Highlights
-
•
Neural stem cells (NSC) from the adult hippocampus easily lose their activity in vitro.
-
•
Inhibition of p38α enables successful long-term culture of adult hippocampus NSC.
-
•
Inhibition of p38α can maintain a high neurogenic capacity for NSC.
-
•
Neurogenic competence-related microRNAs are upregulated in NSC by p38α inhibition.
-
•
In vitro expanded NSC by p38α inhibition are beneficial against brain damage.
Abbreviations: DG, dentate gyrus; NSC, neural stem cells; RGL, radial glia-like cells; SGZ, subgranular zone; SVZ, subventricular zone
Keywords: p38α, Neurosphere, Neural stem cell, Adult hippocampus
Abstract
Neural stem cells (NSC) from the adult hippocampus easily lose their activity in vitro. Efficient in vitro expansion of adult hippocampus-derived NSC is important for generation of tools for research and cell therapy. Here, we show that a single copy disruption or pharmacological inhibition of p38α enables successful long-term neurosphere culture of adult mouse hippocampal cells. Expanded neurospheres with high proliferative activity differentiated into the three neuronal lineages under differentiating conditions. Thus, inhibition of p38α can maintain adult hippocampal NSC activity in vitro.
1. Introduction
In the adult mammalian brain, neurogenesis persists throughout life at least in two regions, the subventricular zone (SVZ) adjacent to the lateral ventricle and the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG). In the SVZ of the lateral ventricles, GFAP- and nestin-expressing radial glia-like precursors produce new interneurons for the olfactory bulb and oligodendrocytes for the corpus callosum. Also in the SGZ of the DG, new granule neurons and astrocytes are continuously generated [1]. Multipotent neural stem cells (NSC), defined as cells that can self-renew and differentiate into the three neuronal lineages (neuron, astrocyte and oligodendrocyte), have been proposed to be the source of adult neurogenesis in not only the SVZ but also the SGZ [2]. To evaluate this notion, neurosphere assays using cells isolated from the SVZ and the SGZ have been performed [3,4]. The neurosphere assay is a simple retrospective assay to identify the cell stemness such as self-renewal and differentiation capacity [5]. Cells from the SVZ can form neurospheres that continually expand and give rise to neurons and glial cells. On the other hand, neurospheres from the hippocampus including the SGZ region fail to expand and exclusively differentiate into astrocytes without BDNF, indicating that the SGZ contains neural progenitor cells but not neural stem cells [3,4]. Thereafter, it has been clearly demonstrated that non-radial SOX2+ cells, GFAP+ radial glia-like (RGL) cells and PTEN-sensitive nestin+ RGL cells function as NSC in the adult hippocampus [6–8]. Thus, it is currently an indisputable fact that resident NSC are the source of adult hippocampal neurogenesis. Likewise, it has been demonstrated that extracellular high K+ or application of Noggin can maintain hippocampus-derived NSC activity in a neurosphere culture system in vitro [9,10]. Establishment of efficient in vitro expansion of NSC from the adult hippocampus is needed to better understand the nature of adult hippocampal neurogenesis and could give an insight into therapeutic technology using brain stem cells.
One of the stress-activated protein kinases, p38 plays a crucial role in various inflammatory diseases and apoptosis of various types of cells including stem cells [11,12]. It has been shown that p38 functions as a negative regulator in proliferation of NSC from embryonic brain [13]. Likewise, it has been very recently demonstrated that miR-17/106 enhances neurogenic competence in an embryonic stem cell-derived neurosphere culture system via direct inhibition of p38α [14]. These findings tempt up to consider that inhibition of p38α accompanied by changes in microRNA expression can control the fate decision of NSC. However, the relationships between p38α and adult hippocampus-derived NSC activity has not been shown to date. We have investigated various pathophysiological roles of p38α using p38α+/− mice because p38α gene deficiency results in lethality in homozygous embryonic mice [15–17]. The p38α+/− mouse is a useful tool for studying the role of p38α at least in certain disease models.
In the present study, we showed that a single copy disruption or pharmacological inhibition of p38α enables successful long-term neurosphere culture of adult hippocampus NSC. Then, the expression of neurogenic or gliogenic competence-related microRNAs (miRs) in those neurospheres was evaluated by miR array analysis. We also elucidated the possible adaptation of p38 inhibitor-treated neurospheres to brain injury.
2. Materials and methods
2.1. Mice
All animal procedures conformed to the Japanese regulations for animal care and use, following the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science, and were approved by the Animal Care and Use Committee of Chiba University. Male mice heterozygous for targeted disruption of the p38α gene (>F6) [18] were crossed with C57BL/6J female mice (Tokyo Experimental Animal Co., Tokyo, Japan) to generate p38α+/− and p38α+/+ (wild type (WT)) mice. Genotyping by PCR analysis of tail-derived DNA was performed according to our previous report [19].
