Abstract
Several species of the genus Liposcelis are common insect pests that cause serious qualitative and quantitative losses to various stored grains and processed grain products. They also can contaminate foods, transmit pathogenic microorganisms and cause allergies in humans. The common occurrence of multi-species infestations and the fact that it is difficult to identify and discriminate Liposcelis spp. make accurate, rapid detection and discriminatory tools absolutely necessary for confirmation of their identity. In this study, PCR primers and probes specific to different Liposcelis spp. were designed based on nucleotide sequences of the cytochrome oxidase 1 (CO1) gene. Primer sets ObsCo13F/13R, PeaCo15F/14R, BosCO7F/7R, BruCo5F/5R, and DecCo11F/11R were used to specifically detect Liposcelis obscura Broadhead, Liposcelis pearmani Lienhard, Liposcelis bostrychophila Badonnel, Liposcelis brunnea Motschulsky and Liposcelis decolor (Pearman) in multiplex endpoint PCRs, which amplified products of 438-, 351-, 191-, 140-, and 87-bp, respectively. In multiplex TaqMan qPCR assays, orange, yellow, red, crimson and green channels corresponding to reporter dyes 6-ROXN, HEX, Cy5, Quasar705 and 6-FAM specifically detected L. obscura, L. brunnea, L. bostrychophila, L. pearmani and L. decolor, respectively. All developed primer and probe sets allowed specific amplification of corresponding targeted Liposcelis species. The development of multiplex endpoint PCR and multiplex TaqMan qPCR will greatly facilitate psocid identification and their management. The use of APCs will streamline and standardize PCR assays. APC will also provide the opportunity to have all positive controls in a single tube, which reduces maintenance cost and labor, but increases the accuracy and reliability of the assays. These novel methods from our study will have applications in pest management, biosecurity, quarantine, food safety, and routine diagnostics.
Introduction
Psocids (booklice or barklice) have caused problems as pests of stored grains and grain products for the last 50 years [1–2]. As far back as over one decade ago, psocids became recognized as serious stored-product pests in some parts of Australia [3]; in the United States they are recognized as pests of substance that cause grain weight loss by consuming the germ and endosperm [4–6]. Psocids are pests because they contaminate food and vector human pathogens [7–9], trigger allergies in humans [10], are not easily controlled by insecticides that are effective against other stored-product pests [11–16], and because the commodities infested by psocids can be rejected for export [6, 17].
Psocids, which belong to the genus Liposcelis (Insecta; Psocodea; Liposcelididae), are most frequently encountered infesting stored products [18]. Psocid infestations usually comprise more than one Liposcelis species [14, 19]. Furthermore, stored-product psocids are able to coexist for a long time on the commodity they infest but this phenomenon is related to species composition [20]
Management of Liposcelis species is difficult because of their differential response to commonly used insecticides and the fumigant phosphine [21–23]; this makes the correct identification of psocids extremely important for successful control. Identification and discrimination of Liposcelis species is done largely by morphological characterization. This task is complicated by the fact that adults are small (ca. 1 mm), and different species are morphologically similar. Discriminating among immature (nymphal) stages of Liposcelis is even more difficult. As a result, identification of both adult and immature stages is usually conducted by specialists or taxonomists, experts who are quite rare [6, 24–29]. Rapid and accurate methods for identifying Liposcelis species are needed for more effective integrated management.
The current availability of nucleic acid-based detection and identification technologies permits rapid, sensitive, and accurate discrimination among morphologically identical species. Conventional or standard endpoint PCR, SYBR Green qPCR and isothermal based methods for identification of psocids have been reported [30–33]. TaqMan probe based qPCR assays have been developed for highly accurate bioforensic detection/identification of plant pathogens [34–35]. However, the cost per reaction of TaqMan qPCR is slightly higher ($1.1) than that of SYBR green based assays ($0.93) [36]. To the best of our knowledge, there are no published studies on the use of multiplex TaqMan qPCR for identification of Liposcelis species.
Molecular assays can now be further enhanced using synthetic biology approaches, such as the development of clonable artificial systems for reproducing natural biological processes [37]. Development of multi-target artificial positive PCR controls (APCs) speeds molecular processing and improves biosafety [37]. Their use allows detection of cross contamination during PCR assays without compromising sensitivity and specificity. The APC approach is a unique way to amplify PCR diagnostic products of different sizes for clear discrimination, and has the advantage to allow rearrangement of the targets, periodic updates (insertion and deletion of new targets), monitoring quality control and international or regional exchange among research and diagnostic centers without the need for sanitary permissions or labels which are time consuming and hard to obtain from respective national and international authorities.
In this work, we developed accurate and rapid (short cycle) multiplex endpoint and multiplex TaqMan qPCR assays for the simultaneous identification and discrimination of five morphologically identical Liposcelis species, namely, L. brunnea, L. decolor, L. bostrychophila, L. pearmani and L. obscura. We also designed and developed customized synthetic PCR APCs. These tools provide capability for applications in insect diagnostics, discrimination and management, population monitoring and agricultural biosecurity.
Materials and Methods
Ethics statement
No specific permission from any government agency or relevant regulatory body was required for the collection of these insects at all of the sites listed below. Collection sites included private facilities that had insect-infested stored grain on site and for which the owners gave us full permission to collect insects. Other collection sites were university research or agricultural research facilities for which we had full permission to collect insects. None of the field sites or the collections of insects from them involved endangered or protected species.
Insects
Eleven psocid species, L. brunnea, L. decolor, L. bostrychophila, L. pearmani, L. obscura, Liposcelis entomophila (Enderlein), Liposcelis fusciceps Badonnel, Liposcelis rufa Broadhead, Liposcelis corrodens Heymons, Liposcelis paeta Pearman, and Lepinotus reticulatus Enderlein were collected in 2004–2009 from different storage types and locations in the United States (Table 1); and reared on a mixture of 93% cracked hard red winter wheat, 5% Rice Krispies (Kellogg Company) and 2% wheat germ (wt/wt) [38] at the Oklahoma State University Stored Product Entomology Laboratory, Stillwater, OK. Lepinotus inquilinus von Heyden and Lepinotus patruelis Pearman were obtained from Illinois State University, Normal, IL, USA. Voucher specimens of 100 females each of L. rufa, L. pearmani, L. bostrychophila, L. decolor, L. entomophila, L. paeta, L. brunnea, L. fusciceps, L. corrodens, L. obscura and L. reticulatus preserved in 95% ethyl alcohol were deposited at the K. C. Emerson Entomology Museum at Oklahoma State University under lot numbers 101, 103, 106, 107, 110, 111, 114, 116, 118, 119 and 120, respectively [30].
Table 1. Details of psocid species.
| Species | Collection locality | Storage type (commodity) | Year collected |
|---|---|---|---|
| Liposcelis brunnea | CGAHR Manhattan, KS, USA | Grain elevator (wheat) | 2006 |
| Liposcelis decolor | CGAHR Manhattan, KS, USA | Steel bins (wheat) | 2006 |
| Liposcelis bostrychophila | CGAHR Manhattan, KS, USA | Grain elevator (wheat) | 2006 |
| Liposcelis pearmani | SPREC Stillwater, OK, USA | Animal feed mill | 2008 |
| Liposcelis obscura | Golden, OK, USA | Warehouse (peanuts) | 2009 |
| Liposcelis entomophila | CGAHR Manhattan, KS, USA | Steel bins (wheat) | 2004 |
| Liposcelis fusciceps | West Lafayette, IN, USA | Animal feed mill | 2006 |
| Liposcelis rufa | SPREC Stillwater, OK, USA | Steel bins (wheat) | 2008 |
| Liposcelis corrodens | CGAHR Manhattan, KS, USA | Grain elevator (wheat) | 2006 |
| Liposcelis paeta | CGAHR Manhattan, KS, USA | Grain elevator (wheat) | 2006 |
| Lepinotus reticulatus | CGAHR Manhattan, KS, USA | Steel bins (wheat) | 2004 |
Information on where different psocid species used in the study were collected.
CGAHR is Center for Grain and Animal Health Research; SPREC is the Stored Product Research and Education Center.
