Abstract
Combat trauma wounds with invasive fungal infections (IFIs) are often polymicrobial with fungal and bacterial growth, but the impact of the wound microbiology on clinical outcomes is uncertain. Our objectives were to compare the microbiological features between IFI and non-IFI wounds and evaluate whether clinical outcomes differed among IFI wounds based upon mold type. Data from U.S. military personnel injured in Afghanistan with IFI wounds were examined. Controls were matched by the pattern/severity of injury, including blood transfusion requirements. Wound closure timing was compared between IFI and non-IFI control wounds (with/without bacterial infections). IFI wound closure was also assessed according to mold species isolation. Eighty-two IFI wounds and 136 non-IFI wounds (63 with skin and soft tissue infections [SSTIs] and 73 without) were examined. The time to wound closure was longer for the IFI wounds (median, 16 days) than for the non-IFI controls with/without SSTIs (medians, 12 and 9 days, respectively; P < 0.001). The growth of multidrug-resistant Gram-negative rods was reported among 35% and 41% of the IFI and non-IFI wounds with SSTIs, respectively. Among the IFI wounds, times to wound closure were significantly longer for wounds with Mucorales growth than for wounds with non-Mucorales growth (median, 17 days versus 13 days; P < 0.01). When wounds with Mucorales and Aspergillus spp. growth were compared, there was no significant difference in wound closure timing. Trauma wounds with SSTIs were often polymicrobial, yet the presence of invasive molds (predominant types: order Mucorales, Aspergillus spp., and Fusarium spp.) significantly prolonged the time to wound closure. Overall, the times to wound closure were longest for the IFI wounds with Mucorales growth.
INTRODUCTION
During military operations in Afghanistan, a surge in wound invasive fungal infections (IFIs) was observed among personnel with combat-related trauma. Between 2009 and 2011, 6.8% of the casualties admitted to Landstuhl Regional Medical Center (LRMC) (Germany) and transferred to a military hospital in the United States were diagnosed with IFIs (1–3). As with civilian trauma cases (4–7), combat-related IFIs are characterized by high mortality (7.8% crude rate) (8) and substantial morbidity, including high-level amputations (i.e., hip disarticulation or hemipelvectomy) (8, 9).
Since recognition of this emergent infection, combat-related IFIs have been described in case investigations, including development of a trauma-related IFI classification scheme (2, 8). Further analyses have examined risk factors, clinical outcomes, and the effect of an early IFI diagnosis practice guidance (10, 11). In a recent analysis, IFI case patients had significantly greater residual limb shortening, more amputation level revisions, and a longer time to initial wound closure than a control patient group without fungal-infected wounds matched on injury pattern (12).
As part of the combat-related IFI evaluation, results from wound cultures have been collected; however, the association of specific mold types with respect to treatment and outcome has not been examined. On a per patient basis, cultures predominantly grew organisms of the order Mucorales (34%) and Aspergillus spp. (31%), frequently growing more than one mold type (8). Moreover, >50% of the IFI extremity wounds had secondary/concurrent bacterial skin and soft tissue infections (SSTIs) (12). Bacterial coinfections related to IFIs have been described after natural disasters as well. Following a tornado in Missouri, 13 residents developed trauma-related IFIs with bacterial organisms isolated from the incident wounds of 10 patients (77%) (4).
The literature suggests that the pathophysiology and outcome differ among various mold infections (13). Furthermore, the current clinical practice often partly depends upon which mold groups are present. Our objective was to determine whether the clinical outcome (i.e., time to wound closure) varied based upon wound microbiological findings, in addition to antifungal therapy effects.
MATERIALS AND METHODS
Study population and data sources.
The Trauma Infectious Disease Outcomes Study (TIDOS) is an observational cohort study of infectious complications among military personnel injured during deployment in Iraq and Afghanistan. The full details of this project have been previously published (14). Data were collected from U.S. military personnel who sustained combat-related injuries in Afghanistan (June 2009 to August 2011), were medically evacuated to LRMC, and subsequently transferred to a military hospital in the United States: Walter Reed Army Medical Center and National Naval Medical Center (National Capital Region) and San Antonio Military Medical Center (Texas). As part of TIDOS, bacterial isolates are collected at all levels of care and stored in a microbiological repository. This study was approved by the Infectious Disease Institutional Review Board of the Uniformed Services University of the Health Sciences (Maryland).
Demographics, clinical history, injury patterns, surgical management, and treatment were obtained through the Department of Defense Trauma Registry (15). The results of fungal/bacterial cultures and histopathological examinations were captured through a review of the supplemental TIDOS infectious disease module. Multidrug-resistant bacteria and SSTIs were identified according to National Healthcare Safety Network definitions (16, 17). Antimicrobial susceptibility testing of bacterial isolates was performed using the BD Phoenix (BD Biosciences, Sparks, MD) or Vitek (bioMérieux Inc., Hazelwood, MO) system, in addition to either disc diffusion or Etest methods (18). Antifungal susceptibility testing of mold isolates was not performed.
Case and comparative wound selection criteria.
The IFI cases were identified based on the finding of recurrent tissue necrosis following at least two operative debridements subsequent to the initial procedure, in addition to histopathological evidence (i.e., tissue containing fungal hyphae angioinvasion or observed fungal elements) and/or mold growth from tissue cultures (2, 8). The histopathology specimens were assessed by two surgical pathologists. Case data obtained from infectious disease and trauma surgery services were evaluated. The IFI cases were restricted to extremity wounds. A comparative group of extremity wounds in patients without IFIs was identified from the overall combat casualty study population and matched by blood products transfused within first 24 h, injury pattern (e.g., fractures and amputations), and injury severity score (ISS) (19). The non-IFI wounds were stratified based upon bacterial infection status. Due to the occurrence of personnel with multiple injuries, non-IFI patients may provide more than one wound to the control groups (e.g., one wound with a SSTI and another without).
