Abstract
Objective
Previous studies on cerebellar infarcts have been largely restricted to acute infarcts in patients with clinical symptoms, and cerebellar infarcts have been evaluated with the almost exclusive use of transversal MR images. We aimed to document the occurrence and 3D-imaging patterns of cerebellar infarcts presenting as an incidental finding on MRI.
Methods
We analysed the 1.5 Tesla MRI, including 3D T1-weighted datasets, of 636 patients (mean age 62 ± 9 years, 81% male) from the SMART-Medea study. Cerebellar infarct analyses included an assessment of size, cavitation and gliosis, of grey and white matter involvement, and of infarct topography.
Results
One or more cerebellar infarcts (mean 1.97; range 1–11) were detected in 70 out of 636 patients (11%), with a total amount of 138 infarcts identified, 135 of which showed evidence of cavitation. The average mean axial diameter was 7 mm (range 2–54 mm), and 131 infarcts (95%) were smaller than 20 mm. Hundred-thirty-four infarcts (97%) involved the cortex, of which 12 in combination with subcortical white matter. No infarcts were restricted to subcortical branches of white matter. Small cortical infarcts involved the apex of a deep (pattern 1) or shallow fissure (pattern 2), or occurred alongside one (pattern 3) or opposite sides (pattern 4) of a fissure. Most (87%) cerebellar infarcts were situated in the posterior lobe.
Conclusions
Small cerebellar infarcts proved to be much more common than larger infarcts, and preferentially involved the cortex. Small cortical infarcts predominantly involved the posterior lobes, showed sparing of subcortical white matter and occurred in characteristic topographic patterns.
Keywords: Cerebellum, Cerebrovascular disease, MRI
Highlights
-
•
Small cerebellar infarcts far more common than large cerebellar infarcts
-
•
Cerebellar infarcts occurred in characteristic imaging patterns.
-
•
Small cortical infarct cavities spared subcortical white matter.
-
•
Patterns of small cortical infarct cavities suggestive of small arterial territories
1. Introduction
Twenty to 30% of all ischaemic strokes occur in the territory of the vertebrobasilar (posterior) circulation, including the cerebellum (Bogousslavsky et al., 1993; Flossmann and Rothwell, 2003; Förster et al., 2011; Hong et al., 2009; Khan et al., 2007). Large cerebellar infarcts have traditionally been classified in function of affected arterial perfusion territories (Edlow et al., 2008; Caplan et al., 2005; Marinković et al., 1995; Hartkamp et al., 2013; Tatu et al., 1996), i.e. into either PICA- (posterior inferior cerebellar artery) (Hartkamp et al., 2013; Kumral et al., 2005a), AICA- (anterior inferior cerebellar artery) (Kumral et al., 2006), and/or SCA- (superior cerebellar artery) territory infarcts (Kumral et al., 2005b; Lee and Kim, 2013). Small (<2 cm) cerebellar infarcts increasingly have come to the attention with the advent of MRI in clinical practice. Although these ‘non-territorial’ infarctions were originally classified according to border zones in between cerebellar perfusion territories (Amarenco et al., 1993; Amarenco et al., 1994; Canaple and Bogousslavsky, 1999), many infarcts do not fit into these classification systems (Canaple and Bogousslavsky, 1999; De Cocker et al., 2013), and recently a more reproducible classification method has been proposed based on cerebellar topography (De Cocker et al., 2013; Schmahmann et al., 1999). This ‘functional’ topographic classification has the potential to offer better clinical–radiological correlation, since there is now evidence for a topographic organization of cerebellar functions, with sensorimotor functions primarily found in the anterior lobe and cognitive processing (non-motor function) represented in the posterior lobe (De Cocker et al., 2013; Schmahmann et al., 2009).
When detected in the acute stage of infarction, small cerebellar infarcts usually occur in combination with other acute brain infarcts, which could indicate that small cerebellar infarcts often escape clinical attention if occurring in isolation (Canaple and Bogousslavsky, 1999; Terao et al., 2005). Still, these small cerebellar infarcts can be detected later on as an incidental finding if MRI studies are performed (De Cocker et al., 2013; De Cocker et al., 2014). If so, they tend to present as a cerebellar ischemic cavity, which have been recently found to selectively affect the cerebellar cortical grey matter and therefore have been designated as cerebellar cortical cavities (De Cocker et al., 2014). Until now, however, clinical studies on cerebellar infarcts have been largely restricted to the evaluation of infarcts in the acute stage in patients with clinical symptoms. In addition, cerebellar infarcts have been investigated with the almost exclusive use of transverse images (usually diffusion- and T2-weighted MRI), which are usually sufficient for diagnostic purposes but only provide poor contrast between grey and white matter and do not allow for a 3-dimensional (3D) infarct assessment. As a result, it remains largely unknown to which degree small and large cerebellar infarcts involve the cerebellar grey and white matter. In addition, unlike large cerebellar infarcts which obviously occur in an arterial distribution pattern, the precise distribution patterns of small cerebellar infarcts remain undetermined. Finally, it is not known to which degree infarcts involve the different cerebellar lobes and lobules.
The purpose of the present study was to precisely investigate the presence, topography and imaging patterns of cerebellar infarcts, which were studied irrespective of clinical symptoms, in general in the chronic stage of infarction. This was achieved by the evaluation of 636 patients with arterial disease in which the MRI protocol included 3D-T1 weighted images.
