Abstract
The meta-analysis was conducted to investigate the impact of gamma-glutamyl carboxylase (GGCX) on maintenance warfarin dose. 8 studies were included, focusing on the impact of GGCX single nucleotide polymorphisms (SNPs) on mean daily warfarin dose (MDWD). GGCX (rs699664; AA versus GG, GA versus GG, A versus GG) and GGCX (rs12714145; GA versus GG, AA versus GG, A versus GG) showed no significant differences on mean daily warfarin dose (MDWD). This meta-analysis was the first to report the relationship between GGCX SNPs and MDWD in Chinese populations. No evidence could be found in the relationship between SNPs of GGCX (rs699664 and rs12714145) and maintenance warfarin dose.
Abbreviations: CI, confidence interval; SD, standard deviation; SNPs, single nucleotide polymorphisms; GGCX, gamma-glutamyl carboxylase; CYP2C9, cytochrome P450 complex subunit 2C9; VKORC1, vitamin K epoxide reductase complex subunit 1; CYP4F2, cytochrome P450 complex subunit 4F2; EPHX1, epoxide hydro-lase 1 INR, International Normalized Ratio; MDWD, mean daily warfarin dose; WMD, weight mean difference
Keywords: Warfarin, GGCX, Gene polymorphisms, Systematic review, Meta-analysis, Chinese
Introduction
Warfarin is a coumarin anticoagulant administered via the oral route and is used widely for the treatment of thrombotic diseases. It has a narrow therapeutic window and considerable differences in dosing requirements in different ethnic groups and individuals. Hence, warfarin dose should be given according to the International Normalized Ratio (INR) (van Walraven et al., 2006). Moreover, if dosing is inappropriate, adverse effects such as severe bleeding and recurrence of thromboembolism, especially at the initial treatment phase, can occur (Wysowski et al., 2007).
Several studies have demonstrated that gene polymorphisms are significantly associated with warfarin dose (Lal et al., 2006, Pavani et al., 2012, Rathore et al., 2011). Moreover, meta-analyses have revealed the genes vitamin K epoxide reductase complex subunit 1 (VKORC1), cytochrome P450 complex subunit 2C9 (CYP2C9) and cytochrome P450 complex subunit 4F2 (CYP4F2) to be significantly associated with warfarin dose (Jorgensen et al., 2012, Liang et al., 2011). Recent studies have shown a small-to-moderate significant relationship between the gamma-glutamyl carboxylase (GGCX) genotype and warfarin dose (Chen et al., 2005, Choi et al., 2011, Guo, 2011, Huang et al., 2011, Kimura et al., 2007, King et al., 2010, Krishna Kumar et al., 2014, Liang et al., 2013, Wadelius et al., 2005, Liu, 2013).
GGCX resides in the membranes of endoplasmic reticulum and is composed of 758 amino acids (Suttie et al., 1980). It is located on chromosome 2p12 and spans a genomic region of ≈ 13 kb consisting of 15 exons (Suttie et al., 1980). GGCX is a key enzyme in the vitamin K cycle. GGCX oxidizes reduced vitamin K to vitamin K-2 and 3-epoxide while adding a carboxyl residue to the gamma carbon on selected glutamic acids; then, functional clotting factors II, VII, IX, and X and other vitamin K-dependent proteins are produced (Gage and Eby, 2004, Rost et al., 2004, Shikata et al., 2004). Researchers revealed the importance of GGCX genes in anticoagulation in 1997 (Wu et al., 1997). In 2004, Shikata et al. (2004) found that polymorphisms in the gene coding for GGCX had a significant impact on warfarin dose. Since then, several studies in various populations have found a significant difference in warfarin doses among diverse GGCX genotypes (Chen et al., 2005, Choi et al., 2011, Guo, 2011, Huang et al., 2011, Kimura et al., 2007, King et al., 2010, Krishna Kumar et al., 2014, Liang et al., 2013, Wadelius et al., 2005, Liu, 2013), but the role of diverse GGCX genotypes is controversial in Chinese population. Carriers of GGCX (rs699664) AA in a Chinese population were believed to need a higher dose of warfarin (Liang et al., 2013), but the opposite result was also found (Guo, 2011). Moreover, the result was indicated that carriers of GGCX (rs12714145) AA required a higher warfarin dose (Huang et al., 2011), but Liu YQ et al. demonstrated that carriers of GGCX (rs12714145) AA required a lower warfarin dose (Liu YQ 2013). In addition, several studies showed no association between polymorphisms in the GGCX gene and warfarin dose (Lou, 2012, Wang et al., 2008, Zhong et al., 2012).
