Abstract
Current influenza virus vaccines provide solid protection from infection with viruses that are well matched with the vaccine strains. However, they do not protect efficiently against drifted or shifted strains. We developed an antigen based on the conserved stalk domain of the influenza virus hemagglutinin and tested its efficacy as a vaccine in a mouse virus challenge model. Although the antigen lacked the correct conformation of the native stalk domain and was not recognized by a panel of neutralizing stalk-reactive antibodies, it did induce considerable protection against H1N1, H5N1 and H6N1 challenge strains. Protection was enhanced when mice had pre-existing immunity against the stalk domain. Since pre-existing immunity is also present in the human population, we hypothesize that a similar antigen could show efficacy in humans as well.
Introduction
Seasonal influenza virus infections cause significant morbidity and mortality worldwide. Current vaccines are effective against well-matched circulating strains. However, these vaccines need to be updated on an annual basis due to constant antigenic drift of human influenza viruses [1]. The prediction of the strains for seasonal influenza virus vaccines is largely based on the surveillance of circulating viruses. If predictions are wrong and the vaccine and the circulating strains are mismatched, vaccine efficacy drops significantly [2]. In addition pandemics occur at irregular intervals and seasonal vaccines are ineffective against those novel viruses [3, 4].
The strain specificity of current inactivated influenza virus vaccines is caused by the immuno-dominance of membrane distal globular head domain of the viral hemagglutinin (HA), the major surface glycoprotein and major immunogen of the virus [5]. Antibodies directed against this domain are generally strongly neutralizing. However, the globular head domain has a high antigenic plasticity - changes in this domain are responsible for most of the antigenic drift of influenza viruses [6, 7]. The membrane proximal stalk domain of the HA is more conserved but immuno-subdominant. The discovery of rare antibodies that bind to the stalk domain and are able to neutralize influenza viruses of divergent subtypes has highlighted the value of the stalk domain as a target for broadly protective vaccines [8–14]. However, due to the immunodominant nature of the globular head domain, stalk-reactive antibodies are usually not induced or boosted by regular inactivated influenza virus vaccines [15–18]. A possible solution for this problem would be the use of headless HAs - stalk-only antigens. Here we describe the production of H1 based trimeric headless HA in an insect cell expression system and its efficacy against influenza virus challenge in the mouse model.
Methods and Materials
Cells and viruses
Madin-Darby Canine Kidney (MDCK) cells were grown in Dulbecco’s Modified Eagles Medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS, HyClone) and a penicillin-streptomycin mix (100 units/ml of penicillin and 100 μg/ml of streptomycin, Gibco). Influenza viruses A/Puerto Rico/8/34 (PR8, H1N1), A/Netherlands/602/09 (NL09, pandemic H1N1), A/Vietnam/1203/04 (H5N1, 6:2 re-assortant with PR8 and polybasic cleavage site removed from HA [19]), A/mallard/Sweden/81/02 (H6N1, 7:1 re-assortant with PR8) and cold adapted A/Ann Arbor/60/66 (H2N2) were grown in 8–10 day old embryonated chicken eggs (Charles River Laboratories) at 37°C (H1N1, H5N1, H6N1) or 33°C (c.a. H2N2) for 48 and 72 hours respectively. Viruses were then titered on MDCK cells in a standard plaque assay in the presence of tosyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin. Virus substrates for ELISAs were produced by pelleting virus from clarified allantoic fluid through a phosphate buffered saline (PBS, pH7.4, Gibco) 30% sucrose cushion via ultracentrifugation at 25,000 rpm for 2 hours using a Beckman SW28 rotor. Virus was then resuspended in PBS and the concentration was measured using the Bradford method. Virus inactivation was performed as described before [20].
Sf9 cells were propagated in TNM-FH medium (Gemini Bio-Products) containing penicillin-streptomycin mix and 10% FBS. BTI-TN5B1-4 cells (Vienna Institute of BioTechnology subclone [21]) were grown in serum free SFX medium (HyClone). Recombinant baculoviruses expressing the hexahistidine-tagged full length PR8 HA ectodomain (PR8 HA) and the Strep-Tag II-tagged full length A/California/04/09 HA (Cal09 ST HA), A/PR/8/34 HA (PR8 ST HA), A/Fort Monmouth/1/47 (FM1 ST HA) and cH6/1 HA [22] (cH6/1 ST HA) ectodomains were propagated on Sf9 cells as described before [23]. A recombinant baculovirus expressing the soluble PR8 headless HA (HL HA) was generated by cloning the codon optimized gene coding for the ectodomain of the headless HA (as described in [24]) with a C-terminal thrombin cleavage site, a T4 phage fibritin foldon trimerization domain and a hexahistine tag (Fig. 1) into a modified [23] pFastBac Dual vector (Invitrogen). The gene was amplified by polymerase chain reaction (PCR) and cloned using BamHI and NotI restriction enzymes (NEB). Primer sequences are available upon request.
