Abstract
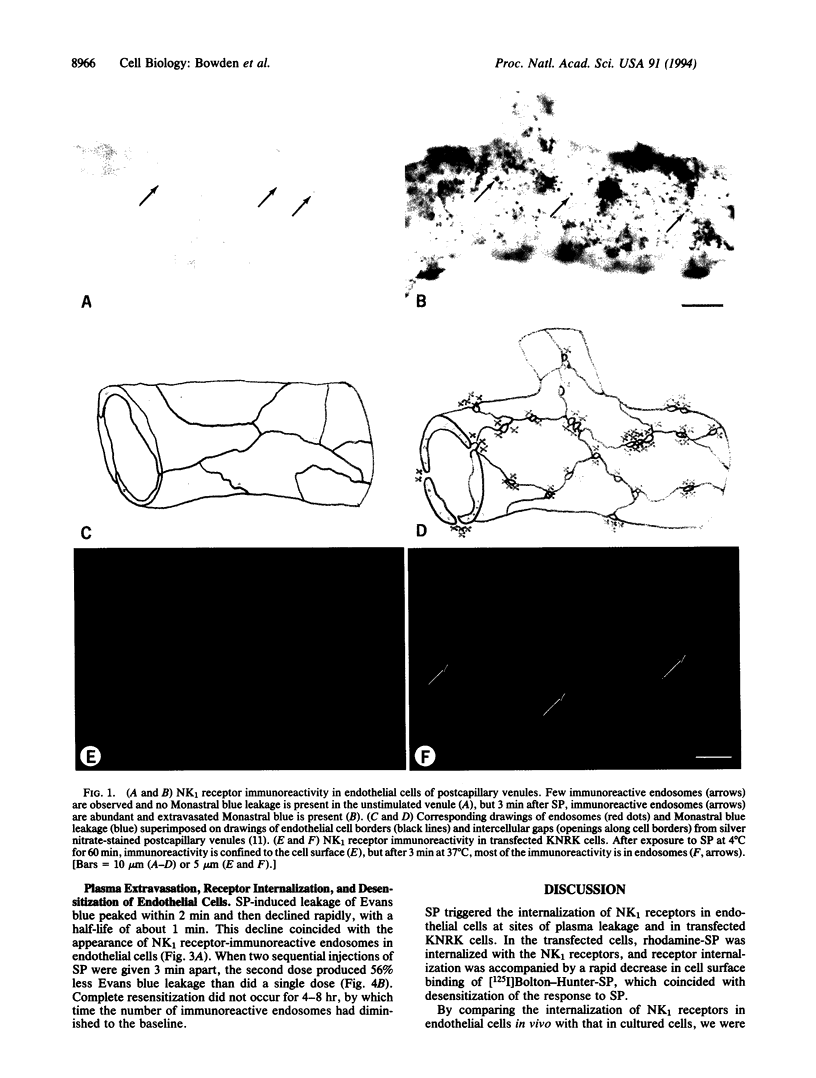
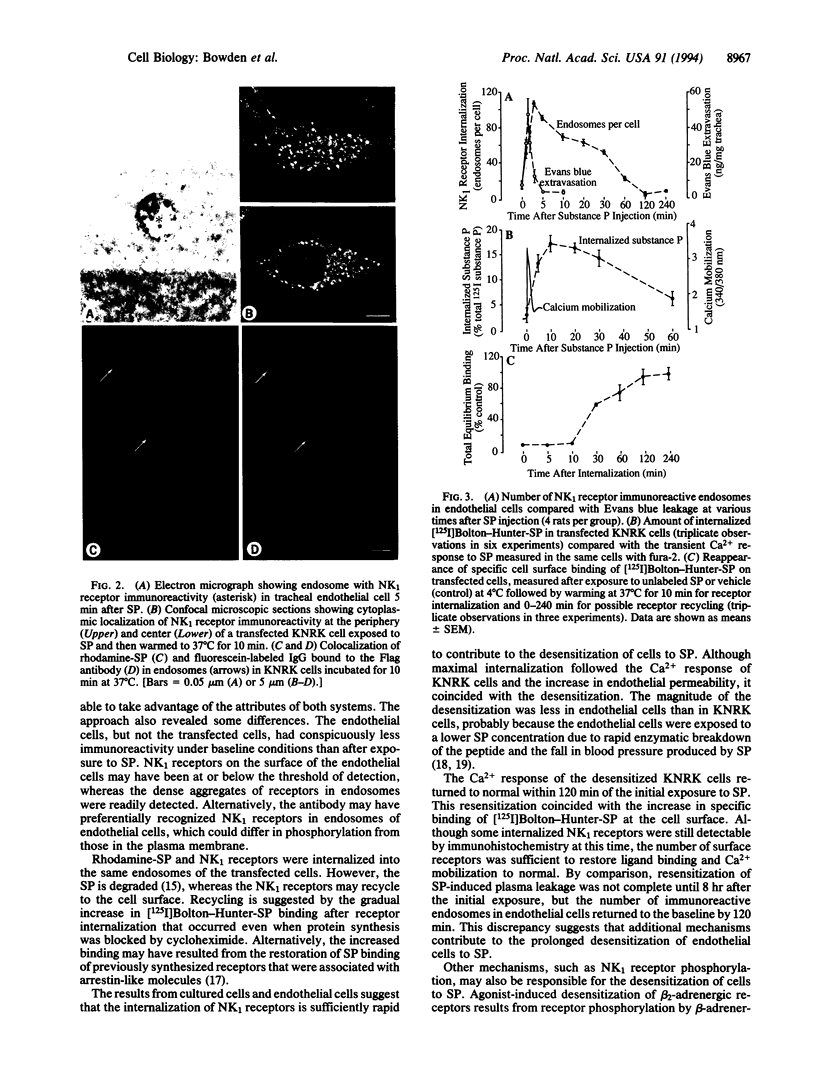
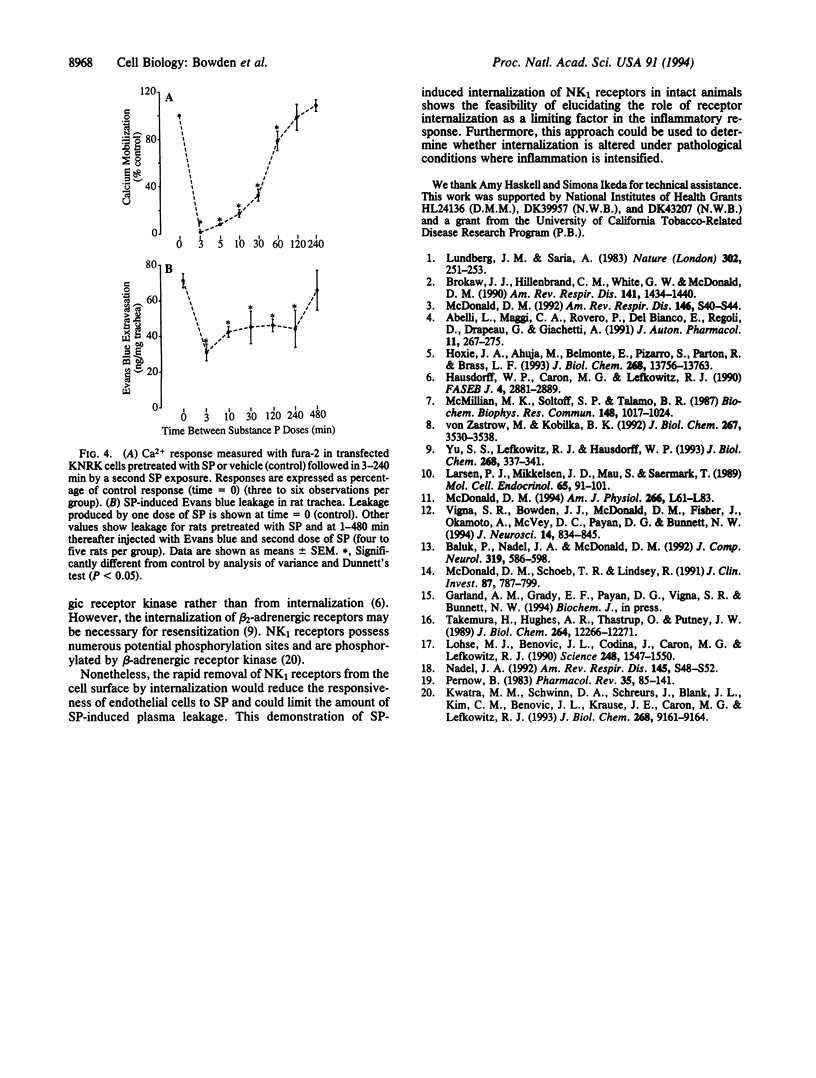
Substance P (SP) can cause plasma leakage at sites of inflammation by binding to neurokinin type 1 (NK1) receptors on the surface of endothelial cells. Internalization after ligand binding could reduce the number of NK1 receptors on the cell surface and thus participate in the desensitization and resensitization of the inflammatory response to SP. By using an antibody to the receptor, we directly observed SP-induced internalization of NK1 receptors into endosomes in endothelial cells of postcapillary venules in the rat tracheal mucosa. In the absence of SP, an average of 15 immunoreactive endosomes were present per endothelial cell. After an intravenous injection of SP, the number of immunoreactive endosomes peaked at 107 per cell at 3 min and gradually returned to the baseline by 120 min. In parallel experiments we observed that when cultured cells transfected with the NK1 receptor were exposed to rhodamine-SP and an antibody to an extracellular Flag epitope of the NK1 receptor, the SP was internalized with the receptor antibody. Both in the cultured cells and in the endothelial cells of intact animals, the prompt SP-induced internalization was accompanied by rapid, long-lasting desensitization to SP. These studies suggest that internalization of NK1 receptors by endothelial cells may be one of the mechanisms that limit the amount of plasma leakage at sites of inflammation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelli L., Maggi C. A., Rovero P., Del Bianco E., Regoli D., Drapeau G., Giachetti A. Effect of synthetic tachykinin analogues on airway microvascular leakage in rats and guinea-pigs: evidence for the involvement of NK-1 receptors. J Auton Pharmacol. 