Until now, meta-analyses have focused on the impact of CYP2C9 genotypes, VORCK1 genotypes and CYP4F2 genotypes on warfarin dose (Jorgensen et al., 2012, Liang et al., 2011, Yang et al., 2010). Moreover, meta-analyses (Jorgensen et al., 2012, Yang et al., 2010) have stated that polymorphisms of VKORC1 and CYP2C9 genes in different ethnicities are significantly different in relation to warfarin dose. Although have recently published meta-analysis on GGCX (Sun, Y., 2015), the impact of GGCX genotypes on warfarin dose in Chinese populations has not been investigated by meta-analyses and it is still controversial. Here, we investigated the effects of individual GGCX genotype on mean daily warfarin dose (MDWD) in Chinese populations by meta-analyses.
Materials and methods
Search strategy
Studies written in English were searched on PubMed, EMBASE and the Cochrane Database of Systematic Reviews databases. Studies written in Chinese were searched for in ChainInfo, Chinese Biomedical Literature Database (CBM), and Chinese National Knowledge Infrastructure (CNKI) databases, or identified through a search for print periodicals. We searched for articles published before 8 March 2015.
Keywords were “warfarin” plus any of the following: “gene”, “genotype”, “genetics”, “allele”, “polymorphism”, “variant”, “pharmacogenetics”, “γ-glutamyl carboxylase”, “gamma-glutamyl carboxylase”, “glutamate carboxylase”, and “GGCX”. If there was unclear or missing information in any of the studies, we contacted the authors via telephone or e-mail to obtain additional information. The search strategy is shown in Table 1.
Table 1.
Search strategy.
| Number | Search terms |
|---|---|
| 1 | Warfarin* |
| 2 | s-Warfarin |
| 3 | r-Warfarin |
| 4 | Coumadin |
| 5 | Marevan |
| 6 | Panwarfin |
| 7 | Gene |
| 8 | Genotype |
| 9 | Genetics |
| 10 | Allele* |
| 11 | Polymorphism* |
| 12 | Variant* |
| 13 | Pharmacogenetics |
| 14 | γ-Glutamyl carboxylase |
| 15 | Gamma-glutamyl carboxylase |
| 16 | Glutamate carboxylase |
| 17 | GGCX |
| 18 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 |
| 19 | 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 |
| 20 | 14 OR 15 OR 16 OR 17 |
| 21 | 18 AND 19 AND 20 |
Study selection
To be included, studies had to satisfy the following criteria: (i) patients received warfarin treatment; (ii) at least one of the following GGCX gene single nucleotide polymorphisms (SNPs) was tested: rs699664, rs12714145; and (iii) warfarin maintenance dose was mentioned along with the target gene(s). (iv) All patients were Chinese. There were no specific limits on the clinical characteristics (indication of warfarin, age, body weight, height) of patients, INR range, or use of other drugs. Studies were excluded if they met the following criteria: (i) articles were reviews, letters, abstracts or case reports and (ii) literatures were published repeatedly. (iii) Patients included were no Chinese population.
Data extraction
Research data were extracted and sorted by two reviewers (ZJH and TL) independently. Information such as the year of publication, indication of warfarin, target INR, male ratio, age, gene frequencies, and MWMD was extracted from selected studies. Then, ZJH and TL checked the integrity and accuracy of extracted data, and resolved differences by discussion. In case of disagreements, other researchers read the literature and decided whether or not to include the study. If the study provided median values and interquartile ranges instead of mean ± standard deviation (SD) values, we calculated approximate mean ± SD values as described by Hozo et al. (2005) and the Cochrane Handbook for Systematic Reviews of Interventions.
Quality assessment
The quality of the selected studies included was conducted by two reviewers (ZJH and TL) independently. The quality assessment criteria were based on the criteria predefined by Little et al. (2002) and was shown in Table 2. Moreover, “++” would be recorded, if the study met most of the above criteria; “+”would be recorded, if the study met some of the criteria; and “−” would be recorded, if the study met few or no criteria (Little et al., 2002).
Table 2.
Quality assessment criteria.
| 1. Analytic validity of genotyping | |
| 1.1 | Types of samples |
| 1.2 | Timing of sample collection |
| 1.3 | The genotyping method used |
| 1.4 | The quality control measures |
| 2. Selection of study subjects | |
| 2.1 | Geographic area |
| 2.2 | Recruitment period |
| 2.3 | Recruitment methods |
| 2.4 | Exclusion criteria for cases and controls, |
| 2.5 | Number of sample |
| 2.6 | Mean age (SD) or age range of study subjects |
| 2.7 | Sex ratio |
| 3. Population stratification | |
| 3.1 | Identified Potential correlates of the genotype |
| 3.2 | Taken Potential correlates into consideration in design or analysis |
| 4. Statistical issues | |
| 4.1 | No. of subjects included in the analysis |
| 4.2 | Method of analysis |
| 4.3 | Software used to perform the analysis |
| 4.4 | Confidence intervals |
Statistical analyses
In the meta-analysis, we defined carriers of rs699664 GG, rs12714145 GG as a reference group in SNP genetic models. Then, warfarin maintenance doses (mean ± SD) associated with GGCX (rs699664) and (rs12714145) genotypes were normalized using the method of Lindh et al. (2009) by dividing the mean maintenance dose in each reference group. We also defined carriers of GGCX rs699664 GA or GGCX rs699664 AA as “rs699664 A carriers”, carriers of GGCX (rs12714145) GA or GGCX (rs12714145) AA as “rs12714145 A carriers”, and conducted the normalization procedure described above (Liang et al., 2011, Yang et al., 2010).