Clinical outcomes.
The timing (in days) of the initial wound closure after injury was at the treating surgeon's discretion and is based upon the appearance of the wound, the patient's clinical status, and the microbiological results (12). The methods of closure included delayed primary closure, skin grafts (split-thickness and full-thickness), flaps (free and rotation), and commercial dermal matrix substitutes (Integra; Integra LifeSciences Corp., Plainsboro, NJ).
Statistical analysis.
Comparisons between categorical variables were conducted with chi-square and Fisher's exact tests, while nonparametric tests (Wilcoxon rank sum and log-rank t tests) were used to examine the overall distribution of continuous variables. The time following injury to initial wound closure relative to wound microbiology was analyzed in a Kaplan-Meier plot. Wound closure was also assessed with regard to the timing of antifungal initiation/duration in a Cochran-Mantel-Haenszel test. Statistical analysis was performed with SAS version 9.3 (SAS, Cary, NC). Significance was defined as a P value of <0.05.
RESULTS
Study population.
From the combat trauma population evacuated from Afghanistan and admitted to LRMC during the study period, 54 IFI case patients were matched to 69 non-IFI control patients. All patients were injured via a blast mechanism with the majority of injuries sustained while on foot patrol (Table 1). Additionally, both case patient and control patient groups experienced a substantial proportion of traumatic amputations of the lower extremities and open fractures. Injury severity was high for both groups, as indicated by the ISS, intensive care unit admissions, shock index (≥1.5), and large-volume (>20 units) transfusions of blood products in the first 24 h.
TABLE 1.
Demographics, injury circumstances, and trauma characteristics of combat-injured U.S. service members (2009-2011)
| Characteristica | Results for: |
P value | |
|---|---|---|---|
| IFIb: case patients (n = 54) | Non-IFI: control patients (n = 69) | ||
| Demographics | |||
| Age (median [IQR]) | 22.9 (21.7−26.2) | 23.2 (21.3−25.2) | 0.85 |
| Male (no. [%]) | 54 (100) | 68 (99) | 1.00 |
| Injury circumstances (no. [%]) | |||
| BLAST injury | 54 (100) | 69 (100) | |
| Foot patrol | 50 (93) | 61 (88) | 1.00 |
| LRMC ISS (median [IQR])c | 21 (18−25) | 21 (17−24) | 0.43 |
| Traumatic amputations (no. [%]) | |||
| Lower extremity | 46 (85) | 59 (86) | 1.00 |
| Above/through the knee | 42 (78) | 55 (80) | 0.80 |
| Below the knee | 4 (7) | 4 (6) | 0.73 |
| Upper extremity | 8 (15) | 4 (6) | 0.13 |
| Both lower/upper extremity | 7 (13) | 3 (4) | 0.10 |
| Fractures (no. [%]) | 0.87 | ||
| None | 13 (24) | 14 (20) | |
| Closed | 5 (9) | 6 (9) | |
| Open ± closed | 36 (67) | 49 (71) | |
| Blood productsd transfused within first 24 h (no. [%]) | 0.50 | ||
| <10 units | 2 (4) | 4 (6) | |
| 10−20 units | 17 (31) | 28 (40) | |
| >20 units | 35 (65) | 37 (54) | |
| Shock index (no. [%])e | 0.36 | ||
| <1 | 16 (30) | 20 (29) | |
| 1−1.49 | 17 (31) | 28 (40) | |
| ≥1.5 | 35 (65) | 37 (54) | |
| Admittance to LRMC ICU | 53 (98) | 64 (93) | 0.23 |
| Admittance to U.S. MTF ICU | 52 (96) | 59 (86) | 0.07 |
ICU, intensive care unit; IQR, interquartile range; ISS, injury severity score; LRMC, Landstuhl Regional Medical Center; MTF, military treatment facility.
IFI, invasive fungal wound infection.
Wounded U.S. military personnel are evacuated from the combat zone to the LRMC, the U.S. military hospital in Germany, prior to transfer to the United States.
Packed red blood cells plus whole blood.
Shock index: heart rate/systolic blood pressure.
Wound microbiology.
The case patients had a total of 170 extremity wounds, of which 82 had fungal infections (IFI wounds). Among the control patients, there were 136 extremity wounds, including 63 (46%) and 73 (54%) wounds with and without SSTIs, respectively. When the IFI wounds were compared to the non-IFI wounds with SSTIs, there was no significant difference in the proportion of bacteria (overall and multidrug-resistant) identified during the infection workup or the number of recurrent SSTIs at the same anatomic site (Table 2).
TABLE 2.