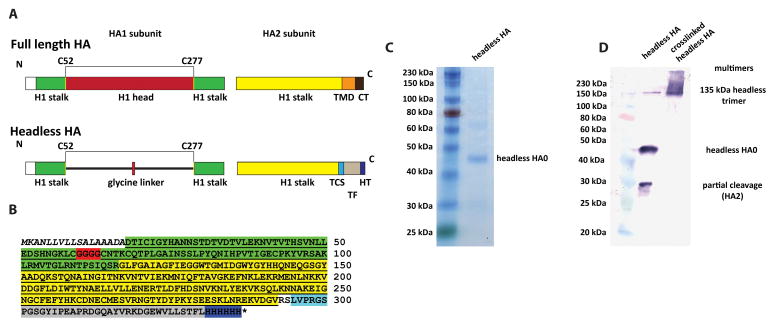
Fig. 1. Headless HA construct design and characterization.
A Schematic of full length and headless HA. Cysteins 52 and 277 (H3 numbering) are indicated on the N- and C-terminus of the globular head domain/tetra-glycine linker in yellow. (TMD= transmembrane domain; CT= cytoplasmic tail; TCS= thrombin cleavage site; TF= T4 foldon; HT= hexahistidine tag). B Sequence of the soluble headless HA construct. The signal peptide is shown in italics, the HA1 part of the headless HA is highlighted in green. This part is interrupted by a tetra-glycine linker (highlighted in red) which replaces the globular head domain. The HA2 part of the stalk domain is highlighted in yellow. The HA2 sequence is followed by a thrombin cleavage site (light blue), a T4 fibritin foldon trimerization domain (grey) and a C-terminal hexahistidine tag (dark blue). C shows purified headless HA on a Coomassie stained reducing SDS-PAGE. D shows purified headless HA on a Western blot under reducing conditions (left lane) and cross-linked under reducing conditions (right lane). The Western blot reveals a degradation product at approximately 27 kDa which is not visible on the Coomassie stained SDS-PAGE. Cross-linking with BS3 results in a trimeric species and a high molecular weight smear. Different protein species are indicated in C and D.
Recombinant protein production
Recombinant proteins were produced and purified as described before [23, 25]. Briefly, BTI-TN5B1-4 cells were infected with recombinant baculoviruses at a multiplicity of infection of 10 in serum free SFX insect cell medium. Supernatants were harvested 72 hours post infection and recombinant protein was purified via Ni-NTA (Qiagen) (PR8 HA, HL HA) or via Strept-Actin (Qiagen) resin (Cal09 ST HA) [22, 23, 25].
Enzyme linked immunosorbent assay and microneutralization assay
A panel of monoclonal antibodies (mABs) were used in an enzyme linked immunosorbent assay (ELISA) to assess the correct folding of full length and headless PR8 HA. To exclude denaturation on the plate due to hydrophobic interactions between protein and plate, the proteins were captured via their hexahistidine tag on Ni-NTA HiSorb plates (Qiagen) and ELISAs were performed as described before [13]. Murine mAbs IB11, 6F12 [9], BD3, GG3 [26] and KB2 [27] and the Fab fragment of human mAb CR6261 [10] bind only to the correctly folded stalk domain and were used to probe for conformational integrity. MAb PY102 [28] binds to the PR8 head domain only and was used as control mAb that should only bind to full length PR8 HA but not to HL HA. Finally a human mAb against the long alpha helix (LAH) was used as control for binding in general because it recognizes both folded and misfolded stalk domains. Starting concentrations for mAbs were 30 ug/ml except for CR6261 for which the starting concentration was 10 ug/ml.
ELISAs for mouse serum analysis were carried out as described in [22]. Purified PR8 (H1N1), H2N2 and H5N1 viruses (coating concentration 5 ug/ml) or purified Cal09 ST HA protein (coating concentration 2 ug/ml) were used as substrate. The Cal09 ST HA, PR8 ST HA, FM1 ST HA and cH6/1 ST HA feature a GCN4pII trimerization domain and a C-terminal Strep-Tag II. These substrates were chosen to avoid measuring reactivity against the immunogenic T4 foldon or the hexahistidine tag which are present on the PR8 HL HA antigen [29]. For ELISAs with PR8 ST HA, FM1 ST HA and cH6/1 ST HA an endpoint titer analysis was performed. An established cut-off of 0.1 absorption at 490nm was used for analysis.
Micro-neutralization assays with NL09 and PR8 viruses were performed as described [15] with the following modifications: To make the assay more sensitive, the amount of virus per well was reduced from 200 to 100 plaque forming units and serum was present during the full incubation time (24 hours).