DNA isolation
The identity of each species of psocids was confirmed using decisive morphological traits (Dr. E. Mockford confirmed the identity of all psocid species used in our study). Genomic DNA from 10–20 individuals of each species was extracted using the Blood and Tissue Kit (Qiagen, Valencia, CA). A prepGEM kit (ZyGEM Corporation Ltd, Hamilton, New Zealand) was used for rapid isolation of DNA from a single insect of each species. The DNeasy Plant Mini Kit (Qiagen) and QIAprep Spin Miniprep Kit (Qiagen) were used to isolate DNA from dry cracked wheat pieces (‘Jagger’ variety) and cloned plasmid DNA from overnight grown bacterial cultures carrying the target sequences of each of the respective species. All procedures were performed following the manufacturer’s instructions. DNA concentrations were determined using a NanoDrop v.2000 spectrophotometer (Thermo Fisher Scientific Inc., Worcester, MA).
Liposcelis CO1 region amplification
The amplification of unknown CO1 regions (not available in GenBank) of L. obscura, L. pearmani, L. bostrychophila and L. decolor was approached by performing PCR with combinations of previously reported universal primers for insects [39–42]. Reagents, PCR conditions and electrophoresis for amplification of CO1 gene region of the above listed species using universal primer sets were previously described by Arif et al. [30]. The purification of amplified PCR products was accomplished using either Illustra GFX PCR DNA or the Gel Band Purification Kit (GE Healthcare Biosciences, Piscataway, NJ) following the manufacturer’s instructions. All PCR products were directly sequenced using an Applied Biosystems DNA Analyzer (Model # 3730) at the Oklahoma State University Nucleic Acids and Proteins Core Facility. Sense primers were: UEA1d, UEA1, LCO, Ron, and UEA3 and anti-sense primers were: Nancy, HCO, UEA6, UEA8 and UEA10. Sense and anti-sense of above primer combinations were made and tested across the COI gene region of the above species (S1 Fig). The primer sets Ron/UEA8 for L. bostrychophila, L. pearmani and L decolor, LCO1490/HCO2198 for L. bostrychophila, LCO1490/UEA6 for L. obscura, and LCO1490/Nancy for L. pearmani, were used for sense and anti-sense strand sequencing. The gene sequences generated using these universal primers combinations were submitted to GenBank under the accession numbers of KP012572, KP012571, KP012569 and KP012570.
Species specific primer and probe design
The CO1 gene sequences of individual Liposcelis species obtained using universal sense and anti-sense primers were aligned using CLUSTALX2 [43] to generate the consensus sequence. Sense and anti-sense primers for multiplex endpoint and TaqMan qPCR with the respective probes for each species were designed within the newly obtained CO1 gene regions (Tables 2 and 3) using Primer3 [44], following parameters previously reported by Arif and Ochoa-Corona [45]. The primers and probe for L. brunnea were designed using accession GenBank: GU569291 (Tables 2 and 3). Prediction of internal structure and self-dimer formation of each primer and probe were examined in silico using Primer3 “ANY” score and the mFold output [46]. Primer sets ObsCo13F/ObsCo13R (product size 438 bp), PeaCo15F/PeaCo14R (product size 351 bp), BosCO7F/BosCO7R (product size 191 bp), BruCo5F/BruCo5R (product size 140 bp), and DecCo11F/DecCo11R (product size 87 bp) for L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor, respectively, were designed to perform single and multiplex endpoint PCR amplifications (Table 2). A second group of primer sets BruCo5F/BruCo5R (140 bp), BosCo8F/BosCo8R (131 bp), PeaCo14F/PeaCo14R (115 bp), ObsCo12F/ObsCo12R (100 bp), and DecCo11F/DecCo11R (87 bp) for L. brunnea, L. bostrychophila, L. pearmani, L. obscura and L. decolor, respectively, were designed to perform single and multiplex TaqMan qPCR (Table 2). The probes were labeled using different reporter dyes: 6-FAM, HEX, 6-ROXN, Cy5 (Integrated DNA Technologies, Inc., Coralville, IA) and Quasar705 (Biosearch Technologies, Inc., Novato, CA) (Table 3).
Table 2. Details of primers.
| Target Liposcelis species | Primer Name | Primer Sequence (5’-3’) | Length (bp) | GC% | *Tm | **ΔG | ^ any | ^^ 3’ | Intended use |
|---|---|---|---|---|---|---|---|---|---|
| Liposcelis brunnea | BruCo5F | GGGGTTTTAGGGTTTGTGGTA | 21 | 47.2 | 60.0 | 0.8 | 2 | 2 | Endpoint/qPCR multiplex |
| BruCo5R | AATGTCGCCAACCATCTAAAG | 21 | 42.9 | 59.1 | 0 | 4 | 2 | Endpoint/qPCR multiplex | |
| BruCo6F | TTTATGCGATAGGAGCGATTG | 21 | 42.9 | 60.2 | 0.5 | 4 | 2 | Single PCR | |
| BruCo6R | CATCCAACCCGACAGTAAACA | 21 | 47.7 | 60.7 | 1.0 | 3 | 0 | Single PCR | |
| Liposcelis bostrychophila | BosCo7F | CGATCCCTACCGGAGTTAAAG | 21 | 52.4 | 60.0 | 0.7 | 4 | 1 | Endpoint PCR multiplex |
| BosCo7R | TGGGCAACAACATAGTATCTATCG | 24 | 41.7 | 60.3 | 0.7 | 6 | 4 | Endpoint PCR multiplex | |
| BosCo8F | ATGCTCTCAATCGGAGCTCTAG | 22 | 50.0 | 60.1 | 0.7 | 6 | 4 | qPCR multiplex | |
| BosCo8R | ACTTTAACTCCGGTAGGGATCG | 22 | 50.0 | 60.7 | 0.9 | 4 | 2 | qPCR multiplex | |
| BosCo9F | TGGGCTAATCTCTCACATCATCT | 23 | 43.5 | 60.1 | 0.9 | 3 | 3 | Single PCR | |
| Liposcelis decolor | DecCo10F | CCGGCTTTTGGTATTATTTCAC | 22 | 40.9 | 59.8 | 0.7 | 4 | 1 | Single PCR |
| DecCo10R | ATTATCCCCATTACCCCAAA | 20 | 40.0 | 58.0 | 0.9 | 3 | 0 | Single PCR | |
| DecCo11F | CGAGCTTATTTTACTTCTGCGACT | 24 | 41.7 | 60.4 | 0.9 | 4 | 1 | Endpoint/qPCR multiplex | |
| DecCo11R | TGATCCATACAACGTAGCTAGTCA | 24 | 41.7 | 58.9 | 1.0 | 6 | 2 | Endpoint/qPCR multiplex | |
| Liposcelis obscura | ObsCo12F | GGACAGGGTGGACGGTTTATC | 21 | 57.1 | 62.8 | 1.0 | 3 | 1 | qPCR multiplex |
| ObsCo12R | CTGATTCCTGCTAAATGAAGAGAG | 24 | 41.7 | 58.8 | 0.9 | 3 | 0 | qPCR multiplex | |
| ObsCo13F | AGCTATTGCTCACGGAGGATA | 21 | 47.6 | 59.0 | 0 | 6 | 4 | Endpoint PCR multiplex | |
| ObsCo13R | CCAATTGCGGACATAGCATAA | 21 | 42.9 | 60.8 | 1.0 | 6 | 1 | Endpoint PCR multiplex | |
| Liposcelis pearmani | PeaCo14F | CTGACTTTTTCCCCCTTCACT | 21 | 47.6 | 59.6 | 0.9 | 3 | 1 | qPCR multiplex |
| PeaCo14R | GCCAGGGTGTGAGATTCTAAA | 21 | 47.6 | 59.2 | 0.9 | 5 | 3 | Endpoint/qPCR multiplex | |
| PeaCo15F | TGGTGTGTGAGCAGGTATGGT | 21 | 52.4 | 61.5 | 0.8 | 2 | 0 | Endpoint PCR multiplex |
Sequences of Liposcelis species specific primers and their thermodynamic features used for endpoint PCR and real-time TaqMan qPCR.
*The melting temperature of the primer calculated using Primer 3;
**Plot ΔG value in plot calculated by mFOLD;
^ The self-complementarity score of the oligo (tendency of oligo to anneal to itself or form a secondary structure) calculated using Primer 3;
^^3’ self-complementarity of the oligo (tendency to form a primer-dimer with itself) calculated using Primer3.