Bacteriology, management, and outcomes among combat-injured U.S. service members (2009-2011)
| Characteristica | Results for: |
P value 1c | P value 2d | ||
|---|---|---|---|---|---|
| IFIb case wounds (n = 82) | Non-IFI wounds |
||||
| With SSTIs (n = 63) | Without SSTIs (n = 73) | ||||
| SSTI (no. [%]) | |||||
| Bacteria identified during infection workup | 52 (63) | 34 (54) | NAe | 0.194 | NA |
| MDROs identified during infection workup | 25 (30) | 17 (27) | NA | 0.416 | NA |
| Recurrent SSTI at same anatomic site | 9 (11) | 6 (10) | NA | 0.738 | NA |
| Time from injury to wound closure (median days [IQR])f | 16 (11−22) | 12 (9–18) | 9 (6–13) | 0.008 | <0.001 |
| OR visits at LRMC and U.S. MTFs (median [IQR]) | 9 (6–12) | 7 (5–10) | 6 (5–7) | 0.010 | 0.002 |
| Type of initial wound closure (no. [%])g | 0.883 | 0.828 | |||
| STSG + FTSG + Integra | 29 (35) | 18 (29) | 17 (23) | ||
| DPC | 48 (59) | 36 (57) | 46 (63) | ||
| Rotational flap | 1 (1) | 0 | 0 | ||
| Free flap | 2 (2) | 2 (3) | 2 (3) | ||
| Surgical amputations/revisions (no. [%])h | 63 (77) | 45 (71) | 26 (36) | 0.460 | <0.001 |
DPC, delayed primary closure; FTSG, full-thickness skin graft; IQR, interquartile range; LRMC, Landstuhl Regional Medical Center; MDRO, multidrug-resistant organism; MTF, military treatment facility; OR, operating room; STSG, split-thickness skin graft.
IFI, invasive fungal wound infection.
P value 1: compares IFI case wounds and non-IFI wounds with SSTIs.
P value 2: compares non-IFI wounds with and without SSTIs.
NA, not applicable.
Two non-IFI wounds with SSTIs were missing the time to wound closure data.
Two fungus-infected wounds, 7 non-IFI wounds with SSTIs, and 8 non-IFI wounds without SSTIs were missing type of closure data.
Operative procedures occurred at the U.S. MTFs.
The most common molds identified in the IFI case wounds were from the order Mucorales (35%) and the genera Aspergillus (29%) and Fusarium (21%) (Table 3). Mucor spp. represented the majority of molds identified from the wounds with order Mucorales growth (15 wounds [18%]). In addition, five wounds (6%) grew Saksenaea vasiformis. For wounds with Aspergillus growth, Aspergillus terreus and Aspergillus flavus were the predominant species with eight (10%) and seven (9%) wounds growing these organisms, respectively. While non-IFI wounds did not have fungal infections, the wounds were occasionally colonized (i.e., organisms isolated from earlier surveillance cultures and not during infection workups) by molds, with Mucorales and Aspergillus spp. contributing 1% and 3%. Both the IFI and non-IFI wounds were frequently polymicrobial (average of 2.9 and 2.4 bacterial organisms per culture for the IFI and non-IFI SSTI wounds, respectively), with growth of Enterococcus spp., Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter spp. being predominant. Furthermore, 35% and 41% of the IFI and non-IFI wounds with SSTIs, respectively, reported growth of multidrug-resistant Gram-negative rods (MDR-GNR).
TABLE 3.
Microbiological findings by wound typea
| Microbiological characteristic | Results for: |
||
|---|---|---|---|
| IFIb case wounds | Non-IFI woundsc |
||
| With SSTIsd | Without SSTIs | ||
| Total wounds (no. [%]) | 82 | 63 | 73 |
| Days from injury to first culture with mold growth (median [IQRe])f | 6 (3−9) | 4 (3−8) | 2 (2−2) |
| Growth of molds (no. [%]) | |||
| Mucoralesg | 29 (35) | 1 (2) | 1 (1) |
| Mucor spp. | 15 (18) | 1 (2) | 0 |
| Saksenaea vasiformis | 5 (6) | 0 | 0 |
| Rhizopus spp. | 1 (1) | 0 | 0 |
| Aspergillusg | 24 (29) | 3 (5) | 1 (1) |
| A. terreus | 8 (10) | 1 (2) | 0 |
| A. flavus | 7 (9) | 0 | 0 |
| A. niger | 2 (2) | 0 | 0 |
| A. fumigatus | 1 (1) | 0 | 0 |
| Fusarium | 17 (21) | 0 | 0 |
| Other molds | 27 (33) | 6 (10) | 1 (1) |
| More than 1 mold | 22 (27) | 1 (2) | 0 |
| Days from injury to first culture with bacterial growth (median [IQR])h | 6 (3−9) | 8 (5−17) | 15 (7−24) |
| Growth of bacteria/yeast (no [%]) | |||
| Candida spp. | 11 (13) | 5 (8) | 0 |
| Staphylococcus aureusi | 3 (4) | 5 (8) | 1 (1) |
| Methicillin-resistant S. aureus | 2 (2) | 4 (6) | 0 |
| Coagulase-negative Staphylococcus | 13 (16) | 9 (14) | 6 (8) |
| Enterococcus spp. | 37 (45) | 22 (35) | 0 |
| Vancomycin-resistant enterococci | 2 (2) | 1 (2) | 0 |
| Escherichia coli | 19 (23) | 17 (27) | 1 (1) |
| Klebsiella spp. | 2 (2) | 2 (3) | 0 |
| Pseudomonas aeruginosa | 16 (20) | 12 (19) | 2 (3) |
| Acinetobacter spp. | 16 (20) | 18 (29) | 2 (3) |
| Other Gram-negative rods | 13 (16) | 7 (11) | 1 (1) |
| Multidrug-resistant Gram-negative rods | 29 (35) | 26 (41) | 4 (6) |
Wounds may have growth of more than one type of organism so the sum may be >100%.
IFI, invasive fungal wound infection.
Non-IFI wounds may have growth of mold without meeting the clinical requirement to be classified as an IFI case wound (i.e., recurrent wound necrosis); therefore, these wounds are considered to be colonized with mold.
SSTI, skin and soft tissue infections.
IQR, interquartile range.