Comparison of the normalized warfarin dose needed in each SNP between the reference group and non-reference group was conducted. In polymorphisms of the GGCX (rs699664) gene, the normalized warfarin dose of GA carriers, AA carriers, or A carriers was compared with those of GG carriers. Similarly, the normalized warfarin dose of carriers of GGCX (rs12714145) GA, GGCX (rs12714145) AA, and GGCX (rs12714145) A was compared with those of carriers of GGCX (rs12714145) GG. The weight mean difference (WMD) obtained for each comparison did not indicate absolute but instead relative differences in maintenance dose for the normalization procedure. That is, a mean difference of 0.1 indicated an increase in warfarin dose of 10% (Lindh et al., 2009).
Revman v5.2.3 (Cochrane Collaboration) was applied to assess the relationship between gene polymorphisms and MDWD. The method of inverse variance was applied to “weight” each study, and was inversely proportional to the size of the SD value. WMD was used to express the effect of polymorphisms of the GGCX gene in each Forest plot. Summing up each WMD was equal to the total WMD, thereby reflecting the total influence on warfarin dose in the studies included in the meta-analysis. Heterogeneity in each genetic group of the meta-analysis was tested using Cochran's Q test (Mantel–Haenszel chi-square test) and expressed by p or I2 values. If I2 > 50%, heterogeneity was demonstrated (Jorgensen et al., 2012), and a random-effects model or fixed-effect model was chosen. Significance was assessed using the Z test. If p < 0.05, the difference in each comparison of GGCX SNPs was significant (Jorgensen et al., 2012).
Sensitivity analyses were conducted to ascertain if the results were stable and to discover the source of heterogeneity, thereby deleting the included studies one-by-one in a certain order.
In the meta-analysis, Stata SE12.0 was also used to conduct Begg's test (Begg and Mazumdar, 1994), Egger's test (Egger et al., 1997) and regression analyses. Potential publication bias was examined by the latter two methods. Regression analyses were conducted to find the source of heterogeneity, including: year of publication, number of participants, male ratio, mean age, interaction of drugs. In regression analyses, “1” was assigned if interacting drugs were used in participants, otherwise “0” was assigned.
Results
Identification of studies
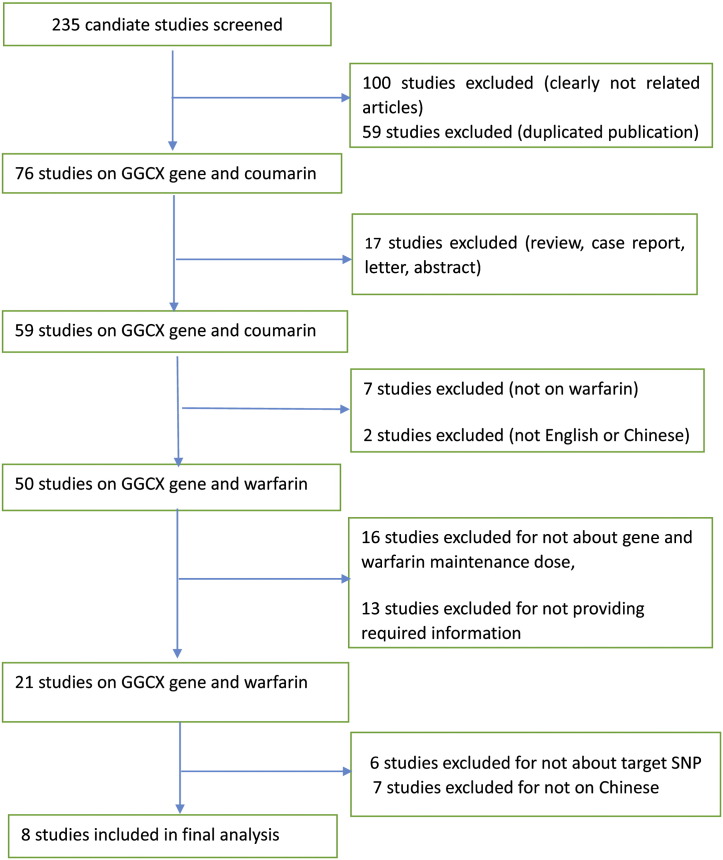
The process of literature screening is illustrated in Fig. 1. Initially, 235 studies were retrieved. Of these, only 8 eligible studies involving 3258 patients were included. Among them, one study (Zhong et al., 2012) included in the meta-analysis provided median values and interquartile ranges instead of mean ± SD values. We estimated mean ± SD values by referring to the formula in Cochrane Handbook for Systematic Reviews of Interventions and described by Hozo et al. (2005) to avoid bias.
