Abstract
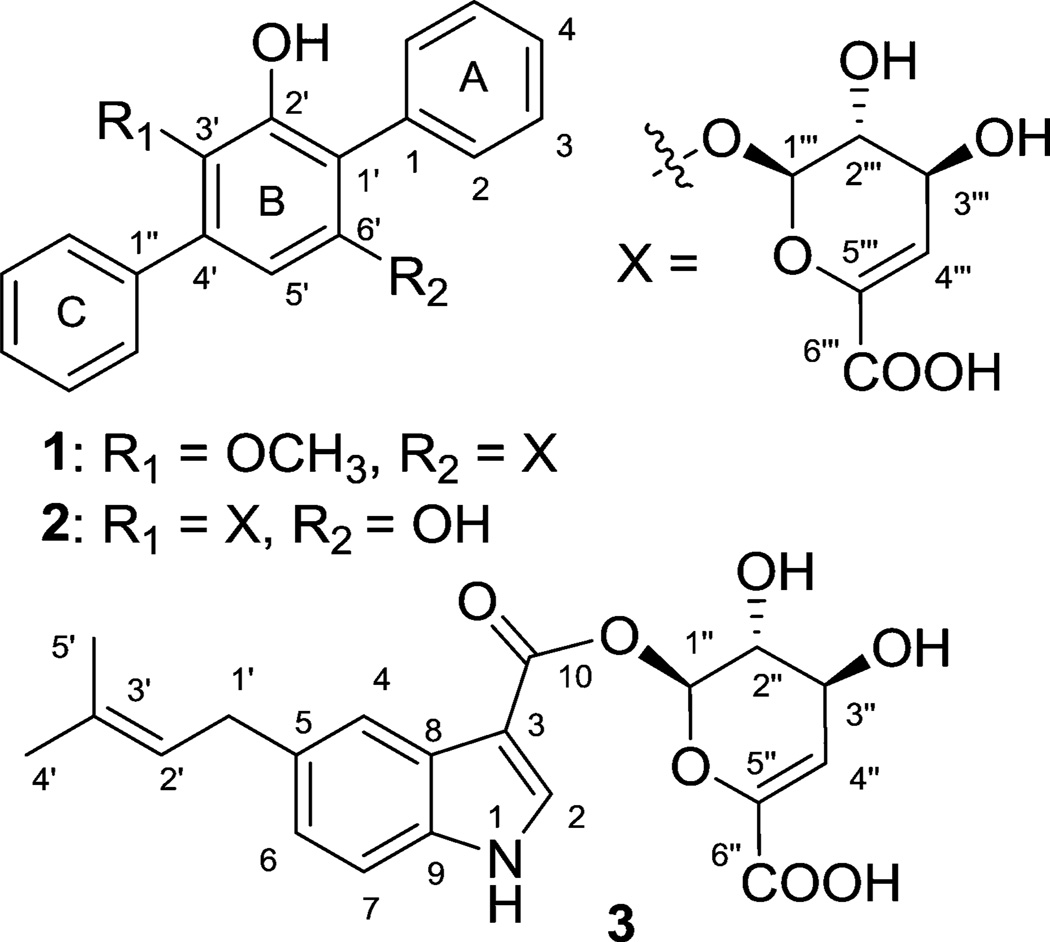
Terfestatins B (1) and C (2), new p-terphenyls bearing a novel unsaturated hexuronic acid (4-deoxy-α-l-threo-hex-4-enopyranuronate), a unique β-d-glycosyl ester of 5-isoprenylindole-3-carboxylate (3) and the same rare sugar, and two new hygromycin precursors, were characterized as metabolites of the coal mine fire isolate Streptomyces sp. RM-5–8. EtOH damage neuroprotection assays using rat hippocampal-derived primary cell cultures with 1, 2, 3 and echoside B (a terfestatin C-3′-β-d-glucuronide from Streptomyces sp. RM-5– 8) revealed 1 as potently neuroprotective, highlighting a new potential application of the terfestatin scaffold.