P = 0.782 for IFI wounds versus non-IFI wounds with SSTIs.
Wounds also grew mold that was not otherwise specified.
P = 0.003 for IFI wounds versus non-IFI wounds with SSTIs.
Includes both methicillin-resistant and methicillin-susceptible.
A total of 49 IFI case patients had wound cultures with fungal growth (1.3 average molds per culture). The remaining five patients (9%) had histopathological evidence of fungal tissue invasion, but wound cultures failed to grow a mold. Examination of histopathological fungal evidence from the 53 IFI case wounds that did not grow Mucorales found aseptate fungal morphology in tissue samples from 4 wounds. Among the IFI wounds that grew the predominant molds, Enterococcus spp. was the most frequently isolated bacterial organism (Table 4). Furthermore, IFI case wounds also commonly grew Acinetobacter spp., Pseudomonas spp., and E. coli, with 33 to 47% of Gram-negative rods being multidrug-resistant. Notably, few wounds grew Staphylococcus aureus (4 to 10%).
TABLE 4.
Microbiological findings from cultures from invasive fungus-infected case woundsa
| Microbiological characteristic | Mucorales | Aspergillus | Fusarium | Other moldsb |
|---|---|---|---|---|
| Total wounds with growth | 29 | 24 | 17 | 27 |
| Coinfection with (no. [%]) | ||||
| Mucoralesc | NAd | 5 (21) | 5 (29) | 4 (15) |
| Mucor spp. | NA | 5 (21) | 4 (24) | 2 (7) |
| Saksenaea vasiformis | NA | 0 | 0 | 0 |
| Rhizopus spp. | NA | 0 | 0 | 1 (4) |
| Aspergillusc | 5 (17) | NA | 5 (29) | 5 (19) |
| A. terreus | 3 (10) | NA | 3 (18) | 2 (7) |
| A. flavus | 0 | NA | 0 | 1 (4) |
| A. niger | 1 (3) | NA | 2 (12) | 0 |
| A. fumigatus | 1 (3) | NA | 0 | 0 |
| Fusarium | 5 (17) | 5 (21) | NA | 2 (7) |
| Candida spp. | 4 (14) | 3 (13) | 2 (12) | 5 (19) |
| Staphylococcus aureuse | 3 (10) | 1 (4) | 0 | 0 |
| Methicillin-resistant S. aureus | 1 (3) | 1 (4) | 0 | 0 |
| Coagulase-negative Staphylococcus | 5 (17) | 2 (8) | 2 (12) | 5 (19) |
| Enterococcus spp. | 18 (62) | 12 (50) | 9 (53) | 9 (33) |
| Vancomycin-resistant enterococci | 1 (3) | 0 | 0 | 1 (4) |
| Escherichia coli | 6 (21) | 7 (29) | 2 (12) | 7 (26) |
| Klebsiella spp. | 1 (3) | 1 (4) | 0 | 1 (4) |
| Pseudomonas aeruginosa | 5 (17) | 8 (33) | 3 (18) | 3 (11) |
| Acinetobacter spp. | 10 (34) | 4 (17) | 5 (29) | 5 (19) |
| Other Gram-negative rods | 5 (17) | 8 (33) | 5 (29) | 5 (19) |
| Multidrug-resistant Gram-negative rods | 11 (38) | 8 (33) | 8 (47) | 11 (41) |
Cultures may have growth of more than one type of fungus. A total of 49 IFI case patients had wound cultures with fungal growth, while the other 5 case patients had histopathological fungal evidence with no mold isolated on culture.
Other molds include unidentified molds (7; 26%), Mycelia sterilia (6; 22%), Alternaria spp. (5; 19%), Ulocladium spp. (4; 15%), Penicillium spp. (6; 22%), Acrophialophora fusispora (3; 11%), and Beauveria spp. (1; 4%).
Wounds also grew mold that was not otherwise specified.
NA, not applicable.
Includes both methicillin-resistant and methicillin-susceptible.
Although serial cultures were recorded (from the same wound on more than 1 day), their collection was not standardized. Regarding serial cultures with order Mucorales or Aspergillus spp. growth, approximately 70% grew mold only on 1 day (data not shown). Of all IFI wounds with serial mold growth, approximately 55% grew mold in cultures collected within 3 days or less; whereas one wound grew mold for more than 1 month. In addition, Mucorales grew in 50% of these wounds, Aspergillus spp. in 39%, and Mucorales plus Aspergillus spp. in 11%.
Therapeutic management.
A significantly larger proportion of the IFI case patients received antifungal therapy than the non-IFI control patients (91% versus 19%; P < 0.001) who likely received antifungals as empirical therapy due to the high-risk injury pattern (10). More IFI case patients received dual therapy than controls (59% versus 9%; P < 0.001). Twelve (22%) IFI case patients received triple antifungal therapy (i.e., liposomal amphotericin, triazole, and echinocandin). The majority of patients in both groups received broad-spectrum antibiotics. Specifically, 94% and 93% of case patients received vancomycin and carbapenems, respectively. Regarding the control patients with SSTIs, 73% received vancomycin and 70% were prescribed carbapenems.
Among the IFI case patients, 64% received their first antifungal agent >7 days following injury (Table 5). Moreover, 67% of the IFI case patients continued antifungal therapy for >3 weeks. When wound mycology was considered, 58% of the IFI case patients with Mucorales growth began antifungal therapy >7 days postinjury and 79% were prescribed antifungals for >3 weeks (not significantly different from the patients with non-Mucorales growth). On a per wound basis, wounds with Mucorales growth had significantly more operating room (OR) visits than wounds with non-Mucorales growth (P = 0.003).
