Abstract
Background and Aims
The heterogeneous nature of breast cancer can make decisions on adjuvant chemotherapy following surgical resection challenging. Oncotype DX is a validated gene expression profiling test that predicts the likelihood of adjuvant chemotherapy benefit in early-stage breast cancer. The aim of this study is to determine the costs of chemotherapy in private hospitals in France, and evaluate the cost-effectiveness of Oncotype DX from national insurance and societal perspectives.
Methods
A multicenter study was conducted in seven French private hospitals, capturing retrospective data from 106 patient files. Cost estimates were used in conjunction with a published Markov model to assess the cost-effectiveness of using Oncotype DX to inform chemotherapy decision making versus standard care. Sensitivity analyses were performed.
Results
The cost of adjuvant chemotherapy in private hospitals was estimated at EUR 8,218 per patient from a national insurance perspective and EUR 10,305 from a societal perspective. Cost-effectiveness analysis indicated that introducing Oncotype DX improved life expectancy (+0.18 years) and quality-adjusted life expectancy (+0.17 QALYs) versus standard care. Oncotype DX was found cost-effective from a national insurance perspective (EUR 2,134 per QALY gained) and cost saving from a societal perspective versus standard care. Inclusion of lost productivity costs in the modeling analysis meant that costs for eligible patients undergoing Oncotype DX testing were on average EUR 602 lower than costs for those receiving standard care.
Conclusions
As Oncotype DX was found both cost and life-saving from a societal perspective, the test was considered to be dominant to standard care. However, the delay in coverage has the potential to erode the quality of the French healthcare system, thus depriving patients of technologies that could improve clinical outcomes and allow healthcare professionals to better allocate hospital resources to improve the standard of care for all patients.
Introduction
Breast cancer is the second most common cancer in the world and, by far, the most frequently occurring cancer in women with an estimated 1.67 million new cases diagnosed in 2012 (resulting in 522,000 deaths).[1] The figures represent approximately one in four of all cancer cases.[1] Approximately 54,000 new cases were diagnosed in France in 2012, representing 33% of all new cancer diagnoses in women.[2, 3] Encouragingly, however, screening practices now mean that most cases are identified at an early stage and, as a result, the utilization of adjuvant chemotherapy following surgical resection has increased in recent years.
Breast cancer is a heterogeneous disease and prognosis, survival and recurrence rates can vary widely. They are influenced by a number of factors including disease stage at diagnosis (based on tumor size, lymph node involvement and distant metastases), presence of particular molecular markers including, in particular, the estrogen and progesterone receptors (ER and PR, respectively) and the human epidermal growth factor receptor 2 (HER2). Decisions on whether to use adjuvant chemotherapy in patients with early, invasive, operable breast cancer have traditionally relied on such clinical, pathologic and biological markers. However, these indicators are imperfect in terms of reproducibility and lack of standardization (for example with the Ki-67 marker), leaving room for interpretation. Current estimates indicate that more than 60% of patients with hormone receptor positive breast cancer receive adjuvant chemotherapy. However several studies have demonstrated that only 4–5% of patients are likely to benefit from chemotherapy.[4] Further, chemotherapy is often associated with many short and long-term side effects, leading to significant costs and serious psychological sequelae for patients, such as anxiety and emotional distress.[5] In addition, chemotherapy can have an impact on the patient’s professional life. Evidence indicates that absenteeism from work is twice as high in women receiving chemotherapy than in those who do not, and can lead to early retirement in some cases.[6] Lost productivity has been estimated to make up more than one-quarter of the total costs of chemotherapy for breast cancer, making it the single biggest cost component, and an important part of any cost evaluation.[7]
Gene expression profiling and immunohistochemistry tests aim to improve the targeting of chemotherapy in breast cancer by more accurately identifying those patients who will benefit most, based on knowledge of biologic features of cancer that indicate increased likelihood of rapid growth, recurrences and/or metastasis. The Oncotype DX Breast Cancer Test (Genomic Health Inc., Redwood City, CA, USA), is one such assay and has been shown to successfully predict the likelihood of chemotherapy benefit as well as distant recurrence 10 years after diagnosis in patients with early-stage, node-negative and node-positive ER-positive breast cancer. The validity of Oncotype DX has been demonstrated in a number of clinical studies both for prognosis and prediction of likelihood of chemotherapy benefit.[8–12] A number of studies evaluating the impact of the assay on adjuvant therapy decisions in patients with ER+ early breast cancer have demonstrated that knowledge of the Oncotype DX Recurrence Score (RS) affects management of patients. Importantly, the studies reveal that every second patient originally recommended adjuvant chemotherapy plus endocrine treatment is recommended endocrine treatment alone after knowledge of the RS.[13–16] Although not currently reimbursed in France, the National Institute for Health and Care Excellence (NICE) in the UK indicated that Oncotype DX has the most robust evidence of the tests currently available for breast cancer.[17] Its use to inform decision making in adjuvant chemotherapy is supported by several guidelines on best clinical practice, including those from European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and St. Gallen.[18–21]
In 2012, a cost-effectiveness evaluation was published that investigated the health economic implications of introducing the Oncotype DX in public hospitals in France.[22] However, there is a paucity of data on the costs of chemotherapy and the cost-effectiveness of introducing gene expression profiling or expanded immunohistochemistry tests in private hospitals in the French setting. This is of particular concern given that, in 2012, private hospitals performed 43% of surgical interventions for breast cancer and 28% of chemotherapies for all cancers.[23] The aims of the present study, therefore, were two-fold: to evaluate the cost of adjuvant chemotherapy for early stage breast cancer from societal and national insurance perspectives in private hospitals, and to perform a cost-effectiveness evaluation investigating the use of Oncotype DX to guide chemotherapy decision making versus standard approaches in eligible patients with early-stage breast cancer in private hospitals in France.