2.2. Animal models
Male WT and p38α+/− C57BL/6J mice aged 12–15 weeks were used for each experiment. In epilepsy experiments, mice were intraperitoneally injected with kainate (20 mg/kg) (day 0), pulse-labeled with 5-bromo-2′-deoxyuridine (BrdU) on day 7 and sacrificed. For evaluation of changes in the number of nestin+SOX2+BrdU+ cells in the SGZ of DG after epilepsy, three observers blinded to experimental conditions counted the cell in sections covering the entire rostrocaudal axis of the DG and added up numbers. Then, the average value of three totalized counts was determined as a final cell number in the SGZ of DG per mouse. In cold injury experiments, the scalp of WT mice under anesthesia was incised with a fine blade, and a steel rod precooled in liquid nitrogen for 30 s was directly attached to the left side of the bony skull for 6 s (day 0). Then, a single cell suspension of neurospheres labeled with PKH26 (Sigma–Aldrich, St. Louis, MO) was intravenously injected into mice (106 cells/body) 24 h after the operation. Thereafter, mice were sacrificed on day 7. The injured areas in sections stained with HE were quantified using Macromax MVC-DU (GOKO, Kanagawa, Japan).
2.3. Neurosphere culture
The hippocampi dissected from 12 to 15-week old male WT or p38α+/− C57BL/6J mice were processed with a neural dissociation kit (Miltenyi Biotech, Gladbach, Germany), and a single cell suspension was passed through a 40-μm cell strainer (BD Biosciences, San Jose, CA). The resulting cells were incubated in DMEM/HamF12 medium supplemented with N2 (Life Technologies, Carlsbad, CA), retinoic acid-free B27 (Life Technologies), 100 U/ml penicillin/streptomycin in the presence of 25 ng/ml murine FGF-2 (Peprotech, Rocky Hill, NJ) and 25 ng/ml human EGF (Life Technologies). In some experiments, a p38α inhibitor, UR-5269 (Ube Industries Co., Ube, Japan), was added to WT neurospheres at a final concentration of 1 μM every three days. For single cell-derived sphere-forming assay, a limiting diluted single cell suspension was applied to a 96-well plate, and the well containing one cell was marked and investigated. For engraftment of a single cell suspension of neurospheres, expanded neurospheres (60th passage) in the presence of a p38α inhibitor were used. In brief, Accutase (Life Technologies)-dissociated single cells were neutralized, passed through a 40-μm cell strainer and treated with PKH26 (4 × 10−6 M) of PKH26 Red Fluorescent Cell Linker Kits for General Cell Membrane Labeling (Sigma–Aldrich) for 3 min at room temperature. After washing the cells with the medium three times, the resulting cells were suspended at a concentration of 5 × 106 cells/ml in PBS.
2.4. Immunostaining
Neurospheres at passage 60 under growth conditions were washed with HBSS, fixed and treated with primary antibodies, anti-SOX2 (Santa Cruz Biotech, Santa Cruz, CA) and anti-nestin (Sigma–Aldrich). Neurospheres (60th passage) plated on poly l-lysine-coated 8-well Lab-Tek chambers (Thermo Scientific, Waltham, MA) in FGF-2/EGF-free medium were stimulated with 10 ng/ml PDGF-AA (Peprotech), 100 ng/ml BMP2 (Peprotech) or 5 μM forskolin (Sigma–Aldrich) for 5 days, fixed, and treated with primary antibodies, anti-DCX (Santa Cruz) for neuroblasts/neural precursors, Milli-Mark FluoroPan Neuronal Marker (Millipore, Billerica, MA) for neurons, anti-GFAP (Sigma–Aldrich) for astrocytes, anti-NG2 (Abcam, Cambridge, UK) for oligodendrocyte precursors, anti-O4 (Millipore) for oligodendrocytes, anti-MAP2 (Sigma–Aldrich) for neurons, anti-glutamate (Sigma–Aldrich) and/or anti-GABA (Sigma–Aldrich). In some experiments, frozen brain sections (30-μm thickness) were treated with primary antibodies, anti-SOX2, anti-BrdU (Abcam), anti-MAP2 and/or anti-GFAP. Each primary reaction was followed by reaction with an appropriate fluorescence-conjugated second antibody. Fluorescent signals were observed by a fluorescent microscopy (AXIO Imager A2, Carl Zeiss, Oberkochen, Germany) and a confocal laser scanning microscope (FV10i, Olympus, Tokyo, Japan).
2.5. Flow cytometric analysis
Neurospheres at passage 60 under growth conditions were labeled with EdU for 6 h, washed with HBSS, dissociated with Accutase and fixed. Then, a single cell suspension was subjected to detection of EdU according to the instructions of a Click-iT Imaging Kit (Life Technologies) and immunostaining. The resulting cells were analyzed with a FACSCantoII (BD Biosciences). Positively stained cells were gated using negative control cells incubated with appropriate secondary reagent/antibodies. Data were collected and analyzed with FACSDiva (BD Biosciences) and FlowJo 9.6.2 software (TreeStar, Ashland, OR).
2.6. MicroRNA array
WT neurospheres at passage 2, p38α+/−-neurospheres at passage 60 and p38 inh-neurospheres at passage 60 under growth conditions were thoroughly washed with HBSS and immersed in FGF-2/EGF-free medium for 3 h, and total RNA prepared. In each sample, three different cell pools varying in the preparation timing of primary neurospheres were subjected to RNA extraction, and resulting RNA samples were mixed. Then, samples were sent to Filgen Co. (Nagoya, Japan) for miR array analysis.