Polyacrylamide gel and Western blot analysis
HL HA protein was analyzed on sodium dodecyl sulfate polyacrylamide gels (SDS PAGE, 10% polyacrylamide, Mini PROTEAN TGX gels, Bio-Rad) under reducing conditions. The extent of trimerization was analyzed using a cross-linking reagent (bis-[sulfosuccinimidyl]suberate (BS3 - Fisher Scientific)) as specified by the manufacturer and as described in detail before [23, 30]. BS3 has a spacer length of 11.4 Å and can cross-links primary amines present on lysine side chains. Cross-linked HL HA was analyzed by Western blot using primary anti-rabbit polyclonal antibody 3951 (raised against influenza virus particles lacking HA1 [31]), a secondary anti-rabbit alkaline phosphatase conjugated antibody (Santa Cruz Biotechnology) and 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT, Biorad) as substrate.
Animals and challenge experiments
All experiments were performed with female six to eight week old BALB/c mice in accordance with the Icahn School of Medicine at Mount Sinai Institutional Animal Care and Use Committee guidelines. Mice were anesthetized by intraperitoneal injection of 100ul of ketamine/xylazine (0.15 mg ketamine and 0.03 mg xylazine) for all procedures involving vaccination or virus infection and a humane endpoint of 25% weight loss was used in all challenge experiments. Challenge experiments were performed with the following groups: One set of mice was completely naive (Naive, n=4–5). A second group (prime-only, cH9/1-BSA-BSA, n=4–5) was vaccinated with 40 μg of a plasmid encoding for cH9/1 HA (an H9 head domain on top of an H1 PR8 stalk domain) administered via intramuscular (i.m.) electroporation into the calf muscle using a TriGrid delivery system (Ichor Medical Systems) [22]. This group was then boosted twice in three week intervals with bovine serum albumin (BSA) adjuvanted with polyI:C (PIC) (10 ug BSA + 10 ug of PIC intranasaly (i.n.) and 10 ug BSA + 10 ug of PIC i.m.). The third group (cH9/1+HL+HL, n=9–10) was primed as described for the second group and then received two boosts of HL HA (10 ug HL HA + 10 ug of PIC i.n. and 10 ug HL HA + 10 ug of PIC i.m.). A fourth group (HL + HL, n=9) received no prime and only the two HL vaccinations as described for the cH9/1+HL+HL group. Finally, a fifth group (Pos. contr., n=5) received two vaccinations of 1 ug of inactivated challenge virus in PBS via i.m. injection (non-adjuvanted) in a three week interval. Four weeks after the last vaccination animals were intranasally challenged with 5 murine lethal doses 50 (mLD50) of PR8 (H1N1), H5N1 or H6N1 virus. Group three (HL+HL) was only used in the homologous PR8 challenge experiment. An additional group of cH9/1+HL+HL vaccinated mice was treated with 300 μg of anti-CD8+ mAb from hybridoma 2.43 48 and 24 hours prior to infection to deplete cytotoxic T-cells following an established protocol [22, 32]. Weight of all animals was monitored daily for a 14 day time period after infection and animals that lost 20% or more of their initial body weight were scored dead and euthanized according to the institutional guidelines.
Results
Expression and characterization of soluble headless HA
To generate headless (HL) HA, the globular head domain was replaced by a tetraglycine linker as described by Steel and colleagues (Fig 1A and B) [24]. The transmembrane domain was then replaced by a thrombin cleavage site, a T4 foldon trimerization domain and a C-terminal hexahistine tag (Fig. 1A and B) to allow for expression of soluble trimers [23]. This construct was cloned into a baculovirus transfer vector, and the recombinant baculovirus was generated and used to express the HL HA in BTI-TN5B1-4 insect cells. The protein was characterized via SDS-PAGE and a major band at approximately 45 kDa was observed. This band corresponds to the predicted molecular weight of uncleaved HL HA (HA0, 37.5 kDa plus four predicted N-linked glycans) (Fig. 1C). A Western blot analysis revealed the presence of a small proportion of cleaved product which is most likely HA2 (Fig. 1D). The cross-linked HL HA formed trimers and higher molecular weight multimers (Fig. 1D).