Materials and Methods
Cost of chemotherapy
A retrospective database analysis was performed to evaluate both direct and indirect costs associated with adjuvant chemotherapy in women with ER+, HER2−, early-stage breast cancer. This study was submitted and approved by the French ethics committee and a National Institutional Review Board (CPP Ile de France 3). Retrospective data from 106 patient files were anonymized and de-identified prior to analysis. Patient records were collected from seven private hospitals belonging to the group Générale de Santé in France: Hôpital Privé La Louvière (Lille), Hôpital Privé Villeneuve-d’Ascq (Villeneuve-d’Ascq), Hôpital Privé Hôpital Jean Mermoz (Lyon), Hôpital Privé Clairval (Marseille), Hôpital Privé des Peupliers (Paris), Hôpital Privé Drôme Ardèche (Guilherand-Granges) and Hôpital Privé Paul d’Egine (Champigny-sur-Marne). Resource data were extracted from medical records of female patients who have undergone surgery for breast cancer from January 2008 to January 2013. Inclusion criteria were: 1) women who received all cycles of chemotherapy within the same private hospital, and 2) women with ER+, negative HER2 status and no node involvement. Patients with incomplete medical files were excluded. Data were collected from the start of therapy (including the pre-chemotherapy period) to the end of adjuvant chemotherapy treatment (Table 1). Costs associated with other interventions such as radiotherapy and other treatments such as endocrine therapy were not included.
Table 1. Data collected for the retrospective analysis of chemotherapy costs.
| Time period | Information collected |
|---|---|
| Baseline information of patient characteristics and pre-chemotherapy procedures | Patient characteristics |
| Age | |
| Socio-professional group | |
| Body weight | |
| Height | |
| Body surface area | |
| TNM (tumor, node, metastasis) classification | |
| Ki-67 status | |
| HER2 status | |
| ER and PR status | |
| Pre-chemotherapy tests and procedures | |
| Central venous access implantation | |
| Electrocardiogram | |
| Laboratory tests | |
| Functional heart tests | |
| Oncologist consultations | |
| Information collected for each chemotherapy cycle | Chemotherapy regimen (chemotherapy agents, cumulative dose, start date, and end date) |
| Prophylactic agents (prophylactic treatment and cumulative dose) | |
| Side effects (medications taken including hospitalizations and consultations) | |
| Visits (number of general practitioner and specialist visits) | |
| Hospitalizations (start date, end date, admission service, reason of admission, type of admission (day care or complete hospitalization) link to adverse events | |
| Laboratory tests (hospital, home or laboratory, list of items tested) | |
| Home care (date and reason) | |
| Transport (ambulance, taxi, personal car, patient transport service ambulance, public transport, voucher from social insurance, number of kilometers between home and hospital) | |
| Sick leave (start date and end date) |
Costs were estimated in 2013 Euros (EUR) using the French tariff system T2A (“tarification à l'activité”). Fixed T2A payments were recorded covering drug costs (EUR 25), excluding expensive products, and administration costs (EUR 272.48), using the results of the national cost scale (Echelle Nationale de Coûts, 2011) defining T2A payments.[23] Costs associated with expensive medications not covered by the T2A tariff system were added separately. Physician consultations and medication costs were extracted from lists of tariffs available from the national health insurance system (Caisse Nationale de l'Assurance Maladie). Hospitalisation costs were determined using the “Groupe Homogène de Malades” (GHM codes are analogous to DRG codes) and the corresponding unit costs from the national scale of costs.[23] Medication costs were based on daily or cumulative dosage and the package size with the least expensive unit cost was used wherever possible. Treatment related to prophylactic or symptomatic prescriptions could be delivered by hospitals or pharmacy. Missing values were imputed for cumulative/daily doses using another prescription of the study for the same treatment or from standard prescription, recommended by ANSM (Agence National de Sécurité du Médicament et des produits de santé).[24] The cost of administration of growth factors by nurses at the patient’s home included an injection fee and compensation for the nurse travel cost, and assumed an average distance of 6.8 km.[25] Cancer is categorized as a long-term condition (“affection longue durée”) for which patients are entitled to 100% reimbursement of healthcare costs. It was assumed that patients received transport vouchers, reimbursed by national insurance, for taxi, ambulance or patient transport service.
A human capital approach was used to estimate costs associated with lost workplace productivity based on sick leave payments from the national health insurance system. Costs were estimated as the number of sick-leave days multiplied by the average daily salary for women (EUR 60.53 per day in 2012).[26] In cases of missing values, lost productivity costs were imputed based on existing data. For presentation, costs were categorized as follows: monitoring costs (consultation, laboratory tests and pre-chemotherapy), drug and administration costs (prophylactic prescriptions and chemotherapy management), side effect costs (drug-related hospitalizations and consultations), and transport and absenteeism costs.
Cost-effectiveness analysis
Cost-effectiveness analysis was performed using a published Markov model designed to compare the cost-effectiveness of using the Oncotype DX Recurrence Score (RS) to guide chemotherapy decision making with standard care.[22] The proportions of patients recommended adjuvant chemotherapy using either standard care or the Oncotype DX RS were derived from a meta-analysis of nine international studies on the decision impact associated with Oncotype DX testing.[13] The meta-analysis showed reductions in utilization of chemotherapy of 22% and 7.5% for patients with low risk and intermediate risk of recurrence, respectively. For patients with a high risk of recurrence, Oncotype DX testing led to an increase of 5.6% in the prescribing of chemotherapy relative to standard care. Based on this therapy allocation, the model made projections of long-term costs and clinical outcomes based on landmark data from a prospective clinical trial comparing chemotherapy and hormonal therapy or hormone therapy alone (National Surgical Adjuvant Breast and Bowel Project [NSABP] B20 trial).[8] Three-year survival after metastatic recurrence of breast cancer came from a prospective study conducted in three French hospitals between 2001 and 2006 (mean 35.8 months, 95% confidence interval 31.7 to 39.1 months).[27] Overall mortality rates were obtained from the French National Demographic Institute.[28]
The analysis was performed from the “collective perspective” defined by the Haute Autorité de Santé (HAS), which includes all direct costs by the national insurance, private insurance or patients. Costs for the Oncotype DX test were based on the list price (EUR 3,180). Estimates of costs associated with chemotherapy were obtained from the retrospective study described above. The cost of distant recurrence events was based on the data published by Bonneterre et al. in 2005.[29] Other model inputs were consistent with the previously published cost-effectiveness analysis of Oncotype DX in the French setting.[22] Both life expectancy and quality-adjusted life expectancy were evaluated by the model. Published utility scores (consistent with previous cost-effectiveness analyses of Oncotype DX were used to estimate quality-adjusted life expectancy, with chemotherapy associated with a utility decrement of 0.07 and annual utility scores of 0.60 and 0.78 for patients with and without recurrence, respectively.[30–32]
The base case time horizon was set to 30 years (the model had a one year cycle length) to capture long-term recurrence risk. Future costs and clinical benefits were discounted at 4% per annum in line with published recommendations.[33] Probabilistic sensitivity analysis (PSA) was performed by performing 1,000 iterations of the base case analysis with sampling from distributions around patient age, allocation of adjuvant therapy, recurrence rates, post recurrence survival, costs of the test, chemotherapy and recurrence, and health-related quality of life utilities. Multiple one-way sensitivity analyses were performed to identify key drivers of model outcomes. Each input parameter was varied between the upper and lower confidence interval as published. For those parameters where no confidence interval was available, the input value was varied by +/− 25%.