Table 3. Details of TaqMan probes.
| Target Liposcelis species | Probe | Probe Sequence (5’-3’) | Length (bp) | GC% | *ΔG | ^ any | ^^ 3’ | Reporter dye | Spectra (nm) | Quencher dye | Channel used in Rotor Gene | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EX | EM | |||||||||||
| Liposcelis decolor | DecCo11P | TGCTGTTCCTACAGGAATCAAGGTTTT | 27 | 41 | 1.0 | 8 | 0 | 6-FAM | 495 | 520 | BHQ2 | Green |
| Liposcelis brunnea | BruCo5P | TGTTTACTGTCGGGTTGGATGTGGA | 25 | 48 | 0.9 | 3 | 0 | HEX | 535 | 554 | ZEN-IABkFQ | Yellow |
| Liposcelis obscura | ObsCo12P | CCTCAGCTATTGCTCACGGAGGA | 23 | 56 | 0.9 | 6 | 4 | 6-ROXN | 575 | 602 | BHQ2 | Orange |
| Liposcelis bostrychophila | BosCo8P | GGGCATGGATGTGGATAGACGAGC | 24 | 58 | 1.0 | 4 | 2 | Cy5 | 647 | 667 | BHQ2 | Red |
| Liposcelis pearmani | PeaCo14P | AGGGAGTCGGAACCGGGTGAA | 21 | 62 | 0.8 | 5 | 0 | Quasar705 | 690 | 705 | BHQ3 | Crimson |
Liposcelis species specific probes used for multiplex TaqMan qPCR.
*Plot ΔG value in plot calculated by mFOLD;
^ The self-complementarity score of the oligo (tendency of oligo to anneal to itself or form a secondary structure) calculated using Primer 3;
^^3’ self-complementarity of the oligo (tendency to form a primer-dimer with itself) calculated using Primer3; EX is the excitation spectra and EM is the emission spectra.
Multiplex endpoint PCR
Single PCR reactions with all species specific primer combinations were performed according to conditions and components described by Arif et al. [30]. Multiplex PCR assays were carried out in 50 μl of reaction mixtures containing 25 μl of Multiplex PCR Master Mix (Qiagen), 0.2 μM of each primer, 5 μl of Q-solution (Qiagen), 1 μl of DNA template from each species and nuclease free water (Ambion, Austin, TX) to make up the volume. PCR amplifications were accomplished in an Eppendorf thermal cycler (Eppendorf, Hauppauge, NY). The cycling parameters were: 15 min at 95°C to activate the HotStar Taq DNA Polymerase followed by 35 cycles, denaturation at 95°C for 20 sec, annealing at 60°C for 90 sec, extension 72°C for 60 sec and final extension at 72°C for 4 min. Amplified PCR products (20 μl) were electrophoresed and separated in a 2% agarose gel in 1X TAE buffer.
Multiplex TaqMan qPCR
Multiplex TaqMan qPCR reactions were carried out in 25 μl reaction mixtures containing 12.5 μl of Rotor-Gene Multiplex PCR Master Mix (Qiagen), 0.5 μM of each primer, 0.2 μM of each probe, 1 μl of each template DNA and nuclease free water to make up the volume. Positive (cloned plasmid DNA: carrying the target gene fragment) and negative (non-template; water) controls were encompassed in each TaqMan qPCR amplification. Each qPCR reaction was completed in three replicates and standard deviation was calculated. Cycling parameters were: 95°C for 5 min to activate the HotStar Taq Polymerase followed by 40 rapid cycles at 95°C for 15 sec, and 60°C for 15 sec. In case of SsoFast Probes Supermix (Bio-Rad, Hercules, CA), reactions were carried out in 20 μl mixture containing 10 μl of SsoFast Probes Supermix, 0.5 μM of each primer, 0.2 μM of each probe, 1 μl of each template DNA and nuclease free water to make up the volume. Cycling parameters for SsoFast Probes Supermix were: 95°C for 2 min initial denaturation followed by 40 rapid cycles at 95°C for 5 sec, and 60°C for 30 sec. The assays were performed in a Rotor-Gene 6000 thermocycler and data analysis was achieved using the Rotor-Gene 6000 series software 1.7 (Built 87) (Corbett Research, Sydney, Australia) with auto and manual cycle threshold (Ct) of 0.2. Auto gain optimization was performed before first acquisition and dynamic tube-based normalization was used.
Multi target artificial positive control (APC)
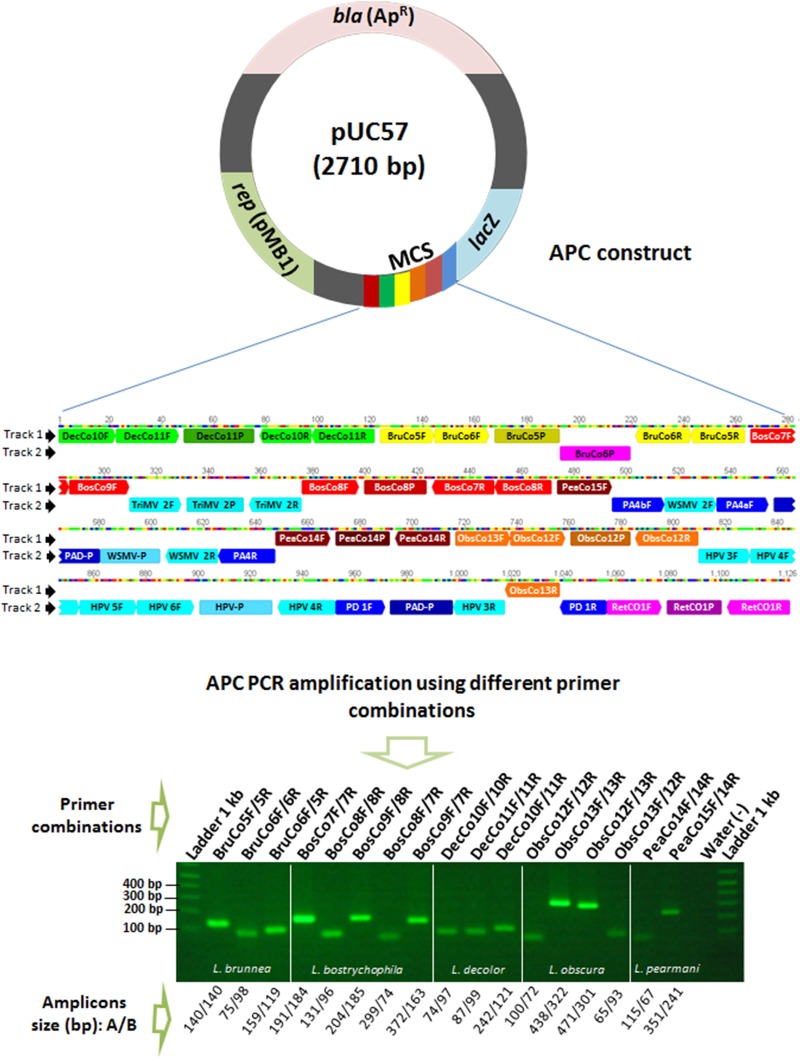
A multi-target APC, generated synthetically (GenScript USA Inc, Piscataway, NJ) was 1126 bp long and composed of tandems of both sense and anti-sense primers and probes including the target sequences of the five Liposcelis species (accession number GenBank:KC555272) in addition to primer and probe sequences of several other phytopathogens (Fig 1). The array of synthetic oligonucleotides was inserted in the multiple cloning site of cloning vector pUC57 (NCBI accession number GenBank:Y14837). PCR generated products obtained with primer sets BruCo6F/BruCo5R (159 bp), BosCo9F/BosCo7R (372 bp), DecCo10F/DecCo11R (242 bp), ObsCo12F/ObsCo13R (471 bp) and PeaCo15F/PeaCo14R (351 bp) were circularized within pCR 2.1-TOPO vector and cloned using TOP10F´ One Shot Chemically Competent cells (TOPO-TA Cloning kit; Invitrogen). The sequences obtained from these cloned DNAs were analyzed in silico for specificity and accuracy.
Fig 1. The multi-target artificial positive control (APC).