2.7. Statistical analysis
Statistical analysis was conducted using Graphpad Prism Version 6 (GraphPad Software, San Diego, CA). Statistical significance was determined by Student’s t-test or analysis of variance (ANOVA) followed by Tukey’s test, and P-values of <0.05 were considered to be significant.
3. Results and discussion
3.1. Characteristics of NSC activity from hippocampus of adult p38α+/− mice
A previous study has demonstrated that the proliferation and neurogenesis of hippocampal progenitor cells are upregulated under the tissue regeneration process after kainate-induced epilepsy [20]. We have also shown that epileptic seizure-induced neuronal loss in the hippocampus was ameliorated in p38α+/− mice compared with wild type (WT) mice [16]. Then, we hypothesized that NSC activity in the hippocampus might be potentiated in p38α+/− mice. In neurogenic regions of the adult brain, different NSC populations commonly express SOX2, which plays a crucial role in maintenance of NSC [21,22]. In addition, nestin is generally recognized as one of NSC markers [1,6,7]. Therefore, we investigated changes in the number of nestin+SOX2+BrdU+ cells in the SGZ of DG of WT and p38α+/− mice 7 days after kainate injection. As shown in Fig. 1A, nestin+SOX2+BrdU+ cells were increased in the two genotypes by epileptic seizures, the induction of which was significantly greater in p38α+/− mice than WT mice. On the other hand, the number of proliferative type I cells evaluated by nestin+GFAP+BrdU+ was not significantly changed in WT mice with or without the kainate treatment. In p38α+/− mice, however, nestin+GFAP+BrdU+ cells were significantly increased by the kainate treatment (data not shown). According to our expectation, at least under this pathophysiological environment, the proliferative activity of hippocampal NSC in p38α+/− mice was higher than that in WT mice. Then, to investigate the NSC activity in each genotype, we conducted EGF- and FGF-responsive neurosphere culture. Neurospheres isolated from the hippocampus of adult WT mice failed to expand, which showed good agreement with previous reports [3,10]. Surprisingly, in contrast, neurospheres isolated from the hippocampus of adult p38α+/− mice (p38α+/−-neurospheres) continually expanded for at least 50 passages (Fig. 1B). Currently, we have confirmed that p38α+/−-neurospheres can expand over 90 passages. Using p38α+/−-neurospheres at passage 60, their characteristics as NSC were evaluated.
Fig. 1.

Successful long-term neurosphere culture of adult hippocampal cells from p38α+/− mouse. (A) Changes in proliferation of nestin+SOX2+ cells in subgranular zone of dentate gyrus in WT and p38α+/− mice. Data are shown as mean ± S.E.M. (n = 6). ∗P < 0.05 (ANOVA followed by Tukey's test). (B) In vitro expansion of neurospheres derived from WT and p38α+/− mice. (C) p38α+/− mouse-derived neurospheres at passage 60 highly express SOX2 and nestin under growth conditions. (D) p38α+/− mouse-derived neurospheres at passage 60 are highly proliferative. Fluorescein-labeled EdU in neurospheres was confirmed by fluorescence microscopy and flow cytometric analysis. (E) p38α+/− mouse-derived neurospheres at passage 60 give rise to three neuronal lineages under differentiating conditions. PDGF induces differentiation of neurospheres into DCX+ (red) and/or Milli-Mark FluoroPan Neuronal Marker+ (green) cells (a); GFAP+ (red) cells (b); and O4+ (red) and/or NG2+ (green) cells (c). BMP2 extensively induces differentiation of neurospheres into GFAP+ (red) cells (d). Forskolin induces differentiation of neurospheres into Glu+ (red) MAP2+ (green) cells (e) and GABA+ (red) MAP2+ (green) cells (f). (F) Percentages of each cell lineage differentiated from neurospheres under PDGF (E-a, b and C) are shown. Data are shown as mean ± S.E.M. (n = 4). Percentages of marker-positive cells per DAPI were evaluated in five randomly selected fields, and their average was determined for each sample. Bars represent 50 μm in (C and D) and 20 μm in (E).
As shown in Fig. 1C, p38α+/−-neurospheres under growth conditions expressed SOX2 and nestin, which are known to be multipotential NSC markers [22]. In addition, p38α+/−-neurospheres of different sizes uniformly were SOX2+nestin+ (Supplemental data Fig. 1A). Immunofluorescent study and flow cytometric analysis showed that a DNA synthesis tracer, EdU, was efficiently incorporated into p38α+/−-neurospheres by pulse administration for 6 h, indicating that p38α+/−-neurospheres are highly proliferative under growth conditions. Likewise, p38α+/−-neurospheres of different sizes were uniformly labeled by EdU, which is similar to the case of SOX2 and nestin expression (Supplemental data Fig. 1AandB). These results suggest that highly proliferative cells expressing NSC markers are enriched in p38α+/−-neurospheres without clonal selection of a single cell-derived neurosphere.