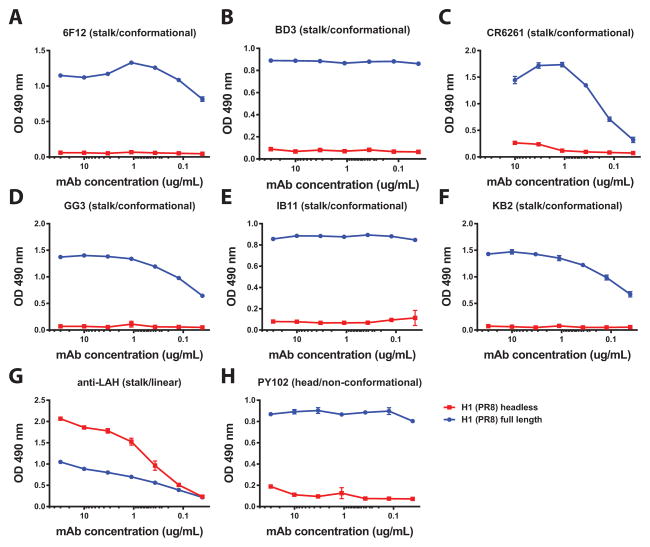
Since neutralizing stalk-reactive antibodies almost exclusively bind to conformational epitopes, we performed a binding analysis with a panel of stalk-reactive mAbs. Importantly, this analysis was done with Ni-NTA plates, which capture the recombinant proteins via their hexahistidine tag. Coating on regular ELISA plates involves strong hydrophobic interactions and can potentially denature fragile proteins. All tested human and murine neutralizing stalk mAbs efficiently bound to soluble full length PR8 HA (Fig. 2 A–F), but they did not react with HL HA. Interestingly, a non-neutralizing human antibody that recognizes the LAH of H1 bound preferentially to HL HA (Fig. 2 G). This data suggests that soluble HL HA does not display most of the neutralizing stalk epitopes in their natural conformation.
Fig. 2. Broadly neutralizing mouse and human stalk-reactive antibodies do not bind to headless HA.
Panels A–F show ELISA binding curves of broadly neutralizing antibodies with conformational epitopes to the full length PR8 H1 ectodomain (‘H1 (PR8) full length’) and to headless HA (‘H1 (PR8) headless’). All antibodies show good binding to the full length protein but do not bind to the recombinant headless HA. G A non-neutralizing human monoclonal antibody that recognizes a linear epitope on the long alpha helix of the HA2 subunit preferentially recognizes headless HA. H Anti PR8 head antibody PY102 was used as control and only binds to the full length PR8 protein. ELISAs were performed using NiNTA plates which capture the recombinant protein via the hexahistidine tag. Using these plates circumvents protein denaturation that can occur on regular ELISA plates due to hydrophobic interactions.
Headless HA immunogens induce cross-reactive antibodies against group 1 HA expressing influenza viruses in mice
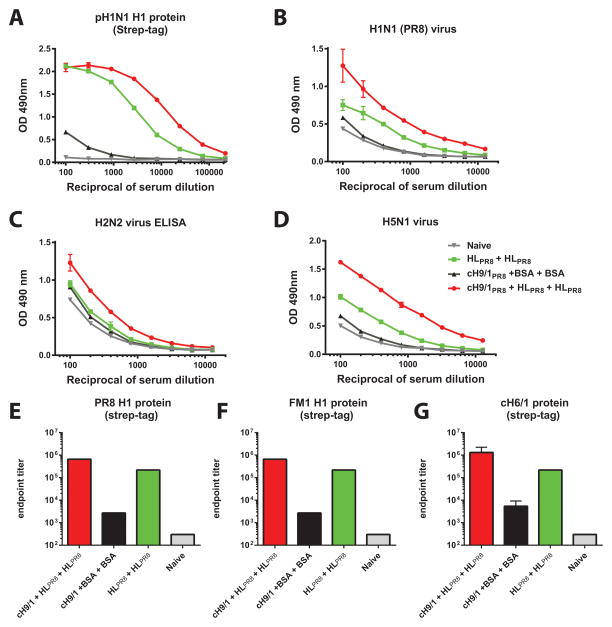
We hypothesized that HL HA constructs would induce a stalk-based antibody response that contributes to protection from challenge. To assess presence of cross-reactive antibodies, we performed ELISA assays with a full-length recombinant Cal09 H1 ectodomain protein and with purified viruses as substrate. Importantly, the Cal09 HA protein was purified using a Strep-Tag II and had a GCN4pII trimerization domain to avoid measuring false-positive cross-reactivity towards the hexahistidine tag and T4 foldon used in the vaccine constructs. Good ELISA reactivity of the stalk-primed HL HA vaccinated animals (cH9/1+HL+HL) was observed against Cal09, PR8, FM1 and cH6/1 HA protein (Fig. 3A, E–G) as well as against PR8 (H1N1), H2N2 and H5N1 virus preparations (Fig. 3C–D). Unprimed animals that received HL HA twice (HL+HL) also had good reactivity against all four substrates, but the titers were significantly lower than the titers of the primed group. Reactivity of naive and prime-only mice (cH9/1+BSA+BSA) was low (Fig. 3). We then assessed whether these antibodies exhibited neutralizing activity. However, all sera tested negative in microneutralization assays against pH1N1 and PR8 using several protocols (data not shown).
