Abstract
Background and Objectives
Cladribine is a cytotoxic drug which ameliorates the clinical course of relapsing-remitting multiple sclerosis. In addition to cytotoxicity, the mode of action may include immunomodulatory mechanisms. This in vitro study was designed to investigate cladribine’s effects on cell function after the removal of cladribine to distinguish cytotoxic versus immunomodulatory effects.
Methods
Cells were incubated in the absence or presence of cladribine (1×10-8 M to 1×10-5 M) for 72 h. Cladribine was removed from the cell culture and surviving peripheral blood mononuclear cells were cultured up to 58 days to determine the immunomodulatory effects of cladribine on cell function (e.g., proliferation and cytokine release).
Results
In the long-term, brief cladribine exposure did not impair the proliferation of surviving peripheral blood mononuclear cells. However, it induced an anti-inflammatory shift in the cytokine milieu with significantly enhanced release of IL-4 (Days 9 and 44, p<0.01; Day 58, p<0.05) and IL-5 (Day 9, p<0.01), resulting in an increased IL-4/INF-gamma ratio (Days 9 and 44, p<0.01; Day 58, p<0.05). Additionally, a trend towards an increased IL-10 production was observed. No changes were found in the production of IFN-gamma, TNF-alpha, IL-6, IL-8, IL-17A, IL-23 or NGF-beta.
Conclusions
In vitro cladribine exposure induces a sustained anti-inflammatory shift in the cytokine profile of surviving peripheral blood mononuclear cells. This immunomodulatory action might contribute to cladribine’s beneficial effects in the treatment of multiple sclerosis.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system which affects more than two million patients worldwide and is the most frequent cause of non-traumatic disabilities in young adults [1,2]. Due to the fact, that there is still no curative treatment for MS available pharmaceuticals aim for a reduction in relapse rate, disability progression, neurological degeneration and a symptomatic treatment of disease symptoms [3].
Clinical phase III trials have demonstrated a beneficial effect on the clinical course of relapsing remitting MS for at least 10 compounds. The mechanism of action of these drugs is considered either immunomodulatory (e.g., interferon-beta, glatirameracetate) or immunosuppressive (e.g., mitoxantrone, cyclophosphamide and teriflunomide) [4–6].
Cladribine, a synthetic purine nucleotide analogue of desoxyadenosine is one of the medications that has shown clinical efficacy in a phaseIII clinical trial in RRMS [6,7]. The compound is cytotoxic to proliferating and non-proliferating lymphocytes, as well as to monocytes and monocyte-derived dendritic cells [8–11]. It is currently used as part of therapy against malignant haematological diseases (e.g., hairy cell leukaemia, chronic lymphatic leukaemia, chronic and acute myeloid leukaemia and non-Hodgkin lymphomas) [12].
In its phase III clinical trial in RRMS patients, oral cladribine was administered in two or four courses of treatment within the first 48 weeks, followed by two additional courses during Weeks 48 and 52. This brief treatment resulted in a significant reduction of the relapse rate, magnetic resonance imaging (MRI) activity and disability progression in RRMS patients [7,13]. Despite meeting its primary outcome parameter during the phaseIII clinical trial, the Food and Drug Administration and the European Medicine Agency refused marketing authorisation for cladribine because of its benefit risk profile [14]. Compared with placebo, lymphopenia and Herpes zoster virus infections were more likely to occur in patients receiving cladribine. Furthermore, a slightly higher risk of developing secondary malignancies had not been ruled out [7,15].
The beneficial effects observed in RRMS patients persisted for many months after the cessation of cladribine exposure, which was limited to two or four courses within the first 48 weeks followed by two courses during Weeks 48 and 52 [7,13]. Therefore, we hypothesized that in addition to its direct cytotoxic effects the compound’s mode of action may also encompass immunomodulatory mechanisms. Such effects might include alterations in cytokine patterns of cells that survived the initial cladribine treatment. Indeed, cyclophosphamide and mitoxantrone, two other immunosuppressive drugs, are well known to have additional immunomodulatory effects [16–18].
For cladribine several studies demonstrated reductions in the release of pro- and anti-inflammatory cytokines, chemokines, and adhesion molecules, as well as a decrease in the cellular migratory capacity of treated cells [19–21]. However, these experiments were performed in the presence of cladribine; therefore, toxic effects rather than modulation of cell function could account for the observed changes. The current in vitro study was designed to explore whether cladribine exerts long-term immunomodulatory effects in addition to immediate cytotoxicity.
Material and Methods
Cell isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of male and female blood donors (Transfusion Medicine, University Medicine Greifswald, Germany) using standard Ficoll gradient centrifugation. CD4+ cells and CD8+ cells were positively selected using Dynal CD4 Positive Isolation Kit and Dynal CD8 Positive Isolation Kit (Invitrogen GmbH, Karlsruhe, Germany) according to the manufacturer’s instructions. All cells were freshly isolated directly before their use. No cryopreserved cells were used in any experiment.
Cell culture
Cells were cultured in RPMI-1640 growth medium (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) supplemented with 1% glutamine, 1% penicillin, 1% streptomycin and 10% human AB serum (Department of Transfusion Medicine, University Medicine Greifswald, Germany). They were stored in an incubator at 37°C and 5% carbon dioxide under a saturated steam atmosphere.
Cells were stimulated with either plate-bound anti-CD3 (clone HIT3, 1μg/ml, BD Biosciences Cat# 555336, RRID: AB_395742) and plate-bound anti-CD28 (clone CD28.2, 1μg/ml, BD Biosciences Cat# 555725, RRID: AB_396068) antibodies (BD Labware, Franklin Lakes, NJ, USA) or phytohaemagglutinin (PHA, 1μg/ml) or remained unstimulated. In all experiments PBMCs, CD4+ cells and CD8+ cells were incubated in the presence (1×10-8M to 1×10-5M) or absence of cladribine [Leustatin (1mg/ml cladribine, sodium chloride, sodium phosphate dibasic heptahydrate), Janssen-Cilag GmbH, Neuss, Germany or Litak (2mg/ml cladribine, sodium chloridhydroxid, hydrochloric acid) Lipomed GmbH, Weil am Rhein, Germany]. The latter was only used for the survival experiments represented in Fig 1A and 1B. Cladribine was provided in dissolved form and was further diluted to the appropriate concentration with RPMI-1640 growth medium (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) containing 1% glutamine, 1% penicillin, 1% streptomycin and 10% AB serum. Cladribine was added to the culture medium directly before use. Exposed cells were treated with cladribine for 72 h. In all experiments, PBMCs, CD4+ cells and CD8+ cells were seeded with a cell density of 200,000 cells per well.