NSC possess the ability to generate neurons and glial cells [1]. Along with this notion, p38α+/−-neurospheres treated with PDGF gave rise to the three neuronal lineages (Fig. 1E-a, mature neurons and neuroblasts; Fig. 1E-b, astrocytes; Fig. 1E-c, oligodendrocytes/and their precursor cells), indicating that p38α+/−-neurospheres are multipotential. Approximately 40% of p38α+/−-neurospheres at passage 60 showed neurogenic capacity (Fig. 1F). It is well known that neurosphere-expanded cells markedly lose their neurogenic capacity after an extended number of passages [23]. Furthermore, primary neurosphere-expanded cells derived from the adult mouse hippocampus are restricted to differentiate into astrocytes without an appropriate neurotrophic factor [3,4]. Indeed, also in the present study, adult WT mice-derived hippocampal neurospheres at passage 5 exclusively showed GFAP-like immmunoreactivity in response to PDGF (Supplemental Fig. 1C). As useful differentiating agents for NSC, BMP2 and forskolin are known to promote differentiation of NSC into astrocytes and functional neurons, respectively [24,25]. To better understand the differentiating potential of putative NSC from p38α+/− mice, p38α+/−-neurospheres were treated with BMP2 and forskolin. As shown in Fig. 1E-d, BMP2 efficiently induced astrocytogenesis of p38α+/−-neurospheres. Likewise, forskolin promoted differentiation of p38α+/−-neurospheres into glutamatergic and GABAergic neurons (Fig. 1E-e and -f). Together, these results indicate that a single copy gene disruption of p38α extensively expands NSC from the adult mouse hippocampus in vitro. Then, to elucidate whether pharmacological inhibition of p38α can recapitulate the phenomena observed in p38α+/−-neurospheres, a neurosphere culture prepared from the hippocampus of adult WT mice was performed in the presence of a p38α-specific inhibitor, UR-5269 [17].
3.2. Effect of p38α inhibitor on NSC activity from hippocampus of adult WT mice
As shown in Fig. 2A, p38 inhibitor-treated WT neurospheres (p38 inh-neurospheres) continually expanded for at least 50 passages, as did p38α+/−-neurospheres. Currently, we have confirmed p38 inh-neurospheres could expand over 80 passages. Then, p38 inh-neurospheres at passage 60 were subjected to confirmation of NSC activity. p38 inh-neurospheres expressing SOX2 and nestin were highly proliferative under growth conditions (Fig. 2B and C). Flow cytometric analysis revealed that approximately 70% of proliferative cells defined as EdU+ were SOX2+nestin+ in both the case of p38α+/−-neurospheres and p38 inh-neurospheres, whose characteristics are similar to the case of in vitro expansion of non-radial SOX2+ NSC sorted from the adult hippocampus (Fig. 2D) [6]. In conjunction with the results of Figs. 1D and 2C, nearly 50% of total neurosphere-expanded cells were SOX2+nestin+EdU+ in both cases, suggesting that each conditioned neurosphere has high self-renewal capability. This notion was supported by the single cell-derived sphere-forming assay. As shown in Fig. 2E, the ratio of clonally expanded neurospheres showed good parallelism with the ratio of SOX2+nestin+EdU+ cells in each conditioned neurosphere.
Fig. 2.

Inhibition of p38α in vitro maintains activity of NSC from hippocampus of adult WT mouse. (A) In vitro expansion of neurospheres derived from WT mice in the presence of p38 inhibitor (1 μM). (B) Expanded neurospheres at passage 60 highly express SOX2 and nestin under growth conditions. (C) EdU+-proliferative activity of expanded neurospheres at passage 60 was confirmed by flow cytometric analysis. (D) Flow cytometric evaluation of SOX2+nestin+EdU+ cells in 60th passage neurospheres under different conditions (neurospheres from p38α+/− mice; WT neurospheres treated with p38 inhibitor). Data are shown as mean ± S.E.M. (n = 6). n.s., not significant by Student’s t-test for unpaired values. (E) Single cell-derived sphere-forming activity of 60th passage neurospheres under two conditions. (F) p38 inhibitor-treated neurospheres at passage 60 give rise to three neuronal lineages under differentiating conditions. PDGF induces differentiation of neurospheres into DCX+ (red) and/or Milli-Mark FluoroPan Neuronal Marker+ (green) cells (a); GFAP+ (red) cells (b); and O4+ (red) and/or NG2+ (green) cells (c). BMP2 induces differentiation of neurospheres into GFAP+ (red) cells (d). Forskolin induces differentiation of neurospheres into Glu+ (red) MAP2+ (green) cells (e) and GABA+ (red) MAP2+ (green) cells (f). (G) Percentages of each cell lineage differentiated from neurospheres under PDGF (F-a, b and C) are shown. (H) Percentages of GFAP+ cells differentiated from 60th passage neurospheres under two conditions in response to BMP2. (I) Percentages of MAP2+ cells differentiated from 60th passage neurospheres under two conditions in response to forskolin. Data are shown as mean ± S.E.M. (n = 4) in (G, H and I). Percentages of marker-positive cells per DAPI were evaluated in five randomly selected fields, and their average was determined for each sample in (G, H and I). ∗P < 0.05 (Student’s t-test for unpaired values) in (H and I). Bars represent 50 μm in (B) and 20 μm in (F).
