Abstract
The importance of antimicrobial stewardship is increasingly recognized, yet data from community hospitals are limited. Despite an initially low acceptance rate, an Infectious Diseases physician-led program at a 70-bed rural hospital was associated with a 42% decrease in anti-infective expenditures and susceptibility improvement in Pseudomonas aeruginosa over 3 years.
Keywords: antimicrobial resistance, antimicrobial stewardship program, ASP, community hospital, value of infectious diseases physician
Antimicrobial stewardship programs (ASPs) in large medical centers have demonstrated repeated success at decreasing the development of antimicrobial resistance, guiding appropriate antibiotic use, and reducing healthcare costs [1, 2]. However, given that 45% of hospitals in the United States have fewer than 100 beds, there is clearly a need for community hospital stewardship [3]. The establishment of an ASP in a community hospital can be challenging due to the lack of multidisciplinary resources. The current literature describing successful community antimicrobial stewardship involves pharmacist-led programs, often with infectious disease (ID) training [4–6]. The value of an ID physician outside of patient care is widely recognized, but there are minimal data regarding their role in small community hospitals [7]. In addition, the impact of an ASP on antimicrobial resistance in the community hospital setting is largely unknown. In this study, we describe the successful implementation of a physician-led ASP without formal involvement of a clinical pharmacist at a 70-bed, community hospital and its impact on antimicrobial resistance.
Setting
This study was conducted at a 70-bed community, nonteaching hospital with 3 operating rooms and 6 intensive care beds located in a rural Virginia community approximately 50 miles from the University of Virginia Medical Center (UVaMC). The majority of patients were admitted to a hospitalist service (other inpatient services were surgical subspecialties, physicians with outpatient practices, pediatrics, and obstetrics). The ASP was established in February of 2010 by a board certified ID physician who also served as medical director of the UVaMC ASP.
The Program
The ASP physician leader (PL) was hired by the community hospital to establish an ASP and as a financial pro forma and paid a flat fee for annual services to the University of Virginia Department of Medicine at an annual rate of $75 000 beginning 1 January 2010 and would escalate by 5% on the anniversary date with an initial 2-year commitment, which was later extended. The PL traveled to the community hospital once weekly and reviewed electronic medical records for all patients receiving systemic antimicrobials; this included physician notes, laboratory/radiology data, and selection and duration of antimicrobials. Interventions were documented prospectively and maintained independent of the medical record. The PL provided face-to-face recommendations to hospitalists, inpatient nurse practitioners, and other providers as indicated in addition to visiting Clinical Microbiology and Pharmacy to discuss any ongoing issues. The PL served as chair and member of the Infection Prevention and Control (IP&C) and Pharmacy and Therapeutics committees, respectively. Other duties included assistance in the design of order sets, which included antimicrobials, leadership for antimicrobial formulary changes, and providing an annual lecture on antimicrobial selection. The PL was available by pager to medical staff during most days of the year and frequently took questions by phone regarding antimicrobial selection, but formal consults were not performed. For the majority of the study period, there was no onsite ID-trained physician when the PL was not present.
Analysis
A recommendation acceptance rate was estimated by retrospective, in-depth chart audit of interventions using ASP documentation as well as pharmacy records and physician notes. Thirty-five cases were reviewed beginning in April of each year (2010, 2011, 2012) to determine whether ASP recommendations were accepted, either in full or partially. The rate was calculated (intervention accepted (partial or full)/total reviewed interventions) for each year. Adverse outcomes from partially or fully accepted interventions were also examined. Interventions were categorized as one or more of the following: discontinue antibiotics, eliminate duplicate therapy, narrow spectrum, broaden spectrum, change intravenous (IV) to oral (PO), duration of therapy, and additional non-antimicrobial interventions (laboratory/radiographic testing, specialty consultation [including a formal Infectious Diseases Consultation, which often required transfer], or transfer). Susceptibility for Pseudomonas aeruginosa was determined using the VITEK2-automated system and breakpoints per the Clinical and Laboratory Standards Institute for the first isolate per patient per year and represented inpatient and outpatient cultures. Antibiotic susceptibility changes were defined as statistically significant (P < .05) by χ2 analysis for any organism and antibiotic combination [8, 9]. Antimicrobial costs for all systemic antibacterials and antifungals from January 2008 to December 2012 were obtained from pharmacy dispensing records. Expenditures were calculated per patient day for each year, and cost savings were determined by calculating antimicrobial expenditure savings for 2011 and 2012 compared to 2010. Daily-defined dose per 1000 patient days (DDD/1000) were calculated for each antibiotic using the 2015 World Health Organization DDD guidelines [10].