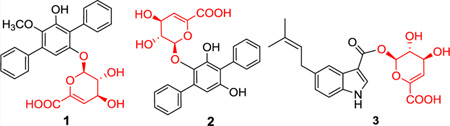
The p-terphenyl-derived natural products are composed of a linear 1,4-diaryl-substituted benzene core often further modified via enzymatic modification. While substituted p-terphenyls are fairly common as fungal metabolites,1 only 13 have been reported from bacteria,2 six of which (terfestatin A3 and echosides A–E4) exist as simple glucosides or glucuronides. Consistent with the diverse range of biological activities reported for p-terphenyl metabolites,5 terfestatin A was identified as a modulator of auxin signaling in Arabidopsis, while the echosides were noted as DNA topoisomerase inhibitors.3,4 Beginning with the pioneering work of Hamilton and co-workers,6 the p-terphenyl core scaffold has also been recognized as a unique small molecule-based α helix mimic and subsequently exploited for the development of synthetic protein–protein interaction inhibitors.7
As part of an effort to explore the microbial diversity and corresponding metabolic potential of actinomycetes associated with thermal vents emanating from underground coal mine fires in Appalachia,8 herein we report the discovery of two new p-terphenyl glycosides [terfestatins B (1) and C (2)] from the Ruth Mullins coal fire-affiliated isolate Streptomyces sp. RM-5–8. Both metabolites bear a novel unsaturated hexuronic acid (4-deoxy-α-l-threo-hex-4-enopyranuronate), a sugar also found appended as a β-d-glycosyl ester of 5-isoprenylindole-3-carboxylate (3) produced by the same strain (Figure 1). While this may implicate the presence of a uniquely permissive glycosyltransferase, it is important to note that there are only two reported unsaturated hexuronic acids among the >3400 known naturally occurring glycosylated bacterial natural products.2,9 Furthermore, 1 displayed notable activity in an EtOH damage neuroprotection assay using rat hippocampal-derived primary cell cultures. As neurodegeneration and gliotoxicity are hallmarks of protracted EtOH dependence,10 this work highlights a new terfestatin activity of clinical significance.
Figure 1.
Structures of new compounds 1–3 isolated from Streptomyces sp. RM-5–8
Nine actinomycete strains were purified from the second-generation plates deriving from a single soil sample collected near a Ruth Mullins underground coal mine fire thermal vent located in Perry County, KY.11 Metabolic profiling of these strains implicated Streptomyces sp. RM-5–8 as capable of unique metabolite production. A seed culture of Streptomyces sp. RM-5–8 was subsequently used to inoculate 6 L of medium A, and large-scale fermentation continued for 7 days at 28 °C and 200 rpm. The culture broth was filtered, and the recovered mycelial cake was extracted with acetone while the recovered aqueous filtrate was subjected to solid-phase extraction (XAD-16 resin, 800 g) and the resin subsequently washed with water and then extracted with methanol to obtain crude extract. The mycelial acetone and solid-phase methanolic crude extracts were combined, dried, and subjected to progressive chromatography (silica gel column chromatography, Sephadex LH-20 column chromatography, and semipreparative C18 HPLC) to yield 1 (9 mg, yield: 1.5 mg/L), 2 (10 mg, yield: 1.7 mg/L), and 3 (4 mg, yield: 0.7 mg/L). In addition, two new hygromycin A precursors 4 (15 mg, yield: 2.5 mg/L; Figure S1) and 5 (5 mg, yield: 0.8 mg/L, Figure S1), and seven known compounds were identified including the major product hygromycin A (107 mg/L) (see the Supporting Information).