Fig. 3. ELISA reactivity of sera from headless HA vaccinated mice to divergent influenza virus substrates.
Stalk-specific reactivity of sera from animals that were vaccinated twice with headless HA (HL+HL) is shown in light green squares and reactivity from mice that were DNA primed with a cH9/1 HA expressing plasmid and then vaccinated twice with headless HA (cH9/1+HL+HL) is shown as red spheres. Sera from naive control mice (Naive) are shown as grey triangles and sera from prime-only mice (cH9/1+BSA+BSA) are shown as black triangles. A Reactivity to Cal09 H1 HA (featuring a GCN4pII trimerization domain and a Strep-Tag II). B Reactivity to purified PR8 (H1N1) virus, C reactivity to purified H2N2 virus and D reactivity to purified H5N1 virus. E–G Endpoint titers against PR ST, FM1 ST and cH6/1 ST HAs.
Headless HA protects against homologous and heterosubtypic influenza virus challenge in a mouse model
Next, we tested whether vaccination with soluble headless HA induces protective immunity in the mouse model. Naive mice (Naive) were used as negative control group and animals vaccinated with inactivated challenge virus were used as positive control group (Pos. contr.). One group of mice received two vaccinations with HL HA (HL+HL) to assess the protective effect of HL HA in previously naive mice. In addition we studied the effect of HL HA vaccination in a stalk-primed population. This is important since most humans have low levels of antibodies with specificities in the stalk domain. These antibodies can be boosted by vaccination or infection [33–35]. Therefore we primed one group of mice with a cH9/1 DNA vaccine. This construct induces low levels of stalk-reactive antibodies against the H1 stalk. It also induces antibodies towards the H9 head but, as shown before, these head antibodies are not cross-reactive to the PR8 (H1N1), H5N1 and H6N1 viruses used in this study and do not interfere with viral challenge [22]. These stalk-primed mice were then boosted twice with HL HA (cH9/1+HL+HL). An additional control group was stalk primed as well but received booster vaccinations with irrelevant protein (cH9/1+BSA+BSA).
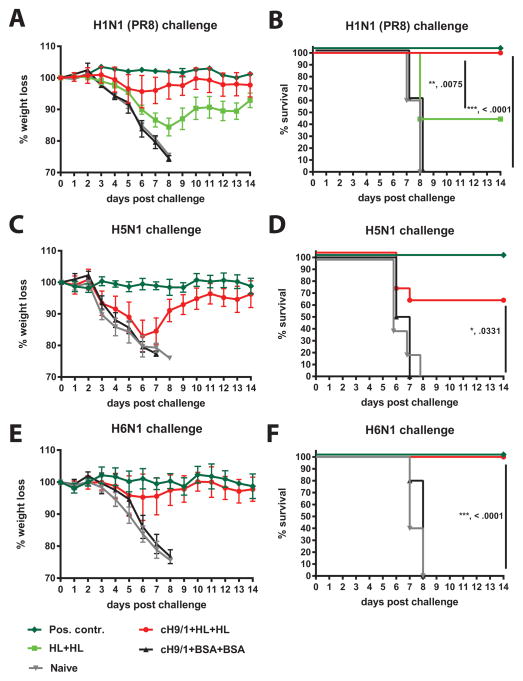
Upon challenge with PR8, naive and cH9/1+BSA+BSA mice lost weight rapidly and succumbed to infection on day 8 post infection (Fig. 4A and B). Positive control animals were completely protected from morbidity and mortality. Unprimed animals vaccinated with HL HA (HL+HL) showed an intermediate weight loss phenotype and had a survival rate of 44.4% (4 out of 9 mice). Stalk-primed HL HA vaccinated animals (cH9/1+HL+HL) showed an average maximum weight loss of 4.3% on day 6 post infection and were completely protected from mortality (Fig. 4A and B). Interestingly, no loss of protection or increased weight loss in CD8+ T-cell depleted cH9/1+HL+HL vaccinated mice was observed as compared to the non-depleted group (S. Fig. 1).
Fig. 4. Vaccination with headless HA protects from homologous and heterosubtypic virus challenge in mice.
A shows body weight loss upon challenge with homologous PR8 (H1N1) virus. Animals were vaccinated twice with headless HA (HL+HL, light green squares) or were DNA primed with a cH9/1 HA expressing plasmid (stalk-priming) and then vaccinated twice with headless HA (cH9/1+HL+HL, red spheres). Control groups included naive animals (Naive, grey triangles), prime-only controls that received a boost with irrelevant proteins (cH9/1+BSA+BSA, black triangles) and positive control animals that received inactivated matched challenge virus (Pos. contr., dark green diamonds). Survival of the animals in A is shown in B. Similar groups of mice were challenged with heterosubtypic H5N1 (C, D) or H6N1 (E, F) virus.