Fig. 1.
Process of literature screening.
Study characteristics
Of the eligible studies, four studies (Guo, 2011, Lou, 2012, Liu, 2013, Wenhui et al., 2014) were in Chinese, and the other four were in English. Moreover, seven studies focused on analyses of the GGCX (rs699664) polymorphism (Liu Y, et al., 2010; Guo, 2011, Zhong et al., 2012, Lou, 2012, Liu, 2013, Wenhui et al., 2014, Li et al., 2015) and three studies on GGCX (rs12714145) (Guo, 2011, Huang et al., 2011, Liu, 2013). The quality scores of all included studies were “++”. Characteristics of the included studies are shown in Table 3.
Table 3.
Characteristics of the studies included in the meta-analysis.
| STUDY |
INR target range |
Indication of warfarin |
Number |
Male ratio (%) |
Mean ages (years) |
Frequency (%) |
Quality score |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 699664 |
rs12714145 |
|||||||||||
| GG | GA | AA | GG | GA | AA | |||||||
| Lou Y 2012 | 1.5–3.0 | HVR,AF,PE | 488 | 44.7 | 56.7 ± 12.3 | 45.5 | 42 | 12.5 | ++ | |||
| Guo G 2011 | 1.5–3.0 | HVR | 300 | 46 | 47.9 ± 12.5 | 42.5 | 48.93 | 8.57 | 36.75 | 51.59 | 11.66 | ++ |
| Liu YQ 2013 | 1.5–2. 5 | HVR | 176 | 46.6 | 44.44 ± 12.33 | 31.8 | 51.1 | 17.1 | 31.8 | 51.1 | 17.1 | ++ |
| Huang S 2011 | 1.8–3.0 | AF,HVR, DVT | 217 | 41.5 | 51.3 ± 7.5 | − | − | − | 38.7 | 53.9 | 7.4 | ++ |
| LI Wenhui 2014 | 1.5–3.0 | HVR | 280 | 49.3 | 56.76 ± 11.59 | 42.5 | 48.93 | 8.57 | − | − | − | ++ |
| Liu Y 2010 | 1.8–3.0 | HVR | 794 | 43.7 | 46.82 ± 11.95 | 45.4 | 45.7 | 9 | − | − | − | ++ |
| Li S 2015 * | 1.5–2.5 | HVR | 158 | 26.4 | 47.65 ± 11.20 | 39.2 | 45.6 | 15.2 | − | − | − | ++ |
| Zhong S 2012 | 1.8–3.0 | HVR | 845 | 56.6 | 47.9 | 45.3 | 45.3 | 8.9 | − | − | − | ++ |
AF = Atrial fibrillation, DVT = deep vein thrombosis, PE = Pulmonary Embolism, HVR = heart valve replacement.
“−”meaning no data.“*” meaning warfarin dose (md/week).
Meta analyses
GGCX (rs699664) polymorphism and warfarin dose
First, the relationship between polymorphisms of the GGCX (rs699664) gene and warfarin dose was analyzed (Fig. 2). Compared with GG carriers, no significant difference was found in each comparison of GGCX (rs699664) (GA versus GG: p = 0.88; WMD = 0.00; 95% CI: − 4% to 5%; AA versus GG: p = 0.86; WMD = − 0.01; 95% CI: − 13% to 11%; A versus GG: p = 0.60; WMD = 0.02; − 4% to 7%). Significant heterogeneity was detected by the Q-test and I2 in each meta-analysis in polymorphisms of the rs699664 gene (p < 0.05, I2 > 50% for all), so a random-effects model was used).
Fig. 2.
Forest plots were used to express the influence of polymorphisms of the GGCX (rs699664) gene upon warfarin dose. The warfarin dose required for carriers of (I) rs699664 AA versus carriers of rs699664 GG, (II) rs699664 GA versus carriers of rs699664 GG, and (III) rs699664 A (rs699664 GA or rs699664 AA) versus carriers of rs699664 GG is shown. Mean (SD): mean ± standard deviation of normalized warfarin doses associated with each genotype. WMD: weight mean difference; and CI: confidence interval.