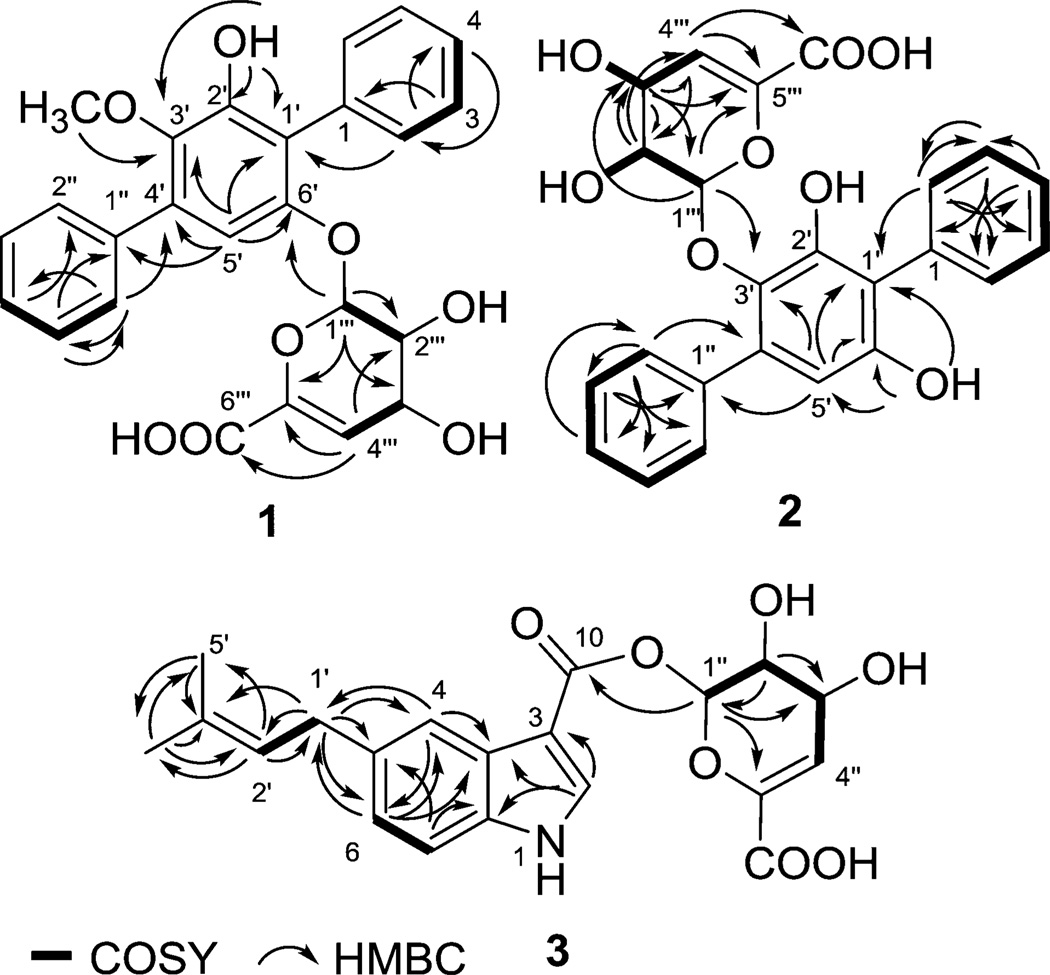
Compounds 1 and 2 were obtained as white amorphous powders, and their molecular formulas were established by HR-ESI-MS as C25H22O8 [m/z 451.1379 (M + H)+] and C24H20O8 [m/z 435.1089 (M − H)−], respectively, indicating 15 degrees of unsaturation and a difference of a single methylene between the two metabolites. 1H and 13C NMR data (Table 1) revealed characteristic features of p-terphenyl-derived compounds, including two monosubstituted benzene rings (rings A and C, Figure 1) with key H-2/H-3, H-3/H-4, H-2″/H-3″, and H-3″/H-4″ COSY correlations and H-3/C-1, H-2/C-4, H-4/C-2, H-3″/C-1″, H-2″/C-4″, and H-4″/C-2″ HMBC correlations (Figure 2). Common features of the shared pentasubstituted benzene core (ring B) included a singlet aromatic proton signal (H-5′) with HMBC correlations to four aromatic quaternary carbons (C-1′, C-3′, C-6′ and C-1″) and a C-2′-OH with HMBC correlations to C-1′, C-2′, and C-3′. Compounds 1 and 2 diverge structurally via their C-3′ and C-6′ substitution patterns (ring B). Among the key signatures used to delineate these distinctions was the 2J HMBC correlation between the 3′-OCH3 and C-3′ as support of the C-3′ methoxy assignment in 1 (absent in 2, C-6′–OH, δH 9.21).
Table 1.