Top, APC made by a custom synthesized DNA insert containing tandems of forward, reverse and probe complement priming sequences ligated into the multiple cloning site (MCS) in the vector pUC57. Each amplified PCR product using APC has a unique identifiable sequence. Bottom, the product sizes of the targets differ from the product size and sequences amplified using genomic DNA. A/B, where A is amplicon size generated using genomic DNA of target species and B is amplicon size generated using APC (shown in picture). Different colors at track 1 are the indications of reporter dyes wavelengths (excitation and emission spectra) detected by different channels of real-time qPCR for particular species of Liposcelis as shown in Table 3. Primers and probes sequences indicated by track 1 were used in this study. Primers and probes sequences indicated by track 2 are from fungi, viruses and insect and were not used in this study. Lists of primer and probe sequences are given in Tables 2 and 3, respectively.
Sensitivity assays
The sensitivity and efficiency of each primer set used in endpoint and multiplex TaqMan qPCR were assessed after 10-fold serial dilutions of the developed APC carrying all the target sequences of each primer and probe of the five Liposcelis species. Dilutions of purified APC DNA ranged from 1 ng to 1 fg per reaction. Both endpoint and multiplex TaqMan qPCR assays were also tested to amplify the DNA extracted from an individual insect.
Results
Primer and probe specificity
The use of insect CO1 universal primer combinations allowed the amplification of unknown regions of the CO1 gene of L. decolor, L. bostrychophila, L. pearmani and L. obscura. Specific primers (Table 2) and probes (Table 3) were then designed from the amplified and sequenced CO1 gene regions. These forward and reverse primers were tested in combination against targeted Liposcelis species. The primers were selected based on the product size of each amplified combination (Table 2). For example, primer set PeaCo14F/PeaCo14R, specific for L. pearmani, generated an amplicon of 115 bp, which was ideal for single or multiplex TaqMan qPCR, but this set was not suitable for endpoint multiplex PCR when product size was evaluated among other four amplicons. When PeaCo14F was replaced with PeaCo15F, the amplicon size increased to 351 bp, a size appropriate for multiplex endpoint PCR (Figs 1 and 2).
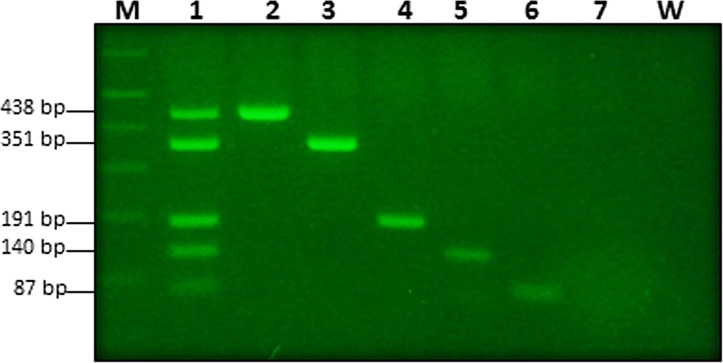
Fig 2. Multiplex endpoint PCR.
Assay was performed with insect genomic DNA and primer sets ObsCo13F/13R (438 bp), PeaCo15F/14R (351 bp), BosCO7F/7R (191 bp), BruCo5F/5R (140 bp), and DecCo11F/11R (87 bp) for L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor, respectively. Lane 1: PCR reaction with all five species genomic DNA. Lanes 2–6: single species genomic DNA viz. L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor, respectively. Lanes M and W are 1 kb ladder and non-template control (water), respectively.
The specificity of the twenty primer (Table 2) was tested in silico and in vitro. The alignment of primer sequences against the GenBank database using BLASTn showed that none of the primers matched with 100% query coverage and 100% identity, because they are newly contributed CO1 gene sequences. However, the primers designed specifically for L. brunnea matched (100%) with accession GenBank:GU569291, as expected. The in vitro specificity assays were performed (using PCR) with each primer set against a panel of near-neighbors including L. inquilinus, L. patruelis, L. reticulatus, L. brunnea, L. bostrychophila, L. decolor, L. rufa, L. entomophila, L. paeta, L. fusciceps, L. obscura, L. pearmani, and L. corrodens (results not shown). No cross reactivity was observed with non-target species in the exclusivity panel. All primer sets amplified only the corresponding Liposcelis species and generated the expected PCR product size with genomic DNA and APC (Fig 1). All primer sets also yielded appropriate negative results when tested for cross reactivity against DNA of the insect Homalodisca vitripennis (Germar) (an out-group) and of cracked wheat grain (a host).
Multiplex endpoint PCR
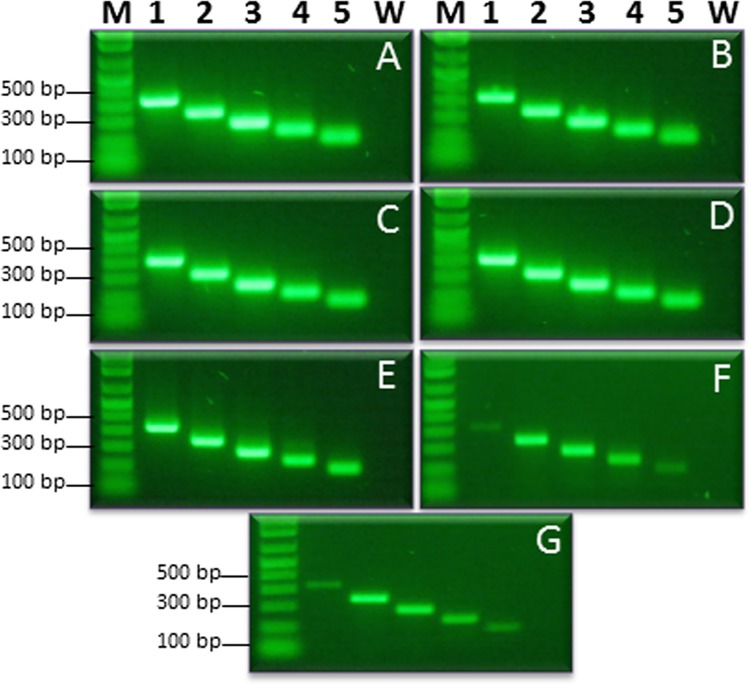
Multiplex endpoint PCRs for detection of L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor were performed using primer sets ObsCo13F/13R, PeaCo15F/14R, BosCO7F/7R, BruCo5F/5R, and DecCo11F/11R, which amplified products of 438-, 351-, 191-, 140-, and 87-bp, respectively (Fig 2 and S2 Fig). Each primer set specifically amplified the corresponding target Liposcelis species. The sensitivity of each primer set was checked with serially diluted APCs. Each primer set detected as little as 1 fg of target APC (Fig 3). The developed multiplex endpoint PCR gave positive results when tested against crude genomic DNA extracted from an individual insect (S2 Fig). No cross reactivity was observed against the non-targeted and/or closely related species of genus Liposcelis.
Fig 3. Endpoint PCR sensitivity assays with individual primer set.
Endpoint PCR performed with individual primer set using 10-fold serially diluted multi target artificial positive control (APC) starting at (A) 1 ng, (B) 100 pg, (C) 10 pg, (D) 1000 fg, (E) 100 fg, (F) 10 fg, and (G) 1 fg. Lane 1 to 5 are primer sets, ObsCo13F/13R (322 bp), PeaCo15F/14R (241 bp), BosCO7F/7R (184 bp), BruCo5F/5R (140 bp), and DecCo11F/11R (99 bp), respectively. Lanes M is a 1 kb ladder and W is a non-template control (water).