Results
Cost of adjuvant chemotherapy in early-stage breast cancer
A total of 106 patients were included in the retrospective analysis of chemotherapy costs, with a mean age of 53.2 years (Table 2 and S1 File). The most frequently prescribed treatment protocol (47.5% of all cycles) in the private hospital setting was a combination of three cycles of epirubicine, cyclophosphamide and fluorouracil (5-FU) followed by three cycles of docetaxel (27.6% of cycles). For managing side effects, the most commonly prescribed agents were anti-emetics (92% of cycles) and growth factors (58% of cycles). Over the 577 cycles reported, a total of 32 re-hospitalizations occurred involving 19% of patients, primarily in surgical wards (25%) and medicine services (25%).
Table 2. Summary of patient characteristics in the chemotherapy costing analysis.
| N | Mean (standard deviation) | |
|---|---|---|
| Age (years) | 106 | 53.2 (11.3) |
| Height (cm) | 106 | 161.4 (7.4) |
| Weight (kg) | 106 | 64.9 (13.4) |
| Surface area (m2) | 106 | 1.7 (0.2) |
| Working status (%) | 92 | |
| Working | 62.1 | |
| Not working | 10.8 | |
| Retired | 27.1 | |
| Tumor stage (%) | 100 | |
| 1 | 50.0 | |
| 2 | 50.0 | |
| TNM staging (%) | 106 | |
| T1n0 | 49.0 | |
| T2n0 | 47.1 | |
| T3n0 | 3.7 | |
| HER2 negative (%) | 106 | 100 |
| ER positive (%) | 106 | 0 |
| PR positive (%) | 106 | 83.1 |
| Chemotherapy cycles under protocol (%) | 106 | |
| Docetaxel | 27.6 | |
| Docetaxel + C | 13.5 | |
| Docetaxel + C + E | 3.1 | |
| Doxorubicine + C | 2.6 | |
| Epirubicine | 0.2 | |
| Epirubicine + C | 3.3 | |
| Epirubicine + C + 5FU | 47.5 | |
| Epirubicine + 5FU | 0.2 | |
| Paclitaxel | 1.7 | |
| Paclitaxel + C | 0.3 |
C, cyclophosphamide; E, epirubicin; 5FU, fluorouracil; HER, human epidermal growth factor receptor; ER, estrogen receptor; PR, progesterone receptor.
The analysis demonstrated that the mean cost of chemotherapy in the private hospital setting was approximately EUR 8,218 from a healthcare payer perspective (direct costs only) (Table 3). Approximately 62% of the study population were employed (Table 2) and 90.7% of these patients took sick leave during the chemotherapy treatment period. Factoring this lost productivity into the analysis showed that the mean costs of adjuvant chemotherapy were EUR 10,305 from a societal perspective (Table 3). Absenteeism was the main driver of costs from both the healthcare payer and societal perspectives (Table 3), accounting for approximately 24% and 39% of total costs, respectively. The other main cost driver was prophylactic prescriptions, which contributed 30% of payer costs and 24% of total costs from the societal perspective.
Table 3. Cost of adjuvant chemotherapy by components for societal and payer perspective.
| Mean cost (standard deviation) [EUR] | |||
|---|---|---|---|
| N | Payer perspective | Societal perspective | |
| Administration chemotherapy | 106 | 1,549.81 (385.79) | 1,549.81 (385.79) |
| Chemotherapy drug | 106 | 302.69 (85.35) | 302.69 (85.35) |
| Prophylactic prescription | 106 | 2,440.39 (2249.97) | 2,440.39 (2249.97) |
| Side effect management | 106 | 687.64 (2192.31) | 687.64 (2192.31) |
| Monitoring | 106 | 616.62 (147.33) | 616.62 (147.33) |
| Transport | 106 | 624.84 (512.32) | 714.46 (608.48) |
| Absenteeism | 106 | 1,996.37 (1724.38) | 3,993.39 (3449.32) |
| Total (including absenteeism) | 106 | 8,218.37 (3784.40) | 10,305.01 (4,979.21) |
| Total (without absenteeism) | 106 | 6,222.00 (3181.09) | 6,311.62 (3,215.39) |
All costs are expressed in 2013 Euros (EUR).
Cost-effectiveness analysis of Oncotype DX to guide chemotherapy decision making
Using Oncotype DX to guide adjuvant chemotherapy decision making was associated with improvements in both life expectancy and quality-adjusted life expectancy compared with standard care (Table 4). Using the Oncotype DX RS was associated with an improvement in mean discounted life expectancy of approximately 0.18 years versus standard care in eligible patients, due primarily to more appropriate allocation of chemotherapy for high risk patients. When the analysis captured health-related quality of life, Oncotype DX was associated with a benefit of approximately 0.17 QALY versus standard care, due to chemotherapy sparing in low risk patients in addition to survival benefits in high risk patients.
Table 4. Summary cost-effectiveness results for Oncotype DX versus standard care to inform adjuvant chemotherapy decision making in French private hospitals.
| Oncotype DX | Standard care | Difference | |
|---|---|---|---|
| Life expectancy (years) | 14.60 | 14.42 | +0.18 |
| Quality-adjusted life expectancy (QALYs) | 11.32 | 11.16 | +0.17 |
| Direct costs (EUR) | 11,489.81 | 11,137.36 | +352.45 |
| Direct plus indirect costs (EUR) | 12,322.91 | 12,924.88 | −601.97 |
| ICER from a healthcare payer perspective | EUR 2,134.36 per QALY gained | ||
| ICER from a societal perspective | Oncotype DX is dominant to standard care (cost and life saving) | ||
All costs are expressed in 2013 Euros (EUR). QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratio.