1H and 13C NMR (400 and 100 MHz) Data for Terfestatins B (1) and C (2) in DMSO-d6 (J in Hz)
| no. | terfestatin B (1) | terfestatin C (2) | ||
|---|---|---|---|---|
| δH | δC, type | δH | δC, type | |
| 1 | 133.4, C | 134.4, C | ||
| 2, 6 | 7.41, m | 130.9, CH | 7.33, m | 130.8, CH |
| 3, 5 | 7.35, m | 127.5, CH | 7.38, m | 127.3, CH |
| 4 | 7.29, m | 126.7, CH | 7.24, m | 126.2, CH |
| 1' | 119.9, C | 116.0, C | ||
| 2' | 148.3, C | 148.5, C | ||
| 3' | 141.0, C | 133.0, C | ||
| 4' | 133.1, C | 132.8, C | ||
| 5' | 6.76, s | 108.8, CH | 6.39, s | 107.4, CH |
| 6' | 150.0, C | 151.5, C | ||
| 1'' | 137.6, C | 137.7, C | ||
| 2'', 6'' | 7.62, d, (7.5) | 128.6, CH | 7.54, dd (7.2, 1.2) | 128.6, CH |
| 3'', 5'' | 7.46, t (7.5) | 128.4, CH | 7.45, t (7.3) | 128.4, CH |
| 4'' | 7.35, m | 127.5, CH | 7.35, m | 127.4, CH |
| 1''' | 5.54, d (4.6) | 99.1, CH | 4.83, d (2.2) | 99.5, CH |
| 2''' | 3.46, m | 69.9, CH | 3.70, br s | 68.1, CH |
| 3''' | 3.91, br s | 66.2, CH | 3.81, br s | 63.9, CH |
| 4''' | 5.87, br s | 112.6, CH | 5.97, d (4.4) | 110.7, CH |
| 5''' | 140.6, C | 140.3, C | ||
| 6''' | 163.2, C | 162.9, C | ||
| 3'-OCH3 | 3.30, s | 60.3, CH3 | ||
| 2'-OH | 8.91, s | 8.28, s | ||
| 6'-OH | 9.21, s | |||
| 2''-OH | 5.37, br s | 5.53, d (4.3) | ||
| 3''-OH | 4.35, br s | 5.68, d (6.6) | ||
Figure 2.
1H,1H-COSY (–) and selected HMBC (→) correlations of compounds 1–3.
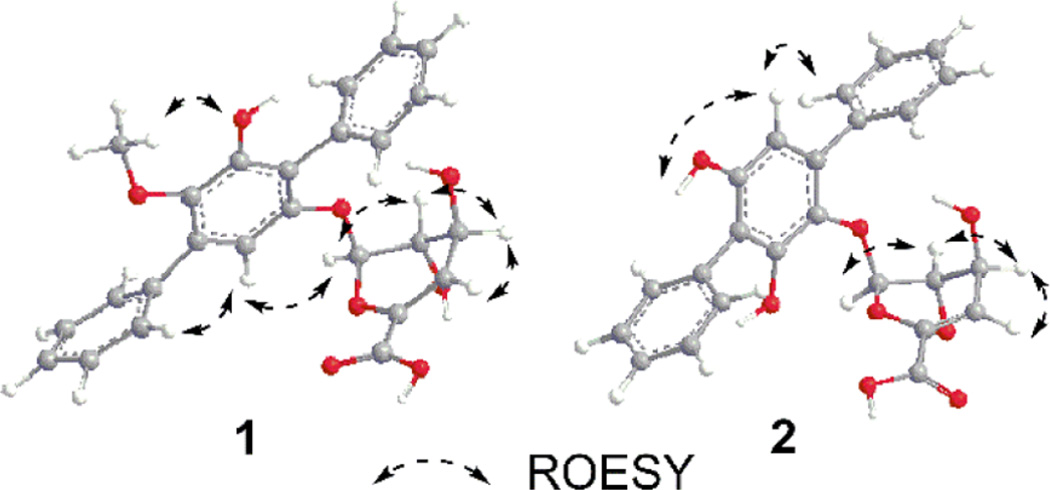
Most notably, both 1 and 2 bear an appended rare sugar assigned as 4-deoxy-α-l-threo-hex-4-enopyranuronate based upon key COSY (Figure 2, H-1‴/H-2‴ and H-3‴/H-4‴), HMBC correlations (Figure 2; from H-1‴ to C-2‴, C-3‴ and C-6‴; from H-4‴ to C-2‴, C-5‴ and C-6‴) and ROESY (Figure 3). The corresponding glycosyl regiospecificity was also found to differ between 1 (C-6′, supported by a key H-1‴/C-6′ HMBC correlation) and 2 (C-3′, supported by a key H-1‴/C-3′ HMBC correlation). The terfestatin naming convention was adopted for compounds 1 and 2 based upon the core scaffold structural similarity compared to the first reported microbial metabolites in this family.3
Figure 3.
Key ROESY correlations of terfestatins B (1) and C (2).