Multiplex TaqMan qPCR
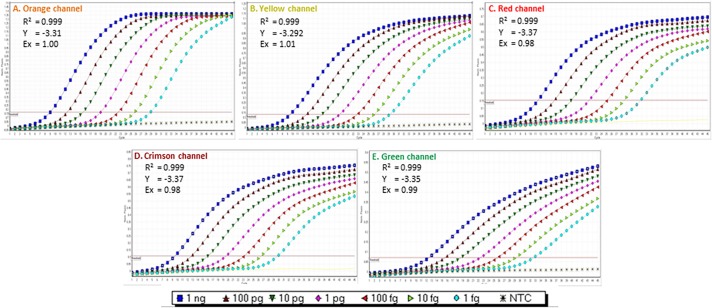
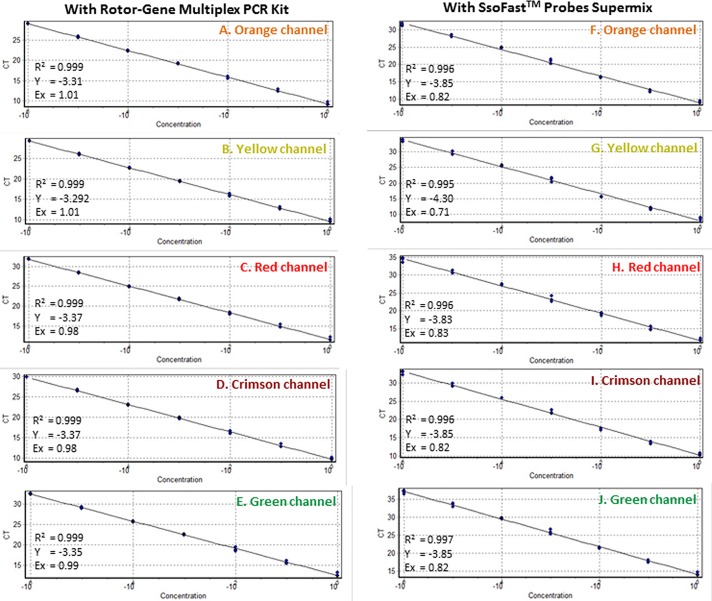
The product sizes resulting from the use of primer sets in multiplex TaqMan qPCR ranged from 87 bp to 140 bp (Fig 1). The excitation/emission spectra (nm) of each probe labeled with 6-FAM (495/520 nm), HEX (535/554 nm), 6-ROXN (575/602 nm), Cy5 (647/667 nm) and Quasar705 (690/705 nm) was different from those of the others, avoiding the overlapping of spectra that could be detected by a corresponding channel leading to false positive results. All channels, whether green, yellow, orange, red or crimson, specifically detected the fluorescence that resulted from its corresponding reporter dye (Table 3). The efficiency of multiplex TaqMan qPCR was evaluated by performing a sensitivity assay with 10-fold serially diluted APC carrying the target sequences of all primer and probe sets (Tables 2 and 3, Figs 4 and 5). The primer and probe sets ObsCo12F/12R/12P; BruCo5F/5R/5P; BosCo8F/8R/8P; PeaCo14F/14R/14P; and DecCo11F/11R/11P, detected down to 1 fg of APC (Table 4, Fig 4) with Ct values of 29.09, 29.36, 31.83, 29.87 and 32.62, respectively. The low Ct value and ideal linear correlation (R2; 0.999), slope (Y; -3.37 to -3.29) and reaction efficiency (Ex; 0.98–1.01) using the Rotor-Gene Multiplex qPCR kit showed high efficiency and accuracy for each primer and probe set in multiplex TaqMan qPCR (Table 4). A low standard deviation among replicates of each dilution within each primer and probe set (Table 4) also indicates the assays are highly accurate. When sensitivity was assessed using SsoFast Probe Mix (a master mix recommended for single TaqMan qPCR) as little as 1 fg of plasmid DNA (Table 4) was detected, indicating good compatibility and thermodynamics. However, reaction efficiency was not as good as using the Rotor-Gene Multiplex qPCR kit (Table 4, Fig 5).
Fig 4. Multiplex TaqMan qPCR sensitivity assay.
Assay was performed with 10-fold serially diluted multi target artificial positive control (APC) from 1 ng to 1 fg using primer and probe sets (A) ObsCo12F/12R/12P (72 bp), (B) BruCo5F/5R/5P (140 bp), (C) BosCo8F/8R/8P (96 bp), (D) PeaCo14F/14R/14P (67 bp), and (E) DecCo11F/11R/11P (99 bp). Different channels viz. orange, yellow, red, crimson and green corresponding to the different reporter dye (excitation/emission spectra in nm) viz. 6-ROXN (575/602), HEX (535/554), Cy5 (647/667) and Quasar705 (690/705) and 6-FAM (495/520), respectively.
Fig 5. Multiplex TaqMan qPCR standard graphs.
Standard graphs were generated using 10-fold serially diluted multi target artificial positive control (APC) from 1 ng to 1 fg using the primer and probe sets BruCo5F/5R/5P (140 bp), BosCo8F/8R/8P (96 bp), DecCo11F/11R/11P (99 bp), ObsCo12F/12R/12P (72 bp) and PeaCo14F/14R/14R (67 bp). Different channels viz. orange, yellow, red, crimson and green corresponding to different reporter dye (excitation/emission spectra in nm) viz. 6-ROXN (575/602), HEX (535/554), Cy5 (647/667) and Quasar705 (690/705) and 6-FAM (495/520).
Table 4. Comparative multiplex TaqMan qPCR sensitivity assays value.
| Template conc. per reaction | Ct(SD) value of corresponding channel using Rotor-Gene Multiplex qPCR kit | Ct(SD) value of corresponding channel using SsoFast Probe Supermix | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orange | Yellow | Red | Crimson | Green | Orange | Yellow | Red | Crimson | Green | ||
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.996 | 0.995 | 0.996 | 0.996 | 0.997 | |
| Y | -3.31 | -3.29 | -3.37 | -3.37 | -3.35 | -3.85 | -4.30 | -3.83 | -3.85 | -3.85 | |
| Ex | 1.01 | 1.01 | 0.98 | 0.98 | 0.99 | 0.82 | 0.71 | 0.83 | 0.82 | 0.82 | |
| *Ct(SD) values | 1 ng | 9.32(0.36) | 9.68(0.36) | 11.71(0.37) | 9.76(0.32) | 12.66(0.43) | 9.13(0.36) | 8.56(0.43) | 11.93(0.29) | 10.41(0.31) | 14.20(0.41) |
| 100 pg | 12.52(0.29) | 12.84(0.28) | 14.92(0.39) | 12.97(0.35) | 15.73(0.37) | 12.11(0.11) | 11.63(0.18) | 14.78(0.17) | 13.33(0.15) | 17.46(0.11) | |
| 10 pg | 15.70(0.30) | 16.00(0.34) | 18.19(0.31) | 16.21(0.35) | 18.91(0.50) | 16.28(0.16) | 15.70(0.11) | 19.28(0.19) | 17.55(0.03) | 21.58(0.11) | |
| 1 pg | 19.17(0.13) | 19.39(0.12) | 21.74(0.17) | 19.75(0.19) | 22.55(0.13) | 20.89(0.56) | 21.12(0.71) | 23.30(0.76) | 21.97(0.53) | 25.86(0.60) | |
| 100 fg | 22.42(0.12) | 22.65(0.13) | 24.98(0.17) | 23.07(0.14) | 25.73(0.16) | 24.74(0.11) | 25.59(0.17) | 27.26(0.15) | 25.90(0.07) | 29.68(0.15) | |
| 10 fg | 25.84(0.21) | 26.09(0.17) | 28.48(0.17) | 26.59(0.20) | 29.23(0.20) | 28.34(0.40) | 29.80(0.59) | 31.03(0.48) | 29.57(0.50) | 33.47(0.50) | |
| 1 fg | 29.09(0.10) | 29.36(0.08) | 31.83(0.08) | 29.87(0.02) | 32.62(0.11) | 31.48(0.38) | 33.43(0.36) | 34.25(0.69) | 32.78(0.47) | 36.85(0.51) | |
| NTC | - | - | - | - | - | - | - | - | - | - | |
Average Ct values of sensitivity assays using 10-fold serial dilutions of a multi-target artificial positive control (APC) from 1 ng to 1 fg with primer and probe sets BruCo5F/5R/5P, BosCo8F/8R/8P, DecCo11F/11R/11P, ObsCo12F/12R/12P and PeaCo14F/14R/14P.
R2, linear correlation; Y, slope; Ex, reaction efficiency; SD, standard deviation; the Ct (threshold cycle) values are average of three replicates.
A small difference in Ct values was observed when multiplex TaqMan qPCR reactions were performed in separate tubes using genomic DNA extracted from either a single Liposcelis species (DNA from individual species was added in a multiplex qPCR) or a mixture of all the five-species of Liposcelis (DNA from all species was added in a single multiplex qPCR). The Ct values (individual species/ targeted species all together) in multiplex TaqMan qPCR corresponding to different channels were orange (20.06/23.37), yellow (21.64/21.85), red (20.76/20.67), crimson (27.09/31.53), and green (23.64/23.37). The reaction efficiency (0.95–1.0), linear correlation (0.999), and slope (-3.44 to -3.32) of all other channels (Table 5) were within an optimal range. A repeat experiment using new extracted genomic DNA of all targeted insects produced the same results (Table 5). DNA isolated from an individual insect was detected using this multiplex TaqMan qPCR.