1991 Aug;11(4):267–275. doi: 10.1111/j.1474-8673.1991.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Baluk P., Nadel J. A., McDonald D. M. Substance P-immunoreactive sensory axons in the rat respiratory tract: a quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol. 1992 May 22;319(4):586–598. doi: 10.1002/cne.903190408. [DOI] [PubMed] [Google Scholar]
- Brokaw J. J., Hillenbrand C. M., White G. W., McDonald D. M. Mechanism of tachyphylaxis associated with neurogenic plasma extravasation in the rat trachea. Am Rev Respir Dis. 1990 Jun;141(6):1434–1440. doi: 10.1164/ajrccm/141.6.1434. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Hoxie J. A., Ahuja M., Belmonte E., Pizarro S., Parton R., Brass L. F. Internalization and recycling of activated thrombin receptors. J Biol Chem. 1993 Jun 25;268(18):13756–13763. [PubMed] [Google Scholar]
- Kwatra M. M., Schwinn D. A., Schreurs J., Blank J. L., Kim C. M., Benovic J. L., Krause J. E., Caron M. G., Lefkowitz R. J. The substance P receptor, which couples to Gq/11, is a substrate of beta-adrenergic receptor kinase 1 and 2. J Biol Chem. 1993 May 5;268(13):9161–9164. [PubMed] [Google Scholar]
- Larsen P. J., Mikkelsen J. D., Mau S., Saermark T. Binding and internalization of a iodinated substance P analog by cultured anterior pituitary cells. Mol Cell Endocrinol. 1989 Aug;65(1-2):91–101. doi: 10.1016/0303-7207(89)90169-x. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983 Mar 17;302(5905):251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- McDonald D. M. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol. 1994 Jan;266(1 Pt 1):L61–L83. doi: 10.1152/ajplung.1994.266.1.L61. [DOI] [PubMed] [Google Scholar]