Compound 3 was isolated as a white amorphous powder, and its molecular formula was established by HR-ESI-MS as C20H21NO7 [m/z 388.1395 (M + H)]. The analysis of the 1H/13C and gHSQC NMR data suggested the presence of two methyl, one methylene, nine methine, and eight quaternary carbon signals (Table S2, Supporting Information). The characteristic aromatic proton signals at δH 8.10 (s), 7.97 (d, 7.8), 7.25 (s), and 7.00 (d, 7.8) were diagnostic of a 5-substituted indole moiety, which displayed HMBC correlations from H-2 (δH 8.10, s) to C-3 (δC 105.3), C-8 (δC 123.7), and C-9 (δC 136.8); from H-4 (δH 7.25, s) to C-6 (δC 122.5) and C-8; from H-6 (δH 7.00, d) to C-4 (δC 111.3) and C-9; and from H-7 (δH 7.97, d) to C-5 (δC 135.9) and C-8. Support for the C-5 isoprenyl assignment derived from a key COSY correlation between H-1′/H-2′ and HMBC correlations from H2-1′ (δH 3.40, 2H) to C-4 (δC 111.3), C-5 (δC 135.9), C-6 (δC 122.5), C-2′ (δC 123.8), and C-3′ (δC 131.3) and from H-2′ (δH 5.34) to C-1′ (δC 33.8), C-4′ (δC 17.6), and C-5′ (δC 25.5), as well as two singlet methyl signals (H3-4′ and H3-5′) correlated with C-2′ (δC 123.8) and C-3′ (δC 131.3). Notably, the same 4-deoxy-α-l-threo-hex-4-enopyranuronate found within 1 and 2 was also detected in 3 (Table S2 and Figure 1). However, distinct from 1 and 2, the corresponding lower anomeric proton chemical shift (δH 6.21, H″-1) in 3 and the key HMBC correlation with the C-10 carboxylic acid implicated a less common glycosyl ester attachment. Interestingly, a similar 4-deoxy-α-l-threo-hex-4-enopyranuronate glycosyl ester of the angiotensin converting inhibitor A58365 was previously discovered as part of a broad microbial bioconversion screening effort.12
Two additional new natural hygromycin precursors [prehygromycin (4) and 4′-epi-prehygromycin (5); Figure S1] and seven known compounds [echoside B,4 hygromycin A,13 4″-epi-hygromycin, 14 5-methoxyhygromycin,15 5-methoxy-4″-epi-hygromycin,15 geldanamycin,16 17-O-demethylgeldanamycin;17 Figure S1] were isolated and identified on the basis of NMR, MS, and comparison with literature precedent (see the Supporting Information). Of these, echoside B was recently reported as a metabolite of Streptomyces sp. LZ35 and differs from 1 via the nature of the appended sugar (where echoside B is the corresponding C-3′-β-d-glucuronide).4
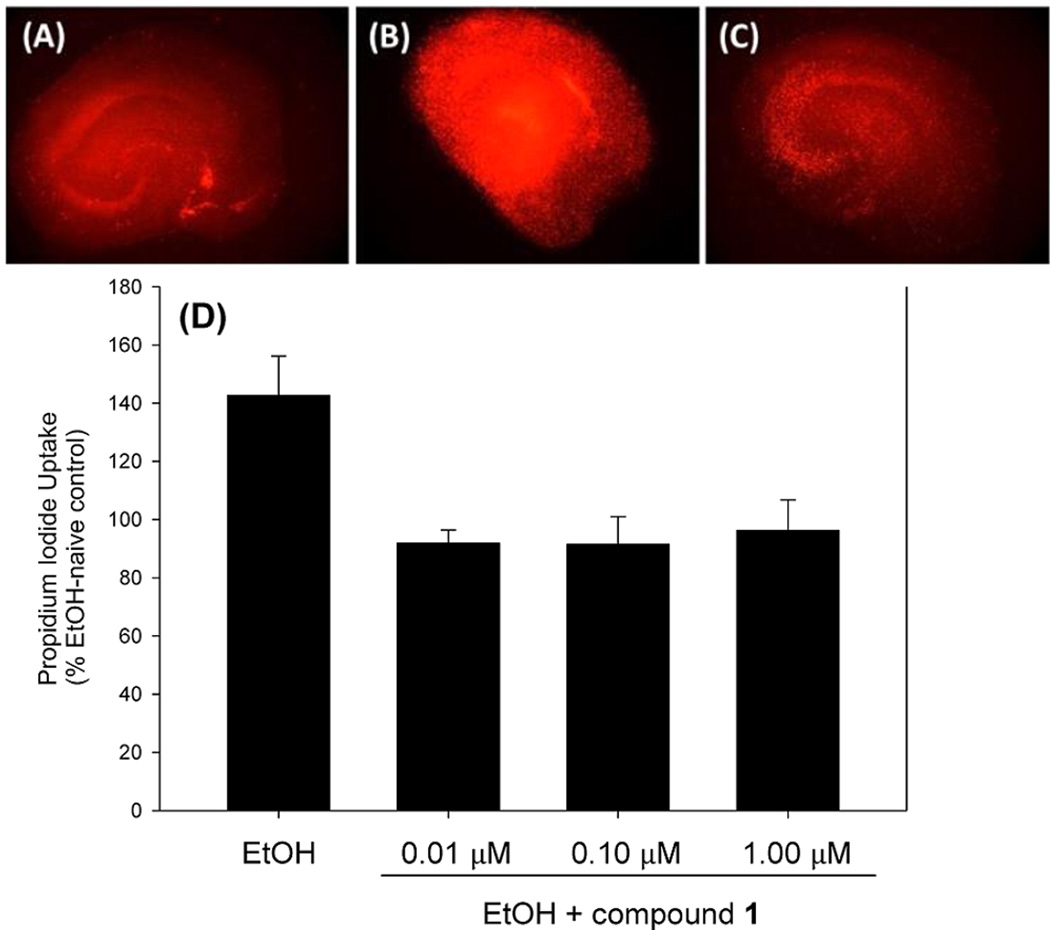