2. Materials and methods
2.1. Subjects
Data were used from the Second Manifestations of ARTerial disease-Memory, depression and aging (SMART-Medea) study, a cohort study in patients with a history of arterial disease (Gerritsen et al., 2011; Grool et al., 2011). The SMART-Medea study is an ancillary study to the SMART-MR study, which has been described in more detail elsewhere (Geerlings et al., 2010; Geerlings et al., 2009). In brief, between May 2001 and December 2005, 1309 patients newly referred to the University Medical Center Utrecht for treatment of symptomatic atherosclerotic disease (manifest coronary artery disease, cerebrovascular disease, peripheral arterial disease or abdominal aortic aneurysm) without MRI contraindications were enrolled in the SMART-MR study. Exclusion criteria were: age ≥ 80 years, diagnosis of a terminal malignancy, lack of independence in daily activities (Rankin scale > 3), and lack of fluency in Dutch or referral back to the referring specialist. Between April 2006 and May 2009, 710 participants had follow-up measurements including 3D T1-weighted MR images to assess hippocampal volumes as part of the SMART-Medea study.
During a one day visit to the University Medical Center, Utrecht, a physical examination, ultrasonography of the carotid arteries, blood and urine samplings, neuropsychological and depression assessment, and a 1.5 Tesla brain MRI scan were performed. Questionnaires were used for assessing demographics, risk factors and medical history, medication use, functioning, psychosocial vulnerability and stress factors, and depressive symptoms.
The SMART-MR and SMART-Medea studies were approved by the ethics committee, and written informed consent was obtained from all participants.
2.2. Study sample
Of the 710 patients included, 44 did not have an MRI scan and 30 did not have adequate data for segmentation. Thus, for the present study, we included the 636 patients in whom an adequate 3D-T1 Fast Field Echo (FFE) sequence was performed (Knoops et al., 2010), which proved to be helpful for optimal characterization of cerebellar infarct patterns, as discussed elsewhere (De Cocker et al., 2014).
2.3. Magnetic resonance protocol
The MR scans were performed on a 1.5-Tesla whole-body system (Gyroscan ACS-NT, Philips Medical Systems, Best, The Netherlands). The protocol consisted of a 3D T1-weighted Fast Field Echo (FFE) (repetition time (TR)/echo time (TE): 7.0/3.2 ms; flip angle 8°, FOV 240 mm; matrix size 240 × 256; slice thickness 1.0 mm; no gap; 170 slices). In addition, transverse T1-weighted (TR/TE: 235/2 ms; flip angle, 80°), transverse PD- and T2-weighted (TR/TE:2200/11 and 2200/100 ms; turbo factor 12), transverse FLAIR (TR/TE/inversion time (TI): 6000/100/2000 ms), and inversion recovery (IR) (TR/TE/TI: 2900/22/410 ms) sequences were performed (field of view (FOV) 230 × 230 mm; matrix size, 180 × 256; slice thickness 4.0 mm; no gap; 38 slices).
2.4. Image analysis
2.4.1. Cerebellum
All MR image analyses were performed by a neuroradiologist with 6 years of experience (LDC) who was blinded to clinical details. Cerebellar infarcts were rated visually and, wherever there was doubt, cerebellar signal alterations were revalued in a consensus meeting with a neuroradiologist with 12 years of experience (JH). For the analysis of number and location of cerebellar infarcts, transverse T2-weighted images (T2WI) were used as a screening modality (Cormier et al., 1992), since these are known to be more sensitive than FLAIR for the evaluation of the posterior fossa (De Cocker et al., 2013; Moreau et al., 2012). T2-hyperintensities were only considered to represent an infarct if a normal fissure, a perivascular space, or another alternative structure could be excluded. Infarcts detected on T2WI were correlated with FLAIR- and 3D T1-weighted images to assess the presence of cavitation. Cavitation was present if intralesional fluid intensity on T2WI could be confirmed on FLAIR- and/or 3D T1-weighted images (T1WI). The longest and shortest diameter of each infarct was measured on axial T2WI, and the mean axial diameter was calculated as the mean of both values. The topography of each cerebellar infarct was precisely defined and categorized into affected lobes and lobules by one experienced observer (LDC) according to the revised Larsell classification, using the three-dimensional MRI atlas of the human cerebellum in stereotaxic space by Schmahmann et al. (1999), as discussed elsewhere (De Cocker et al., 2013). In addition, for each infarct encountered, the relative involvement of cortex and white matter was evaluated on T1WI. A distinction was made between infarcts involving the cortex, the subcortical branches of white matter (which are situated centrally within cerebellar folia), the deep regions of the cerebellum consisting of deep white matter and cerebellar nuclei, and infarcts involving any combination of the former. For each infarct involving the cerebellar cortex, its position was evaluated relative to the adjacent fissure(s) (De Cocker et al., 2013). This fissure was named if it concerned one of the three major cerebellar fissures (primary fissure in between anterior and posterior lobe, posterior superior fissure, and great horizontal fissure), while all other fissures were classified under unnamed fissures (De Cocker et al., 2013; Schmahmann et al., 1999). Furthermore, all fissures were subdivided in large and small fissures. Large fissures were defined as fissures extending up to the cortex covering the deep white matter, while small fissures were defined as fissures not reaching the deep white matter. Distinction was made between infarcts occurring in the apex of a large (pattern 1) or a small fissure (pattern 2), infarcts occurring more superficially alongside one (pattern 3) or opposite sides (pattern 4) of a fissure (Figs. 1 and 2), and infarcts bridging multiple fissures.
Fig. 1.