Heterosubtypic challenge experiments were conducted with H5N1 and H6N1 viruses. Here we tested protection of naive, positive control, prime-only (cH9/1+BSA+BSA) and stalk-primed HL HA boosted (cH9/1+HL+HL) groups. Naive and prime-only groups lost weight rapidly upon H5N1 and H6N1 challenge and showed no survival (Fig. 4 C–F). No morbidity or mortality was observed for positive control animals with both challenge viruses. Stalk-primed HL HA vaccinated animals (cH9/1+HL+HL) were partially protected from H5N1 induced morbidity and mortality with a survival rate of 60% (6 out of 10 mice) (Fig. 4C and D). In contrast, stalk-primed HL HA vaccinated mice were completely protected from mortality after H6N1 challenge and showed only moderate weight loss with a maximum of 4.7% on day 6 post infection (Fig. 4E and F).
Discussion
Several approaches to design protective HA stalk immunogens have been made in the past [24, 31, 36–40]. The major obstacle for a successful antigen is the misfolding of important neutralizing epitopes in the absence of the globular HA head domain. Although this problem has been overcome by an alternative - but more complex - universal influenza virus vaccine strategy involving chimeric HAs [22, 41, 42], the simplicity of the HL HA approach remains appealing. Our data shows that vaccination with soluble recombinant HL HA partially protects animals from challenge. This data reflects earlier findings reported by Mallajosyula and colleagues with an engineered bacterially expressed stalk-construct that protected completely against mortality, but not morbidity, after homologous challenge [39]. Large proportions of the human population have been exposed to influenza viruses through infection and/or vaccination and have low levels of stalk reactive antibodies [33, 34, 43]. These antibodies could be selectively boosted using an HL HA immunogen. Here, we mimicked this pre-existing immunity to the stalk domain in the mouse model without inducing immunity against the globular head domain of HA, the neuraminidase or the conserved internal proteins. We primed animals with a cH9/1 HA construct [22, 27] which induced low levels of anti-H1 stalk antibodies. H9 head antibodies, which are also induced by this construct, do not cross-react with any of the challenge viruses (H1N1, H5N1 or H6N1). Interestingly, mice that were primed and then vaccinated with HL HA showed minimal weight loss and full protection against H1N1 virus challenge. While cross-reactive antibodies against H1N1 and other group 1 HA expressing viruses were present in vaccinated mice, no neutralizing activity could be detected. This was an expected finding since the HL HA is not displaying (most) neutralizing epitopes in the right conformation. However, other mechanisms of protection, including antibody dependent cell-mediated cytotoxicity (ADCC), complement dependent cytotoxicity (CDC) and cytotoxic T-cells may have contributed to protection. While CD8+ T-cells were not necessary to prevent weight loss in our model they might still contribute to protection. It will therefore be important to study the CD4+ and CD8+ T-cell response to HL HA in future studies. The significant roles of both ADCC and CDC in heterosubtypic and stalk-based immunity have been highlighted recently [44–48]. Importantly, no enhanced disease was observed in the absence of neutralizing antibodies. Protection was also observed against H5N1 (partial protection) and H6N1 (full protection), two group 1 HA subtypes that occasionally cause zoonotic infections in humans. This demonstrates the potential of HL HA as universal influenza vaccine candidate in a primed population. Despite the good protection observed with soluble HL HA in the mouse model several practical issues need to be addressed before this vaccine candidate can progress into clinical development. While other novel broadly protective influenza virus vaccine candidates are platform independent [49, 50] HL HA needs to be expressed as recombinant protein in vitro or by viral vectors or DNA vaccines in vivo. In addition, the presence of a bacteriophage derived trimerization domain and a purification tag on recombinant HL HA might be an obstacle on the path to clinical trials and immunogens that lack these features might need to be developed. In our experimental set up we vaccinated via the i.n. and i.m. route simultaneously. This protocol might be hard to implement in clinical trials and i.n.-only or i.m.-only vaccination regimens need to be explored. Furthermore, group 1 stalk based vaccines usually induce immunity only against other group 1 HA expressing viruses [22, 33, 34]. To cover all influenza viruses it is likely that also a group 2 and an influenza B HL HA would need to be developed. While approaches to design group 2 HL HAs have been moderately successful so far [36, 38] no attempts to generate an influenza B HL HA have been published yet. While additional research to overcome these obstacles is needed HL HA constructs represent an interesting and valuable approach to develop novel broadly protective influenza virus vaccines.
Supplementary Material
Acknowledgments
We thank Jens C. Krause for providing the anti-LAH mAb, John Steel for the original headless HA constructs and Natalie Pica for help with preliminary experiments. This work was funded by the National Institutes of Health program project grant 1P01AI097092-01A1.