Table 5. Multiplex TaqMan qPCR assays with Liposcelis genomic DNA.
| Template conc. per reaction | Ct(SD) value of corresponding channel with genomic DNA | Ct(SD) value of corresponding channel with genomic DNA (performed after 8 months) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Orange | Yellow | Red | Crimson | Green | Orange | Yellow | Red | Crimson | Green | ||
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Y | -3.319 | -3.364 | -3.384 | -3.444 | -3.433 | -3.370 | -3.178 | -3.368 | -3.266 | -3.388 | |
| Ex | 1.00 | 0.98 | 0.97 | 0.95 | 0.96 | 0.98 | 1.06 | 0.98 | 1.02 | 0.97 | |
| Ct(SD) values | 1 ng | 9.01(0.05) | 10.55(0.06) | 11.12(0.09) | 9.52(0.05) | 13.61(0.03) | 9.38(0.02) | 9.56(0.27)* | 11.39(0.15) | 10.33(0.17) | 12.68(0.20) |
| 100 pg | 12.29(0.05) | 14.03(0.06) | 14.56(0.04) | 13.08(0.02) | 17.02(0.11) | 12.75(0.04) | 11.66(0.02) | 14.72(0.11) | 13.67(0.04) | 16.07(0.06) | |
| 10 pg | 15.64(0.04) | 17.28(0.06) | 17.86(0.06) | 16.41(0.08) | 20.48(0.07) | 16.12(0.03) | 14.84(0.02) | 18.13(0.08) | 16.86(0.05) | 19.45(0.09) | |
| With single species | 20.06(0.08) | 21.64(0.13) | 20.76(0.11) | 27.09(0.17) | 23.64(0.41) | 24.29(0.25) | 18.54(0.19) | 19.68(0.02) | 25.67(0.13) | 22.65(0.21) | |
| With all five species | 23.37(0.32) | 21.85(0.15) | 20.67(0.15) | 31.53(1.23) | 23.37(0.33) | 22.27(0.05) | 17.57(0.02) | 19.19(0.01) | 28.34(0.87) | 22.30(0.12) | |
| NTC | - | - | - | - | - | - | - | - | - | - | |
Average Ct values using genomic DNA of Liposcelis brunnea, L. bostrychophila, L. decolor, L. obscura and L. pearmani with primer and probe sets BruCo5F/5R/5P, BosCo8F/8R/8P, DecCo11F/11R/11P, ObsCo12F/12R/12P and PeaCo14F/14R/14P, respectively.
*Due to error in fluorescence, this value has not been included in analysis; R2, linear correlation; Y, slope; Ex, reaction efficiency; SD, standard deviation; the Ct values are average of three replicates; Orange, yellow, red, crimson and green color corresponding to L. obscura, L. brunnea, L. bostrychophila, L. pearmani and L. decolor, respectively.
Multi-target artificial positive control
A unique multi-target APC was designed, with all the target primer and probe sequences, to amplify a product for each primer set that would have a size compatible for different PCR techniques (Fig 1). The size difference in the amplified products allows visualization of products of the APC that can be used distinctly for endpoint and real-time qPCR. The probe sequences were adjusted to be between the forward and reverse primers for qPCR (Fig 1). The developed APC also carries sense and anti-sense primer sequences of other pathogens including the viruses High plains virus, Wheat streak mosaic virus and Triticum mosaic virus, and the fungi Pythium aphanidermatum and Pythium deliense. To check the reproducibility of assays using the APC, PCR amplifications using all 17 Liposcelis primer combinations were repeated three times. All results obtained were accurate and reproducible (Fig 1). The APC was used in endpoint PCR and to generate standard graphs for multiplex qPCR (Figs 3–5). The multiplex TaqMan qPCR showed 0.999 linear correlations, -3.37 to -3.292 slopes and 0.98 to 1.01 reaction efficiencies.
Discussion
We have developed and validated two methods for the simultaneous detection and discrimination of L. brunnea, L. decolor, L. bostrychophila, L. pearmani and L. obscura using multiplex versions of both endpoint and real-time TaqMan qPCR. The developed primer sets can also be used for species-specific single endpoint or real-time TaqMan qPCR. Furthermore, we have designed and developed a single, unique multi-target and clonable APC that mimics these five species of genus Liposcelis.
In pest management, biosecurity, quarantine and routine diagnostics, accuracy, reliability and sensitive discriminatory capabilities are important [35, 47]. New segments of the Liposcelis CO1 region were amplified using endpoint PCR from L. decolor, L. bostrychophila, L. pearmani and L. obscura using a combination of previously reported primers. The generated sequences were used to design species specific primers and probes from signature diagnostic targets. A conserved region of the COI gene which is reliable for identification purposes, was previously used for the detection of L. entomophila, L. corrodens and L. reticulatus [30, 32, 48–50] and led us to use a primer combination approach based on previously reported universal primers for insects (S1 Fig), which facilitated the amplification of the unknown CO1 gene regions of L. decolor, L. bostrychophila, L. pearman and L. obscura. This approach would be suitable for the amplification of CO1 or unknown gene regions of other species. Subsequently, specific primers and probes for multiplex endpoint and real-time TaqMan qPCR were designed for detection and discrimination of the five Liposcelis species. All primers and probes were designed to have minimal secondary structure, delta G values equal or close to zero for increased sensitivity and compatibility, and reduced thermodynamic interference during PCR [45]. A second approach using target specific primers was used to determine the best reverse and forward primer sets with maximum PCR yield or fluorescence, a capability of amplifying PCR products with different sizes, and good compatibility during multiplex endpoint PCR and real-time TaqMan qPCR for better discrimination of the five Liposcelis targets (Table 2 and Fig 1). Previously, Arif et al. [36] used the primer combination approach to develop a primer and probe set having broad range detection capabilities for High plains virus variants.
All the primer sets that were developed are highly specific for their corresponding targets and no cross amplification was detected. All primer sets showed high specificity in multiplex endpoint PCR (Fig 2). Each individual primer set used in multiplex endpoint PCR detected as little as 1 fg of plasmid DNA (APC) carrying the multi-targets for all the primer sets (Fig 3). Saccaggi et al. [51] developed multiplex endpoint PCR for mealybug species (Hemiptera: Pseudococcidae) Planococcus ficus, Planococcus citri and Pseudococcus longispinus and reported high accuracy.
The described qPCR assays can be performed simultaneously (multiplex) and individually using a Rotor-Gene 6000 thermocycler. Different florescent reporter dyes (6-FAM, HEX, 6-ROXN, Cy5 and Quasar705) were selected based on their wavelength to avoid overlapping of wavelengths, which could lead to false positives. The multiplex TaqMan qPCR detected down to 1 fg of APC using Sso Fast Probes Master Mix, which is intended for single qPCR reactions. This result confirmed the high accuracy and compatibility that exists among the primers and probes when performing in multiplex qPCR. The developed assays are capable of detecting and discriminating each target species from the other species of genus Liposcelis that were used. This is the first published study on detection and discrimination of stored-grain pest species of any kind using multiplex qPCR methods.
The four main pest species of psocids worldwide are L. bostrychophila, L. decolor, L. entomophila, and L. paeta. The method we have developed includes only two of these species, namely, L. bostrychophila and L. decolor. Given the method we have developed, similar research can be conducted to enable simultaneous identification of the aforementioned four main pest psocid species. Moreover, the APC can be modified to insert the new complement sequences of primers and/or probes designed for specific identification of L. entomophila and L. paeta.