Compounds 1–3 and echoside B were subsequently evaluated in an EtOH damage neuroprotection assay using rat hippocampal-derived primary cell cultures. Forty-eight hours of exposure to 100 mM EtOH in cell culture medium produced significant increases in uptake of propidium iodide, a highly polar nucleic acid intercalating agent that labels degenerating cells in the primary neuronal and glial cell layers of hippocampal cell cultures. Mean increases of approximately 145% of control levels were observed with each replication. Exposure of EtOH naïve or EtOH-exposed cultures to compounds 2 and 3 and echoside B for the 48 h incubation period did not significantly reduce propidium iodide uptake into cultures. In contrast, coexposure of cultures to compound 1 with EtOH produced a significant reduction in propidium iodide uptake. Post hoc analysis demonstrated that this reversal of EtOH-induced propidium iodide uptake was observed with coexposure to 0.01, 0.10, and 1.0 μM compound 1 (Figure 4). Exposure of EtOH-naïve cultures to compound 1 did not alter propidium iodide uptake.
Figure 4.
EtOH damage neuroprotection assay (propidium iodide uptake in rat-derived organotypic hippocampal slice primary cell cultures): (A) DMSO control; (B) 48 h exposure to 100 mM EtOH; (C) 48 h exposure to 100 mM EtOH with 10 nM 1; (D) dose response with 48 h exposure to EtOH (100 mM) in the absence or presence of 1. *P < 0.001 vs control; **P < 0.001 vs EtOH; F(4,35) = 4.629, P < 0.01.
Protracted intake of EtOH is known to produce cytotoxicity in the central nervous system of prenatal, adolescent, and adult individuals via myriad diverse mechanisms.10 While compounds that influence inflammatory cascades and osmotic disturbance have been found to reduce this form of insult in preclinical models, none are effective in countering the adverse effects of chronic EtOH dependence in a clinical population. Additionally, loop diuretics such as furosemide produce orthostatic hypotension that may be markedly enhanced by concomitant EtOH intake,18 while drugs approved for the treatment of alcohol dependence also lack efficacy against the neurodegenerative effects of binge-like or chronic ethanol exposure. Thus, the identification of novel compounds, such as 1, with the potential to address the debilitating neurodegenerative aspects of EtOH dependence offer significant translational potential.