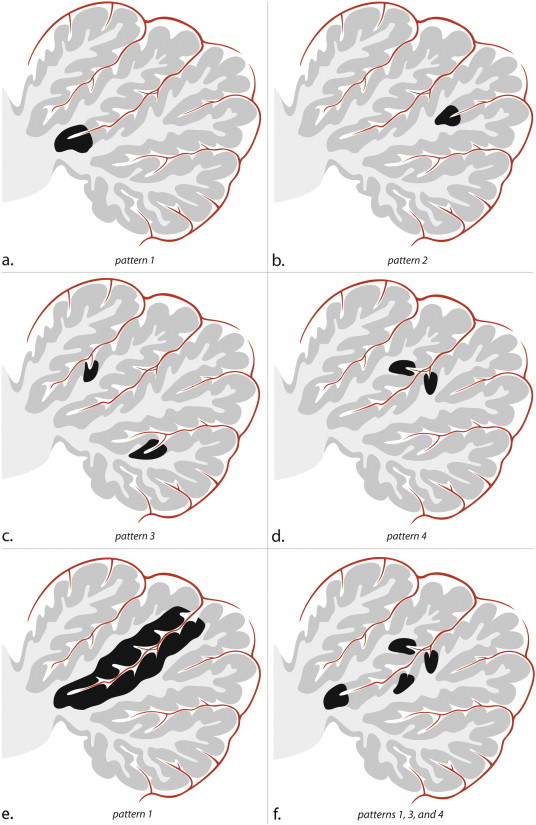
Schematic sagittal drawings illustrate a single cerebellar lobe, its arterial supply, and the patterns of small infarcts. Within the depicted cerebellar lobe, multiple folia are separated by fissures. The folia, which consist of the cortex and subcortical white matter, converge towards the deep white matter of the cerebellum. In the fissures, an arterial branch is present which gives rise to cortical arteries. (a) Pattern 1 corresponds to infarcts involving the apex of a large fissure, (b) pattern 2 corresponds to infarcts involving the apex of a shallow fissure, (c) two infarcts corresponding to pattern 3, and (d) one infarct involving opposite sides of a fissure, indicative of pattern 4. (e) Infarct involving the entire cortical coating of a deep cerebellar fissure; notice this also is a pattern 1 infarct since it involves the apex of a deep fissure. This infarct likely resulted from the occlusion of the arterial branch in the cerebellar fissure. (f) Combinations of small infarcts commonly occur alongside the same fissure. (a–f) Notice the sparing of both subcortical and deep white matter in each cortical infarct.
Fig. 2.
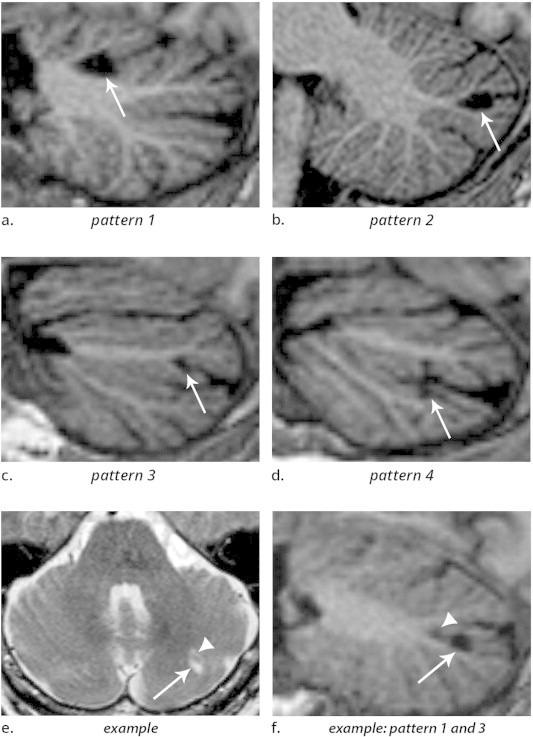
(a–d) 3D T1-weighted images (sagittal reconstructions) of the brain, providing excellent contrast between grey matter and white matter, show the four patterns by which cerebellar infarcts (arrows) typically affect the cerebellar cortex. (e) Example of two cerebellar infarcts adjacent to each other (arrow and arrowhead), detected on transverse T2WI. (f) Sagittal T1-weighted image of the same two infarcts shows how the topography of one infarct corresponds to pattern 3 (arrow), while the other infarct (arrowhead) corresponds to a small pattern 1 infarct. Notice the sparing of the subcortical and deep white matter in each infarct. Images are cropped to display the cerebellum only.
2.4.2. Brain stem
The brain stem was visually searched for infarcts by two trained investigators and a neuroradiologist. All raters were blinded for the history and diagnosis of the patient. Discrepancies in rating were re-evaluated in a consensus meeting. Infarcts were defined as focal hyperintensities on T2WI of at least 3 mm in diameter.
2.5. Data analysis
For the study sample (n = 636), frequency distributions of cerebellar infarct characteristics were calculated. Next, chi-square testing was performed to estimate the proportion of cerebellar infarcts in those with brain stem infarcts. Statistical analyses were performed with SPSS 20.0 (IBM Corp., Armonk, NY, USA).
3. Results
The mean age of the population was 62 years (SD = 9; range 33–83 years) and 81% were men. One or more cerebellar infarcts (mean 1.97; range 1–11) were detected in 70 out of 636 patients (11%), with a total amount of 138 infarcts identified. Forty-four cerebellar infarcts (32%) occurred in isolation, while 94 infarcts (68%) occurred in combination with one (n = 24; 17%) or more (n = 94; 68%) other cerebellar infarcts, with a maximum of 11 cerebellar infarcts encountered in one patient.