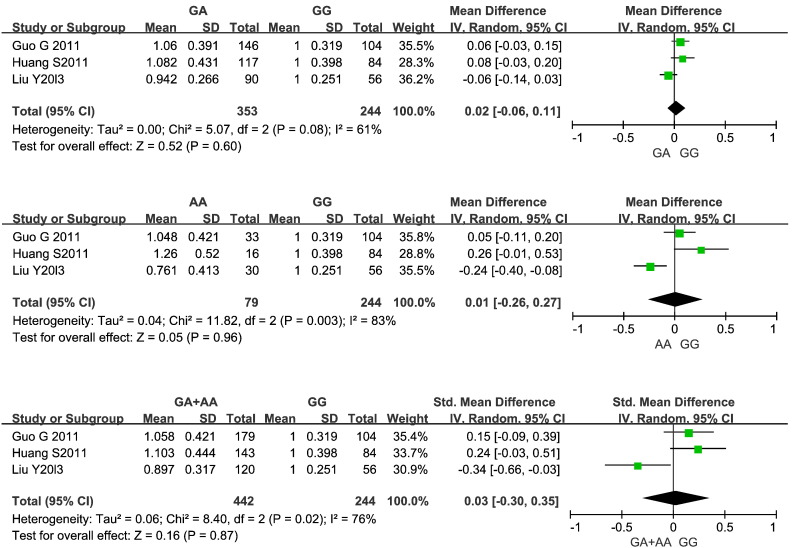
GGCX (rs12714145) polymorphism and warfarin dose
Results of each meta-analysis on groups of GGCX (rs12714145) genotypes are shown in Fig. 3. No significant difference was found in each comparison of GGCX (rs12714145) genotype groups (GA versus GG: p = 0.60; WMD = 0.02; 95% CI: − 6% to 11%; AA versus GG: p = 0.96; WMD = 0.01; − 26% to 27%; A versus GG: p = 0.87; WMD = 0.03; − 30% to 35%). Due to significant heterogeneity detected by the Q-test and I2 (p < 0.05, I2 > 50% for all), a random-effects model was applied in each comparison for polymorphisms of the rs12714145 gene.
Fig. 3.
Forest plots were used to express the influence of polymorphisms of the GGCX (rs12714145) gene upon warfarin dose. The warfarin dose required for carriers of (I) rs12714145 GA versus carriers of rs12714145 GG, (II) rs12714145 AA versus carriers of rs12714145 GG, and (III) rs12714145 A (rs12714145 GA or rs12714145 AA) versus carriers of GG is shown. Mean (SD): mean ± standard deviation of normalized warfarin doses associated with each genotype. WMD: weight mean difference; and CI: confidence interval.
Sensitivity analyses
Sensitivity analyses were conducted by reducing one of the studies at a time in a certain order first. The following results were found: (i) except for rs12714145 (A versus GG), heterogeneity in each meta-analysis could not be removed; and (ii) there was no one but GGCX (rs12714145 A versus GG) to be found significant difference for revision between results and original outcomes. When we deselected the study of Liu Y (Y Liu YQ, 2013), significance was detected by the Z test (p > 0.05) in the comparison of rs12714145 A versus GG.
Meta-regression analyses were conducted to examine the influence of potential sources of heterogeneity. No significant association was found between clinical characteristics and heterogeneity in studies (all p > 0.05). Data are shown in Table 4.
Table 4.
Results of meta-regression analyses.
| _ES | Coef. | Std. err. | t | P > t | I squared_res (%) | Adj R-squared (%) |
|---|---|---|---|---|---|---|
| Year | − 0.01 | 0.0243 | − 0.41 | 0.7 | 82.33 | − 19.11 |
| Number | − 0.0001 | 0.0001 | 0.62 | 0.562 | 79.59 | − 15.31 |
| Mrate | − 0.0029 | 0.0043 | 0.68 | 0.528 | 79.9 | − 12.44 |
| Mage | − 0.0033 | 0.0081 | − 0.41 | 0.698 | 82.08 | − 21.55 |
| Comedication | 0.1221 | 0.0673 | 1.81 | 0.129 | 68.49 | 38.83 |
Publication bias
Publication bias for the association between GGCX and warfarin dose in each meta-analysis as identified by Begg's test or Egger's test (data not shown) was not observed.
Discussion
Warfarin is the most common anticoagulant drug. It is used widely in patients who have undergone heart-valve replacement, or in those suffering from deep-vein thrombosis or pulmonary embolism. However, warfarin use is limited because: (i) it has a narrow therapeutic window; (ii) dose requirements vary widely in different ethnic groups and individuals; and (iii) of adverse effects (thromboembolism, bleeding events) due to under-dosing or over-dosing of warfarin. Hence, individual dose adjustments are a challenge in the clinical application of warfarin.
The influence of genes on warfarin dose has been used to explain individual variability. Polymorphisms of CYP2C9 and VKOCR1 genes are significantly associated with warfarin maintenance dose, which could explain more than half of the variability in warfarin dose, in combination with clinical factors such as age, ethnicity, smoking status, and interaction with other drugs (Gage and Lesko, 2008, Wadelius et al., 2005). Moreover, the US Food and Drug Administration (FDA) has suggested use of an equation that includes genetic factors (VKORC1 and CYP2C9 genes) and clinical characteristics to predict the warfarin maintenance dose, which could explain 53% of the variability in the warfarin dose (Gage and Lesko, 2008). In recent years, pharmacogenetic models have suggested that additional genetic factors are present that could influence warfarin dose (Gage and Lesko, 2008). Moreover, GGCX genetic variations have been postulated to have a profound influence on the anticoagulation action of warfarin and contribute to individual variability in Chinese population (Yiu YQ, 2013; Wenhui et al., 2014). But Li S et al. confirmed that polymorphism of the GGCX gene did not affect the maintenance dose of warfarin (Li S et al., 2015). Recently, Sun Y et al. indicated that GGCX may be one of factors affecting the dose of warfarin requirement in different ethnicities (Sun et al., 2015), while, whether the GGCX gene should be used to predict warfarin dose in Chinese population is controversial. Thus, conducting a meta-analysis focusing on polymorphisms of the GGCX gene and inter-individual warfarin dose in Chinese population would be important. We analyzed the relationship between GGCX polymorphisms and warfarin maintenance dose to predict the warfarin dose more accurately.