Footnotes
Conflict of interest
The Icahn School of Medicine at Mount Sinai has filed several patent applications regarding influenza virus vaccines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–9. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 2.Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, et al. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11:153. doi: 10.1186/1741-7015-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palese P. Influenza: old and new threats. Nat Med. 2004;10:S82–7. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 4.Krammer F. Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol J. 2015 doi: 10.1002/biot.201400393. [DOI] [PubMed] [Google Scholar]
- 5.Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol. 2013;3:521–30. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton NS, Sachs D, Chen CJ, Hai R, Palese P. Genome-wide mutagenesis of influenza virus reveals unique plasticity of the hemagglutinin and NS1 proteins. Proc Natl Acad Sci U S A. 2013;110:20248–53. doi: 10.1073/pnas.1320524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhard W, Yewdell J, Frankel ME, Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981;290:713–7. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- 8.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67:2552–8. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan GS, Krammer F, Eggink D, Kongchanagul A, Moran TM, Palese P. A pan-h1 anti-hemagglutinin monoclonal antibody with potent broad-spectrum efficacy in vivo. J Virol. 2012;86:6179–88. doi: 10.1128/JVI.00469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friesen RH, Lee PS, Stoop EJ, Hoffman RM, Ekiert DC, Bhabha G, et al. A common solution to group 2 influenza virus neutralization. Proc Natl Acad Sci U S A. 2014;111:445–50. doi: 10.1073/pnas.1319058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–6. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 13.Henry Dunand CJ, Leon PE, Kaur K, Tan GS, Zheng NY, Andrews S, et al. Preexisting human antibodies neutralize recently emerged H7N9 influenza strains. J Clin Invest. 2015;125:1255–68. doi: 10.1172/JCI74374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16:265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margine I, Hai R, Albrecht RA, Obermoser G, Harrod AC, Banchereau J, et al. H3N2 influenza virus infection induces broadly reactive hemagglutinin stalk antibodies in humans and mice. J Virol. 2013;87:4728–37. doi: 10.1128/JVI.03509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One. 2011;6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krammer F, Pica N, Hai R, Tan GS, Palese P. Hemagglutinin Stalk-Reactive Antibodies Are Boosted following Sequential Infection with Seasonal and Pandemic H1N1 Influenza Virus in Mice. J Virol. 2012;86:10302–7. doi: 10.1128/JVI.01336-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steel J, Lowen AC, Pena L, Angel M, Solorzano A, Albrecht R, et al. Live attenuated influenza viruses containing NS1 truncations as vaccine candidates against H5N1 highly pathogenic avian influenza. J Virol. 2009;83:1742–53. doi: 10.1128/JVI.01920-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krammer F, Albrecht RA, Tan GS, Margine I, Hai R, Schmolke M, et al. Divergent H7 immunogens offer protection from H7N9 virus challenge. J Virol. 2014;88:3976–85. doi: 10.1128/JVI.03095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, Grabherr R. Trichoplusia ni cells (High Five) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol Biotechnol. 2010;45:226–34. doi: 10.1007/s12033-010-9268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krammer F, Pica N, Hai R, Margine I, Palese P. Chimeric hemagglutinin influenza virus vaccine constructs elicit broadly protective stalk-specific antibodies. J Virol. 2013;87:6542–50. doi: 10.1128/JVI.00641-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krammer F, Margine I, Tan GS, Pica N, Krause JC, Palese P. A carboxy-terminal trimerization domain stabilizes conformational epitopes on the stalk domain of soluble recombinant hemagglutinin substrates. PLoS One. 2012;7:e43603. doi: 10.1371/journal.pone.0043603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steel J, Lowen AC, TTW, Yondola M, Gao Q, Haye K, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1:1–9. doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margine I, Palese P, Krammer F. Expression of Functional Recombinant Hemagglutinin and Neuraminidase Proteins from the Novel H7N9 Influenza Virus Using the Baculovirus Expression System. J Vis Exp. 2013 doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heaton NS, Leyva-Grado VH, Tan GS, Eggink D, Hai R, Palese P. In vivo bioluminescent imaging of influenza a virus infection and characterization of novel cross-protective monoclonal antibodies. J Virol. 2013;87:8272–81. doi: 10.1128/JVI.00969-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hai R, Krammer F, Tan GS, Pica N, Eggink D, Maamary J, et al. Influenza viruses expressing chimeric hemagglutinins: globular head and stalk domains derived from different subtypes. J Virol. 2012;86:5774–81. doi: 10.1128/JVI.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinca L, Neuwirth S, Schulman J, Bona C. Induction of antihemagglutinin antibodies by polyclonal antiidiotype antibodies. Viral Immunol. 1993;6:75–84. doi: 10.1089/vim.1993.6.75. [DOI] [PubMed] [Google Scholar]