- McDonald D. M. Infections intensify neurogenic plasma extravasation in the airway mucosa. Am Rev Respir Dis. 1992 Nov;146(5 Pt 2):S40–S44. doi: 10.1164/ajrccm/146.5_Pt_2.S40. [DOI] [PubMed] [Google Scholar]
- McDonald D. M., Schoeb T. R., Lindsey J. R. Mycoplasma pulmonis infections cause long-lasting potentiation of neurogenic inflammation in the respiratory tract of the rat. J Clin Invest. 1991 Mar;87(3):787–799. doi: 10.1172/JCI115082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Talamo B. R. Rapid desensitization of substance P- but not carbachol-induced increases in inositol trisphosphate and intracellular Ca++ in rat parotid acinar cells. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1017–1024. doi: 10.1016/s0006-291x(87)80233-4. [DOI] [PubMed] [Google Scholar]
- Nadel J. A. Regulation of neurogenic inflammation by neutral endopeptidase. Am Rev Respir Dis. 1992 Feb;145(2 Pt 2):S48–S52. doi: 10.1164/ajrccm/145.2_Pt_2.S48. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol Rev. 1983 Jun;35(2):85–141. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Vigna S. R., Bowden J. J., McDonald D. M., Fisher J., Okamoto A., McVey D. C., Payan D. G., Bunnett N. W. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J Neurosci. 1994 Feb;14(2):834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. S., Lefkowitz R. J., Hausdorff W. P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993 Jan 5;268(1):337–341. [PubMed] [Google Scholar]
- von Zastrow M., Kobilka B. K. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J Biol Chem. 1992 Feb 15;267(5):3530–3538. [PubMed] [Google Scholar]