In response to PDGF, p38 inh-neurospheres gave rise to the three neuronal lineages (Fig. 2F-a, mature neurons and neuroblasts; Fig. 2F-b, astrocytes; Fig. 2F-c, oligodendrocytes and their precursor cells). However, astrocytes bearing fibrous processes and oligodendrocytes bearing many extended processes were rarely observed under a PDGF-induced differentiation state. Most importantly, PDGF-induced neurogenic competence was markedly enhanced in p38 inh-neurospheres (Fig. 2G). BMP2 induced astrocytogenesis of p38 inh-neurospheres, in which typical morphological features of astrocytes bearing fibrous processes were observed (Fig. 2F-d). However, BMP2-induced astrocytogenesis in p38 inh-neurospheres was markedly less than that in p38α+/−-neurospheres (Fig. 2H). On the other hand, forskolin efficiently promoted terminal differentiation of p38 inh-neurospheres into glutamatergic and GABAergic neurons (Fig. 2F-e and -f). Likewise, forskolin-induced neurogenesis in p38 inh-neurospheres was significantly greater than that in p38α+/−-neurospheres (Fig. 2I). These results clearly indicate that pharmacological inhibition of p38α can recapitulate the effect of genetic inhibition of p38α and expand NSC from the adult WT mouse hippocampus in vitro. Notably, the neurogenic but not gliogenic potential of p38 inh-neurospheres was markedly higher than that of p38α+/−-neurospheres. To evaluate the mechanisms underlying the enhanced neurogenesis, miR array was performed.
3.3. Evaluation of changes in neurogenic and gliogenic competence-related miRs
It is well known that neurospheres heterologously express markers for the three neuronal lineages even under growth conditions [21,26]. Although SOX2 and nestin are uniformly and highly expressed in both p38α+/−-neurospheres and p38 inh-neurospheres with a variety of sizes under growth conditions, they simultaneously expressed DCX and NG2 in a heterogenous sphere population pattern. In p38α+/−-neurospheres, DCX-like immunoreactivity (LI) and NG2-LI were observed. On the other hand, NG2-LI but not DCX-LI was rarely detected in p38 inh-neurospheres (Supplemental Fig.1DandE). These findings reflect their differentiating capacity in response to differentiating agents (Fig. 1F and 2G). Then, to elucidate the intrinsic fate commitment of each type of neurosphere, RNA from the neurospheres was subjected to miR array.
As shown in Fig. 3A, the expression of miR-17-5p, miR-106a-5p and miR-106b-5p, which can restore neurogenic competence in gliogenic neural stem progenitor cells, was equally upregulated in both p38α+/−-neurospheres and p38 inh-neurospheres compared with WT neurospheres. In particular, p38α is a specific target of miR-17, which means that miR-17 functions as an upstream molecule of p38α [14]. In our case, a single copy disruption or pharmacological inhibition of p38α upregulated miR-17 expression. Therefore, downregulation of p38α expression or p38α activity may influence miR-17 expression by a negative feedback pathway, although the precise mechanism remains unknown. Other neurogenic competence-related miRs, 26b-5p and 9-5p, were also upregulated in both p38α+/−-neurospheres and p38 inh-neurospheres compared with WT neurospheres (Fig. 3B). However, the expression of miR-124a-3p, which affects neural lineage differentiation in concert with miR 9-5p, was upregulated in p38α+/−-neurospheres but not p38 inh-neurospheres (Supplemental Fig. 2) [27,28]. On the other hand, the expression of plausible gliogenic competence-related miRs such as 23a-3p, 24-3p and 138-5p was not changed in p38α+/−-neurospheres but was downregulated in p38 inh-neurospheres compared with WT neurospheres (Fig. 3C) [29–31]. These results suggest that the high neurogenic potential of both p38α+/−-neurospheres and p38 inh-neurospheres from the adult hippocampus shows good agreement with the upregulation of neurogenic competence-related miRs, and that the downregulation of gliogenic competence-related miRs especially in p38 inh-neurospheres may force them to differentiate into neurons predominantly. Further study is needed to confirm the direct contribution of candidate miRs to the specific characteristics of neurospheres.
Fig. 3.
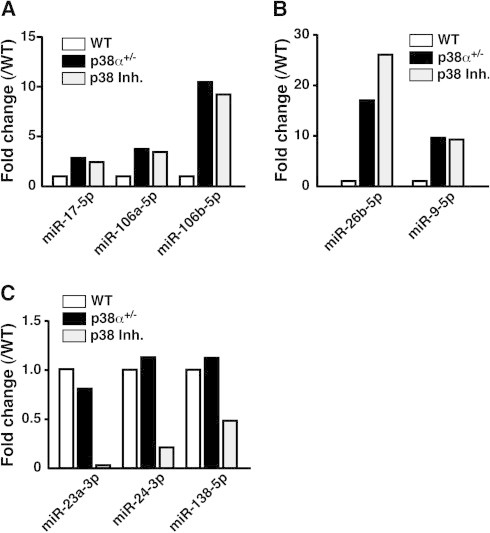
Changes in neurogenic and gliogenic competence-related microRNAs in three different neurospheres. Expression level of each molecule was normalized to the signal obtained in WT neurospheres and expressed as fold change. Changes in neurogenic competence-related miRs are shown in (A) and (B). Changes in gliogenic competence-related miRs are shown in (C). Similar results were obtained in two independent experiments.