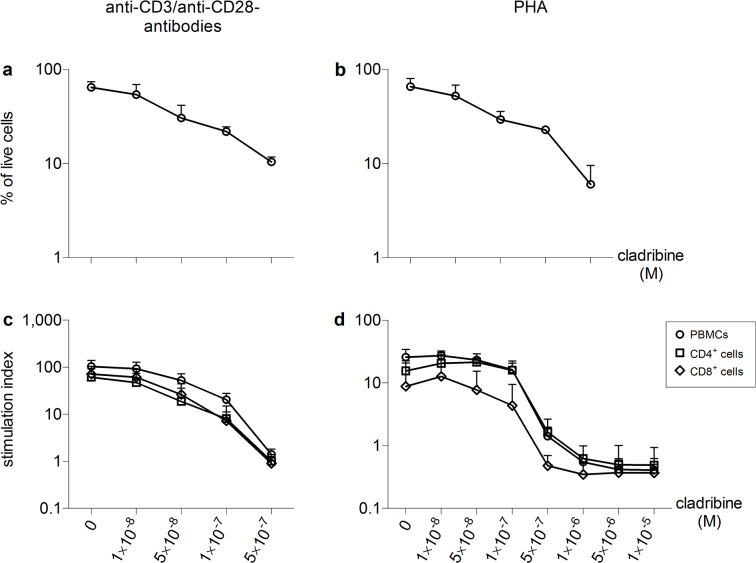
Fig 1. In vitro effects of cladribine on cell survival of PBMCs and proliferation of PBMCs, CD4+ cells and CD8+ cells Human immune cells were stimulated with anti-CD3/anti-CD28 antibodies (a, n = 4; c, n = 6) or PHA (b, n = 4; d, n = 3) in the absence or presence of cladribine (1×10−8 M to 1×10−5 M) for 72 h.
Determination of cell survival in PBMCs (a, b) was performed by standard trypan blue exclusion method. Proliferation was determined separately in PBMC, CD4+ cells and CD8+ cells (c, d) by the incorporation of tritiated thymidine. Stimulation indices were calculated as the ratios of the counts per minute of stimulated samples to unstimulated and untreated samples. Data are depicted as mean + standard deviation (SD).
In short-term experiments, cell survival was determined by counting of live cells using the standard trypan blue exclusion method in single use C-Chip Neubauer improved counting chambers (NanoEnTec Newton, MA, USA). A total number of four independent experiments were performed. Proliferation was determined by assessing the incorporation of tritiated thymidine. After 72 h, tritiated thymidine was added to the culture and incorporation was quantified using a Phosphorimager (Molecular Dynamics, Sunnyvale, USA). Proliferation was evaluated as the stimulation index, which was calculated as the ratio of counts per minute of sample with stimulation to counts per minute of untreated sample without stimulation. A total number of six (stimulation with anti-CD3/anti-CD28 antibodies) and three (PHA stimulation) independent experiments were performed with a minimum of three technical replicates per independent experiment. Apoptosis was assessed using Annexin V and propidium iodide staining after 12, 24, 48 and 72 h (FITC Annexin V Apoptosis Detection Kit, BD Pharmingen, USA) according to the manufacturer’s instructions. Apoptosis was quantified on a FACScan (Becton Dickinson, Franklin Lakes, NJ, USA). Three independent experiments were performed with a minimum of three technical replicates per independent experiment.
For long-term experiments, stimulated PBMCs that were exposed to cladribine (1×10-8M to 5×10-7M) for the initial 72 h, were washed three times to remove cladribine and transferred into cladribine-free medium to which 50U/ml interleukin (IL)-2 had been added. Cells were cultured for 9 to 58 additional days. At Days 9, 16, 23, 30, 44 and 58, cell numbers were equilibrated and cells were re-stimulated with plate-bound anti-CD3/anti-CD28 antibodies or PHA as described. The re-stimulation was performed in the absence of cladribine and after a 20 h IL-2–free resting phase. Forty-eight hours later, cell proliferation was determined through the incorporation of tritiated thymidine, which was measured using a Microbeta2 Plate Counter (PerkinElmer, Waltham, MA, USA). Supernatants were collected for cytokine analysis. A total number of five (stimulation with anti-CD3/anti-CD28 antibodies) and four (PHA stimulation) independent experiments were performed. Number of technical replicates at Days 9, 16, 23, 30, 44 and 58 were adapted to cell availability with a minimum number of three technical replicates per independent experiment.
Cytokines
Cytokine concentrations were determined using a multiplex assay according to the manufacturer’s instructions (FlowCytomix Human Th1/Th2 11 Plex Kit, FlowCytomix Human IL17-A, IL-23 and NGF Kit, Bender MedSystems GmbH, Vienna, Austria). A FACScan was used for quantification (Beton Dickinson, Franklin Lakes, NJ, USA). Data were evaluated using the Flow Cytomix Pro 2.2 software (Bender MedSystems GmbH, Vienna, Austria). The IL-4/Interferon-gamma (IFN-γ) ratio was defined as the ratio of IL-4 [pg/ml] to IFN-γ [pg/ml].
Statistical analysis
This study was designed as an exploratory study, therefore no predefined primary or secondary endpoints were used and statistical significance was not adjusted for multiple testing. Because not all data sets showed Gaussian distributions, the Kruskal-Wallis test and Dunn's Multiple Comparison test were used in all analyses (Prism 5.0 software, GraphPad Software inc., San Diego, CA, USA). P-values <0.05 were considered statistically significant. Data from short-term experiments and proliferation data from long-term experiments are depicted as mean + standard deviation. Cytokine data are presented as box plot diagrams.