Evaluation of costs from a healthcare payer perspective showed that the use of Oncotype DX was associated with an increase in mean costs of approximately EUR 352 per patient relative to standard care (Table 4). The acquisition costs of the test (EUR 3,180) were offset by substantial reductions in chemotherapy costs (EUR 1,508 due to chemotherapy sparing in low risk patients) and lower costs associated with distant recurrence (EUR 1,319 due to recurrence events avoided). Cost-effectiveness, expressed as an incremental cost-effectiveness ratio (ICER) of incremental costs divided by incremental effectiveness (QALYs), showed that Oncotype DX is likely to be considered highly cost-effective from a healthcare payer perspective with an ICER of approximately EUR 2,134 per QALY gained versus standard care.
When costs were evaluated from a societal perspective, using Oncotype DX to guide chemotherapy decision making was found to be cost saving versus standard care (Table 4). Inclusion of lost productivity costs in the modeling analysis meant that costs for eligible patients undergoing Oncotype DX testing were on average EUR 602 lower than costs for those receiving standard care. This was primarily due to days off work associated with chemotherapy and management of side effects. As Oncotype DX was both cost and life saving from a societal perspective, Oncotype DX was considered to be dominant to standard care and no ICER was calculated based on societal costs.
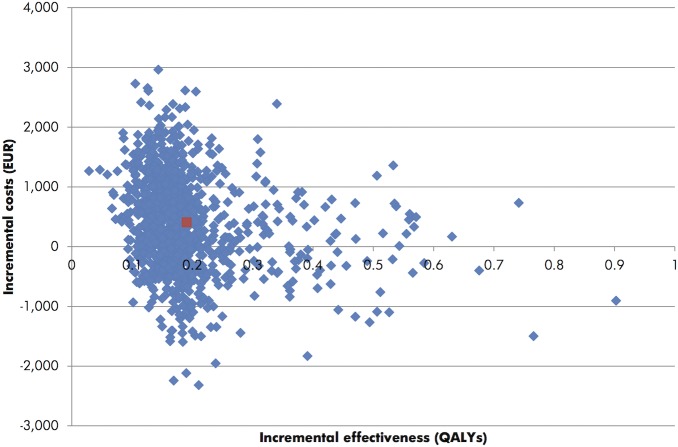
Probabilistic sensitivity analysis with sampling from distributions around key model input with 1,000 iterations (Fig 1) showed that there was 30% probability that Oncotype DX would be dominant to standard care (cost and life saving). Assuming a willingness to pay of EUR 30,000 per QALY gained, all 1,000 iterations indicated that Oncotype DX would be cost-effective relative to standard care in the French private hospital setting.
Fig 1. Cost-effectiveness scatterplot of the probabilistic sensitivity analysis.
The cost-effectiveness scatterplot shows incremental costs (€) versus incremental effectiveness expressed in quality-adjusted life years (QALYs) for the comparison of Oncotype DX with standard care. Each blue point represents one iteration of the probabilistic sensitivity analysis (with data based on sampling from distributions around clinical and cost parameters). The red point indicates the mean (of 1,000 iterations).
One-way sensitivity analysis showed that Oncotype DX remained cost-effective (or dominant) versus standard care with variation in a broad range of base case input parameters (Table 5). Decreasing the time horizon to 10 years increased the ICER for Oncotype DX versus standard care to EUR 14,772.57 per QALY gained as the long-term benefits (in terms of recurrence events avoided) were not fully captured at this time horizon. Varying the costs and risk of recurrence also had a notable impact on the ICER. When upper 95% confidence limit of recurrence costs was used, Oncotype DX became dominant to standard care. The same was true when the bounds of the upper 95% confidence intervals for the relative risks of recurrence were used in the analysis. Other one-way sensitivity analysis had a limited effect on the cost-effectiveness of Oncotype DX versus standard care.
Table 5. Summary of one-way sensitivity analysis outcomes for Oncotype DX testing versus standard care.
| Quality-adjusted life expectancy (QALYs) | Direct costs (EUR) | ICER*/Outcomes | |||||
|---|---|---|---|---|---|---|---|
| Oncotype DX | Standard care | Difference | Oncotype DX | Standard care | Difference | ||
| Base case | 11.32 | 11.16 | 0.17 | 11,489.81 | 11,137.36 | +352.45 | 2,134.36 |
| Time horizon 10 years | 6.27 | 6.22 | 0.05 | 8,407.83 | 7,677.39 | +730.44 | 14,772.57 |
| Time horizon 20 years | 9.79 | 9.67 | 0.12 | 10,589.40 | 10,171.99 | +417.41 | 3,476.99 |
| Time horizon 40 years | 11.58 | 11.41 | 0.17 | 11,636.39 | 11,287.70 | +348.69 | 2,003.20 |
| Discount rate 0% | 17.44 | 17.14 | 0.30 | 15,228.66 | 15,355.73 | -127.07 | DOMINANT |
| Discount rate 8% | 8.12 | 8.02 | 0.10 | 9,509.94 | 8,870.69 | +639.25 | 6,268.62 |
| Chemotherapy costs UL | 11.32 | 11.16 | 0.17 | 11,617.57 | 11,411.48 | +206.09 | 1,248.04 |
| Chemotherapy costs LL | 11.32 | 11.16 | 0.17 | 11,362.01 | 10,863.14 | +498.87 | 3,021.02 |
| Recurrence costs UL | 11.32 | 11.16 | 0.17 | 29,210.95 | 32,201.15 | -2,990.20 | DOMINANT |
| Recurrence costs LL | 11.32 | 11.16 | 0.17 | 7,999.83 | 6,989.08 | +1,010.75 | 6,120.87 |
| Relative risk of recurrence UL | 11.44 | 11.24 | 0.21 | 10,285.62 | 10,352.64 | -67.02 | DOMINANT |
| Relative risk of recurrence LL | 11.21 | 11.09 | 0.13 | 12,537.98 | 11,820.41 | +717.57 | 5,651.85 |
| Net change in chemotherapy use UL in low RS group | 11.32 | 11.16 | 0.17 | 11,376.21 | 11,137.36 | +238.84 | 1,435.46 |
| Net change in chemotherapy use LL in low RS group | 11.32 | 11.16 | 0.16 | 11,836.95 | 11,137.36 | +699.59 | 4,337.26 |
| Net change in chemotherapy use UL in intermediate RS group | 11.32 | 11.16 | 0.17 | 11,371.16 | 11,137.36 | +233.79 | 1,404.67 |
| Net change in chemotherapy use LL in intermediate RS group | 11.32 | 11.16 | 0.16 | 11,607.84 | 11,137.36 | +470.48 | 2,871.70 |
| Net change in chemotherapy use UL in high risk group | 11.36 | 11.16 | 0.20 | 11,248.41 | 11,137.36 | +111.04 | 551.70 |
| Net change in chemotherapy use LL in high risk group | 11.29 | 11.16 | 0.13 | 11,731.22 | 11,137.36 | +593.86 | 4,604.02 |
| 10 year risk of recurrence UL | 11.27 | 11.08 | 0.19 | 11,928.15 | 11,740.32 | +187.83 | 992.25 |
| 10-year risk of recurrence LL | 11.37 | 11.23 | 0.14 | 11,030.52 | 10,489.27 | +541.45 | 3,872.44 |
| Survival post recurrence UL | 11.20 | 11.03 | 0.17 | 12,720.28 | 12,367.83 | +352.45 | 2,134.54 |
| Survival post recurrence LL | 11.45 | 11.29 | 0.17 | 10,187.78 | 9,835.33 | +352.45 | 2,134.18 |
QALY, quality-adjusted life year; EUR, 2013 Euros; ICER, incremental cost-effectiveness ratio
* ICERs are presented in EUR per QALY gained; RS, Recurrence Score.