The positive controls were essentially developed for assessment of PCR reliability. These controls are challenging to obtain for rare insects or microbes that are exotic and/or emerging pests that pose a potential biosafety risk as regulated insects or infectious pathogens. A functional, clonable multi-target, synthetic, and artificial positive control, custom made of synthetic DNA inserts containing tandems of forward and reverse complement priming sequences, was designed de novo and inserted and circularized into a plasmid vector. The product size of amplicons generated from APC with each primer set can vary from amplicons generated using genomic DNA. However, the amplicons size for APCs can be determined at the time of the APC design (Fig 1) which can facilitate discrimination between amplicons generated using genomic DNA and APC, if cross contamination occurred. Moreover, each PCR product generated using APC has a unique identifiable sequence. For example, primer set PeaCO14F and PeaCo14R generate a 115 bp amplicon with genomic DNA of L. pearmani and 67 bp using APC (Fig 1). The suitability of customized synthetic DNA was demonstrated in silico by analyzing the thermodynamics of the selected primers and in vitro by PCR [37].
This approach can be used for incorporating hundreds of primer and probe sequences in a clone to use as positive control for a large number of insects, pathogens and other target of interest. The use of this APC will speed-up PCR processing, and will increase accuracy and reliability, minimize costs and will remove the biosafety risks associated with in vivo positive controls. This kind of positive control can also be shipped or exchanged among different national and regional diagnostic laboratories, and can be used as a reference for multiple PCR assays where insect and/or pathogen are regulated due to geographical distribution.
Supporting Information
Location of different reported universal CO1 primers within the CO1 gene region used to amplify the CO1 region of five species of genus Liposcelis.
(TIF)
Endpoint multiplex PCR performed with individual insect crude DNA and primer sets ObsCo13F/13R (438 bp), PeaCo15F/14R (351 bp), BosCO7F/7R (191 bp), BruCo5F/5R (140 bp), and DecCo11F/11R (87 bp) for L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor, respectively. Lane 1: PCR reaction with all five species genomic DNA. Lane 2–6: Single species genomic DNA viz. L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor, respectively. Lane M and W are 1 kb ladder and non-template control (water), respectively.
(TIF)
Acknowledgments
We thank Jacqueline Fletcher and Ulrich Melcher (OSU) for critically reviewing this manuscript. We extend our appreciation to E. Mockford, who confirmed the identity of the psocid species used in the study and provided Lepinotus inquilinus and Lepinotus patruelis. This work was supported by the Oklahoma Agricultural Experiment Station, Oklahoma State University (project numbers OKL02695 and OKL02773). The mention of trade names or commercial products in this publication does not imply recommendation or endorsement by Oklahoma State University.
Data Availability
Data are available within the paper and its Supporting Information files.
Funding Statement
This work was funded by Oklahoma Agricultural Experiment Station, Oklahoma State University (project numbers OKL02695 and OKL02773).
References
- 1. Phillips TW, Throne JE. Biorational approaches to managing stored-product insects. Annu Rev Entomol. 2010; 55: 375–397. 10.1146/annurev.ento.54.110807.090451 [DOI] [PubMed] [Google Scholar]
- 2. Turner BD, Maude-Roxby H, Pike V. Control of the domestic insect pest Liposcelis bostrychophila (Badonnel) (Psocoptera): an experimental evaluation of the efficiency of some insecticides. Int Pest Contr. 1991; 33: 153–157. [Google Scholar]
- 3. Rees DP. Psocoptera (psocids) as pests of bulk grain storage in Australia: a cautionary tale to industry and researchers In: Credland PF, Armitage DM, Bell CH, Cogan PM, Highley E, editors. Proceedings of the 8th International Working Conference on Stored Product Protection. CAB International, Wallingford, UK; 2003. pp. 59–64. [Google Scholar]
- 4. Phillips TW, Throne JE. Biorational approaches to managing stored-product insects. Annu Rev Entomol. 2010; 55: 375–397. 10.1146/annurev.ento.54.110807.090451 [DOI] [PubMed] [Google Scholar]
- 5. McFarlane JA. Damage to milled rice by psocids. Trop Stored Prod Inf. 1982; 44: 3–10. [Google Scholar]
- 6. Kučerová Z. Weight losses of wheat grains caused by psocid infestation (Liposcelis bostrychophila: Liposcelididae: Psocoptera). Plant Prot Sci. 2002; 38: 103–107. [Google Scholar]
- 7. Trematerra P, Stejskal V, Hubert J. The monitoring of semolina contamination by insect fragments using the light filth method in an Italian mill. Food Control. 2011; 22: 1021–1026. [Google Scholar]
- 8. Obr S. Psocoptera of food-processing plants and storages, dwellings and collections of natural objects in Czecholslovakia. Acta Entomol Boehm. 1978; 75: 226–242. [Google Scholar]
- 9. Kalinovic I, Rozman V, Liska A. Significance and feeding of psocids (Liposcelididae, Psocoptera) with microorganisms In: Lorini I, Bacaltchuk B, Beckel H, Deckers D, Sundfeld E, dos Santos JP, et al. , editors. Proceedings of the 9th International Working Conference on Stored Product Protection. Brazilian Post-harvest Association—Associação Brasileira de Pós-Colheita (ABRAPOS); 2006. pp. 1087–1094. [Google Scholar]
- 10. Turner BD, Staines NA, Brostoff J, Howe CA, Cooper K. Allergy to psocids In: Wildey KB, editors. Proceedings of the International Conference on Insect Pests in the Urban Environment (ICIPUE). ICIPUE, Heriot-Watt University, Edinburgh, Scotland; 1996. pp. 609. [Google Scholar]
- 11. Wang JJ, Zhao ZM, Li LS. Induced tolerance of the psocid Liposcelis bostrychophila (Psocoptera: Liposcelididae) to controlled atmosphere. Int J Pest Manage. 1999; 45: 75–79. [Google Scholar]
- 12. Beckett S, Morton R. The mortality of three species of Psocoptera, Liposcelis bostrychophila Badonnel, Liposcelis decolor (Pearman), and Liposcelis paeta Pearman, at moderately elevated temperatures. J Stored Prod Res. 2003; 39: 103–115. [Google Scholar]
- 13. Nayak MK, Collins PJ, Pavic H, Kopittke RA. Inhibition of egg development by phosphine in the cosmopolitan pest of stored products Liposcelis bostrychophila (Psocoptera: Liposcelididae). Pest Manag Sci. 2003; 59: 1191–1196. [DOI] [PubMed] [Google Scholar]
- 14. Nayak MK, Daglish GJ. Combined treatments of spinosad and chlorpyrifos-methyl for management of resistant psocid pests (Psocoptera: Liposcelididae) of stored grain. Pest Manag Sci. 2007; 63: 104–109. [DOI] [PubMed] [Google Scholar]
- 15. Kavallieratos NG, Athanassiou CG, Hatzikonstantinou AN, Kavallieratou HN. Abiotic and biotic factors affect efficacy of chlorfenapyr for control of stored-product insect pests. J Food Prot. 2011; 74: 1288–1299. 10.4315/0362-028X.JFP-10-575 [DOI] [PubMed] [Google Scholar]
- 16. Athanassiou CG, Arthur FH, Kavallieratos NG, Throne JE. Efficacy of spinosad and methoprene, applied alone or in combination, against six stored-product insect species. J Pest Sci. 2011; 84: 61–67. [Google Scholar]
- 17. Nayak MK. Psocid and mite pests of stored commodities: small but formidable enemies In: Lorini I, Bacaltchuk B, Beckel H, Deckers D, Sundfeld E, dos Santos JP, et al. , editors. Proceedings of the 9th International Working Conference on Stored Product Protection. Brazilian Post-harvest Association—Associação Brasileira de Pós-Colheita (ABRAPOS); 2006. pp. 1061–1073. [Google Scholar]
- 18. Rees DP. Insects of stored products. CSIRO Publishing, Collingwood, Victoria; 2004. [Google Scholar]
- 19. Opit GP, Bonjour EL, Beeby R. Seasonal abundance and distribution of psocids in an animal feed warehouse In: Athanassiou CG, Adler CS, Trematerra P, editors. Proceedings of the International Organisation for Biological and Integrated Control West Palaearctic Region Section Working Group on Integrated Protection of Stored Products; 2011. pp. 111–122. [Google Scholar]