3.4. Transplantation of p38 inh-neurospheres into brain
Application of a p38α-specific inhibitor to neurospheres from the adult hippocampus is an easy way to induce in vitro expansion of NSC with high self-renewal and neurogenic capacity. Then, we elucidated whether p38 inh-neurospheres can efficiently differentiate into neurons in vivo. We employed a cold injury model because extensive gliosis secondary to blood–brain barrier breakdown occurs in this traumatic injury, producing a gliogenic microenvironment in the brain [32]. Then, we evaluated whether p38 inh-neurospheres could maintain neurogenesis in such a brain environment. Intravenously transplanted NSC can be delivered into the damaged central nervous system by the very late antigen (VLA)-4-mediated mechanism, like T cells infiltrate into inflammatory lesions [33]. VLA-4 is an integrin heterodimer consisting of α4 and β1. We have confirmed that the expression of integrin α4 is upregulated in p38 inh-neurospheres compared with WT neurospheres, although the expression of integrin β1 is consistent between them (Supplemental Fig.3A). Therefore, a single cell suspension of p38 inh-neurospheres was labeled with PKH and intravenously injected 24 h after the onset of cold injury.
As shown in Fig. 4A-a, MAP2+PKH+ cells were obviously observed in marginal areas of the lesion site 7 days after engraftment. On the other hand, GFAP+PKH+ cells were observed as a small population in marginal areas of the lesion site (Fig. 4A-b). Likewise, not SOX2+PKH+ but SOX2+PKH-/endogenous SOX2+ cells were observed in marginal areas of the lesion site (Fig. 4A-c). These results suggest that p38 inh-neurospheres give rise to neurons efficiently in vivo. Surprisingly, transplantation of p38 inh-neurospheres suppressed the size of injury (Fig. 4B). As shown in Fig. 4C and D, the maximal depth of injury and the injured area in transplanted mice were significantly less than those in control mice. Interestingly, transplanted PKH+ cells were condensed in the border zone and the marginal area of the lesion site, and migration of endogenous reactive astrocytes to the lesion site was inhibited in transplanted mice (Supplemental Fig.3BandC). Hence, in concert with tissue regeneration by neurogenesis of transplanted NSC, the transplanted cells may act to protect neurons from gliosis via previously proposed mechanisms [34].
Fig. 4.

Transplantation of adult hippocampal NSC in vitro expanded by p38 inhibitor. Expanded NSC labeled with PKH in PBS, and cell-free PBS were intravenously injected into mice bearing a traumatic cortical injury. (A) PKH+ (red) MAP2+ (green) are obviously observed (a), although PKH+ (red) GFAP+ (green) cells are observed as a small cell population (b). PKH+ (red) SOX2+ (green) are rarely observed (c). Z-stack analysis was performed by a confocal laser scanning microscope. (B) Transplantation of expanded NSC ameliorated the size of the injury, confirmed by HE staining. Accordingly, the maximal depth of injury from the cortical surface (C) and calculated injured area (D) were decreased by transplantation. We confirmed that the length of both the anterior–posterior axis and the lateral axis of the injured cortical surface were not significantly different between the two groups. Data are shown as mean ± S.E.M. (n = 4) in (C and D). ∗P < 0.05 (Student’s t-test for unpaired values) in (C and D).
The present study demonstrated that p38 inh-neurospheres possess a high neurogenic capacity in vitro and in vivo. Likewise, we have estimated that nearly 70% of neurons differentiated from p38 inh-neurospheres by forskolin were GABAnergic. These findings tempt us to consider that a single cell suspension of p38 inh-neurospheres engrafted in the hippocampus may function as an GABAnergic interneurons. Thus, as a next step, to evaluate the therapeutic effect of p38 inh-neurospheres on epileptic seizure is needed.
4. Conclusion
Inhibition of p38α can maintain activity of NSC from the adult hippocampus in vitro, the way of which is useful to generate a great number of NSC for reparative therapy of brain damage.
Author contributions
K.Y., K.N. and Y.K. developed the concept and designed the experiments. K.Y., K.N., T.S. and Y.K. performed the experiments. YK wrote the paper.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research ((B), 24390137 to Y.K.) and for Challenging Exploratory Research (25670256 to Y.K.), and by the Takeda Science Foundation for Visionary Research (to Y.K.). We thank Dr. Wendy Gray for editing our manuscript.
Contributor Information
Kento Yoshioka, Email: kent.y.mb@gmail.com.
Kana Namiki, Email: k_kana_0531@yahoo.co.jp.
Tatsuhiko Sudo, Email: sudo@riken.jp.
Yoshitoshi Kasuya, Email: kasuya@faculty.chiba-u.jp.
Appendix A. Supplementary data
Figure S1. Under growth conditions, p38α+/−-neurospheres of different sizes uniformly are SOX2+nestin+ (A) and labeled by EdU (B). Adult WT mice-derived hippocampal neurospheres at passage 5 exclusively show GFAP-like immmunoreactivity in response to PDGF (C). Under growth conditions, p38α+/−-neurospheres express DCX and NG2 in a heterogenous sphere population pattern (D). On the other hand, p38 inh-neurospheres obviously express DCX but not NG2 (E).
Figure S2. Venn diagram of the number of microRNAs upregulated (>2, vs WT) or downregulated (<0.5, vs WT) by a single copy disruption or pharmacological inhibition of p38α in neurospheres.