TABLE 5.
Surgical and medical management of patients with invasive fungus-infected case woundsa
| Characteristicb | Presence of fungus |
P value | ||
|---|---|---|---|---|
| Mucorales | Non-Mucorales | Total | ||
| Total no. of patientsc | 19 | 26 | 45 | |
| OR visits at LRMC and U.S. MTFs (median [IQR])d | 10.5 (9.0–14.0) | 9.0 (6.0–11.0) | 9.0 (6.0–12.0) | 0.003 |
| Type of antifungal usagee | ∼1.0 | |||
| Single | 2 (10.5) | 2 (7.7) | 4 (8.9) | |
| Double | 13 (68.4) | 19 (73.1) | 32 (71.1) | |
| Triple | 4 (21.1) | 5 (19.2) | 9 (20) | |
| Timing of receipt of first antifungal following injury | ||||
| Median days (IQR) | 8.0 (6.0–10.0) | 9.5 (7.0–12.0) | 9.0 (6.0–11.0) | 0.25 |
| Received antifungals within (no. [IQR]): | 0.43 | |||
| ≤7 days | 8 (42.1) | 8 (30.8) | 16 (35.6) | |
| >7 days | 11 (57.9) | 18 (69.2) | 29 (64.4) | |
| Duration of antifungal use | ||||
| Days (median [IQR]) | 31.0 (22.0–44.0) | 28.5 (19.0–47.0) | 31.0 (20.0–45.0) | 0.88 |
| Receipt of antifungals (no. [%]): | 0.20 | |||
| ≤21 days | 4 (21.1) | 11 (42.3) | 15 (33.3) | |
| >21 days | 15 (78.9) | 15 (57.7) | 30 (66.7) | |
Data are on a per patient basis.
IQR, interquartile range; LRMC, Landstuhl Regional Medical Center; MTF, military treatment facility; OR, operating room.
Extreme observations and outliers were excluded from the antifungal analysis. Overall patient totals are 5 for single, 32 for double, and 12 for triple.
OR visit data are on a per wound basis from the population of 45 patients (29 wounds with Mucorales and 48 wounds without Mucorales).
Single, amphotericin B or voriconazole; double, amphotericin B plus a triazole; triple, amphotericin B, a triazole, and an echinocandin.
Time to initial wound closure.
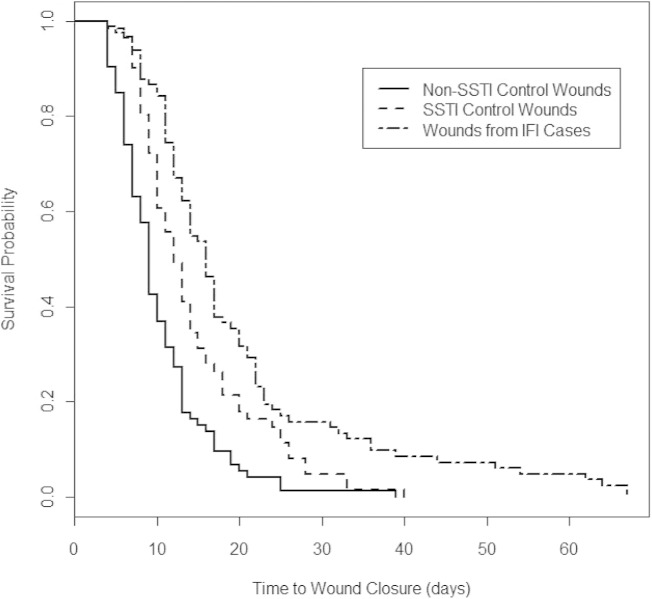
Overall, IFI wounds had a significantly higher number of OR visits (surrogate for surgical procedures; P = 0.01) and a longer postinjury duration to wound closure than non-IFI wounds with SSTIs (P = 0.008) (Table 2). Furthermore, non-IFI wounds with SSTIs had greater numbers of OR visits and longer times to wound closure than control wounds without any bacterial or fungal infection (P = 0.002 and P < 0.001, respectively). A significantly longer duration to wound closure following injury for the IFI case wounds was confirmed (P < 0.001) (Fig. 1).
FIG 1.
Kaplan-Meier survival plots of times to wound closure by wound types. Plots were created using R version 2.13.2 (R Project for Statistical Computing, Vienna, Austria). Log rank chi-square, 41.5 (P < 0.001); Wilcoxon chi-square test, 46.3 (P < 0.001). IFI, invasive fungal wound infection; SSTI, skin and soft tissue wound infection.
