed removal of the device in this patient for suspected retroperitoneal bleeding highlighted the significant haemodynamic benefit provided by Impella.
Transplantation
Impella was used with success in one patient with acute post-infarct cardiogenic shock as a bridge to transplantation. There are anecdotal reports of the use of Impella in similar situations with both the 2.5L and 5L device, and also in transplanted hearts for acute rejection [21, 22]. A recent series [36] has also reported the use of 5L Impella support in 9 patients with cardiogenic shock due to end-stage ischaemic cardiomyopathy (3 patients) and post-ST elevation myocardial infarction (6 patients). This less invasive percutaneous VAD is therefore, a useful additional tool in advanced heart failure management.
We have described the use of the Impella CP 3.8L device in 11 patients—10 for PCI and one BAV. To our knowledge, this is the first series reporting the use of the 3.8L device which has only recently received Conformité Européenne (CE) marking. Notably, despite the larger lumen vascular access (14F vs. 13F for 2.5L), there was no increase in the incidence of vascular complications, and the haemodynamic support was reliable and superior to that provided by the 2.5L device.
We have confirmed that the indications for the Impella device are expanding and that it can be used in acutely unwell patients with a high degree of success. The main limitations of the Impella device include the requirement for large lumen vascular access and closure, and the significantly higher cost of the device as compared to mechanical support using IABP. A recent study has also confirmed cost-effectiveness of Impella [37] compared with IABP, an issue which is crucial in the current economic environment. Moreover, the 2014 European Society of Cardiology guidelines on myocardial revascularisation no longer recommend routine use of IABP in cardiogenic shock, complicating myocardial infarction (class III recommendation). However, mechanical support using devices such as Impella may now be considered for short-term support (class IIb recommendation) in this setting [38].
The main limitation of our study is its retrospective nature. No comparison with IABP use was attempted, as IABP was used in milder degrees of circulatory disturbance whereas Impella was often used as a last resort. This retrospective analysis provides an insight into "real-world" experience with Impella.
Conclusion
We have demonstrated its feasibility and safety in a cohort of higher risk patients with extended and novel indications, and reported for the first time globally the feasibility and safety of the 3.8L device. With increasing experience in the use of Impella, the device may be used to provide invaluable support to increasingly complex patients who would, in turn, have the largest benefit. This could be a subject of future larger studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. No funding or sponsorship was received for this study or publication of this article. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Conflict of interest
V. Venugopal, J. Spiro, A. Zaphiriou, S. Khan, J. N. Townend, P. F. Ludman and S. N. Doshi declare no conflicts of interest.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors. The analysis in this article is based on previously recorded data, and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Maini B, Naidu SS, Mulukutla S, et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: the USpella Registry. Catheter Cardiovasc Interv. 2012;80(5):717–725. doi: 10.1002/ccd.23403. [DOI] [PubMed] [Google Scholar]
- 2.Sjauw KD, Engstrom AE, Henriques JP. Percutaneous mechanical cardiac assist in myocardial infarction. Where are we now, where are we going? Acute Card Care. 2007;9(4):222–230. doi: 10.1080/17482940701534818. [DOI] [PubMed] [Google Scholar]
- 3.Keelan PC, Johnston JM, Koru-Sengul T, et al. Comparison of in-hospital and one-year outcomes in patients with left ventricular ejection fractions ≤40%, 41–49%, and ≥50% having percutaneous coronary revascularization. Am J Cardiol. 2003;91(10):1168–1172. doi: 10.1016/S0002-9149(03)00261-3. [DOI] [PubMed] [Google Scholar]