Discussion
The present study is the first multicenter analysis to report the cost of chemotherapy in patients with early-stage breast cancer treated in private hospitals in France. Seven hospitals from different cities were involved, taking into account differences in management of adjuvant chemotherapy, and all resources related to the management of toxicity, including both prophylactic and symptomatic prescriptions, were accounted for with a high degree of detail. These data are of significant importance, not only because 43% of surgeries for early stage breast cancer are performed at private hospitals, but because the complexity of the reimbursement landscape in France means that health economic analysis will be key in optimizing reimbursement decision in the months and years ahead for both public and private payers. The costing analysis reported in this paper presents a valuable resource for researchers investigating reimbursement issues for patients with early-stage breast cancer. It showed that prophylactic prescriptions and chemotherapy administration costs, along with absenteeism, were the biggest contributors to total costs from both the healthcare payer and societal perspectives.
The utility of these cost estimates was demonstrated in the present cost-effectiveness analysis of using the Oncotype DX recurrence score to guide chemotherapy decision making versus standard care. Based on an international meta-analysis and a previously published model, the modeling study showed that Oncotype DX is likely to improve both life expectancy and quality-adjusted life expectancy relative to standard care in eligible patients with early-stage breast cancer. Moreover, the acquisition costs of the test were largely offset by reductions in chemotherapy costs (chemotherapy sparing) and reductions in distant recurrence. As a result, Oncotype DX is found to be dominant to standard care from a societal perspective and should be considered highly cost-effective by commonly quoted standards from a healthcare payer perspective relative to standard care. The findings of the cost-effectiveness evaluation were consistent with those of previous analyses on Oncotype DX, which have shown that Oncotype DX was also likely to be dominant to standard care (cost and life saving) in the public hospital setting from a societal perspective.[22] Comparable findings in the public and private sectors in France support the generalizability of the present study and are consistent with recent observations that no notable differences exist in the management of breast cancer between public and private centers in France.[34] Moreover, a recent systematic review identified 18 published cost-effectiveness analyses of Oncotype DX, across a range of country-settings, which consistently showed that Oncotype DX is associated with improved clinical outcomes and is either cost-effective or cost saving relative to standard care in women with ER+, HER2−, early-stage breast cancer.
The evaluation of costs was limited to those associated with adjuvant chemotherapy. The objective was not to provide an estimate of the total costs of breast cancer care, but rather to focus on the additional costs of adjuvant chemotherapy, and as a result costs associated with radiotherapy, endocrine therapy, and long-term toxicities were not included. In 2012, Laas et al. published an evaluation of the costs of adjuvant chemotherapy in France from the societal perspective and reported an estimate of EUR 15,740 per patient.[7] This estimate is higher than that reported here (EUR 10,305). However, in the present analysis, all chemotherapy drugs were included in the unique T2A tariff paid to the hospital (no drugs were paid in addition) and, in 2011, agents such as docetaxel were not included in the T2A tariff (but were paid in addition to the T2A tariff). Moreover, there is a notable difference in the mean T2A payments for public (EUR 390.28 in 2011) and private hospitals (EUR 297.48 in 2013) due to the two reimbursement regimens implemented by health authorities, which is likely also to contribute to the difference in total cost estimates in the two studies.
Long-term modeling analyses are inevitably associated with limitations, as assumptions are invariably required to make long-term estimates of costs and clinical outcomes. In the present analysis, a previously published health economic model was used to minimize the potential impact of unvalidated assumptions and model (structural) uncertainty. A potential short-coming of the modeling analysis is that it used decision impact data (on how Oncotype DX influences decision on adjuvant chemotherapy) from an international meta-analysis. In the absence of country-specific decision impact data, one-way sensitivity analyses were performed that showed altering the decision for patients with a low, intermediate or high risk of recurrence based on RS within plausible ranges, did not alter the conclusions of the analysis (Oncotype DX remained cost-effective or cost saving). Further, even though the model captured more than short-term adverse effects of chemotherapy treatment, it did not take into account long-term adverse-events such as abnormal heart function or impaired cognitive function. This limitation is conservative, as the chemotherapy sparing benefits associated with Oncotype DX are not fully captured within the present modeling framework. Similarly, a conservative assumption was made in that no utility increment associated with the use of the Oncotype DX test was captured in the analysis. It has been shown that the use of the Oncotype DX breast cancer assay increases patients and physicians confidence in their decision and decreases anxiety in patients.[35] It would therefore be reasonable to assume that the value of the information delivered by gene expression tests such as Oncotype DX should translate into quality of life benefits. In addition, the cost of recurrence was estimated from a healthcare payer perspective and may, therefore, represent an underestimate in the societal analysis.[29] Although the clinical validity of the Oncotype DX test has been evaluated retrospectively in several studies involving over 3,300 patients, prospective clinical data (level 1 evidence) are not yet available, though three prospective trial are currently ongoing. The findings from these studies may offer a more robust evidence base not only for the test itself, but also for future health economic evaluations.