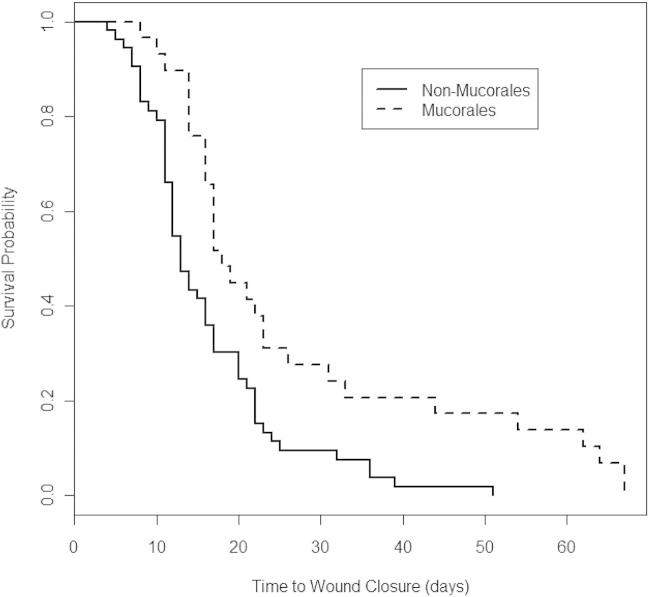
Among the IFI cases, wounds with Mucorales growth had significantly longer durations from injury to wound closure than non-Mucorales growth wounds (median of 17 versus 13 days, respectively; P < 0.01) (Fig. 2). The median times to closure for wounds with Mucorales growth in a single culture and repeated cultures were 17 and 19 days, respectively. For wounds with Aspergillus growth in single and repeated cultures, wound closures occurred in medians of 14 and 17 days, respectively. Regardless of whether the wound grew Mucorales or Aspergillus in either a single culture or repeated cultures, there was no significant difference in the timing of wound closure (P = 0.22). When wounds were stratified by Mucorales or non-Mucorales growth, the timing of the antifungal initiation (odds ratio, 1.9; 95% confidence interval [CI], 0.7 to 5.2) and the duration of use (odds ratio, 1.6; 95% CI, 0.6 to 4.4) were also not statistically associated with a shorter duration to wound closure.
FIG 2.
Kaplan-Meier survival plots of times to wound closure based on the presence of Mucorales among wounds with invasive fungal infections. Plots were created using R version 2.13.2 (R Project for Statistical Computing, Vienna, Austria). The analysis included 29 wounds with and 53 wounds without Mucorales growth. Log rank chi-square test, 10.4 (P = 0.001); Wilcoxon chi-square test, 9.7 (P = 0.002).
DISCUSSION
Wound bacterial infections, especially with multidrug-resistant organisms, can also lead to poor outcomes, and many IFI wounds have concurrent/secondary bacterial SSTIs. Furthermore, IFI patients tend to be more severely injured, often sustaining multiple traumatic amputations, which complicate attempts to ascertain the effects of IFIs and SSTIs on outcomes. Our primary aim was to determine whether IFI patients have more critical illnesses and worse outcomes than the larger cohort simply because an IFI is a marker of more severe injuries. Furthermore, does the pathophysiology from the mold infection itself contributes to the increased morbidity, delaying wound closure, and, if so, to what degree, and does morbidity differ by mold type, duration of positivity, or the polymicrobial nature of infection?
To best answer these questions, we carried out a case-control study on a per-wound level. The IFI wounds were matched to control wounds by ISS, along with blood transfusion requirements and extremity injury patterns, providing a combat trauma wound population with risk factors similar to those of patients who ultimately develop IFIs. Moreover, to fully assess the specific impact of fungal infection, we utilized two types of comparative wounds (i.e., with/without bacterial SSTIs). The outcome of time to wound closure was selected since this action is the result of the surgeon's judgment based on wound appearance and the patient's clinical status. Thus, a longer time to wound closure is a surrogate indicator for clinical evidence of infection. Overall, the time to wound closure was longer for IFI wounds than for control wounds with SSTIs (P = 0.008) (Table 2) and for those without either bacterial or fungal infections (P < 0.001) (Fig. 1). Although the differences in median wound closure times were only 4 and 7 days when the IFI wounds were compared to the non-IFI wounds with/without SSTIs, respectively, the delay was still indicative of the greater clinical impact of fungal infections. It should be noted that physicians may be conservative with regard to wound closure in cases where an IFI is apparent. A further limitation is that observer bias may have influenced wound closure timing when the type of mold (i.e., Mucorales) was already known due to the perception of pathogenicity. Nevertheless, the impact on wound closure is corroborated by the greater numbers of OR visits among the patients with IFI wounds than of the SSTI controls in our analysis and the significant association of IFI wounds and proximal amputation revisions along with early complication rates found in a prior analysis (12).
Our investigation confirms the complexity of fungus-infected wounds, which may provide an explanation for the significantly longer time to wound closure for the IFI wounds. Specifically, of the 82 IFI wounds, 27% were infected with more than one mold type, and 63% grew bacteria. The fact that 35% of the IFI wounds grew MDR-GNR highlights the challenges of managing these infections.
As IFI clinical awareness among military physicians expanded, efforts for earlier diagnosis, such as a local clinical practice guidance, were implemented (10). This effort led to earlier diagnosis and treatment, but the effects of screening “high-risk” patients also led to more positive wound cultures for molds (58% of patients who met the screening criteria and were found to not have an IFI but had wound culture mold growth) (10). Thus, an important clinical question in IFI patients with growth of multiple organisms (both fungal and bacterial) is which are pathogenic and require targeted antimicrobial therapy and which are noninvasive wound colonizers? For IFI wounds, the times to the first fungal and bacterial growth were the same, while for the non-SSTI controls, fungal colonization occurred earlier than bacterial (Table 3).
Among the IFI wounds in our analysis, many grew more than one mold type. For instance, combinations of the three predominant mold types (Mucorales, Aspergillus, and Fusarium) each grew from five different IFI wounds. A large proportion of these wounds also grew Enterococcus spp. (50 to 62%) and MDR-GNR (33 to 47%). Furthermore, the 27 IFI wounds that grew other/unidentifiable molds also grew predominant mold types (e.g., 15% grew Mucorales and 19% Aspergillus), in addition to Enterococcus spp. (33%) and MDR-GNR (41%). Given the large number of various copathogen combinations, direct comparisons regarding outcomes or management among particular microbial combinations was not possible.