Ethics statement
Blood donations for buffy coats were obtained from healthy donors at the Department of Transfusion Medicine of the University Medicine Greifswald. Written informed consent was obtained from every blood donor on the date of blood donation stating that blood components which will not be used for clinical purposes may be used for clinical investigations and research in an anonymous manner. The Ethics Committee of the University of Greifswald has approved this procedure (BB014/14). Therefore all buffy coats were obtained from individuals that had given written informed consent for the use of their blood cells in a research project but without re-informing the blood donors about the specific study for which their samples were used. The study was conducted according to the principles expressed in the Declaration of Helsinki.
Results
Short-term effects of cladribine on cell survival, immune cell proliferation and apoptosis
Cell survival of PBMCs was determined by microscopic cell counting and trypan blue exclusion of dead cells. At the highest concentration of 5x107 M cladribine, 10% ± 1.3% (mean ± SD) of cells survived after 72 h when stimulated with anti-CD3/anti-CD28 antibodies and 8% ± 4% (mean ± SD) of cells when stimulated with PHA (Fig 1A and 1B). We assessed the effects of increasing concentrations of cladribine on the proliferation of PBMCs, CD4+ cells and CD8+ cells that were either stimulated with anti-CD3/anti-CD28 antibodies (Fig 1C) or PHA (Fig 1D). When stimulated through their T-cell receptor, 1×10-7M cladribine reduced proliferation of PBMCs by 80% and had a slightly stronger effect on T-cell subsets (Fig 1C). PHA-stimulated cells were strongly inhibited in their proliferation starting at a concentration of 5×10-7M cladribine (Fig 1D). The decrease in the proliferative response was paralleled by a strong increase in the percentage of Annexin V positive cells, showing that nanomolar concentrations of cladribine are capable of inducing apoptotic cell death (Fig 2, Table 1, Table 2 and Table 3).
Fig 2. Apoptosis of stimulated PBMCs, CD4+ cells and CD8+ cells caused by cladribine in vitro PBMCs (a, n = 3), CD4+ cells (b, n = 3) and CD8+ cells (c, n = 3) were stimulated with anti-CD3/anti-CD28 antibodies and were incubated in the absence or presence of cladribine (1×10−8 M to 5×10−7 M) for 12, 24, 48 and 72 h.
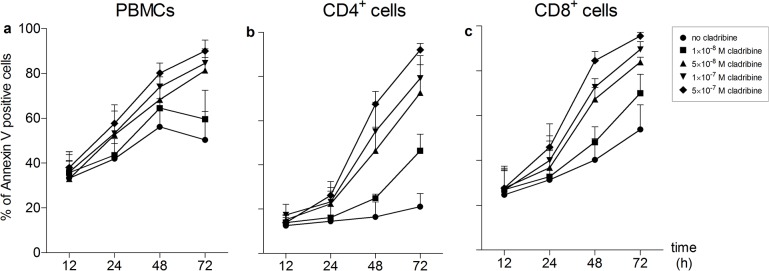
Apoptotic cells were defined as Annexin V positive cells. Data are depicted as mean + standard deviation (SD).
Table 1. Apoptosis of stimulated PBMCs caused by cladribine in vitro.
| Condition | Living cells | Early apoptosis | Late apoptosis | Non-apoptotic death | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean in % | SD | Mean in % | SD | Mean in % | SD | Mean in % | SD | ||
| PBMCs | |||||||||
| 12h | No cladribine | 66.7 | 5.2 | 22.1 | 2.0 | 10.8 | 3.7 | 0.4 | 0.3 |
| 1x10-8 M cladribine | 64.1 | 6.6 | 23.9 | 3.6 | 11.5 | 3.3 | 0.5 | 0.3 | |
| 5x10-8 M cladribine | 67.0 | 5.5 | 22.3 | 3.4 | 10.2 | 2.4 | 0.4 | 0.4 | |
| 1x10-7 M cladribine | 63.5 | 4.8 | 24.2 | 3.1 | 11.4 | 2.5 | 0.8 | 1.0 | |
| 5x10-7 M cladribine | 61.5 | 5.4 | 24.7 | 4.6 | 13.0 | 2.1 | 0.8 | 0.9 | |
| 24h | No cladribine | 59.0 | 6.8 | 27.5 | 7.6 | 13.0 | 3.5 | 0.6 | 0.3 |
| 1x10-8 M cladribine | 57.3 | 6.8 | 28.0 | 6.0 | 14.2 | 4.5 | 0.4 | 0.2 | |
| 5x10-8 M cladribine | 48.5 | 4.2 | 31.7 | 9.3 | 19.4 | 9.6 | 0.4 | 0.3 | |
| 1x10-7 M cladribine | 47.5 | 8.8 | 29.5 | 9.9 | 22.4 | 11.4 | 0.6 | 0.5 | |
| 5x10-7 M cladribine | 42.9 | 7.1 | 30.8 | 10.0 | 25.7 | 13.1 | 0.5 | 0.3 | |
| 48h | No cladribine | 42.3 | 3.9 | 35.4 | 13.2 | 21.9 | 9.8 | 0.4 | 0.5 |
| 1x10-8 M cladribine | 34.4 | 3.7 | 38.8 | 15.1 | 26.4 | 13.0 | 0.4 | 0.5 | |
| 5x10-8 M cladribine | 30.2 | 7.5 | 32.9 | 4.6 | 36.2 | 11.3 | 0.7 | 0.7 | |
| 1x10-7 M cladribine | 25.0 | 6.0 | 32.9 | 10.9 | 41.5 | 14.8 | 0.6 | 0.7 | |
| 5x10-7 M cladribine | 19.1 | 5.7 | 31.3 | 8.3 | 49.1 | 13.0 | 0.5 | 0.5 | |
| 72h | No cladribine | 45.6 | 9.4 | 27.8 | 4.8 | 23.7 | 8.8 | 2.9 | 4.3 |
| 1x10-8 M cladribine | 37.0 | 9.3 | 31.2 | 1.8 | 29.3 | 13.1 | 2.5 | 3.6 | |
| 5x10-8 M cladribine | 16.7 | 4.4 | 36.9 | 11.8 | 45.2 | 17.9 | 1.2 | 1.8 | |
| 1x10-7 M cladribine | 13.6 | 5.3 | 35.5 | 4.6 | 49.6 | 11.6 | 1.2 | 1.9 | |
| 5x10-7 M cladribine | 8.4 | 3.9 | 28.6 | 2.1 | 61.8 | 7.6 | 1.2 | 1.8 | |
PBMCs (a, n = 3) were stimulated with anti-CD3/anti-CD28 antibodies and were incubated in the absence or presence of cladribine (1×10−8 M to 5×10−7 M). Apoptosis was determined after 12, 24, 48 and 72 h by Annexin V and propidium iodide (PI) staining. The table lists the percentages of living cells (Annexin V negative, PI negative cells), cells that are in the state of early apoptosis (Annexin V positive, PI negative cells), cells that are in the state of late apoptosis (Annexin V positive, PI positive cells) and cells that have died a non-apoptotic cell death (Annexin V negative, PI positive cells).