RESULTS
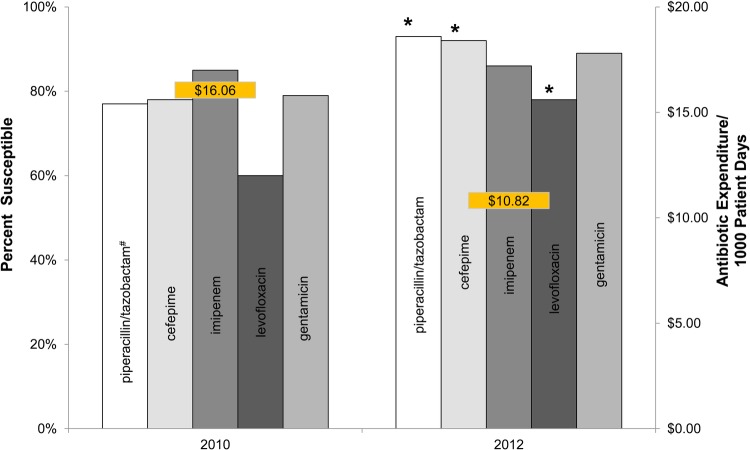
One thousand two hundred seventeen documented interventions were performed (mean 34/month) over 36 months. From the 105 thoroughly reviewed charts the acceptance rate was 40% (11 full, 3 partial/35) in 2010, 80% (22 full, 6 partial/35) in 2011, and 89% (27 full, 4 partial/35) in 2012 (2010 [14 of 35] vs 2011 [28 of 35]; P = .001). The most common recommendations were to narrow spectrum (27%), discontinue antibiotics (21%), and additional non-antimicrobial interventions (laboratory/radiographic testing, specialty consultation [including a formal Infectious Diseases Consultation, which often required transfer], or transfer) (20%). Other interventions included broaden spectrum (15%), eliminate duplicate therapy (9%), duration of therapy (8%), and change IV to PO (2%). No adverse outcomes were identified in any of the cases reviewed when ASP recommendations were accepted. Significant improvement in antimicrobial susceptibilities were observed for P aeruginosa from 2010 to 2012 (n = 81 and 99 for respective years; 2009 data were used for piperacillin/tazobactam because of manufacture susceptibility issue in 2010, n = 70) for levofloxacin (18% change), piperacillin/tazobactam (16% change), and cefepime (14% change) (P ≤ .01 for all; Figure 1). Although no other significant susceptibility changes to intravenous antibiotics were seen during this time, the total number of clinical vancomycin-resistant Enterococcus isolates decreased (2010 [46 isolates/13 380 patient days] vs 2012 [12 of 11 381]; P = .0002). Dose per 1000 patient days for all antimicrobials decreased from 1092 in 2010 to 1014 in 2012. A decrease in DDD/1000 was seen for levofloxacin (172 in 2010, 145 in 2012), piperacillin/tazobactam (158 in 2010, 81 in 2012), and doripenem (23 in 2010, 9 in 2012). An increase in DDD/1000 was seen with cefepime (30 in 2010, 92 in 2012), although cefepime was only added to formulary mid-2010. A decrease in DDD/1000 was also seen with metronidazole (80 in 2010, 50 in 2012) and linezolid (74 in 2010, 12 in 2012) with minimal increase in vancomycin (95 in 2010, 102 in 2012). Anti-infective costs per patient day decreased from $16.06 in 2010 to $11.90 in 2011 and $10.82 in 2012 (Figure 1). The anti-infective expenditures decreased by 32% and 42% in years 2 and 3, respectively, for a total savings of $161 251, which was less than the annual PL fee for services during those years.