In addition to the retrospective costing analysis and cost-effectiveness evaluation, an analysis of revenue from the perspective of private hospitals was performed to help put the present findings in context for healthcare payers. The analysis was deigned to estimate marginal revenue, defined as the revenue generated by one patient with adjuvant chemotherapy, for scenarios with and without the use of the Oncotype DX test based on French data describing patient characteristics and the meta-analysis of Oncotype DX decision impact studies.[4, 13] The analysis showed that, based on an average net profit margin of 2.9% reported for Générale de Santé private hospitals, there would be an estimated revenue loss for private hospitals of at least EUR 3,203 per patient tested as the test is not currently reimbursed.[36] This evaluation did not integrate the potential benefits associated with improved hospital reputation and enhanced attractiveness for patients of using Oncotype DX (due to improved clinical outcomes). Moreover, offering the test is likely to free up resources (e.g. medical personnel, chairs for intravenous infusion, etc.), which could be allocated for the patients who still undergo chemotherapy with higher recurrence scores.
Oncotype DX was chosen for the present analysis as it is supported by a greater evidence base than other gene expression profiling or expanded immunohistochemistry tests.[37] However, several other tests exist that may support improved chemotherapy decision making and beneficial economic outcomes. Although meaningful comparisons of cost-effectiveness between tests are not currently possible in the absence of data from head-to-head decision impact studies, Ward et al. (2013) published a systematic review of clinical and cost-effectiveness of current prognostic tools in guiding the use of adjuvant chemotherapy in patients with early breast cancer performed to support the development of guidelines for the National Institute of Health and Care Excellence (NICE) in the UK.[38] A cost-effectiveness evaluation was performed for Oncotype DX, IHC4, MammaPrint and Mammostrat tests in comparison with standard clinical practice in England and Wales (as evidence for five other tests was too limited: PAM50, NPI+, BCI, BluePrint and Randox). The authors concluded that the clinical evidence base for Oncotype DX was the most robust (and was associated with an ICER GBP 26,940 per QALY gained versus standard care). The authors also noted that treatment guided using IHC4 has the most potential to be cost-effective but that the evidence base to support IHC4 “needs significant further research.”
As highlighted in the 2013 St. Gallen guidelines, there is discord between the reimbursement status in several European countries, including France.[21] Following the French Plan Hôpital reforms in 2007, the government required hospitals to adopt an activity-based Disease Related Groups (DRG) pricing system: the tarification à l'activité (T2A). The approach is based on Groupes Homogènes de Séjour (GHS or uniform hospitalization groups). Hospitals receive a fixed price for treating a patient allocated to a GHS. The GHS price includes all medicine and resources used to treat the patient. However, innovative treatments often do not fall within existing T2A definitions, particularly if the act does not resemble a previously established practice, as can be the case for gene expression profiling. Creating a new T2A takes 2–3 years in the best case. Intermediate funding opportunities exist through regional authorities or project tenders, but these are limited. As a result, the launch of a new product can therefore be directly affected by the ability to obtain funding outside of the T2A system. If no funds are available, public and private hospitals will be reluctant to use the product or may simply be unable to do so. In 2014, the 21 gene assay was funded through a very limited number of hospitals and regional authorities (Agence Régionale de Santé).
The absence of a national funding mechanism allowing the controlled used of innovative tests, such as Oncotype DX, has therefore created a situation of unequal patient access. Similar situations exist in a number of other countries, and this situation raises the question of whether traditional models of reimbursement are adequate to deal with issues such as genetic testing in the public health sphere in the era of personalized medicine.[39, 40] Since 2013, French health reform had a clear focus on patient access to personalized medicine (passed by the French Congress in April 2015), but currently the reimbursement of prognostic tests remain unclear.[41] As reimbursement remains the largest hurdle to the wider adoption of personalized medicine products, alternative payment methods are emerging. Public-private partnerships, such as AstraZeneca-funded testing in non-small cell lung cancer patients to identify those who would respond to its gefitinib, may offer a potential solution for some conditions. Patient advocacy groups may also play a role; for example the Cystic Fibrosis Foundation’s Mutation Analysis Program offered genetic testing to identify those who would benefit from benefit from an orphan drug (ivacaftor). Direct patient payments represent another option, but with the obvious caveat of inequality. The ethical committee from the Ligue Nationale contre le Cancer, one of the leading patient cancer associations in France, underlined that the absence of coverage presents a dilemma where only wealthy patients will be able to afford the test.[42] This report indicates that some oncologists prefer to dissimulate the test availability to patients that may benefit from it, but simply cannot afford it. During the period where the test is not reimbursed, oncologists are still confronted with their obligation of means with respect to all patients. Prescribing chemotherapies to resistant patients that could have been detected with the test can present medico-legal risks as patients could claim a loss of chance, resulting in litigation involving both physicians and health care institutions. Moreover, it creates the potential for unequal access by socioeconomic status, with more affluent patients opting to pay privately for testing, an option that may be beyond the means of other individuals. From an economic perspective, the present T2A French system reimburses adjuvant chemotherapies in all cases, even for resistant patients. To maximize revenues, public and private hospitals are incentivized not to use gene expression profiling or expanded immunohistochemistry tests and continue prescribing chemotherapies even when unnecessary, which represents a clinical, ethical and economic impasse.
Conclusion
Our study provides evidence of the cost-effectiveness of Oncotype DX from a healthcare payer perspective as well as the cost savings from a societal perspective. However, the analysis also raises the questions around reimbursement and access to the test for patients and healthcare professionals, as it is not covered by primary payers such as the social security. The delay in coverage of molecular testing has the potential to erode the quality of the French healthcare system. Budget constraints are likely to mean that tests like Oncotype DX will remain underused in France, thus depriving patients of technologies that could improve clinical outcomes and allow healthcare professionals to better allocate hospital resources to improve the standard of care for all patients.