Table 2. Apoptosis of stimulated CD4+ cells caused by cladribine in vitro.
| Condition | Living cells | Early apoptosis | Late apoptosis | Non-apoptotic death | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean in % | SD | Mean in % | SD | Mean in % | SD | Mean in % | SD | ||
| CD4+ cells | |||||||||
| 12h | No cladribine | 88.4 | 4.7 | 4.4 | 2.9 | 7.0 | 1.9 | 0.3 | 0.2 |
| 1x10-8 M cladribine | 87.2 | 4.7 | 4.9 | 3.5 | 7.7 | 1.3 | 0.3 | 0.2 | |
| 5x10-8 M cladribine | 86.2 | 3.9 | 5.3 | 2.6 | 8.2 | 1.3 | 0.3 | 0.2 | |
| 1x10-7 M cladribine | 84.2 | 2.2 | 5.4 | 2.5 | 10.0 | 0.4 | 0.4 | 0.3 | |
| 5x10-7 M cladribine | 86.9 | 4.5 | 4.8 | 2.6 | 7.9 | 2.1 | 0.3 | 0.3 | |
| 24h | No cladribine | 86.3 | 3.1 | 3.7 | 0.9 | 9.8 | 2.3 | 0.2 | 0.1 |
| 1x10-8 M cladribine | 84.5 | 5.1 | 4.0 | 1.3 | 11.3 | 3.9 | 0.2 | 0.1 | |
| 5x10-8 M cladribine | 78.3 | 5.7 | 7.1 | 3.2 | 14.4 | 3.7 | 0.2 | 0.1 | |
| 1x10-7 M cladribine | 77.3 | 6.6 | 6.8 | 2.7 | 15.6 | 4.6 | 0.3 | 0.1 | |
| 5x10-7 M cladribine | 74.5 | 6.1 | 8.9 | 3.5 | 16.4 | 4.3 | 0.2 | 0.1 | |
| 48h | No cladribine | 82.5 | 11.4 | 5.4 | 5.8 | 12.0 | 5.8 | 0.1 | 0.0 |
| 1x10-8 M cladribine | 74.0 | 5.6 | 8.2 | 4.6 | 17.6 | 1.0 | 0.2 | 0.0 | |
| 5x10-8 M cladribine | 52.7 | 9.6 | 12.2 | 5.7 | 34.9 | 5.5 | 0.2 | 0.1 | |
| 1x10-7 M cladribine | 44.6 | 13.2 | 12.3 | 5.0 | 43.0 | 8.4 | 0.2 | 0.1 | |
| 5x10-7 M cladribine | 32.4 | 7.6 | 11.7 | 3.9 | 55.7 | 3.9 | 0.2 | 0.0 | |
| 72h | No cladribine | 78.6 | 9.7 | 5.8 | 4.5 | 15.4 | 5.2 | 0.2 | 0.1 |
| 1x10-8 M cladribine | 53.4 | 6.7 | 9.6 | 3.3 | 36.7 | 4.6 | 0.3 | 0.1 | |
| 5x10-8 M cladribine | 27.0 | 12.0 | 10.2 | 3.6 | 62.6 | 9.8 | 0.3 | 0.2 | |
| 1x10-7 M cladribine | 20.4 | 9.3 | 9.2 | 3.7 | 70.2 | 9.1 | 0.3 | 0.2 | |
| 5x10-7 M cladribine | 7.7 | 2.7 | 7.2 | 3.0 | 84.9 | 2.3 | 0.2 | 0.2 | |
CD4+ cells (a, n = 3) were stimulated with anti-CD3/anti-CD28 antibodies and were incubated in the absence or presence of cladribine (1×10−8 M to 5×10−7 M). Apoptosis was determined after 12, 24, 48 and 72 h by Annexin V and propidium iodide (PI) staining. The table lists the percentages of living cells (Annexin V negative, PI negative cells), cells that are in the state of early apoptosis (Annexin V positive, PI negative cells), cells that are in the state of late apoptosis (Annexin V positive, PI positive cells) and cells that have died a non-apoptotic cell death (Annexin V negative, PI positive cells).
Table 3. Apoptosis of stimulated CD8+ cells caused by cladribine in vitro.