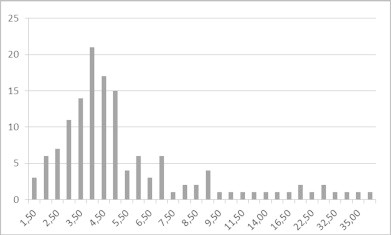
The average mean axial diameter was 6.5 mm (SD = 7.1; range 1.5–54 mm). Hundred-twenty-four (90%) out of 138 infarcts were smaller or equal to 10 mm, while 128 (93%) infarcts were smaller than 15 mm and 131 infarcts (95%) were smaller than 20 mm (Table 1).
Table 1.
Size (in mm, x-axis) and frequency (y-axis) of cerebellar infarcts; notice the overwhelming majority of infarcts smaller than 10 mm.
Hundred-thirty-five infarcts (98%) showed evidence of cavitation on 3D-T1 and/or FLAIR. Seven infarcts (5%) presented on FLAIR as a hypointense cavity without surrounding hyperintensity suggestive of gliosis, while 120 infarcts (87%) did show hyperintense signal suggestive of gliosis. Eleven infarcts (8%) were occult on FLAIR.
Hundred-thirty-four (97%) out of all 138 infarcts involved the cerebellar cortex, uniquely or in part, and are further described as cortical infarcts. The subcortical branch of white matter was spared in 122 out of 134 cortical infarcts (91%), and no infarcts were restricted to subcortical branches of white matter. Eight (6%) out of all 138 infarcts involved the deep regions of the cerebellum, four of which (3%) were large cortical infarcts involving multiple folia and extending into the deep cerebellum and the other four (3%) were smaller than 10 mm and restricted to the deep cerebellum. Three of the latter showed evidence of cavitation and were compatible with lacunes of presumed vascular origin (Wardlaw et al., 2013).
Cortical infarcts involved the apex of a large fissure (pattern 1; n = 63 or 47% of 134 cortical infarcts) or of a small fissure (pattern 2; n = 15 or 11%), or occurred more superficially alongside one side of a fissure (pattern 3; n = 34 or 25%) or opposite sides of a fissure (pattern 4; n = 13 or 10%) (Figs. 1 and 2). Some cortical infarcts involved multiple fissures (n = 9 or 7%), and therefore were not assigned to one of the four described cortical infarct patterns. The latter group included all infarcts with a mean axial diameter above 16 mm. Fifty-three (38%) cortical infarcts were located adjacent to the great horizontal fissure, 34 (25%) adjacent to the posterior superior fissure, nine (7%) to the primary fissure, and 39 (28%) adjacent to one of the unnamed fissures.
According to the lobar classification (De Cocker et al., 2013), hundred-thirty-one infarcts (95%) were situated within the cerebellar hemispheres, three infarcts (2.2%) within the paravermis, two infarcts (1.4%) within the vermis, while two more infarcts (1.4%) involved the combination of cerebellar hemisphere, paravermis and vermis (Table 2). Hundred-nineteen infarcts (87%) were situated within the posterior lobe of the cerebellum, seven infarcts (5%) were situated within the anterior lobe, and another eight infarcts (6%) involved both the anterior and the posterior lobe, while the remainder four infarcts (3%) were restricted to the deep cerebellum and thus did not involve the anterior or posterior lobe (Table 2). With respect to the lobular classification (De Cocker et al., 2013), infarcts involving Crus I were most common (n = 92 or 67%); 33 (24%) infarcts involved Crus I in isolation and another 59 (43%) infarcts involved Crus I in combination with adjacent lobule(s). Crus II was second only to Crus I in order of frequency, and was involved in 54 infarcts (39%), 15 (11%) in isolation and 39 (28%) in combination. In declining frequency, either alone or in combination with other lobules, cerebellar infarcts involved lobule VI (n = 33 or 24%), lobule VIII (n = 13 or 9%), lobule V (n = 12 or 9%), lobule VII (n = 9 or 7%), lobule IX (n = 7 or 5%), lobule IV (n = 5 or 4%), lobule III (n = 4 or 3%) and lobule I/II (n = 1 or 1%), while no infarct was seen to involve lobule X (Table 2).
Table 2.
Lobar and lobular classification of cerebellar infarcts using the functional topographic classification based on a three-dimensional MRI atlas in proportional stereotaxic space; notice the predominant involvement of the posterior lobe over the anterior lobe. In respect to the lobular classification, many infarcts involve the combination of multiple lobules. These infarcts are counted for each involved lobule, and therefore the total sum of involved lobules exceeds the 138 infarcts observed in the present study.
| Lobar Classification | |
|---|---|
| Anterior Lobe | 15 |
| Posterior Lobe | 127 |
| Anterior and Posterior Lobe | 8 |
| Lobular Classification | |
| Anterior Lobe | |
| Lobule I/II | 1 |
| Lobule III | 4 |
| Lobule IV | 5 |
| Lobule V | 12 |
| Posterior Lobe | |
| Lobule VI | 33 |
| Crus I | 92 |
| Crus II | 54 |
| Lobule VII | 9 |
| Lobule VIII | 13 |
| Lobule IX | 7 |
| Lobule X | 0 |
A total of 21 patients showed evidence of brain stem infarcts, seven of whom (33%) showed evidence of cerebellar infarcts; of the 615 patients without brain stem infarcts, 63 (10%) showed evidence of cerebellar infarcts (Pearson Chi square 11.5; p = 0.001).