Supporting Information
Table B1. Distribution of sick leave for working patients. Table B2. ART-Patients characteristics. Table B3. Average dosage of chemotherapy drug per cycle in mg/m² per protocol. Table B4. Patient characteristics by hospital. Table B5. Distribution of side effects per cycle. Table B6. Home care. Table B7. Hospitalization characteristics. Table B8. Income for hospital per cycle. Table B9. Income for hospital by patient. Table B10. Aggregated costs per patient–Insurance perspective. Table B11. Aggregated costs per cycle–Insurance perspective. Table B12. Monitoring–Distribution of cycle with laboratory tests. Table B13. Monitoring–Consultation per cycle. Table B14. Pre-chemotherapy tests. Table B15. Protocols–Number of cycles. Table B16. Average cost by patient–Societal perspective. Table B17. Average cost per cycle–Societal perspective. Table B18. Distribution of chemotherapy strategies and protocols. Table B19. Distribution of cycle and reason for symptomatic prescription. Table B20. Transportation and distribution of transport.
(RTF)
Acknowledgments
The authors would like to thank William Valentine, Juliette Plun-Favreau and Alexandre Vimont for their assistance with the realization of this article. The authors are also grateful to Maya Rejdyck, Adrien Hessenbruch, Nadège Vieillard, Mathilde Rohfritsch, Gaëlle Le Gulludec, Charlotte Dujardin, Pauline Jouany, Jean-Baptiste Paoli, François Amalric, Claudine Althschuller, Isabelle Ferrié-Philippot, Dominique Beal-Ardisson, Mathieu Bosset.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
ALV has received funding from Genomic Health International. No other authors received financial support for the study. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.International Agency for Research on Cancer. Estimated Incidence, Mortality and Prevalence Worldwide in 2012; 2015. Available: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 2014 May 8.
- 2. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013. April;49(6):1374–403. 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 3.Institut National du Cancer (INCa). [Le cancer du sein: état des lieux en 2012]; 2015. Available http://www.e-cancer.fr/publications/61-etat-des-lieux-et-des-connaissances. Accessed 2014 May 8.
- 4. Chereau E, Coutant C, Gligorov J, Lesieur B, Antoine M, Darai E, et al. Discordance with local guidelines for adjuvant chemotherapy in breast cancer: reasons and effect on survival. Clin Breast Cancer 2011. March;11(1):46–51. 10.3816/CBC.2011.n.008 [DOI] [PubMed] [Google Scholar]
- 5. Lim CC, Devi MK, Ang E. Anxiety in women with breast cancer undergoing treatment: a systematic review. Int J Evid Based Healthc 2011. September;9(3):215–35. 10.1111/j.1744-1609.2011.00221.x [DOI] [PubMed] [Google Scholar]
- 6. Peugniez C, Fantoni S, Leroyer A, Skrzypczak J, Duprey M, Bonneterre J. Return to work after treatment for breast cancer: single center experience in a cohort of 273 patients. Bull Cancer 2011. July;98(7):E69–E79. 10.1684/bdc.2011.1401 [DOI] [PubMed] [Google Scholar]
- 7. Laas E, Vataire AL, Aballea S, Valentine W, Gligorov J, Chereau E, et al. Evaluation of the costs and resource use associated with adjuvant chemotherapy for breast cancer in France. J Med Econ 2012;15(6):1167–75. 10.3111/13696998.2012.713414 [DOI] [PubMed] [Google Scholar]
- 8. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006. August 10;24(23):3726–34. [DOI] [PubMed] [Google Scholar]
- 9. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004. December 30;351(27):2817–26. [DOI] [PubMed] [Google Scholar]
- 10. Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 2010. April 10;28(11):1829–34. 10.1200/JCO.2009.24.4798 [DOI] [PubMed] [Google Scholar]
- 11. Habel LA, Shak S, Jacobs MK, Capra A, Alexander C, Pho M, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res 2006;8(3):R25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010. January;11(1):55–65. 10.1016/S1470-2045(09)70314-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornberger J, Chien R. Meta-Analysis of the Decision Impact of the 21-Gene Breast Cancer Recurrence Score in Clinical Practice. Poster session presented at: San Antonio Breast Cancer Symposium, 2010 December 8–12, Texas, USA.
- 14.Holt S, Pudney D, Rolles M, Moe M, Durani S, Khawaja S, and et, al. Results from a prospective clinical study on the impact of Oncotype DX on adjuvant treatment decision in a cohort of 142 UK patients. Poster session presented at: San Antonio Breast Cancer Symposium, 2011 December 6–10, Texas, USA.
- 15.Yamauchi H, Nakagawa C, Yamashige S, Takei H, Yagata H, Yoshida A. Decision impact and economic evaluation of the 21-gene Recurrence Score assay for physicians and patients in Japan. Poster session presented at: the annual European Society for Medical Oncology Congress, 2011 September 23–27, Stockholm, Sweden.
- 16.De Boer RH, Baker C, Speakman D, Mann B. Australian Decision Impact Study: The impact of Oncotype DX Recurrence Score (RS) on adjuvant treatment decisions in hormone receptor positive (HR+), node negative and node positive (N+) early breast cancer (ESBC) in the multidisciplinary clinic. Poster session presented at: San Antonio Breast Cancer Symposium, 2011, December 6–10, Texas, USA.
- 17.National Institute for Health and Clinical Excellence (NICE). Diagnostics Consultation Document. Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat. 2013 Feb. Available: http://www.nice.org.uk/guidance/dg10/documents/gene-expression-profiling-and-expanded-immunohistochemistry-tests-to-guide-the-use-of-adjuvant-chemotherapy-in-early-breast-cancer-management-mammaprint-oncotype-dx-ihc4-and-mammostrat-diagnostics-co2. Accessed 2014 May 8.
- 18. Aebi S, Davidson T, Gruber G, Castiglione M. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010. May;21 Suppl 5:v9–14. 10.1093/annonc/mdq159 [DOI] [PubMed] [Google Scholar]
- 19. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 2007. November 20;25(33):5287–312. [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Breast Cancer; 2013. Available: http://www.nccn.org. Accessed 2014 May 8.
- 21. Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 2013. September;24(9):2206–23. 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vataire AL, Laas E, Aballea S, Gligorov J, Rouzier R, Chereau E. [Cost-effectiveness of a chemotherapy predictive test]. Bull Cancer 2012. October;99(10):907–14. 10.1684/bdc.2012.1652 [DOI] [PubMed] [Google Scholar]
- 23.Agence Technique de l'information sur l'Hospitalisation (ATIH). 2013. Available: http://www.atih.sante.fr/. Accessed 2014 May 8.