Figure 1.
Antimicrobial susceptibility of Pseudomonas aeruginosa with corresponding antimicrobial expenditures of first year compared to third year after establishment of a community antimicrobial stewardship program. Left axis is percentage of organism susceptible from corresponding annual antibiogram. Right axis is annual antimicrobial expenditure in dollar per patient day. *Statistically significant difference in antimicrobial susceptibility from 2010 to 2012 (P ≤ .01); #Piperacillin/tazobactam from 2009 due to unavailable results in 2010.
DISCUSSION
In this study, we describe a physician-led ASP at a 70-bed rural community hospital that demonstrated a decrease in antimicrobial use with associated cost savings and associated with improved antimicrobial sensitivities to P. aeruginosa. Many community hospitals, including this one, may not be able to find and support a clinical pharmacist with the expertise who desires to work in a small hospital and lead an ASP. We describe a potential alternative model where the physician does the review and intervention and is supported by partnership with the pharmacy staff. Having an ID PL may have provided the program with several advantages. One advantage was the ability of the PL to develop trust with local physicians. Several of the admitting physicians completed training before the role of clinical pharmacists and antibiotic stewardship programs were widely recognized. The commitment of the PL to travel to the community hospital despite remote availability of the EMR permitted in-person discussion and contributed to a perceived high level of commitment to the ASP, hospital, and medical staff. The PL also participated in hospital committees, thereby providing an opportunity to interface with other physicians and quality leaders. Another advantage was the PL's ability to give non-antimicrobial-focused recommendations including additional tests, suggest specialty consults, and help facilitate patient transfers to tertiary care hospitals. These suggestions were well received despite the absence of formal consultation from PL responsibilities.
Postprescription audit and feedback were believed to be the most effective stewardship methods in this setting because it provided the opportunity for real-time, face-to-face education. Preauthorization was avoided because antimicrobial stewardship was unfamiliar to the medical staff and ID consults, which is often suggested when there is disagreement, were not generally available. One limitation of postprescription audit was that only patients on antimicrobials were reviewed. However, dialogue and availability of the PL to the microbiology laboratory, infection control, and staff pharmacists allowed for identification of additional high-risk cases that were missed in weekly audits.
Although the number of interventions remained fairly stable each year, the acceptance rate significantly improved after the first year. This delay is an important finding because it took time for the PL, who was only on site 1 day a week, to establish trust and gain the support of other physicians. The first year of the program was also required for the PL to understand and programmatically address specific challenges and establish collaborative hospitalist partnerships to implement diagnosis-specific order sets. We would caution others when establishing similar programs to advise hospital administration that this type of “grace period” may be expected before seeing durable results. Several factors could have increased the rate of intervention acceptance, such as a more regular presence and a dedicated ASP clinical pharmacist, but there was no way to evaluate these factors in this study [6]. The two most common interventions were to narrow spectrum or discontinue antibiotics, which is similar to other reports [11]; however, other recommendations were also frequently given.