4. Discussion
In the present study we investigated the presence and topography of cerebellar infarcts, irrespective of clinical symptomatology, in patients with a history of arterial disease. The results of our study indicate that (very) small cerebellar infarcts (<=2 cm) may be much more common than large cerebellar infarcts, and that most cerebellar infarcts involve the posterior lobe. Small cerebellar cortical cavities, by far the most common type of cerebellar infarction observed, consistently showed sparing of subcortical white matter and occurred in characteristic distribution patterns.
First, (very) small cerebellar infarcts were far more common than large cerebellar infarcts, as the majority (90%) of cerebellar infarcts was smaller or equal to 1 cm. Cerebellar infarcts were often (in 68%) seen in combination with other cerebellar infarct(s). These findings are in line with previous reports on multiple cerebellar infarcts studied in the symptomatic stage of infarction (Canaple and Bogousslavsky, 1999; Terao et al., 2005), one of which showed that very small cerebellar infarcts are present in over 90% of patients with multiple cerebellar infarcts (Canaple and Bogousslavsky, 1999). In addition, the present study now indicates that the same very small infarcts commonly occur in isolation as well, as opposed to previous studies which have studied cerebellar infarcts in the acute/symptomatic stage of infarction (Amarenco et al., 1994; Tohgi et al., 1993). As a result, cerebellar infarcts in general may be much more common than previously thought, as the majority seem to be very small and may be asymptomatic or only present with minor, non-specific or non-focal symptoms, such as dizziness, nausea and vomiting, unsteady gait, and headache (Edlow et al., 2008; Savitz et al., 2007; Compter et al., 2013). Therefore, most cerebellar infarcts could escape clinical attention during the acute stage of infarction.
Second, cerebellar infarcts seem to be mainly a disease of cortical grey matter. All very small infarcts occurred in the cortex and spared subcortical white matter, with the exception of a few deep cavities which were compatible with cerebellar lacunes. All larger cerebellar infarcts primarily involved the cortex as well. In the same way as very small infarcts, larger cerebellar infarcts showed a complete denudation of the cerebellar cortex, albeit with extension into subcortical white matter. Nevertheless, even larger infarcts could spare some subcortical branches of white matter (Fig. 3). These findings on a large scale confirm the white matter sparing observed in a limited number of cerebellar cavities which were recently studied with ex vivo 7 T MRI and histopathological correlation (De Cocker et al., 2014). Considering the cerebellar infarct patterns presented in the present study, the sparing of subcortical white matter seems determined by the cortical and the subcortical architecture of the cerebellum and its arterial supply. In the cerebellum, the white matter resembles a tree (‘the arbor vitae’) with a stem of deep white matter and branches of subcortical white matter (De Cocker et al., 2014; Sedlaczek et al., 2005). These branches and surrounding cortex form cerebellar folia, separated from each other by deeply interdigitating fissures which contain arterial branches. As such, these folia receive arterial supply from arterial branches in two fissures (Figs. 1 and 4) (Akima et al., 1987; Duvernoy et al., 1983; Icardo et al., 1982). We postulate that exactly this dual cortical arterial supply of cerebellar folia might account for the subcortical white matter sparing of the observed cortical infarcts. In larger infarcts caused by a more proximal occlusion of a cerebellar artery, however, arterial branches may be occluded in both the fissure above and beneath the infarcted folium, leaving no collateral arterial supply. Although speculative, this might explain why the subcortical branch of white matter was not consistently spared in larger infarcts.
Fig. 3.
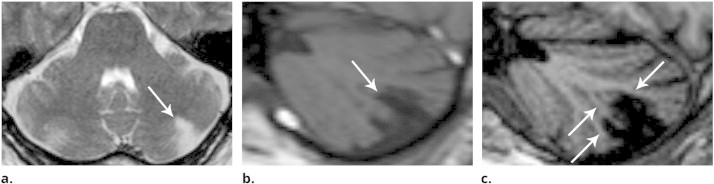
Although this larger infarct spans multiple fissures, it is seen to spare the deep white matter and multiple (but not all) branches of subcortical white matter (arrows in c). (a) Transverse T2-weighted image, (b) sagittal T1-weighted image, (c) 3D-weighted T1 with sagittal reconstructions. Notice the superior contrast between grey and white matter on image (c) compared to image (b).
Fig. 4.
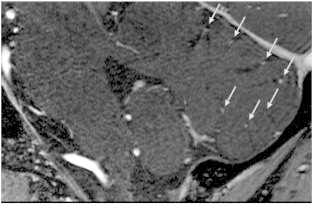
Sagittal contrast-enhanced time-of-flight (TOF) MR angiography at 7 Tesla illustrates how blood vessels (arrows) are seen within each cerebellar fissure. (Image courtesy of Anita Harteveld.)
Third, the majority (87%) of cerebellar infarcts occurred in the posterior lobes of the cerebellar hemispheres. This disproportion may be at least explained by the much larger volume of the cerebellar hemispheres compared to the cerebellar vermis on the one hand, and of the posterior lobes compared to the anterior lobes on the other hand (Schmahmann et al., 1999). Nevertheless, previous reports on cerebellar infarcts in the symptomatic stage have found that infarcts in the SCA-territory, which more or less corresponds to the anterior lobe, are similar in frequency to infarcts in the PICA-territory, which more or less corresponds to the posterior lobe. Thus, the disproportionate involvement of the posterior lobe in the present study seems to suggest that infarcts in the posterior lobe are less often symptomatic or present with minor, non-specific or non-focal symptoms (Searls et al., 2012). This in turn could be related to the topographic organization of cerebellar functions, with sensorimotor functions primarily found in the anterior lobe and cognitive processing (non-motor function) represented in the posterior lobe of the cerebellum (De Cocker et al., 2013; Schmahmann et al., 2009; Stoodley and Schmahmann, 2010).