In summary, the discovery of 1–3 as metabolites of the coal mine fire isolate Streptomyces sp. RM-5–8 further highlights the potential for novel microbial natural product discovery from this unique ecological niche.8,19 The current study reveals the attachment of a rare unsaturated hexuronic acid (4-deoxy-α-l-threo-hex-4-enopyranuronate) to two structurally distinct classes (5-isoprenylindole-3-carboxylate and p-terphenyl). The former offers an uncommon glycosyl ester linkage, while the latter highlights two distinct terphenyl glycosyl regioisomers. As such, this study may implicate Streptomyces sp. RM-5–8 as containing a uniquely permissive glycosyltransferase of potential utility to chemoenzymatic glycodiversification efforts.20 In addition, while the genes encoding metabolites bearing a C-2″-epimer of the corresponding unsaturated hexuronic acid (the 4,5-unsaturated-α-d-mannuronic acid within capuramycins) have recently been reported,21 the biosynthetic pathway(s) for unsaturated hexuronic acid construction and/or attachment remain(s) uncharacterized. Furthermore, the demonstrated specific functional cytoprotection by 1 against EtOH toxicity in a primary brain cell culture model establishes, for the first time, the p-terphenyl scaffold as a neuroprotective agent within this context. The lack of such activity in 2 or echoside B also implicates the importance of both the specifically unique structure and corresponding regiospecificity of the attached unsaturated hexuronic acid. The fact that compounds 1–3 lacked representative activity against bacteria, fungi, or cancer cell lines (see Supporting Information) further supports the perceived specificity and limited general toxicity of 1.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by the University of Kentucky College of Pharmacy, the University of Kentucky Markey Cancer Center, and the National Center for Advancing Translational Sciences (UL1TR000117).
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental procedures and spectral data of isolated compounds. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.5b01203.
The authors declare the following competing financial interest: J.S.T. is a cofounder of Centrose (Madison, WI).
REFERENCES
- 1.Liu JK. Chem. Rev. 2006;106:2209–2223. doi: 10.1021/cr050248c. [DOI] [PubMed] [Google Scholar]
- 2.Laatsch H. AntiBase: The Natural Compound Identifier. Weinheim: Wiley-VCH; 2014. [Google Scholar]
- 3.(a) Yamazoe A, Hayashi K-i, Kuboki A, Ohira S, Nozaki H. Tetrahedron Lett. 2004;45:8359–8362. [Google Scholar]; (b) Yamazoe A, Hayashi K-i, Kepinski S, Leyser O, Nozaki H. Plant Physiol. 2005;139:779–789. doi: 10.1104/pp.105.068924. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hayashi K, Yamazoe A, Ishibashi Y, Kusaka N, Oono Y, Nozaki H. Bioorg. Med. Chem. 2008;16:5331–5344. doi: 10.1016/j.bmc.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 4.Deng J, Lu C, Li S, Hao H, Li Z, Zhu J, Li Y, Shen Y. Bioorg. Med. Chem. Lett. 2014;24:1362–1365. doi: 10.1016/j.bmcl.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 5.(a) Kamigauchi T, Sakazaki R, Nagashima K, Kawamura Y, Yasuda Y, Matsushima K, Tani H, Takahashi Y, Ishii K, Suzuki R, Koizumi K, Nakai H, Ikenishi Y, Terui YJ. Antibiot. 1998;51:445–450. doi: 10.7164/antibiotics.51.445. [DOI] [PubMed] [Google Scholar]; (b) Yun BS, Lee IK, Kim JP, Yoo ID. J. Antibiot. 2000;53:114–122. doi: 10.7164/antibiotics.53.114. [DOI] [PubMed] [Google Scholar]; (c) Burton JF, Cain BF. Nature. 1959;184:1326–1327. doi: 10.1038/1841326a0. [DOI] [PubMed] [Google Scholar]
- 6.Orner BP, Ernst JT, Hamilton AD. J. Am. Chem. Soc. 2001;123:5382–5383. doi: 10.1021/ja0025548. [DOI] [PubMed] [Google Scholar]
- 7.(a) Che Y, Marshall GR. Expert Opin. Ther. Targets. 2008;12:101–114. doi: 10.1517/14728222.12.1.101. [DOI] [PubMed] [Google Scholar]; (b) Isvoran A, Craciun D, Martiny V, Sperandio O, Miteva MA. BMC Pharmacol. Toxicol. 2013;14:31–41. doi: 10.1186/2050-6511-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Azzarito V, Long K, Murphy NS, Wilson AJ. Nat. Chem. 2013;5:161–173. doi: 10.1038/nchem.1568. [DOI] [PubMed] [Google Scholar]