Figure S3. (A) Changes in mRNA expression of α4 and β1 integrins in both WT- and p38 inh-neurospheres. Using the following primers: 5′-GATGCTGTTGTTGTACTTCGGG-′3 and 5′-ACCACTGAGGCATTAGAGAGC-′3 for α4, 5′-AGACTTCCGCATTGGCTTTG-′3 and 5′-GCTGGTGCAGTTTTGTTCAC-′3 for β1, a real time PCR was performed. Amplification parameters were as follows: one cycle at 95 °C for 7 min followed by 40 cycles at 95 °C for 5 s, 60 °C for 10 s, 72 °C for 30 s. Data were collected and analyzed with PikoReal Software 2.2 (Thermo scientific). Signal values of each gene were normalized by the expression of β-actin, and induction ratio relative to the average values of WT samples was calculated. Data are shown as mean ± S.E.M. (n = 3) ∗P < 0.05, n.s., not significant (Student’s t-test for unpaired values). (B) Transplanted PKH+ cells were condensed in the border zone and the marginal area of the lesion site. (C) Migration of endogenous reactive astrocytes (GFAP+, green) to the lesion site was suppressed in transplanted group (right panel) compared with control group (left panel). The broken line indicates the border of injured area.
References
- 1.Ming G.-L., Song H. Adult neurogenesis in the mammalian brain significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gage F.H. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 3.Bull N.D., Bartlett P.F. The adult mouse hippocampal progenitor is neurogenic but not a stem cell. J. Neurosci. 2005;25:10815–10821. doi: 10.1523/JNEUROSCI.3249-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seaberg R.M., van der Kooy D. Adult rodent neurogenic regions: the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. J. Neurosci. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastrana E., Silva-Vargas V., Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suh H., Consiglio A., Ray J., Sawai T., D’Amour K.A., Gage F.H. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonaguidi M.A., Wheeler M.A., Shapiro J.S., Stadel R.P., Sun G.J., Ming G.-L., Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Encinas J.M., Michurina T.V., Peunova N., Park J.-H., Tordo J., Peterson D.A., Fishell G., Koulakov A., Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker T.L., White A., Black D.M., Wallace R.H., Sah P., Bartlett P.F. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J. Neurosci. 2008;28:5240–5247. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaguidi M.A., Peng C.-Y., Tammy M., Falciglia G., Gobeske Kevin T., Czeisler C., Kessler J.A. Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 2008;28:9194–9294. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S., Boehm J., Lee J.C. P38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat. Rev. Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L., Opalinska J., Vermap A. P38 MAP Kinase regulates stem cell apoptosis in human hematopoietic failure. Cell Cycle. 2007;6:534–537. doi: 10.4161/cc.6.5.3921. [DOI] [PubMed] [Google Scholar]
- 13.Sato K., Hamanoue M., Takamatsu K. Inhibitors of p38 mitogen-activated protein kinase enhance proliferation of mouse neural stem cells. J. Neurosci. Res. 2008;86:2179–2189. doi: 10.1002/jnr.21668. [DOI] [PubMed] [Google Scholar]
- 14.Naka-Kaneda H., Nakamura S., Igarashi M., Aoi H., Kanki H., Tsuyama J., Tsutsumi S., Aburatani H., Shimazaki T., Okano H. The miR-17/106-p38 axis is a key regulator of the neurogenic-to-gliogenic transition in developing neural stem/progenitor cells. Proc. Natl. Acad. Sci. USA. 2014;111:1604–1609. doi: 10.1073/pnas.1315567111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo Y., Amano S., Furuya M., Namiki K., Sakurai K., Nishiyama M., Sudo T., Tatsumi K., Kuriyama T., Kimura S., Kasuya Y. Involvement of p38alpha mitogen-activated protein kinase in lung metastasis of tumor cells. J. Biol. Chem. 2006;281:36767–36775. doi: 10.1074/jbc.M604371200. [DOI] [PubMed] [Google Scholar]
- 16.Namiki K., Nakamura A., Furuya M., Mizuhashi S., Matsuo Y., Tokuhara N., Sudo T., Hama H., Kuwaki T., Yano S., Kimura S., Kasuya Y. Involvement of p38alpha in kainate-induced seizure and neuronal cell damage. J. Recept. Signal Transduct. Res. 2007;27:99–111. doi: 10.1080/10799890701357855. [DOI] [PubMed] [Google Scholar]
- 17.Namiki K., Matsunaga H., Yoshioka K., Tanaka K., Murata K., Ishida J., Sakairi A., Kim J., Tokuhara N., Shibakawa N., Shimizu M., Wada Y., Tokunaga Y., Shigetomi M., Hagihara M., Kimura S., Sudo T., Fukamizu A., Kasuya Y. Mechanism for p38α-mediated experimental autoimmune encephalomyelitis. J. Biol. Chem. 2012;287:24228–24238. doi: 10.1074/jbc.M111.338541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K., Sudo T., Senftleben U., Dadak A.M., Johnson R., Karin M. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102:221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 19.Takanami-Ohnishi Y., Amano S., Kimura S., Asada S., Utani A., Maruyama M., Osada H., Tsunoda H., Irukayama-Tomobe Y., Goto K., Karin M., Sudo T., Kasuya Y. Essential role of p38 mitogen-activated protein kinase in contact hypersensitivity. J. Biol. Chem. 2002;277:37896–37903. doi: 10.1074/jbc.M207326200. [DOI] [PubMed] [Google Scholar]