In our meta-analysis, GGCX (rs699664; AA versus GG, GA versus GG, A versus GG) and GGCX (rs12714145; GA versus GG, AA versus GG, A versus GG) showed no significant differences on MDWD. These results were stable and reliable, involving 3258 patients.
Meanwhile, GGCX (rs11676382) and GGCX (rs6738645) genes were also proved to be associated with warfarin dose. GGCX (rs11676382) CG carriers required lower warfarin dose than GGCX (rs11676382) CC carriers (Guo, 2011), in which the result was consistent with other ethnic groups (Sun et al., 2015), and GGCX (rs6738645) GG required lower warfarin dose than GGCX (rs6738645) CC carriers (Liu et al., 2014). While, only one study referred to GGCX (rs11676382) and GGCX (rs6738645), respectively. GGCX (rs11676382) and GGCX (rs6738645) probably influenced warfarin dose in Chinese. More studies were needed in the research on GGCX (rs11676382) and GGCX (rs6738645) in Chinese.
Recently, Sun et al. indicated that GGCX rs11676382 polymorphism may be one of factors affecting the dose of warfarin requirement in Caucasian (Sun et al., 2015). However, there were not enough evidence indicating that GGCX gene polymorphism could affect warfarin maintain dose in our meta-analysis. Namely, it is still uncertain whether GGCX gene polymorphism is used in the clinical to estimated warfarin maintain dose in Chinese population. More studies in Chinese are needed to explore the relationship between warfarin maintain dose and GGCX gene polymorphism.
We conducted sensitivity analyses and except for rs12714145 (A versus GG), heterogeneity in each meta-analysis could not be removed and no significant difference for revision were found between the results and original outcomes in polymorphisms of GGCX (rs699664) or GGCX (rs12714145) genes. More studies are required to identify the role of polymorphisms of the GGCX (rs12714145) gene and individual variations in warfarin dose.
Meta-regression analyses were conducted to discover the variables associated with heterogeneity. No significant association between the examined clinical characteristics and heterogeneity in the included studies was found. We tried to carry out subgroup analyses to ascertain the influence of clinical factors such as sex, age, body surface area, weight, indication of warfarin, and interaction between drugs because clinical factors have been shown to have a significant association with warfarin dose (Gage and Lesko, 2008, Choi et al., 2011, Zhong et al., 2012, Liu, 2013). However, only one study (Liu YQ, 2013) in the meta-analysis demonstrated a relationship between clinical factors such as different age, sex, indication of warfarin, interaction between drugs and warfarin dose by providing specific data. Therefore, we could not conduct subgroup analyses according to the factors described above.
Recently, researchers have shown that included VKORC1, CYP2C9 and CYP4F2, GGCX and EPHX1 genes can influence the warfarin dose significantly (Jorgensen et al., 2012, Liang et al., 2011, Lindh et al., 2009, Sun et al., 2015, Yang et al., 2010). Moreover, the International Warfarin Pharmacogenetics Consortium (Klein et al., 2009) and FDA (Gage and Lesko, 2008) have suggested other candidate genes such as GGCX have been shown to have a significant effect on warfarin dose, and have been suggested to be used to estimate warfarin dosage using Gage's algorithm for warfarin dosing (available at www.WarfarinDosing.org). Also, other candidate genes have been investigated and might influence warfarin dose, but the relationship between apolipoprotein E and warfarin dose is controversial (Kimmel et al., 2008, Kohnke et al., 2005, Lal et al., 2008, Liang et al., 2013). To estimate the warfarin dose more accurately, more studies are needed to ascertain the influence of other candidate genes.
Our meta-analysis had several advantages. First, this was the first meta-analysis focusing on the influence of polymorphisms of the GGCX gene upon warfarin dose in Chinese population. Second, no publication bias was identified according to Begg's test and Egger's regression test. Third, selection bias was avoided because studies were searched comprehensively in several databases. Finally, no significant difference or need for revision was found in the results for polymorphisms of GGCX (rs699664) and suggesting that our results were stable.
Our meta-analysis had several disadvantages. First, our meta-analysis was based on experimental studies and we could not strictly control the quality of included studies. Second, we converted non-normally distributed statistics (median range) to normally distributed statistics (mean ± SD), which could have been a cause of bias in our meta-analysis. Third, only one eligible study referred to polymorphisms of the GGCX (rs11676382) and GGCX (rs6738645) genes respectively, so we didn't conduct meta-analyses.