Trauma-related IFIs are frequently reported to involve Mucorales (4–8, 13). Therefore, it was important to determine if wounds that grew Mucorales were different from other IFI wounds with regard to outcome or management. Overall, IFI wounds with Mucorales growth did in fact have longer times to wound closure than other IFI wounds (Fig. 2) and a higher number of OR visits, indicative of the need for aggressive surgical care. These data confirm the clinical suspicion of the more pathogenic nature of mucormycosis compared to that of other mold infections (13, 20, 21). Nonetheless, our data also suggest that Mucorales group molds are not the only pathogenic molds of concern in this patient population. Only five of the IFI wounds with Aspergillus spp. and Fusarium spp. grew concomitant Mucorales (Table 4). This highlights the pathogenic nature of these other molds and supports the clinical practice of early empirical therapy with both liposomal amphotericin B and voriconazole, as potential copathogenic molds are not uniformly sensitive to amphotericin B (22, 23).
Empirical dual therapy was used in nearly all cases (>90% of IFI patients), but times to initiation of antifungal therapy and durations were varied. We evaluated these treatment patterns to detect any difference between mucormycosis and non-Mucorales patients, and no difference in either parameter was found. The median durations of antifungal use were 31 days for Mucorales and 28.5 days for other molds. Furthermore, wound closure was not impacted by timing or duration of antifungal use.
One limitation in this study is that fungal organisms do not always grow in culture. Among the IFI case patients, 9% had no fungal culture growth but met the IFI case definition based on histopathology. In particular, of the 53 IFI case wounds that did not have Mucorales growth, 4 wounds had aseptate mold seen on histopathology. This morphology is characteristic of the order Mucorales, so these wounds may have been additional cases of invasive mucormycosis. Nevertheless, since they did not grow Mucorales in culture, they were not included in the Mucorales group for analysis. We feel it was reasonable to limit our study to culture data, as a degree of discordance has been demonstrated between mold culture growth and fungal morphology on histopathology (S. M. Heaton, A. C. Weintrob, K. Downing, B. Keenan, D. Aggarwal, F. Shaikh, D. R. Tribble, and J. Wells, manuscript in preparation).
Our data suggest that IFI wounds may be more complex than wounds without fungal infections. Specifically, times to wound closure for IFI wounds were significantly longer than those for wounds with SSTIs but lacking fungal infection. This complexity is likely due to the magnitude of tissue loss and severity of the blast injury, along with virulence and tissue destruction due to mold infection (11). While this finding highlights the morbidity of molds in trauma wounds in general, the longer times to wound closure for Mucorales infections suggests increased pathogenicity of this mold type in these wounds. The presence of bacteria, including MDR-GNR, in the IFI wounds is an important feature as well. Future studies to further analyze the role of bacteria in IFI wounds should include elucidating any pathogenic role of enterococci, as well as analyzing changing microbiology over time. Presently, we are assessing the methodology related to histopathology (i.e., frozen sections and special stains) and molecular PCR-based assays with regard to diagnosis of IFIs.
ACKNOWLEDGMENTS
This work (IDCRP-024) was supported by the Infectious Disease Clinical Research Program (IDCRP), a Department of Defense program executed through the Uniformed Services University of the Health Sciences. This project has been funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Inter-Agency Agreement Y1-AI-5072, and the Department of the Navy under the Wounded, Ill, and Injured Program (HU001-10-1-0014).
We are indebted to the Infectious Disease Clinical Research Program Trauma Infectious Disease Outcomes Study team of clinical coordinators, microbiology technicians, data managers, clinical site managers, and administrative support personnel for their tireless hours to ensure the success of this project.
The views expressed are those of the authors do not necessarily reflect the official views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, the National Institutes of Health or the Department of Health and Human Services, Brooke Army Medical Center, Walter Reed National Military Medical Center, Landstuhl Regional Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense, or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organization does not imply endorsement by the U.S. Government.
REFERENCES
- 1.Paolino KM, Henry JA, Hospenthal DR, Wortmann GW, Hartzell JD. 2012. Invasive fungal infections following combat-related injury. Mil Med 177:681–685. doi: 10.7205/MILMED-D-11-00364. [DOI] [PubMed] [Google Scholar]
- 2.Warkentien T, Rodriguez C, Lloyd B, Wells J, Weintrob A, Dunne J, Ganesan A, Li P, Bradley W, Gaskins LJ, Seillier-Moiseiwitsch F, Murray CK, Millar EV, Keenan B, Paolino K, Fleming M, Hospenthal DR, Wortmann GW, Landrum ML, Kortepeter MG, Tribble DR. 2012. Invasive mold infections following combat-related injuries. Clin Infect Dis 55:1441–1449. doi: 10.1093/cid/cis749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radowsky JS, Strawn AA, Sherwood J, Braden A, Liston W. 2011. Invasive mucormycosis and aspergillosis in a healthy 22-year-old battle casualty: case report. Surg Infect (Larchmt) 12:397–400. doi: 10.1089/sur.2010.065. [DOI] [PubMed] [Google Scholar]