| Condition | Living cells | Early apoptosis | Late apoptosis | Non-apoptotic death | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean in % | SD | Mean in % | SD | Mean in % | SD | Mean in % | SD | ||
| CD8+ cells | |||||||||
| 12h | No cladribine | 76.3 | 14.6 | 7.6 | 4.4 | 15.6 | 10.1 | 0.5 | 0.4 |
| 1x10-8 M cladribine | 73.9 | 13.2 | 9.2 | 4.5 | 16.3 | 8.5 | 0.6 | 0.5 | |
| 5x10-8 M cladribine | 73.2 | 13.4 | 10.5 | 5.3 | 15.9 | 8.1 | 0.3 | 0.3 | |
| 1x10-7 M cladribine | 74.4 | 12.7 | 10.4 | 5.6 | 14.9 | 7.3 | 0.3 | 0.4 | |
| 5x10-7 M cladribine | 73.7 | 12.6 | 11.3 | 5.4 | 14.7 | 7.4 | 0.4 | 0.4 | |
| 24h | No cladribine | 70.2 | 13.8 | 8.0 | 3.4 | 21.7 | 13.0 | 0.2 | 0.1 |
| 1x10-8 M cladribine | 69.1 | 13.1 | 9.8 | 2.8 | 20.9 | 12.8 | 0.3 | 0.3 | |
| 5x10-8 M cladribine | 64.8 | 12.8 | 11.4 | 2.5 | 23.2 | 12.4 | 0.5 | 0.7 | |
| 1x10-7 M cladribine | 61.7 | 11.3 | 11.9 | 5.5 | 26.1 | 14.7 | 0.3 | 0.3 | |
| 5x10-7 M cladribine | 55.6 | 11.6 | 13.8 | 5.8 | 29.9 | 13.9 | 0.7 | 0.9 | |
| 48h | No cladribine | 60.9 | 15.5 | 8.6 | 4.1 | 30.3 | 11.6 | 0.2 | 0.2 |
| 1x10-8 M cladribine | 52.8 | 13.9 | 11.0 | 4.5 | 36.1 | 9.4 | 0.1 | 0.1 | |
| 5x10-8 M cladribine | 33.7 | 11.3 | 12.8 | 3.3 | 53.3 | 8.3 | 0.2 | 0.1 | |
| 1x10-7 M cladribine | 28.0 | 9.1 | 12.5 | 1.3 | 59.3 | 7.9 | 0.2 | 0.1 | |
| 5x10-7 M cladribine | 16.1 | 7.6 | 10.6 | 1.0 | 73.1 | 7.2 | 0.2 | 0.1 | |
| 72h | No cladribine | 49.2 | 14.0 | 8.6 | 1.9 | 41.9 | 12.2 | 0.3 | 0.4 |
| 1x10-8 M cladribine | 31.3 | 8.3 | 10.7 | 3.1 | 57.8 | 10.1 | 0.3 | 0.3 | |
| 5x10-8 M cladribine | 17.0 | 4.6 | 8.4 | 2.1 | 74.3 | 2.8 | 0.3 | 0.3 | |
| 1x10-7 M cladribine | 11.0 | 3.9 | 6.8 | 2.3 | 82.0 | 3.2 | 0.2 | 0.3 | |
| 5x10-7 M cladribine | 4.7 | 2.2 | 5.1 | 2.0 | 89.9 | 1.8 | 0.3 | 0.3 | |
CD8+ cells (a, n = 3) were stimulated with anti-CD3/anti-CD28 antibodies and were incubated in the absence or presence of cladribine (1×10−8 M to 5×10−7 M). Apoptosis was determined after 12, 24, 48 and 72 h by Annexin V and propidium iodide (PI) staining. The table lists the percentages of living cells (Annexin V negative, PI negative cells), cells that are in the state of early apoptosis (Annexin V positive, PI negative cells), cells that are in the state of late apoptosis (Annexin V positive, PI positive cells) and cells that have died a non-apoptotic cell death (Annexin V negative, PI positive cells).
Long-term effects of cladribine on the proliferation of surviving PBMCs
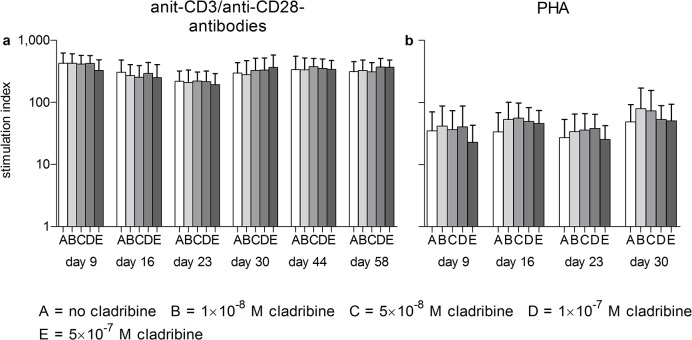
To evaluate whether in vitro treatment with cladribine alters the cytokine profile of surviving cells, proliferation and cytokines were measured after cladribine had been removed from the cell culture medium. Therefore, cladribine was removed from the cell culture after 72 h of incubation, and PBMCs were cultured for up to 58 days in cladribine-free medium supplemented with 50U/ml of the survival factor IL-2. Surviving cells were re-stimulated with anti-CD3/anti-CD28 antibodies or PHA 9 to 58 days after the removal of cladribine. IL-2 was removed from the cell culture 20 h before re-stimulation. Despite the cytotoxic effects observed in short-term treatment (Figs 1 and 2) initial exposure to cladribine did not impair the proliferation of surviving cells (Fig 3).
Fig 3. In vitro effects of initial cladribine treatment on the proliferation of PBMCs stimulated at Days 9, 16, 23, 30, 44 and 58 after transfer into cladribine-free medium PBMCs were initially incubated in the absence or presence of cladribine (1×10−8 M to 5×10−7 M) for 72 h.
Cells were washed three times and then transferred into cladribine-free medium. Cells were restimulated with anti-CD3/anti-CD28 antibodies (a, n = 5) or PHA (b, n = 4) for 48h at Days 9, 16, 23, 30, 44 and 58; proliferation was determined by the incorporation of tritiated thymidine. Stimulation indices were defined as the ratios of the counts per minute of stimulated samples to unstimulated and untreated samples. Data are depicted as mean + standard deviation (SD).