For multiple reasons, the described imaging patterns of cerebellar cortical cavities could be compatible with infarcted cortical arterial territories (Akima et al., 1987). First, the locations of cortical infarcts centred around a cortical groove or around the apex of a fissure, seemed to correspond to the territory around the entry point of a cortical artery (Fig. 1) (Duvernoy et al., 1983; Icardo et al., 1982). Secondly, multiple small cortical infarcts were often seen grouped together along the same fissure (Figs. 1f and 2e–f), suggesting the occlusion of multiple cortical arteries running in this fissure. Thirdly, cortical infarcts involving the apex (patterns 1 and 2, Figs. 1 and 2) had a more or less triangular shape and were very variable in size. While some of these were very small and restricted to the apex of a fissure (arrowhead in Fig. 2f), others extended along part or even the whole of the cortex covering the fissure (Fig. 1e). This variability in extent could have depended on the site of arterial occlusion; distal occlusions causing cortical infarcts restricted to the apex and more proximal occlusions giving rise to more extensive cortical infarcts. Although this theory remains in part assumptive with only indirect anatomical evidence, the documented cortical infarct patterns could serve as a scientific basis for future research in cerebellar vasculature and ischaemic disease.
Finally, besides the cortical lesions, four small lesions occurred in the deep cerebellar grey/and or white matter, three of which showed evidence of cavitation and were compatible with lacunes of presumed vascular origin (Wardlaw et al., 2013). Since the original post-mortem studies, lacunes of presumed vascular origin are known to be relatively rare in the cerebellum, which was recently confirmed in a combined radiologic–pathologic correlation study and again in the present imaging study (De Cocker et al., 2014; Fisher, 1965).
Strengths of our study include the large number of examined subjects as well as its original design in which cerebellar infarcts were evaluated beyond the exclusive use of the transverse imaging plane in the acute stage of infarction. There are also limitations on both radiological and clinical points. From a radiological perspective, the arterial cerebellar territories are not discussed with reference to the small versus large infarct patterns. Unfortunately, small cerebellar infarcts are difficult to classify according to perfusion territories, as the perfusion territories are widely variable among subjects (Hartkamp et al., 2013; De Cocker et al., 2013). Moreover, it is not always possible to draw a sharp line between complete and incomplete large territorial cerebellar infarcts. Therefore, we opted for the topographic classification of small and large cerebellar infarcts, as proposed in an earlier publication (De Cocker et al., 2013). With respect to this classification, some lobules are more difficult to define than others. We dealt with this to combine lobules I and II, as proposed by the MRI atlas in stereotaxic space (Schmahmann et al., 1999), and to combine lobules VIIIa and VIIIb (De Cocker et al., 2013). Since no infarcts involved lobule X (flocculonodular lobe), all infarcts involving adjacent lobules were reviewed, and again no infarcts involving lobule X were found. Just like the flocculonodular lobe, the anterior and posterior lobes (lobules V and VI) are easy to define due to large intervening fissures, and the same goes for lobules VI, Crus I, and Crus II. Thus, lobules V, VI, Crus I and Crus II are all easy to define and these constituted the vast majority of all involved lobules (191 out of 230 involved lobules in total). Another limitation from an imaging perspective is the study of patients irrespective of symptomatology, without notion of the delay between infarct onset and MRI scan. As a result of this study design, infarcts were in general imaged in the chronic stage, as witnessed by cavitation of almost any observed infarct. Since infarct features, such as oedema, infarct volume and cavitation, differ substantially between the acute and chronic stages, it cannot be excluded that white matter might have been involved to some degree in the acute stage, with (partial) recovery and therefore absence of signal abnormalities in the chronic stage of infarction. Thus, our description of grey and white matter involvement may not hold true for acute cerebellar infarcts. From a clinical perspective, there were no data on which infarcts corresponded to a clinical stroke syndrome and which were asymptomatic. However, the current study was aimed to describe and qualify the different anatomic patterns of infarcts as incidental findings in a large epidemiological study. Also, the clinical significance of the chronic infarcts on cognitive and motor functions was not evaluated and should be investigated in further studies. Finally, the present imaging study did not provide information on vertebrobasilar arterial status or pathophysiological mechanism.
In conclusion, we studied the occurrence and imaging patterns of cerebellar infarcts on MRI irrespective of clinical symptoms. Small cerebellar infarcts proved to be much more common than larger infarcts, and the posterior lobe was involved much more often than the anterior lobe. Small cerebellar infarcts involving the cortex were by far most common. These occurred in four typical distribution patterns, each with sparing of subcortical white matter. Although speculative, a considerable number of these small infarcts could have remained hidden during the acute stage of infarction due to lack of symptomatology or non-specific symptoms, especially if situated in the posterior lobe and if occurring in isolation.
Acknowledgments
Members of the SMART Study Group are: A. Algra, MD, PhD, Y. van der Graaf, MD, PhD, D.E. Grobbee, MD, PhD, and G.E. H.M. Rutten, MD, PhD, Julius Center for Health Sciences and Primary Care; F.L.J. Visseren, MD, PhD, Department of Vascular Medicine; F.L. Moll, MD, PhD, Department of Vascular Surgery; L.J. Kappelle, MD, PhD, Department of Neurology; W.P.T.M. Mali, MD, PhD, Department of Radiology; and P.A. Doevendans, MD, PhD, Department of Cardiology.