- 20.Jessberger S., Römer B., Babu H., Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp. Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Brazel C.Y., Limke T.L., Osborne J.K., Miura T., Cai J., Pevny L., Rao M.S. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 22.Graham V., Khudyakov J., Ellis P., Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 23.Fricker R.A., Carpenter M.K., Winkler C., Greco C., Gates M.A., Björklund A. Site-specific migration and neuronal differentiation of human neural progenitor cells after transplantation in the adult rat brain. J. Neurosci. 1999;19:5990–6005. doi: 10.1523/JNEUROSCI.19-14-05990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima K., Takizawa T., Ochiai W., Yanagisawa M., Hisatsune T., Nakafuku M., Miyazono K., Kishimoto T., Kageyama R., Taga T. BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc. Natl. Acad. Sci. USA. 2001;98:5868–5873. doi: 10.1073/pnas.101109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepski G., Jannes C.E., Nikkhah G., Bischofberger J. cAMP promotes the differentiation of neural progenitor cells in vitro via modulation of voltage-gated calcium channels. Front. Cell. Neurosci. 2013;7:155. doi: 10.3389/fncel.2013.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suslov O.N., Kukekov V.G., Ignatova T.N., Steindler D.A. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc. Natl. Acad. Sci. USA. 2002;99:14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dill H., Linder B., Fehr A., Fischer U. Intronic miR-26b controls neuronal differentiation by repressing its host transcript, ctdsp2. Genes Dev. 2012;26:25–30. doi: 10.1101/gad.177774.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krichevsky A.M., Sonntag K.C., Isacson O., Kosik K.S. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24:857–864. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smirnova L., Gräfe A., Seiler A., Schumacher S., Nitsch R., Wulczyn F.G. Regulation of miRNA expression during neural cell specification. Eur. J. Neurosci. 2005;21:1469–1477. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith B., Treadwell J., Zhang D., Ly D., McKinnell I., Walker P.R., Sikorska M. Large-scale expression analysis reveals distinct microRNA profiles at different stages of human neurodevelopment. PLoS One. 2010;5:e11109. doi: 10.1371/journal.pone.0011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dugas J.C., Cuellar T.L., Scholze A., Ason B., Ibrahim A., Emery B., Zamanian J.L., Foo L.C., McManus M.T., Barres B.A. Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hama H., Kasuya Y., Sakurai T., Yamada G., Suzuki N., Masaki T., Goto K. Role of endothelin-1 in astrocyte responses after acute brain damage. J. Neurosci. Res. 1997;47:590–602. doi: 10.1002/(sici)1097-4547(19970315)47:6<590::aid-jnr4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Pluchino S., Quattrini A., Brambilla E., Gritti A., Salani G., Dina G., Galli R., Del Carro U., Amadio S., Bergami A., Furlan R., Comi G., Vescovi A.L., Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 34.Jäderstad J., Jäderstad L.M., Li J., Chintawar S., Salto C., Pandolfo M., Ourednik V., Teng Y.D., Sidman R.L., Arenas E., Snyder E.Y., Herlenius E. Communication via gap junctions underlies early functional and beneficial interactions between grafted neural stem cells and the host. Proc. Natl. Acad. Sci. USA. 2010;107:5184–5189. doi: 10.1073/pnas.0915134107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Under growth conditions, p38α+/−-neurospheres of different sizes uniformly are SOX2+nestin+ (A) and labeled by EdU (B). Adult WT mice-derived hippocampal neurospheres at passage 5 exclusively show GFAP-like immmunoreactivity in response to PDGF (C). Under growth conditions, p38α+/−-neurospheres express DCX and NG2 in a heterogenous sphere population pattern (D). On the other hand, p38 inh-neurospheres obviously express DCX but not NG2 (E).
Figure S2. Venn diagram of the number of microRNAs upregulated (>2, vs WT) or downregulated (<0.5, vs WT) by a single copy disruption or pharmacological inhibition of p38α in neurospheres.
Figure S3. (A) Changes in mRNA expression of α4 and β1 integrins in both WT- and p38 inh-neurospheres. Using the following primers: 5′-GATGCTGTTGTTGTACTTCGGG-′3 and 5′-ACCACTGAGGCATTAGAGAGC-′3 for α4, 5′-AGACTTCCGCATTGGCTTTG-′3 and 5′-GCTGGTGCAGTTTTGTTCAC-′3 for β1, a real time PCR was performed. Amplification parameters were as follows: one cycle at 95 °C for 7 min followed by 40 cycles at 95 °C for 5 s, 60 °C for 10 s, 72 °C for 30 s. Data were collected and analyzed with PikoReal Software 2.2 (Thermo scientific). Signal values of each gene were normalized by the expression of β-actin, and induction ratio relative to the average values of WT samples was calculated. Data are shown as mean ± S.E.M. (n = 3) ∗P < 0.05, n.s., not significant (Student’s t-test for unpaired values). (B) Transplanted PKH+ cells were condensed in the border zone and the marginal area of the lesion site. (C) Migration of endogenous reactive astrocytes (GFAP+, green) to the lesion site was suppressed in transplanted group (right panel) compared with control group (left panel). The broken line indicates the border of injured area.