Effects of initial cladribine exposure on cytokine release from surviving PBMCs
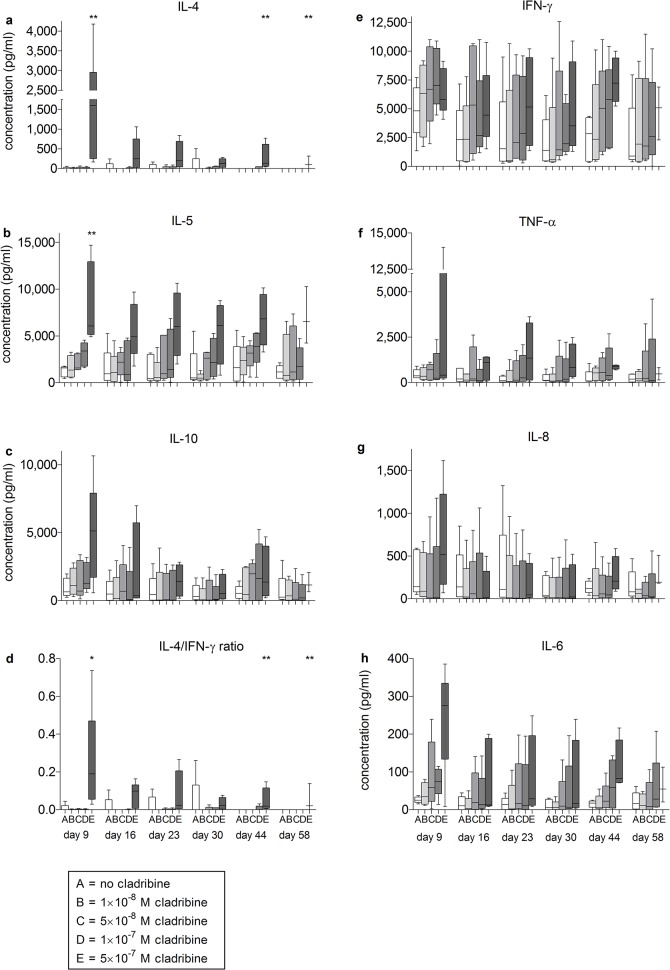
To characterize the surviving cells in more detail, we measured the inducibility of prototypical pro- and anti-inflammatory cytokines (Figs 4 and 5). The release of anti-inflammatory cytokines by PBMCs in response to stimulation with anti-CD3/anti-CD28 antibodies was influenced by initial cladribine treatment in a concentration-dependent manner (Fig 4). Initial exposure to 5×10-7M cladribine had the strongest effects on cytokine release at all investigated time points. There was a statistically significant increase in the cytokine concentration for IL-4 and IL-5 at Day 9 (IL-4 p<0.01 and IL-5 p<0.01), Day 44 (IL-4 p<0.01) and Day 58 (IL-4 p < 0.05); similar trends were observed for IL-10. In contrast, no significant changes were observed regarding IFN-γ, tumor necrosis factor-alpha (TNF-α), IL-8 or IL-6. We also measured the secretion of IL-17A, IL-23 and nerve growth factor beta (NGF-β) at Days 9 and 30. No significant changes were observed (data not shown). To determine the overall effect of this enhanced cytokine production, the IL-4/IFN-γ ratio was determined. This analysis confirmed a shift towards an anti-inflammatory cytokine milieu (Day 9, p < 0.01; Day 44, p < 0.01; Day 58, p < 0.05) (Fig 4).
Fig 4. In vitro effects of initial cladribine treatment on cytokine secretion of PBMCs restimulated at Days 9, 16, 23, 30, 44 and 58 after transfer into cladribine-free medium.
PBMCs from healthy blood donors (n = 5) were incubated in the absence or presence of cladribine. (1×10−8 M to 5×10−7 M) for 72 h. Then cells were washed three times and transferred into cladribine-free medium. Cells were restimulated with anti-CD3/anti-CD28 antibodies for 48 h at days 9, 16, 23, 30, 44 and 58; supernatants were collected and cytokine concentrations were determined. Data are depicted as box plot diagrams. Whiskers represent maximum and minimum values. The IL-4/IFN-γ ratio was defined as the ratio of IL-4 (pg/ml) to IFN-γ (pg/ml). *: p<0.05; **: p<0.01.
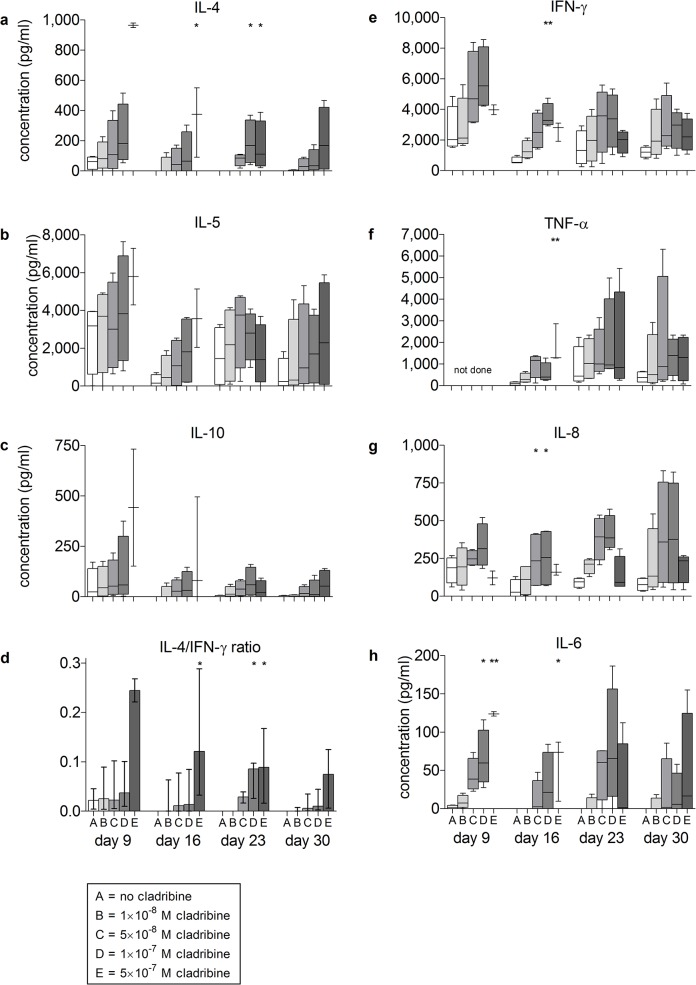
Fig 5. In vitro effects of initial cladribine treatment on cytokine secretion of PBMCs restimulated at Days 9, 16, 23 and 30 after transfer into cladribine-free medium.
PBMCs from healthy blood donors (n = 4) were incubated in the absence or presence of cladribine. (1×10−8 M to 5×10−7 M) for 72 h. Then cells were washed three times and transferred into cladribine-free medium. Cells were restimulated with PHA for 48 h at days 9, 16, 23 and 30; supernatants were collected and cytokine were determined. Data are depicted as box plot diagrams. Whiskers represent maximum and minimum values. The IL-4/IFN-γ ratio was defined as the ratio of IL-4 (pg/ml) to IFN-γ (pg/ml). *: p<0.05; **: p<0.01.
In an additional series of experiments, we stimulated the immune cells with the mitogen PHA instead of anti-CD3/anti-CD28 antibodies. The increase of TH2-type cytokines in cladribine-treated cells was reproduced under these conditions, demonstrating the robustness of this phenomenon (Fig 5).