Conclusion
This was the first meta-analysis focusing on the influence of GGCX polymorphisms on warfarin dose in Chinese population. We showed that polymorphisms of GGCX (rs699664) and GGCX (rs12714145) genes may not be associated with variation of warfarin dose between individuals. In addition to the dosing algorithm for warfarin in Chinese, addition of genotyping of polymorphisms of GGCX in pharmacogenetic algorithms, larger sample sizes, and more ethnic groups are required to support our hypothesis that polymorphisms of the GGCX gene influence warfarin dose.
Funding sources
This work was supported by grants from the National Natural Science Foundation of China (81370629), National and Fujian Provincial Key Clinical Specialty Discipline Construction Program, P.R.C, the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 2015B001) and the Fujian Medical Innovation Project (No. 2014-CX-18).
Potential conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We would like to thank the native English speaking scientists of Elixigen Company for editing our manuscript.
References
- Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Chen L.Y., Eriksson N.R.G., Bentley D., Deloukas P., M W. Gamma-glutamyl carboxylase (GGCX) microsatellite and warfarin dosing. Blood. 2005;106:3673–3674. doi: 10.1182/blood-2005-04-1711. [DOI] [PubMed] [Google Scholar]
- Choi J.R. Proposal of pharmacogenetics-based warfarin dosing algorithm in Korean patients. J. Hum. Genet. 2011;56:290–295. doi: 10.1038/jhg.2011.4. [DOI] [PubMed] [Google Scholar]
- Egger M. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage B.F., Eby C.S. The genetics of vitamin K antagonists. Pharmacogenomics J. 2004;4:224–225. doi: 10.1038/sj.tpj.6500258. [DOI] [PubMed] [Google Scholar]
- Gage B.F., Lesko L.J. Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J. Thromb. Thrombolysis. 2008;25:45–51. doi: 10.1007/s11239-007-0104-y. [DOI] [PubMed] [Google Scholar]
- Guo G. Kunming Medical College; 2011. Yunnan Han population GGCX gene polymorphism and warfarin dose study of the correlation. [Google Scholar]
- Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.W. Influence of GGCX genotype on warfarin dose requirements in Chinese patients. Thromb. Res. 2011;127:131–134. doi: 10.1016/j.thromres.2010.10.027. [DOI] [PubMed] [Google Scholar]
- Jorgensen A.L. Influence of CYP2C9 and VKORC1 on patient response to warfarin: a systematic review and meta-analysis. 2012;7:1–20. doi: 10.1371/journal.pone.0044064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel S.E. Apolipoprotein E genotype and warfarin dosing among Caucasians and African Americans. Pharmacogenomics J. 2008;8:53–60. doi: 10.1038/sj.tpj.6500445. [DOI] [PubMed] [Google Scholar]
- Kimura R. Genotypes of vitamin K epoxide reductase, gamma-glutamyl carboxylase, and cytochrome P450 2C9 as determinants of daily warfarin dose in Japanese patients. Thromb. Res. 2007;120:181–186. doi: 10.1016/j.thromres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- King C.R. Gamma-glutamyl carboxylase and its influence on warfarin dose. Thromb. Haemost. 2010;104:750–754. doi: 10.1160/TH09-11-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T.E. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnke H. Warfarin dose related to apolipoprotein E (APOE) genotype. Eur. J. Clin. Pharmacol. 2005;61:381–388. doi: 10.1007/s00228-005-0936-3. [DOI] [PubMed] [Google Scholar]
- Krishna Kumar D. Effect of CYP2C9, VKORC1, CYP4F2 and GGCX genetic variants on warfarin maintenance dose and explicating a new pharmacogenetic algorithm in South Indian population. Eur. J. Clin. Pharmacol. 2014;70:47–56. doi: 10.1007/s00228-013-1581-x. [DOI] [PubMed] [Google Scholar]
- Lal S. Pharmacogenetics of target genes across the warfarin pharmacological pathway. Clin. Pharmacokinet. 2006;45:1189–1200. doi: 10.2165/00003088-200645120-00004. [DOI] [PubMed] [Google Scholar]
- Lal S. Influence of APOE genotypes and VKORC1 haplotypes on warfarin dose requirements in Asian patients. Br. J. Clin. Pharmacol. 2008;65:260–264. doi: 10.1111/j.1365-2125.2007.03053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Warfarin dosage response related pharmacogenetics in Chinese population. PLoS ONE. 2015;10:e0116463. doi: 10.1371/journal.pone.0116463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R. Influence of CYP4F2 genotype on warfarin dose requirement—a systematic review and meta-analysis. Thromb. Res. 2011;130:38–44. doi: 10.1016/j.thromres.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Liang Y. Association of genetic polymorphisms with warfarin dose requirements in Chinese patients. Genet. Test. Mol. Biomarkers. 2013;17:932–936. doi: 10.1089/gtmb.2013.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh J.D. Influence of CYP2C9 genotype on warfarin dose requirements—a systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2009;65:365–375. doi: 10.1007/s00228-008-0584-5. [DOI] [PubMed] [Google Scholar]