Understanding the effect of quality metrics to control antibiotic overuse and the relationship to prevention of antimicrobial resistance will be central to addressing this modern healthcare crisis [6, 12]. This ASP showed decreased use of piperacillin/tazobactam, levofloxacin, and doripenem and was associated with significant improvements in the susceptibility profile for P aeruginosa to several antimicrobial classes. It is interesting to note that the use of cefepime increased after initiation of ASP, but this finding is difficult to interpret because cefepime was added to formulary midway through 2010. One limitation here was that the PL became chair of IP&C, which may have decreased nosocomial organism spread. However, the significant change in P aeruginosa alone is a compelling indicator organism of antibiotic use rather than infection control as previously demonstrated [2, 13]. Another limitation was that the antibiogram is based on inpatient and outpatient cultures. The source of all clinical cultures was not available for the drafting of this manuscript; therefore, there could have been changes in community practice or patient population which contributed to change in P aeruginosa susceptibility that are not accounted for in the manuscript. A review of hospital exposure for the most recent antibiogram where data were available revealed that 90% (78 of 87) of patients with a P aeruginosa culture had been admitted to the community hospital during the same year, and there have not been major changes identified in outpatient practice in the area since the study period (Written communication 9 March 2015 from Patricia Harris).
The decrease in total antibiotic doses, DDD/1000 patient days, and expenditures over the latter two years of the program demonstrate decrease in use and subsequent cost savings provided by ASPs. The total antibiotic cost per patient day is much lower than in larger hospitals but is comparable with published reports from community hospitals [6]. Justifying the cost of ASPs to hospital administration can be difficult, especially at financially constrained smaller institutions; however, an ID physician committed to stewardship can “pay for themselves” through pharmacy cost savings and other quality metrics not included in our analysis [7]. As healthcare continues to evolve, the complications of antimicrobial use such as Clostridium difficile colitis and infections caused by hospital-acquired, drug-resistant pathogens are less likely to be reimbursed. This change requires that hospitals of all sizes need to seek creative approaches to decrease the consequences of antimicrobial overuse.
CONCLUSIONS
In conclusion, we have described an effective, physician-led ASP associated with a significant improvement in P aeruginosa resistance and decreased antimicrobial expenditure in a community hospital setting.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Standiford HC, Chan S, Tripoli M, et al. Antimicrobial stewardship at a large tertiary care academic medical center: cost analysis before, during, and after a 7-year program. Infect Control Hosp Epidemiol 2012; 33:338–45 [DOI] [PubMed] [Google Scholar]
- 2.Pakyz AL, Oinonen M, Polk RE. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2009; 53:1983–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Hospital Association Resource Center. American Hospital Association Annual Survey. Chicago, IL, Health Forum, 2012 [Google Scholar]
- 4.Behnia M, Logan SC, Fallen L, Catalano P. Nosocomial and ventilator-associated pneumonia in a community hospital intensive care unit: a retrospective review and analysis. BMC Res Notes 2014; 7: 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohl CA, Dodds Ashley ES. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011; 53(Suppl 1):S23–8; quiz S9–30 [DOI] [PubMed] [Google Scholar]
- 6.Trivedi KK, Kuper K. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am 2014; 28:281–9 [DOI] [PubMed] [Google Scholar]
- 7.McQuillen DP, Petrak RM, Wasserman RB, et al. The value of infectious diseases specialists: non-patient care activities. Clin Infect Dis 2008; 47:1051–63 [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing M100-S20. Nineteenth informational supplement. Twentith ed Wayne, PA: Clinical and Laboratory Standards Institute, 2010 [Google Scholar]
- 9.Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data: Microbiology. 2014. Clinical and Laboratory Standards Institute (CLSI), 2014; 34:1–80 [Google Scholar]
- 10.World Health Organization (WHO). Collaborating Centre for Drug Statistics Methodology, Guidelines for ATC classification and DDD assignment. WHO Collaborating Centre for Drug Statistics Methodology Vol. 18th ed Oslo: Norway; Norwegian Institute of Public Health, 2015:288 [Google Scholar]
- 11.Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control 2012; 1:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States. In: (CDC) CfDCaP. 1 ed Vol. 1 Atlanta, GA: 2013; 1–114 [Google Scholar]
- 13.Pakyz AL, Lee JA, Ababneh MA, et al. Fluoroquinolone use and fluoroquinolone-resistant Pseudomonas aeruginosa is declining in US academic medical centre hospitals. J Antimicrob Chemother 2012; 67:1562–4 [DOI] [PMC free article] [PubMed] [Google Scholar]