Discussion
This study is the first to address the question of whether cladribine exerts long-term immunomodulatory effects on the immune cells that survive the initial treatment, in addition to its demonstrated cytotoxicity. The concentration range of cladribine used for our in vitro study was chosen to reflect concentrations that occur in in vivo situations. Patients in the phase III clinical trial of cladribine for the treatment of MS patients received one or two 10 mg cladribine tablets once daily for four or five consecutive days. No plasma concentrations have been reported from this trial. Data are available from early reports by Liliemark et al. who detected peak plasma levels exceeding 100x10-9 M following the oral administration of 0.28 mg/kg cladribine [22]. More recently Munafo et al. presented plasma concentrations of 7x10-8 M after a single tablet of 10 mg oral cladribine. [23].
To explore whether the alteration of cytokine patterns persisted beyond the initial cytotoxic effects of cladribine, human PBMCs were cultured for up to 2 months after an initial 72h exposure to cladribine. Removal of the compound from the culture enabled the experimental separation of sustained immune modulation from the acutely toxic effects of cladribine.
The short-term treatment with cladribine resulted in a dose-dependent decrease of proliferation and a dose- and time-dependent increase of apoptosis. However, these acute effects did not persist in surviving cells: when the drug was removed from the cell culture after the initial 72h, cladribine did not inhibit the proliferation of PBMCs up to 58 days thereafter. Assessing the cytokine levels enabled us to show that initial cladribine treatment results in a sustained alteration of cytokine release in the surviving cells. Treated cells predominantly released anti-inflammatory cytokines in comparison to untreated cells, which was reflected by an increased IL-4/IFN-γ ratio. Cladribine did not change the secretion of NGF-β.
In earlier in vivo studies reductions of chemokines and soluble adhesion molecules were observed after the subcutaneous administration of cladribine [20,21]. Furthermore, Laugel et al. reported decreases in the production of IL-2, IL-10, IFN-γ and TNF-α in T cells that were treated with cladribine in vitro [24]. However, in these studies readouts were performed in the presence of cladribine; therefore, direct cytotoxic drug effects likely account for the reduced cytokine production.
Kraus et al. recently investigated the effects of cladribine on murine and human dendritic cells in vitro. T cells stimulated by murine dendritic cells that had been pre-treated with cladribine produced significantly less IFN-γ and TNF-α and significantly more IL-10. IL-4 production remained unchanged [25]. Together with our observation that cladribine-exposed human T cells produced more IL-4 but did not alter IL-10 production, these data suggest that cladribine exerts its immunomodulatory effects both by directly influencing T cell function and by altering the co-stimulatory function of dendritic cells.
We are aware that an in vitro study only partly reflects the in vivo treatment situation. The effect of cladribine on tissue resident cells and the effects that may occur through repopulation from bone marrow and lymphoid organs are not modelled in our in vitro system. Also, since the cells were obtained from healthy donors they may show a response pattern that differs from cells derived from patients with an ongoing autoimmune disease. Since marketing authorisation for cladribine was not granted in the US and Europe for patients with RRMS the manufacturer withdrew the drug from those markets where cladribine had been introduced. Therefore complementing this study with ex vivo data derived from treated MS patients is not feasible. The statistical approach chosen, was adapted to the exploratory character of the study. Statistical results were not corrected for multiple testing for each cytokine investigated. Therefore, additional studies are needed to confirm our findings. Also, the strongest effects reaching statistical significance were observed only at the highest concentration of cladribine. However, we believe that these data are biologically relevant since these findings were consistently observed using two different stimuli. Furthermore the graphical representation demonstrates a dose dependent induction of cytokines.
Despite these limitations our study is the first to apply an assay that allows the investigation of immunomodulatory effects of cladribine on surviving PBMCs that cannot be explained by direct cytotoxicity. Our data reveal that cells which survive cladribine treatment exhibit an anti-inflammatory phenotype with respect to their cytokine patterns. These findings are in line with the concept that the induction of an anti-inflammatory cytokine pattern contributes to the mechanism of action of immunosuppressive drugs in relapsing remitting MS [16]. Since enhanced induction of IL-4 is a common feature of cyclophosphamide, mitoxantrone and cladribine, it is tempting to speculate that IL-4 induction could be used as a lead marker to select additional immunosuppressive drugs for clinical testing.
In summary, we were able to demonstrate that an initial treatment with cladribine induces a sustained alteration in the cytokine profile of surviving PBMCs in vitro. This cannot be explained by persistent toxic effect after the removal of cladribine because proliferation remained unimpaired and cladribine administration resulted in an induction of IL-4 and IL-5. We conclude that the induction of anti-inflammatory cytokines may contribute to the beneficial effect of cladribine in RRMS patients.
Supporting Information
(XLSX)
Acknowledgments
We thank Andreas Greinacher (Department of Transfusion Medicine, University Medicine Greifswald, Germany) for kindly providing the buffy coats and the AB-serum and Sigrid Peters (Section of Neuroimmunology, Department of Neurology, University Medicine Greifswald, Germany) for her technical assistance.
Data Availability
All source data are supplied in a supplemental Excel file.
Funding Statement
This work was in part supported by an unrestricted research grant from Merck Serono GmbH to AD. The funders have read the manuscript before submission but had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. MK was supported by a scholarship from the German National Academic Foundation and from the DOMAGK program. Sara Bragado Alonso received a scholarship from the Spanish Erasmus program.
References
- 1. Pugliatti M, Rosati G, Carton H, Riise T, Drulovic J, Vecsei L, et al. (2006) The epidemiology of multiple sclerosis in Europe. Eur J Neurol 13: 700–722. [DOI] [PubMed] [Google Scholar]
- 2. Comabella M, Khoury SJ (2012) Immunopathogenesis of multiple sclerosis. Clin Immunol 142: 2–8. 10.1016/j.clim.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Gold R, Chan A, Flachenecker P, Haghikia A, Hellwig K, Kappos L, et al. (2012) DGN / KKNMS Leitlinie zur Diagnose und Therapie der MS. Available: http://wwwdgnorg/images/stories/dgn/leitlinien/LL_MS_Neu/DGN-KKNMS_MS-LL_20120809_frei_neu4pdf 11012013.