- 29.Sliepen K, van Montfort T, Melchers M, Isik G, Sanders RW. Immunosilencing a highly immunogenic protein trimerization domain. J Biol Chem. 2015;290:7436–42. doi: 10.1074/jbc.M114.620534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weldon WC, Wang BZ, Martin MP, Koutsonanos DG, Skountzou I, Compans RW. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graves PN, Schulman JL, Young JF, Palese P. Preparation of influenza virus subviral particles lacking the HA1 subunit of hemagglutinin: unmasking of cross-reactive HA2 determinants. Virology. 1983;126:106–16. doi: 10.1016/0042-6822(83)90465-8. [DOI] [PubMed] [Google Scholar]
- 32.Salem ML, Hossain MS. In vivo acute depletion of CD8(+) T cells before murine cytomegalovirus infection upregulated innate antiviral activity of natural killer cells. Int J Immunopharmacol. 2000;22:707–18. doi: 10.1016/s0192-0561(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 33.Nachbagauer R, Wohlbold TJ, Hirsh A, Hai R, Sjursen H, Palese P, et al. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J Virol. 2014;88:13260–8. doi: 10.1128/JVI.02133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellebedy AH, Krammer F, Li GM, Miller MS, Chiu C, Wrammert J, et al. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc Natl Acad Sci U S A. 2014;111:13133–8. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, et al. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109:2573–8. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bommakanti G, Citron MP, Hepler RW, Callahan C, Heidecker GJ, Najar TA, et al. Design of an HA2-based Escherichia coli expressed influenza immunogen that protects mice from pathogenic challenge. Proc Natl Acad Sci U S A. 2010;107:13701–6. doi: 10.1073/pnas.1007465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bommakanti G, Lu X, Citron MP, Najar TA, Heidecker GJ, ter Meulen J, et al. Design of Escherichia coli-expressed stalk domain immunogens of H1N1 hemagglutinin that protect mice from lethal challenge. J Virol. 2012;86:13434–44. doi: 10.1128/JVI.01429-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallajosyula VV, Citron M, Lu X, Meulen JT, Varadarajan R, Liang X. In vitro and in vivo characterization of designed immunogens derived from the CD-helix of the stem of influenza hemagglutinin. Proteins. 2013;81:1759–75. doi: 10.1002/prot.24317. [DOI] [PubMed] [Google Scholar]
- 39.Mallajosyula VV, Citron M, Ferrara F, Lu X, Callahan C, Heidecker GJ, et al. Influenza hemagglutinin stem-fragment immunogen elicits broadly neutralizing antibodies and confers heterologous protection. Proc Natl Acad Sci U S A. 2014;111:E2514–23. doi: 10.1073/pnas.1402766111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Y, Welsh JP, Swartz JR. Production and stabilization of the trimeric influenza hemagglutinin stem domain for potentially broadly protective influenza vaccines. Proc Natl Acad Sci U S A. 2014;111:125–30. doi: 10.1073/pnas.1308701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, et al. H3 Stalk-Based Chimeric Hemagglutinin Influenza Virus Constructs Protect Mice from H7N9 Challenge. J Virol. 2014;88:2340–3. doi: 10.1128/JVI.03183-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Margine I, Krammer F, Hai R, Heaton NS, Tan GS, Andrews SA, et al. Hemagglutinin Stalk-Based Universal Vaccine Constructs Protect against Group 2 Influenza A Viruses. J Virol. 2013;87:10435–46. doi: 10.1128/JVI.01715-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, et al. Neutralizing Antibodies Against Previously Encountered Influenza Virus Strains Increase over Time: A Longitudinal Analysis. Sci Transl Med. 2013;5:198ra07. doi: 10.1126/scitranslmed.3006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terajima M, Cruz J, Co MD, Lee JH, Kaur K, Wrammert J, et al. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol. 2011;85:13463–7. doi: 10.1128/JVI.05193-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dilillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–51. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jegaskanda S, Weinfurter JT, Friedrich TC, Kent SJ. Antibody-dependent cellular cytotoxicity is associated with control of pandemic H1N1 influenza virus infection of macaques. J Virol. 2013;87:5512–22. doi: 10.1128/JVI.03030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol. 2013;190:1837–48. doi: 10.4049/jimmunol.1201574. [DOI] [PubMed] [Google Scholar]
- 48.Jegaskanda S, Reading PC, Kent SJ. Influenza-specific antibody-dependent cellular cytotoxicity: toward a universal influenza vaccine. J Immunol. 2014;193:469–75. doi: 10.4049/jimmunol.1400432. [DOI] [PubMed] [Google Scholar]
- 49.Kirchenbaum GA, Ross TM. Eliciting broadly protective antibody responses against influenza. Curr Opin Immunol. 2014;28:71–6. doi: 10.1016/j.coi.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167–82. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.