- 4.Neblett Fanfair R, Benedict K, Bos J, Bennett SD, Lo Y-C, Adebanjo T, Etienne K, Deak E, Derado G, Shieh W-J, Drew C, Zaki S, Sugerman D, Gade L, Thompson EH, Sutton DA, Engelthaler DM, Schupp JM, Brandt ME, Harris JR, Lockhart SR, Turabelidze G, Park BJ. 2012. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med 367:2214–2225. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 5.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 6.Vitrat-Hincky V, Lebeau B, Bozonnet E, Falcon D, Pradel P, Faure O, Aubert A, Piolat C, Grillot R, Pelloux H. 2009. Severe filamentous fungal infections after widespread tissue damage due to traumatic injury: six cases and review of the literature. Scand J Infect Dis 41:491–500. doi: 10.1080/00365540902856537. [DOI] [PubMed] [Google Scholar]
- 7.Skiada A, Petrikkos G. 2009. Cutaneous zygomycosis. Clin Microbiol Infect 15(Suppl 5):S41–S45. [DOI] [PubMed] [Google Scholar]
- 8.Weintrob AC, Weisbrod AB, Dunne JR, Rodriguez CJ, Malone D, Lloyd BA, Warkentien T, Wells J, Murray CK, Bradley W, Shaikh F, Shah J, Aggarwal D, Carson ML, Tribble DR. 2015. Combat trauma-associated invasive fungal wound infections: Epidemiology and clinical classification. Epidemiol Infect 143:214–224. doi: 10.1017/S095026881400051X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lundy JB, Driscoll IR. 2014. Experience with proctectomy to manage combat casualties sustaining catastrophic perineal blast injury complicated by invasive mucor soft-tissue infections. Mil Med 179:e347–e350. doi: 10.7205/MILMED-D-13-00493. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd B, Weintrob A, Rodriguez C, Dunne J, Weisbrod A, Hinkle M, Warkentien T, Murray C, Oh J, Millar E, Shah J, Shaikh F, Gregg S, Lloyd G, Stevens J, Carson ML, Aggarwal D, Tribble DR. 2014. Effect of early screening for invasive fungal infections in U.S. service members with explosive blast injuries. Surg Infect (Larchmt) 15:619–626. doi: 10.1089/sur.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez C, Weintrob AC, Shah J, Malone D, Dunne JR, Weisbrod AB, Lloyd BA, Warkentien T, Murray CK, Wilkins K, Shaikh F, Carson ML, Aggarwal D, Tribble DR. 2014. Risk factors associated with invasive fungal infections in combat trauma. Surg Infect (Larchmt) 15:521–526. doi: 10.1089/sur.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewandowski L, Weintrob AC, Tribble DR, Rodriguez CJ, Petfield J, Lloyd BA, Murray CK, Stinner D, Aggarwal D, Shaikh F, Potter BK. 2014. Early complications and outcomes in combat injury-related invasive fungal infections: a case-control analysis, poster 152, p 590. Orthop Trauma Assoc 30th Annu Meet. Orthopaedic Trauma Association, Rosemont, IL: http://ota.org/media/161371/2014-OTA-AM-FP-interior-FINAL-LOW-RES.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ribes JA, Vanover-Sams CL, Baker DJ. 2000. Zygomycetes in human disease. Clin Microbiol Rev 13:236–301. doi: 10.1128/CMR.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tribble DR, Conger NG, Fraser S, Gleeson TD, Wilkins K, Antonille T, Weintrob A, Ganesan A, Gaskins LJ, Li P, Grandits G, Landrum ML, Hospenthal DR, Millar EV, Blackbourne LH, Dunne JR, Craft D, Mende K, Wortmann GW, Herlihy R, McDonald J, Murray CK. 2011. Infection-associated clinical outcomes in hospitalized medical evacuees after traumatic injury: Trauma Infectious Disease Outcome Study. J Trauma 71:S33–S42. doi: 10.1097/TA.0b013e318221162e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eastridge BJ, Jenkins D, Flaherty S, Schiller H, Holcomb JB. 2006. Trauma system development in a theater of war: Experiences from Operation Iraqi Freedom and Operation Enduring Freedom. J Trauma 61:1366–1372; discussion 1372–1373. doi: 10.1097/01.ta.0000245894.78941.90. [DOI] [PubMed] [Google Scholar]
- 16.Division of Healthcare Quality Promotion. 2008. The National Healthcare Safety Network (NHSN) manual. Patient safety component. Protocol multidrug-resistant organism (MDRO) and Clostridium difficile-associated disease (CDAD) module. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 17.Centers for Disease Control and Prevention. 2015. CDC/NHSN surveillance definitions for specific types of infections. http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
- 18.Clinical and Laboratory Standards Institute. 2009. Analysis and presentation of cumulative antimicrobial susceptibility test data; approved guideline, 3rd ed CLSI document M39-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 19.Linn S. 1995. The injury severity score—importance and uses. Ann Epidemiol 5:440–446. doi: 10.1016/1047-2797(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 20.Lewis RE, Lortholary O, Spellberg B, Roilides E, Kontoyiannis DP, Walsh TJ. 2012. How does antifungal pharmacology differ for mucormycosis versus aspergillus? Clin Infect Dis 54(Suppl 1):S67–S72. doi: 10.1093/cid/cir884. [DOI] [PubMed] [Google Scholar]
- 21.Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, Bitar D, Dromer F, Lortholary O, French Mycosis Study Group. 2012. A global analysis of mucormycosis in France: the RetroZygo Study (2005-2007). Clin Infect Dis 54 (Suppl 1) :S35–S43. doi: 10.1093/cid/cir880. [DOI] [PubMed] [Google Scholar]
- 22.Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH. 2011. Overview of treatment options for invasive fungal infections. Med Mycol 49:561–580. doi: 10.3109/13693786.2011.560197. [DOI] [PubMed] [Google Scholar]
- 23.Kriengkauykiat J, Ito JI, Dadwal SS. 2011. Epidemiology and treatment approaches in management of invasive fungal infections. Clin Epidemiol 3:175–191. doi: 10.2147/CLEP.S12502. [DOI] [PMC free article] [PubMed] [Google Scholar]