- 4. Kantarci OH, Pirko I, Rodriguez M (2014) Novel immunomodulatory approaches for the management of multiple sclerosis. Clin Pharmacol Ther 95: 32–44. 10.1038/clpt.2013.196 [DOI] [PubMed] [Google Scholar]
- 5. Filippini G, Del Giovane C, Vacchi L, D'Amico R, Di Pietrantonj C, Beecher D, et al. (2013) Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev 6: CD008933. [DOI] [PubMed] [Google Scholar]
- 6. Bruck W, Gold R, Lund BT, Oreja-Guevara C, Prat A, Spencer CM, et al. (2013) Therapeutic decisions in multiple sclerosis: moving beyond efficacy. JAMA Neurol 70: 1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sorensen P, et al. (2010) A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 362: 416–426. 10.1056/NEJMoa0902533 [DOI] [PubMed] [Google Scholar]
- 8. Carson DA, Wasson DB, Taetle R, Yu A (1983) Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood 62: 737–743. [PubMed] [Google Scholar]
- 9. Carrera CJ, Terai C, Lotz M, Curd JG, Piro LD, Beutler E, et al. (1990) Potent toxicity of 2-chlorodeoxyadenosine toward human monocytes in vitro and in vivo. A novel approach to immunosuppressive therapy. J Clin Invest 86: 1480–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seto S, Carrera CJ, Kubota M, Wasson DB, Carson DA (1985) Mechanism of deoxyadenosine and 2-chlorodeoxyadenosine toxicity to nondividing human lymphocytes. J Clin Invest 75: 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Singh V, Prajeeth CK, Gudi V, Benardais K, Voss EV, Stangel M (2013) 2-Chlorodeoxyadenosine (cladribine) induces apoptosis in human monocyte-derived dendritic cells. Clin Exp Immunol 173: 288–297. 10.1111/cei.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman GR, Beutler E, Saven A (2003) Cladribine in the treatment of hairy-cell leukaemia. Best Pract Res Clin Haematol 16: 101–116. [DOI] [PubMed] [Google Scholar]
- 13. Comi G, Cook SD, Giovannoni G, Rammohan K, Rieckmann P, Sorensen PS, et al. (2013) MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol 260: 1136–1146. 10.1007/s00415-012-6775-0 [DOI] [PubMed] [Google Scholar]
- 14. Comi G, Hartung HP, Kurukulasuriya NC, Greenberg SJ, Scaramozza M (2013) Cladribine tablets for the treatment of relapsing-remitting multiple sclerosis. Expert Opin Pharmacother 14: 123–136. 10.1517/14656566.2013.754012 [DOI] [PubMed] [Google Scholar]
- 15. Cook S, Vermersch P, Comi G, Giovannoni G, Rammohan K, Rieckmann P, et al. (2011) Safety and tolerability of cladribine tablets in multiple sclerosis: the CLARITY (cladribine tablets treating multiple sclerosis orally) study. Mult Scler 17: 578–593. 10.1177/1352458510391344 [DOI] [PubMed] [Google Scholar]
- 16. Vogelgesang A, Rosenberg S, Skrzipek S, Broker BM, Dressel A (2010) Mitoxantrone treatment in multiple sclerosis induces TH2-type cytokines. Acta Neurol Scand 122: 237–243. 10.1111/j.1600-0404.2009.01295.x [DOI] [PubMed] [Google Scholar]
- 17. Comabella M, Balashov K, Issazadeh S, Smith D, Weiner HL, Khoury SJ (1998) Elevated interleukin-12 in progressive multiple sclerosis correlates with disease activity and is normalized by pulse cyclophosphamide therapy. J Clin Invest 102: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith DR, Balashov KE, Hafler DA, Khoury SJ, Weiner HL (1997) Immune deviation following pulse cyclophosphamide/methylprednisolone treatment of multiple sclerosis: increased interleukin-4 production and associated eosinophilia. Ann Neurol 42: 313–318. [DOI] [PubMed] [Google Scholar]
- 19. Kopadze T, Dobert M, Leussink VI, Dehmel T, Kieseier BC (2009) Cladribine impedes in vitro migration of mononuclear cells: a possible implication for treating multiple sclerosis. Eur J Neurol 16: 409–412. 10.1111/j.1468-1331.2008.02433.x [DOI] [PubMed] [Google Scholar]
- 20. Bartosik-Psujek H, Belniak E, Mitosek-Szewczyk K, Dobosz B, Stelmasiak Z (2004) Interleukin-8 and RANTES levels in patients with relapsing-remitting multiple sclerosis (RR-MS) treated with cladribine. Acta Neurol Scand 109: 390–392. [DOI] [PubMed] [Google Scholar]
- 21. Mitosek-Szewczyk K, Stelmasiak Z, Bartosik-Psujek H, Belniak E (2010) Impact of cladribine on soluble adhesion molecules in multiple sclerosis. Acta Neurol Scand 122: 409–413. 10.1111/j.1600-0404.2010.01330.x [DOI] [PubMed] [Google Scholar]
- 22. Liliemark J, Albertioni F, Hassan M, Juliusson G (1992) On the bioavailability of oral and subcutaneous 2-chloro-2'-deoxyadenosine in humans: alternative routes of administration. J Clin Oncol 10: 1514–1518. [DOI] [PubMed] [Google Scholar]
- 23.Munafo A, Tran D, Marcus S, Ammoury N (2005) An open-label randomized three-way crossover study on the absolute oral bioavailability of cladribine tablets administered to subjects with multiple sclerosis. Presented as poster at the 21st Congress of the European Committee for Treatment and Research in Multiple Sclerosis and the 10th Annual Meeting of the Americas Committee for Treatment and Research in Multiple Sclerosis.
- 24. Laugel B, Borlat F, Galibert L, Vicari A, Weissert R, Chvatchko Y, et al. (2011) Cladribine inhibits cytokine secretion by T cells independently of deoxycytidine kinase activity. J Neuroimmunol 240–241: 52–57. [DOI] [PubMed] [Google Scholar]
- 25.Kraus SH, Luessi F, Trinschek B, Lerch S, Hubo M, Poisa-Beiro L, et al. (2013) Cladribine exerts an immunomodulatory effect on human and murine dendritic cells. Int Immunopharmacol. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All source data are supplied in a supplemental Excel file.