- 4.Wallace TW, Berger JS, Wang A, Velazquez EJ, Brown DL. Impact of left ventricular dysfunction on hospital mortality among patients undergoing elective percutaneous coronary intervention. Am J Cardiol. 2009;103(3):355–360. doi: 10.1016/j.amjcard.2008.09.088. [DOI] [PubMed] [Google Scholar]
- 5.Perera D, Stables R, Thomas M, BCIS-1 Investigators et al. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention: a randomized controlled trial. JAMA. 2010;304(8):867–874. doi: 10.1001/jama.2010.1190. [DOI] [PubMed] [Google Scholar]
- 6.Thiele H, Zeymer U, Neumann FJ, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 7.Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW; TandemHeart Investigators Group. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152(3):469.e1–8. [DOI] [PubMed]
- 8.Thiele H, Sick P, Boudriot E, et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26(13):1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 9.Sauren LD, Accord RE, Hamzeh K, et al. Combined Impella and intra-aortic balloon pump support to improve both ventricular unloading and coronary blood flow for myocardial recovery: an experimental study. Artif Organs. 2007;31(11):839–842. doi: 10.1111/j.1525-1594.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 10.Reesink KD, Dekker AL, Van Ommen V, et al. Miniature intracardiac assist device provides more effective cardiac unloading and circulatory support during severe left heart failure than intraaortic balloon pumping. Chest. 2004;126(3):896–902. doi: 10.1378/chest.126.3.896. [DOI] [PubMed] [Google Scholar]
- 11.Cubeddu RJ, Lago R, Horvath SA, Vignola PA, O’Neill W, Palacios IF. Use of the Impella 2.5 system alone, after and in combination with an intra-aortic balloon pump in patients with cardiogenic shock: case description and review of the literature. EuroIntervention. 2012;7(12):1453–1460. doi: 10.4244/EIJV7I12A226. [DOI] [PubMed] [Google Scholar]
- 12.Remmelink M, Sjauw KD, Henriques JP, et al. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70(4):532–537. doi: 10.1002/ccd.21160. [DOI] [PubMed] [Google Scholar]
- 13.Remmelink M, Sjauw KD, Henriques JP, et al. Effects of mechanical left ventricular unloading by Impella on left ventricular dynamics in high-risk and primary percutaneous coronary intervention patients. Catheter Cardiovasc Interv. 2010;75(2):187–194. doi: 10.1002/ccd.22263. [DOI] [PubMed] [Google Scholar]
- 14.Valgimigli M, Steendijk P, Sianos G, Onderwater E, Serruys PW. Left ventricular unloading and concomitant total cardiac output increase by the use of percutaneous Impella Recover LP 2.5 assist device during high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65(2):263–267. doi: 10.1002/ccd.20380. [DOI] [PubMed] [Google Scholar]
- 15.Sjauw KD, Konorza T, Erbel R, et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54(25):2430–2434. doi: 10.1016/j.jacc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 16.O’Neill WW, Kleiman NS, Moses J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 17.Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J. 2009;30(17):2102–2108. doi: 10.1093/eurheartj/ehp292. [DOI] [PubMed] [Google Scholar]
- 18.Seyfarth M, Sibbing D, Bauer I, et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;52(19):1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 19.Lauten A, Engstrom AE, Jung C, et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013;6(1):23–30. doi: 10.1161/CIRCHEARTFAILURE.112.967224. [DOI] [PubMed] [Google Scholar]
- 20.Myers TJ. Temporary ventricular assist devices in the intensive care unit as a bridge to decision. AACN Adv Crit Care. 2012;23(1):55–68. doi: 10.1097/NCI.0b013e318240e369. [DOI] [PubMed] [Google Scholar]
- 21.Chandola R, Cusimano R, Osten M, Horlick E. Use of Impella 5L for acute allograft rejection postcardiac transplant. Thorac Cardiovasc Surg. 2012;60(4):302–304. doi: 10.1055/s-0032-1304553. [DOI] [PubMed] [Google Scholar]
- 22.Rajagopal V, Steahr G, Wilmer CI, Raval NY. A novel percutaneous mechanical biventricular bridge to recovery in severe cardiac allograft rejection. J Heart Lung Transplant. 2010;29(1):93–95. doi: 10.1016/j.healun.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Patanè F, Zingarelli E, Sansone F, Rinaldi M. Acute ventricular septal defect treated with an Impella recovery as a ‘bridge therapy’ to heart transplantation. Interact CardioVasc Thorac Surg. 2007;6(6):818–819. doi: 10.1510/icvts.2007.159558. [DOI] [PubMed] [Google Scholar]