- 24.Assurance maladie. 2015 Available: http://www.ameli.fr/. Accessed 2014 May 8.
- 25.Direction générale de l'action sociale. [Les patients en service de soins infirmiers à domicile. Le coût de leur prise en charge et ses déterminants]; 2015. Available: http://travail-emploi.gouv.fr/IMG/pdf/SSIAD.pdf. Accssed 2014 May 8.
- 26.Institut national de la statistique et des études économiques (INSEE). [Salaires et revenus d'activités]; 2014. Available: http://www.insee.fr/. Accssed 2014 May 8.
- 27. Paviot BT, Bachelot T, Clavreul G, Jacquin JP, Mille D, Rodrigues JM. [Impact of the chemotherapy protocols for metastatic breast cancer on the treatment cost and the survival time of 371 patients treated in three hospitals of the Rhone-Alpes region]. Bull Cancer 2009. October;96(10):929–40. 10.1684/bdc.2009.0920 [DOI] [PubMed] [Google Scholar]
- 28.French National Demographic Institute website. [Espérance de vie, Mortalité, Santé]; 2015. Available: http://www.ined.fr/fr/grands-themes/esperance-de-vie-mortalite-sante/. Accssed 2014 Oct 8.
- 29. Bonneterre J, Bercez C, Bonneterre ME, Lenne X, Dervaux B. Cost-effectiveness analysis of breast cancer adjuvant treatment: FEC 50 versus FEC 100 (FASG05 study). Ann Oncol 2005. June;16(6):915–22. [DOI] [PubMed] [Google Scholar]
- 30. Peasgood T, Ward SE, Brazier J. Health-state utility values in breast cancer. Expert Rev Pharmacoecon Outcomes Res 2010. October;10(5):553–66. 10.1586/erp.10.65 [DOI] [PubMed] [Google Scholar]
- 31. Conner-Spady BL, Cumming C, Nabholtz JM, Jacobs P, Stewart D. A longitudinal prospective study of health-related quality of life in breast cancer patients following high-dose chemotherapy with autologous blood stem cell transplantation. Bone Marrow Transplant 2005. August;36(3):251–9. [DOI] [PubMed] [Google Scholar]
- 32. Milne RJ, Heaton-Brown KH, Hansen P, Thomas D, Harvey V, Cubitt A. Quality-of-life valuations of advanced breast cancer by New Zealand women. Pharmacoeconomics 2006;24(3):281–92. [DOI] [PubMed] [Google Scholar]
- 33.Haute Autorité de Santé (HAS). [Choix méthodologiques pour l'évaluation économique à la HAS]; 2011. Available: http://www.has-sante.fr/portail/jcms/r_1499251/en/choix-methodologiques-pour-l-evaluation-economique-a-la-has. Accssed 2014 May 8.
- 34.Clough KB, Acosta-Marin V, Nos C, Alran S, Rouanet P, Garbay JR, et al. Rates of Neoadjuvant Chemotherapy and Oncoplastic Surgery for Breast Cancer Surgery: A French National Survey. Ann Surg Oncol 2015 Feb 10. [DOI] [PubMed]
- 35. Lo SS, Mumby PB, Norton J, Rychlik K, Smerage J, Kash J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 2010. April 1;28(10):1671–6. 10.1200/JCO.2008.20.2119 [DOI] [PubMed] [Google Scholar]
- 36.Générale de Santé. [Document de référence 2012]; 30-4-2013. Available: www.generale-de-sante.fr/content/download/17666/199840/version/1/file/GDS_Document_de_Reference_2012.pdf. Accssed 2014 May 8.
- 37.National Institute for Health and Clinical Excellence (NICE). Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DX, IHC4 and Mammostrat; 2013. Available: https://www.nice.org.uk/guidance/dg10. Accessed 2015 Apr 24.
- 38. Ward S, Scope A, Rafia R, Pandor A, Harnan S, Evans P, et al. Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review and cost-effectiveness analysis. Health Technol Assess 2013. October;17(44):1–302. 10.3310/hta17440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hall PS, McCabe C. What evidence is there for the reimbursement of personalised medicine? Pharmacoeconomics 2013. March;31(3):181–3. 10.1007/s40273-013-0025-x [DOI] [PubMed] [Google Scholar]
- 40. Katz G, Schweitzer S. Implications of genetic testing for health policy. Yale J of Public Health, Law and Ethics 2010;10(1):90–134. [PubMed] [Google Scholar]
- 41.Ministére des Affaires Sociales et de la Santé. [Stratégie Nationale de Santé]; 23-9-2013. Available: http://www.social-sante.gouv.fr/IMG/pdf/SNS-version-courte.pdf. Accessed 2015 Apr 24.
- 42.Ligue contre le cancer. Comité Ethique et Cancer. [Avis n°21 du 15 janvier 2013.- « De l'équité d'accès et d'information aux tests génomiques: le cas du test prédictif Oncotype DX dans les cancers du sein »]; 2015. Available: http://www.ethique-cancer.fr/phoenixws/detailavis/topic-1/article-89/avis-n-21-du-15-janvier-2013.html. Accessed 2014 May 8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table B1. Distribution of sick leave for working patients. Table B2. ART-Patients characteristics. Table B3. Average dosage of chemotherapy drug per cycle in mg/m² per protocol. Table B4. Patient characteristics by hospital. Table B5. Distribution of side effects per cycle. Table B6. Home care. Table B7. Hospitalization characteristics. Table B8. Income for hospital per cycle. Table B9. Income for hospital by patient. Table B10. Aggregated costs per patient–Insurance perspective. Table B11. Aggregated costs per cycle–Insurance perspective. Table B12. Monitoring–Distribution of cycle with laboratory tests. Table B13. Monitoring–Consultation per cycle. Table B14. Pre-chemotherapy tests. Table B15. Protocols–Number of cycles. Table B16. Average cost by patient–Societal perspective. Table B17. Average cost per cycle–Societal perspective. Table B18. Distribution of chemotherapy strategies and protocols. Table B19. Distribution of cycle and reason for symptomatic prescription. Table B20. Transportation and distribution of transport.
(RTF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.