The authors thank Chris van Kesteren for the drawing of the schematic images (Fig. 1).
References
- Akima M., Nonaka H., Kagesawa M. A study on the microvasculature of the cerebellar cortex. The fundamental architecture and its senile change in the cerebellar hemisphere. Acta Neuropathol. 1987;75(1):69–76. doi: 10.1007/BF00686795. 3434216 [DOI] [PubMed] [Google Scholar]
- Amarenco P., Kase C.S., Rosengart A. Very small (border zone) cerebellar infarcts. Distribution, causes, mechanisms and clinical features. Brain. 1993;116(1):161–186. doi: 10.1093/brain/116.1.161. 8453455 [DOI] [PubMed] [Google Scholar]
- Amarenco P., Lévy C., Cohen A. Causes and mechanisms of territorial and nonterritorial cerebellar infarcts in 115 consecutive patients. Stroke. 1994;25(1):105–112. doi: 10.1161/01.str.25.1.105. 8266355 [DOI] [PubMed] [Google Scholar]
- Bogousslavsky J., Regli F., Maeder P. The etiology of posterior circulation infarcts: a prospective study using magnetic resonance imaging and magnetic resonance angiography. Neurol. 1993;43(8):1528–1533. doi: 10.1212/wnl.43.8.1528. [DOI] [PubMed] [Google Scholar]
- Canaple S., Bogousslavsky J. Multiple large and small cerebellar infarcts. J. Neurol. Neurosurg. Psychiatry. 1999;66(6):739–745. doi: 10.1136/jnnp.66.6.739. 10329747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan L., Wityk R., Pazdera L., Chang H.M., Pessin M., Dewitt L. New England Medical Center Posterior Circulation Stroke Registry II. Vascular lesions. J Clin Neurol. 2005;1:31–49. doi: 10.3988/jcn.2005.1.1.31. 20396470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compter A., Kappelle L.J., Algra A. Nonfocal symptoms are more frequent in patients with vertebral artery than carotid artery stenosis. Cerebrovasc. Dis. 2013;35(4):378–384. doi: 10.1159/000348849. 23635415 [DOI] [PubMed] [Google Scholar]
- Cormier P.J., Long E.R., Russell E.J. MR imaging of posterior fossa infarctions: vascular territories and clinical correlates. RadioGraphics. 1992;12(6):1079–1096. doi: 10.1148/radiographics.12.6.1439013. 1439013 [DOI] [PubMed] [Google Scholar]
- De Cocker L.J., van Veluw S.J., Biessels G.J. Ischaemic cavities in the cerebellum: an ex vivo 7-tesla MRI study with pathological correlation. Cerebrovasc. Dis. 2014;38(1):17–23. doi: 10.1159/000365411. 25171512 [DOI] [PubMed] [Google Scholar]
- De Cocker L.J., van Veluw S.J., Fowkes M. Very small cerebellar infarcts: integration of recent insights into a Functional Topographic Classification. Cerebrovasc. Dis. 2013;36(2):81–87. doi: 10.1159/000353668. 24029219 [DOI] [PubMed] [Google Scholar]
- Duvernoy H., Delon S., Vannson J.L. The vascularization of the human cerebellar cortex. Brain Res. Bull. 1983;11(4):419–480. doi: 10.1016/0361-9230(83)90116-8. 6652521 [DOI] [PubMed] [Google Scholar]
- Edlow J.A., Newman-Toker D.E., Savitz S.I. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7(10):951–964. doi: 10.1016/S1474-4422(08)70216-3. 18848314 [DOI] [PubMed] [Google Scholar]
- Fisher C.M. Lacunes: small, deep cerebral infarcts. Neurol. 1965;15:774–784. doi: 10.1212/wnl.15.8.774. 14315302 [DOI] [PubMed] [Google Scholar]
- Flossmann E., Rothwell P.M. Prognosis of vertebrobasilar transient ischaemic attack and minor stroke. Brain. 2003;126(9):1940–1954. doi: 10.1093/brain/awg197. 12847074 [DOI] [PubMed] [Google Scholar]
- Förster A., Griebe M., Gass A. Recent advances in magnetic resonance imaging in posterior circulation stroke: implications for diagnosis and prognosis. Curr. Treat. Options Cardiovasc. Med. 2011;13(3):268–277. doi: 10.1007/s11936-011-0119-8. [DOI] [PubMed] [Google Scholar]
- Geerlings M.I., Appelman A.P., Vincken K.L. Association of white matter lesions and lacunar infarcts with executive functioning: the SMART-MR study. Am. J. Epidemiol. 2009;170(9):1147–1155. doi: 10.1093/aje/kwp256. 19783584 [DOI] [PubMed] [Google Scholar]
- Geerlings M.I., Appelman A.P., Vincken K.L. Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis. 2010;210(1):130–136. doi: 10.1016/j.atherosclerosis.2009.10.039. 19945704 [DOI] [PubMed] [Google Scholar]
- Gerritsen L., Comijs H.C., Van der Graaf Y. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex Volumes — the SMART Medea study. Biol. Psychiatry. 2011;70(4):373–380. doi: 10.1016/j.biopsych.2011.01.029. 21439552 [DOI] [PubMed] [Google Scholar]
- Grool A.M., Van der Graaf Y., Mali W.P. Location of cerebrovascular and degenerative changes, depressive symptoms and cognitive functioning in later life: the SMART-Medea study. J. Neurol. Neurosurg. Psychiatry. 2011;82(10):1093–1100. doi: 10.1136/jnnp.2010.232413. 21459931 [DOI] [PubMed] [Google Scholar]