- Little J., Bradley L., Bray M.S., Clyne M., Dorman J., DL E. Reporting, appraising, and integrating data on genotype prevalence and gene–disease associations. Am. J. Epidemiol. 2002;156:300–310. doi: 10.1093/oxfordjournals.aje.a000179. [DOI] [PubMed] [Google Scholar]
- Liu Y.Q. Research on correlation between GGCX (rs6738645) polymorphism and warfarin stable dose. Chongqing Med. 2014;43:1184–1186. [Google Scholar]
- Liu Y.Q. Kunming Medical University; 2013. The Research of Individualized Anticoagulation for Warfarin After Heart Valve Replacement. [Google Scholar]
- Lou Y. Peking Union Medical College Chinese Academy of Medical Sciences Tsinghua University; 2012. The Study of Warfarin Maintenance Dose Algorithm in Chinese Han Population. [Google Scholar]
- Pavani A., Naushad S.M, Mishra R.C., Malempati A.R., Pinjala R., Kumar T.R., Kutala V.K. Retrospective evidence for clinical validity of expanded genetic model in warfarin dose optimization in a South Indian population. Pharmacogenomics. 2012;13:869–878. doi: 10.2217/pgs.12.62. [DOI] [PubMed] [Google Scholar]
- Rathore S.S., Agarwal S.K., Pande S., Singh S.K., Mittal T., Mittal B. Pharmacogenetic aspects of coumarinic oral anticoagulant therapies. Indian J. Clin. Biochem. 2011;26:222–229. doi: 10.1007/s12291-011-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost S., Fregin A., Koch D., Compes M., Muller C.R., Oldenburg J. Compound heterozygous mutations in the gamma-glutamyl carboxylase gene cause combined deficiency of all vitamin K-dependent blood coagulation factors. Br. J. Haematol. 2004;126:546–549. doi: 10.1111/j.1365-2141.2004.05071.x. [DOI] [PubMed] [Google Scholar]
- Shikata E., Ieiri I., Ishiguro S., Aono H., Inoue K., Koide T., Ohgi S., Otsubo K. Association of pharmacokinetic (CYP2C9) and pharmacodynamic (factors II, VII, IX, and X; proteins S and C; and gamma-glutamyl carboxylase) gene variants with warfarin sensitivity. Blood. 2004;103:2630–2635. doi: 10.1182/blood-2003-09-3043. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wu Z., Li S., Qin X., Li T., Xie L., Deng Y., Chen J. Impact of gamma-glutamyl carboxylase gene polymorphisms on warfarin dose requirement: a systematic review and meta-analysis. Thromb. Res. 2015:737–747. doi: 10.1016/j.thromres.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Suttie J.W., Canfield L.M., Shah D.V. Microsomal vitamin K-dependent carboxylase. Methods Enzymol. 1980;67:180–185. doi: 10.1016/s0076-6879(80)67025-6. [DOI] [PubMed] [Google Scholar]
- van Walraven C., Jennings A., Oake N., Fergusson D., Forster A.J. Effect of study setting on anticoagulation control: a systematic review and metaregression. Chest. 2006;129:1155–1166. doi: 10.1378/chest.129.5.1155. [DOI] [PubMed] [Google Scholar]
- Wadelius M. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–270. doi: 10.1038/sj.tpj.6500313. [DOI] [PubMed] [Google Scholar]
- Wang T.L. Genetic factors contribute to patient-specific warfarin dose for Han Chinese. Clin. Chim. Acta. 2008;396:76–79. doi: 10.1016/j.cca.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Wenhui L., Ying X.B., Qiang D.J., Ren L., Ying L.S., Gang G. Relationship between genetic polymorphism of GGCX (rs699664) and warfarin dose requirements in Yunnan Han population. Mod. Diagn. Treat. 2014;25:3121–3123. [Google Scholar]
- Wu S.-M. Genomic sequence and transcription start site for the human g-glutamyl carboxylase. Am. Soc. Hematol. 1997;89:4058–4062. [PubMed] [Google Scholar]
- Wysowski D.K., Nourjah P., Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch. Intern. Med. 2007;167:1414–1419. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- Yang L., Ge W., Yu F., Zhu H. Impact of VKORC1 gene polymorphism on interindividual and interethnic warfarin dosage requirement—a systematic review and meta analysis. Thromb. Res. 2010;125:e159–e166. doi: 10.1016/j.thromres.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Zhong S.L. Integrating interacting drugs and genetic variations to improve the predictability of warfarin maintenance dose in Chinese patients. Pharmacogenet. Genomics. 2012;22:176–182. doi: 10.1097/FPC.0b013e32834f45f9. [DOI] [PubMed] [Google Scholar]