- 20. Athanassiou CG, Kavallieratos NG, Throne JE, Nakas CT. Competition among species of stored-product psocids (Psocoptera) in stored grain. PLoS ONE 2014; 9: e102867 10.1371/journal.pone.0102867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nayak MK, Collins PJ, Reid SR. Efficacy of grain protectants and phosphine against Liposcelis bostrychophila, L. entomophila, and L. paeta (Psocoptera: Liposcelidae). J Econ Entomol. 1998; 9: 1208–1212. [Google Scholar]
- 22. Nayak MK, Collins PJ. Influence of concentration, temperature, and humidity on the toxicity of phosphine to the strongly phosphine resistant psocid Liposcelis bostrychophila Badonnel (Psocoptera: Liposcelididae). Pest Manag Sci. 2008; 64: 971–976. 10.1002/ps.1586 [DOI] [PubMed] [Google Scholar]
- 23. Opit GP, Throne JE, Flinn PW. Temporospatial distribution of the psocids Liposcelis entomophila and L. decolor (Psocoptera: Liposcelididae) in steel bins containing wheat. J Econ Entomol. 2009; 102: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 24. Lienhard C. Revision of the western Palaearctic species of Liposcelis Motschulsky (Psocoptera: Liposcelididae). Zool Jb Syst. 1990; 117: 117–174. [Google Scholar]
- 25. Bai XG, Cao Y. Two new findings of the genus Liposcelis from the stored grain in China. J Chin Cereals Oils Assoc. 1997;12: 1–4 [Google Scholar]
- 26. Lienhard C, Smithers CN: Psocoptera—World Catalogue and Bibliography Instrumenta Biodiversitatis V. Museum d'Histoire Naturelle, Geneve; 745 2002. [Google Scholar]
- 27. Kučerová Z, Li Z, Hromádková J. Morphology of nymphs of common stored product psocids (Psocoptera: Liposcelididae). J Stored Prod Res. 2009; 45: 54–60. [Google Scholar]
- 28. Kučerová Z. Stored product psocids (Psocoptera): External morphology of eggs. Eur J Entomol. 2002; 99: 491–503. [Google Scholar]
- 29. Kučerová Z, Li Z, Hromádková J. Morphology of nymphs of common stored-product psocids (Psocoptera, Liposceliididae). J Stored Prod Res. 2009; 45: 54–60. [Google Scholar]
- 30. Arif M, Ochoa-Corona FM, Opit G, Li ZH, Kučerová Z, Stejskal V, et al. PCR and isothermal-based molecular identification of the stored-product psocid pest Lepinotus reticulatus (Psocoptera: Trogiidae). J Stored Prod Res. 2012; 49: 184–188. [Google Scholar]
- 31. Qin M, Li Z, Kučerová Z, Cao Y, Stejskal V. Rapid discrimination of common species of stored Liposcelis (Psocoptera: Liposcelididae) from China and the Czech Republic based on PCR-RFLP analysis. Eur J Entomol. 2008; 105: 713–717. [Google Scholar]
- 32. Yang Q, Kučerová Z, Li Z, Kalinovic I, Stejskal V, Opit G, et al. Diagnosis of Liposcelis entomophila (Insecta: Psocodea: Liposcelididae) based on morphological characteristics and DNA barcodes. J Stored Prod Res. 2012; 48: 120–125. [Google Scholar]
- 33. Li Z, Kučerová Z, Zhao S, Stejskal V, Opit G, Qin M. Morphological and molecular identification of three geographical populations of the storage pest Liposcelis bostrychophila (Psocoptera). J Stored Prod Res. 2011; 47: 168–172. [Google Scholar]
- 34. Ouyang P, Arif M, Fletcher J, Melcher U, Ochoa-Corona FM. Enhanced reliability and accuracy for field deployable bioforensic detection and discrimination of Xylella fastidiosa subsp. pauca, causal agent of citrus variegated chlorosis using Razor Ex technology and TaqMan quantitative PCR. PLoS ONE. 2013; 8: e81647 10.1371/journal.pone.0081647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arif M, Fletcher J, Marek S, Melcher U, Ochoa-Corona FM. Development of a rapid, sensitive and field deployable Razor Ex BioDetection System and qPCR assay for detection of Phymatotrichopsis omnivora using multiple gene targets. Appl Environ Microbiol. 2013; 79: 2312–2320. 10.1128/AEM.03239-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arif M, Aguilar-Moreno GS, Wayadande A, Fletcher J, Ochoa-Corona FM. Primer modification improves rapid and sensitive in vitro and field deployable assays for detection of High plains virus variants. Appl Environ Microbiol. 2014; 80: 320–327. 10.1128/AEM.02340-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caasi DRJ, Arif M, Payton M, Melcher U, Winder L, Ochoa-Corona FM. A multi target, non-infectious and clonable artificial positive control for routine PCR-based assays. J Microbiol Methods. 2013; 95: 229–234. 10.1016/j.mimet.2013.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gautam SG, Opit GP, Giles KL. Population growth and development of the psocid Liposcelis rufa (Psocoptera: Liposcelididae) at constant temperatures and relative humidities. J Econ Entomol. 2010; 103: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 39. Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994; 87: 651–701. [Google Scholar]
- 40. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994; 3: 294–299. [PubMed] [Google Scholar]
- 41. Zhang DX, Hewitt GM. Assessment of the universality and utility of a set of conserved mitochondrial COI primers in insects. Insect Mol Biol. 1997; 6: 143–150. [DOI] [PubMed] [Google Scholar]
- 42. Wilcox TP, Hugg L, Zeh JA, Zeh DW. Mitochondrial DNA sequencing reveals extreme genetic differentiation in a cryptic species complex of neotropical pseudoscorpions. Mol Phylogenet Evol. 1997; 7: 208–216. [DOI] [PubMed] [Google Scholar]
- 43. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and Clustal X version 2. Bioinformatics. 2007; 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 44. Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers In Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. New Jersey, Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 45. Arif M, Ochoa-Corona FM. Comparative assessment of 5’ A/T-rich overhang sequences with optimal and sub-optimal primers to increase PCR yields and sensitivity. Mol Biotechnol. 2013; 55: 17–26. 10.1007/s12033-012-9617-5 [DOI] [PubMed] [Google Scholar]
- 46. Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003; 31: 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dobhal S, Arif M, Olsen J, Mendoza-Yerbafría1 A, Aguilar-Moreno GS, Perez-Garcia1 M, Ochoa-Corona FM Sensitive detection and discrimination of five major plant viruses infecting ornamental plants in nursery plants by multiplex reverse transcriptase PCR. Ann Appl Biol. 2015; 166: 286–296. [Google Scholar]
- 48. Yang Q, Zhao S, Kucerova Z, Stejskal V, Opit G, Qin M, et al. Validation of the 16S rDNA and COI DNA Barcoding technique for rapid molecular identification of stored product psocids (Insecta: Psocodea: Liposcelididae). J Econ Entomol. 2013; 106: 419–425. [DOI] [PubMed] [Google Scholar]
- 49. Yang Q, Zhao S, Kucerova Z, Opit G, Cao Y, Stejskal V, et al. Rapid molucular diagnosis of the stored-product psocid Liposcelis corrodens (Psocoptera: Liposcelididae): species-specific PCR primers of 16S rDNA and COI. J Stored Prod Res. 2013; 54: 1–7. [Google Scholar]
- 50. Li Z, Kucerova Z, Yang Q, Stejskal V. New record of stored product pest Lepinotus reticulatus (Psocoptera: Trogiidae) from China: Identification through scanning electron microscopy and DNA barcode. Afr J Biotechnol. 2012; 11: 15460–15464. [Google Scholar]
- 51. Saccaggi DL, Kruger K, Pietersen G. A multiplex PCR assay for the simultaneous identification of three mealybug species (Hemiptera: Pseudococcidae). Bull Entomol Res. 2008; 98: 27–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Location of different reported universal CO1 primers within the CO1 gene region used to amplify the CO1 region of five species of genus Liposcelis.
(TIF)
Endpoint multiplex PCR performed with individual insect crude DNA and primer sets ObsCo13F/13R (438 bp), PeaCo15F/14R (351 bp), BosCO7F/7R (191 bp), BruCo5F/5R (140 bp), and DecCo11F/11R (87 bp) for L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor, respectively. Lane 1: PCR reaction with all five species genomic DNA. Lane 2–6: Single species genomic DNA viz. L. obscura, L. pearmani, L. bostrychophila, L. brunnea and L. decolor, respectively. Lane M and W are 1 kb ladder and non-template control (water), respectively.
(TIF)
Data Availability Statement
Data are available within the paper and its Supporting Information files.