- 8.(a) Shaaban KA, Wang X, Elshahawi SI, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J. Nat. Prod. 2013;76:1619–1626. doi: 10.1021/np400308w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang X, Shaaban KA, Elshahawi SI, Ponomareva LV, Sunkara M, Zhang Y, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J. Nat. Prod. 2013;76:1441–1447. doi: 10.1021/np400231r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang X, Elshahawi SI, Shaaban KA, Fang L, Ponomareva LV, Zhang Y, Copley GC, Hower JC, Zhan CG, Kharel MK, Thorson JS. Org. Lett. 2014;16:456–459. doi: 10.1021/ol4033418. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shaaban KA, Singh S, Elshahawi SI, Wang X, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. Nat. Prod. Res. 2014;28:337–339. doi: 10.1080/14786419.2013.855932. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang X, Shaaban KA, Elshahawi SI, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J. Antibiot. 2014;67:571–575. doi: 10.1038/ja.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elshahawi SI, Shaaban KA, Kharel MK, Thorson JS. Chem. Soc. Rev. 2015 doi: 10.1039/c4cs00426d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Crews FT, Nixon K. Alcohol Alcohol. (Oxford, U.K.) 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Collins MA, Neafsey EJ. Neurotox. Res. 2012;21:70–78. doi: 10.1007/s12640-011-9276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva LFO, Oliveira MLS, Philippi V, Serra C, Dai SF, Xue WF, Chen WM, O’Keefe JMK, Romanek CS, Hopps SG, Hower JC. Int. J. Coal Geol. 2012;94:206–213. [Google Scholar]
- 12.Mynderse JS, Fukuda DS, Hunt AH. J. Antibiot. 1995;48:425–427. doi: 10.7164/antibiotics.48.425. [DOI] [PubMed] [Google Scholar]
- 13.Kakinuma K, Sakagami Y. Agric. Biol. Chem. 1978;42:279–286. [Google Scholar]
- 14.Wakisaka Y, Koizumi K, Nishimoto Y, Kobayashi M, Tsuji NJ. Antibiot. 1980;33:695–704. doi: 10.7164/antibiotics.33.695. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida M, Takahashi E, Uozumi T, Beppu T. Agric. Biol. Chem. 1986;50:143–149. [Google Scholar]
- 16.(a) DeBoer C, Meulman PA, Wnuk RJ, Peterson DH. J. Antibiot. 1970;23:442–447. doi: 10.7164/antibiotics.23.442. [DOI] [PubMed] [Google Scholar]; (b) Sasaki K, Rinehart KL, Jr, Slomp G, Grostic MF, Olson EC. J. Am. Chem. Soc. 1970;92:7591–7593. doi: 10.1021/ja00729a018. [DOI] [PubMed] [Google Scholar]
- 17.Niu S, Li S, Tian X, Hu T, Ju J, Yang X, Zhang S, Zhang C. Zhongguo Zhongyao Zazhi. 2011;36:1763–1768. [PubMed] [Google Scholar]
- 18.Musini VM, Rezapour P, Wright JM, Bassett K, Jauca CD. Cochrane Database Syst. Rev. 2012;8:CD003825. doi: 10.1002/14651858.CD003825.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Shaaban KA, Singh S, Elshahawi SI, Wang X, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J. Antibiot. 2014;67:223–230. doi: 10.1038/ja.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Gantt RW, Peltier-Pain P, Singh S, Zhou M, Thorson JS. Proc. Natl. Acad. Sci. U.S.A. 2013;110:7648–7653. doi: 10.1073/pnas.1220220110. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Peltier-Pain P, Marchillo K, Zhou M, Andes DR, Thorson JS. Org. Lett. 2012;14:5086–5089. doi: 10.1021/ol3023374. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gantt RW, Peltier-Pain P, Thorson JS. Nat. Prod. Rep. 2011;28:1811–1853. doi: 10.1039/c1np00045d. [DOI] [PubMed] [Google Scholar]; (d) Gantt RW, Peltier-Pain P, Cournoyer WJ, Thorson JS. Nat. Chem. Biol. 2011;7:685–691. doi: 10.1038/nchembio.638. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zhang C, Griffith BR, Fu Q, Albermann C, Fu X, Lee IK, Li L, Thorson JS. Science. 2006;313:1291–1294. doi: 10.1126/science.1130028. [DOI] [PubMed] [Google Scholar]
- 21.Cai W, Goswami A, Yang Z, Liu L, Green KD, Barnard-Britson S, Baba S, Funabashi M, Nonaka K, Sunkara M, Morris AJ, Spork AP, Ducho C, Garneau-Tsodikova S, Thorson JS, Van Lanen SG. J. Biol. Chem. 2015 doi: 10.1074/jbc.M115.646414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.