- 24.Hollander SA, Reinhartz O, Chin C, et al. Use of the Impella 5.0 as a bridge from ECMO to implantation of the HeartMate II left ventricular assist device in a pediatric patient. Pediatr Transplant. 2012;16(2):205–206. doi: 10.1111/j.1399-3046.2011.01578.x. [DOI] [PubMed] [Google Scholar]
- 25.Perera D, Stables R, Clayton T, BCIS-1 Investigators et al. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): a randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation. 2013;127(2):207–212. doi: 10.1161/CIRCULATIONAHA.112.132209. [DOI] [PubMed] [Google Scholar]
- 26.Sassard T, Scalabre A, Bonnefoy E, Sanchez I, Farhat F, Jegaden O. The right axillary artery approach for the Impella Recover LP 5.0 microaxial pump. Ann Thorac Surg. 2008;85(4):1468–1470. doi: 10.1016/j.athoracsur.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Spiro J, Venugopal V, Raja Y, Ludman PF, Townend JN, Doshi SN. Feasibility and efficacy of the 2.5L and 3.8L Impella percutaneous left ventricular support device during high-risk, percutaneous coronary intervention in patients with severe aortic stenosis. Catheter Cardiovasc Interv. 2014. doi:10.1002/ccd.25355. [DOI] [PubMed]
- 28.Brener SJ, Ellis SG, Schneider J, Topol EJ. Frequency and long-term impact of myonecrosis after coronary stenting. Eur Heart J. 2002;23(11):869–876. doi: 10.1053/euhj.2001.2976. [DOI] [PubMed] [Google Scholar]
- 29.Kini A, Marmur JD, Kini S, et al. Creatine kinase-MB elevation after coronary intervention correlates with diffuse atherosclerosis, and low-to-medium level elevation has a benign clinical course: implications for early discharge after coronary intervention. J Am Coll Cardiol. 1999;34(3):663–671. doi: 10.1016/S0735-1097(99)00298-3. [DOI] [PubMed] [Google Scholar]
- 30.Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) Steg PG, James SK, Atar D, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 31.Kunadian B, Dunning J, Das R, et al. External validation of established risk adjustment models for procedural complications after percutaneous coronary intervention. Heart. 2008;94(8):1012–1018. doi: 10.1136/hrt.2007.129197. [DOI] [PubMed] [Google Scholar]
- 32.Dixon SR, Henriques JP, Mauri L. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial US experience. JACC Cardiovasc Interv. 2009;2(2):91–96. doi: 10.1016/j.jcin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Badawi RA, Grise MA, Thornton SN. Impella 2.5 assisted balloon aortic valvuloplasty and percutaneous coronary intervention as a bridge to heart transplantation. J Invasive Cardiol. 2012;24(5):229–230. [PubMed] [Google Scholar]
- 34.Londoño JC, Martinez CA, Singh V, O’Neill WW. Hemodynamic support with impella 2.5 during balloon aortic valvuloplasty in a high-risk patient. J Interv Cardiol. 2011;24(2):193–197. doi: 10.1111/j.1540-8183.2010.00625.x. [DOI] [PubMed] [Google Scholar]
- 35.Martinez CA, Singh V, Londoño JC, et al. Percutaneous retrograde left ventricular assist support for interventions in patients with aortic stenosis and left ventricular dysfunction. Catheter Cardiovasc Interv. 2012;80(7):1201–1209. doi: 10.1002/ccd.24303. [DOI] [PubMed] [Google Scholar]
- 36.Bresson D, Sibellas F, Farhat F, Jegaden O, Kirkorian G, Bonnefoy E. Preliminary experience with Impella Recover((R)) LP5.0 in nine patients with cardiogenic shock: a new circulatory support system in the intensive cardiac care unit. Arch Cardiovasc Dis. 2011;104(8–9):458–464. doi: 10.1016/j.acvd.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Roos JB, Doshi SN, Konorza T, et al. The cost-effectiveness of a new percutaneous ventricular assist device for high-risk PCI patients: mid-stage evaluation from the European perspective. J Med Econ. 2013;16(3):381–390. doi: 10.3111/13696998.2012.762004. [DOI] [PubMed] [Google Scholar]
- 38.Authors/Task Force members. Windecker S, Kolh P, Alfonso F. 2014 ESC/EACTS guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35(37):2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.