- Hartkamp N.S., De Cocker L.J., Helle M. In vivo visualization of the PICA perfusion territory with super-selective pseudo-continuous arterial spin labeling MRI. Neuroimage. 2013;83:58–65. doi: 10.1016/j.neuroimage.2013.06.070. 23820436 [DOI] [PubMed] [Google Scholar]
- Hong J.M., Chung C.-S., Bang O.Y. Vertebral artery dominance contributes to basilar artery curvature and peri-vertebrobasilar junctional infarcts. J. Neurol. Neurosurg. Psychiatry. 2009;80(10):1087–1092. doi: 10.1136/jnnp.2008.169805. 19414436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardo J.M., Ojeda J.L., Garcia-Porrero J.A. The cerebellar arteries: cortical patterns and vascularization of the cerebellar nuclei. Acta Anat. 1982;113(2):108–116. doi: 10.1159/000145545. 7124324 [DOI] [PubMed] [Google Scholar]
- Khan S., Cloud G.C., Kerry S. Imaging of vertebral artery stenosis: a systematic review. J. Neurol. Neurosurg. Psychiatry. 2007;78(11):1218–1225. doi: 10.1136/jnnp.2006.111716. 17287234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoops A.J., Gerritsen L., Van der Graaf Y. Basal hypothalamic pituitary adrenal axis activity and hippocampal volumes: the SMART-Medea study. Biol. Psychiatry. 2010;67(12):1191–1198. doi: 10.1016/j.biopsych.2010.01.025. 20299006 [DOI] [PubMed] [Google Scholar]
- Kumral E., Kisabay A., Ataç C. Spectrum of the posterior inferior cerebellar artery territory infarcts. Clinical-diffusion-weighted imaging correlates. Cerebrovasc. Dis. 2005;20(5):370–380. doi: 10.1159/000088667. 16205055 [DOI] [PubMed] [Google Scholar]
- Kumral E., Kisabay A., Ataç C. Lesion patterns and etiology of ischemia in superior cerebellar artery territory infarcts. Cerebrovasc. Dis. 2005;19(5):283–290. doi: 10.1159/000084496. 15775708 [DOI] [PubMed] [Google Scholar]
- Kumral E., Kisabay A., Ataç C. Lesion patterns and etiology of ischemia in the anterior inferior cerebellar artery territory involvement: a clinical-diffusion weighted-MRI study. Eur. J. Neurol. 2006;13(4):395–401. doi: 10.1111/j.1468-1331.2006.01255.x. 16643319 [DOI] [PubMed] [Google Scholar]
- Lee H., Kim H.-A. Nystagmus in SCA territory cerebellar infarction: pattern and a possible mechanism. J. Neurol. Neurosurg. Psychiatry. 2013;84(4):446–451. doi: 10.1136/jnnp-2012-303177. 23172866 [DOI] [PubMed] [Google Scholar]
- Marinković S., Kovacević M., Gibo H. The anatomical basis for the cerebellar infarcts. Surg. Neurol. 1995;44(5):450–460. doi: 10.1016/0090-3019(95)00195-6. Discussion 460–461. [DOI] [PubMed] [Google Scholar]
- Moreau F., Patel S., Lauzon M.L. Cavitation after acute symptomatic lacunar stroke depends on time, location, and MRI sequence. Stroke. 2012;43(7):1837–1842. doi: 10.1161/STROKEAHA.111.647859. 22733793 [DOI] [PubMed] [Google Scholar]
- Savitz S.I., Caplan L.R., Edlow J.A. Pitfalls in the diagnosis of cerebellar infarction. Acad. Emerg. Med. 2007;14(1):63–68. doi: 10.1197/j.aem.2006.06.060. 17200515 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Doyon J., McDonald D. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10(3 1):233–260. doi: 10.1006/nimg.1999.0459. 10458940 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Macmore J., Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162(3):852–861. doi: 10.1016/j.neuroscience.2009.06.023. 19531371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searls D.E., Pazdera L., Korbel E. Symptoms and signs of posterior circulation ischemia in the New England Medical Center posterior circulation registry. Arch. Neurol. 2012;69(3):346–351. doi: 10.1001/archneurol.2011.2083. 22083796 [DOI] [PubMed] [Google Scholar]
- Sedlaczek O., Grips E., Bäzner H. Infarction of the central cerebellar arbor vitae and transient loss of spatial orientation. Neurol. 2005;65(1):168. doi: 10.1212/01.wnl.0000167537.28213.73. 16009915 [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. 20152963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatu L., Moulin T., Bogousslavsky J. Arterial territories of human brain: brainstem and cerebellum. Neurol. 1996;47(5):1125–1135. doi: 10.1212/wnl.47.5.1125. 8909417 [DOI] [PubMed] [Google Scholar]
- Terao S., Miura N., Osano Y. Multiple cerebellar infarcts: clinical and pathophysiologic features. J. Stroke Cerebrovasc. Dis. 2005;14(5):193–198. doi: 10.1016/j.jstrokecerebrovasdis.2005.05.005. 17904025 [DOI] [PubMed] [Google Scholar]
- Tohgi H., Takahashi S., Chiba K. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku Cerebellar Infarction Study Group. Stroke. 1993;24(11):1697–1701. doi: 10.1161/01.str.24.11.1697. 8236346 [DOI] [PubMed] [Google Scholar]
- Wardlaw J.M., Smith E.E., Biessels G.J. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8. 23867200 [DOI] [PMC free article] [PubMed] [Google Scholar]


