Abstract
Despite increasing reports of human infection, data about the optimal care of Phaeoacremonium infections are missing. We report a case of an infection due to Phaeoacremonium parasiticum and Paraconiothyrium cyclothyrioides, initially localized to skin and soft tissue, in a kidney transplant patient. Despite surgical drainage and excision of the lesion and combination antifungal therapy with voriconazole and liposomal amphotericin B, a disseminated infection involving the lungs and brain developed and led to death. We performed a systematic literature review to assess the general features and outcome of human infections due to Phaeoacremonium species. Thirty-six articles were selected, and 42 patients, including ours, were reviewed. Thirty-one patients (74%) were immunocompromised because of organ or bone marrow transplantation (n = 17), diabetes or glucose intolerance (n = 10), rheumatoid arthritis or Still's disease (n = 4), chronic hematological diseases (n = 3), or chronic granulomatous disease (n = 3). Ten patients (24%) reported initial cutaneous trauma. Skin and soft tissue infections represented 57% of infections (n = 24), and disseminated infections, all occurring in immunocompromised patients, represented 14% of infections (n = 6). The main antifungal drugs used were azoles (n = 41) and amphotericin B (n = 16). Surgical excision or drainage was performed in 64% of cases (n = 27). The cure rate was 67% (n = 28). There were 10% cases of treatment failure or partial response (n = 4), 19% relapses (n = 8), and 7% losses to follow-up (n = 3). The death rate was 19% (n = 8). Management of Phaeoacremonium infections is complex because of slow laboratory identification and limited clinical data, and treatment relies on a combination of surgery and systemic antifungal therapy.
INTRODUCTION
Reports of human diseases related to dark molds are increasing with the expanded spectrum of immunocompromised patients. Phaeohyphomycoses are a heterogenous group of cutaneous, subcutaneous, and systemic infections caused by fungi that are distributed worldwide, with melanized cell walls that develop in the host's tissue as dark-walled septate mycelial elements (1). Phaeoacremonium species, which are found in the environment in soil or in woody plants, as endophytes or as agents of plant disease, particularly in grapevines (2), are included in the phaeohyphomycosis group. Initially described in 1974 as Phialophora parasitica by Ajello et al. (3) and then transferred in 1996 to the new hyphomycete genus Phaeoacremonium by Crous et al., as Phaeoacremonium parasiticum (4), this fungus is a rare cause of human disease, occurring in both immunocompetent and immunosuppressed subjects. Reports of Phaeoacremonium infections are increasing over time, probably because laboratory confirmation of fungal pathogens has improved and because of the increase in immunocompromised conditions in the population. However, clinical and treatment data on Phaeoacremonium infection are scarce.
We describe a case of a disseminated infection due to Phaeoacremonium parasiticum and Paraconiothyrium cyclothyrioides in a kidney transplant recipient. We have performed a systematic review of the literature to identify clinical and mycological features and outcomes of human Phaeoacremonium infections.
CASE REPORT
A 71-year-old man underwent kidney transplantation in December 2011, having undergone hemodialysis for 10 years for end-stage nephroangiosclerosis.
He lived in Guadeloupe, French Caribbean, and was a retired post office employee. He reported regular gardening activity during his spare time. Several years prior to admission, he had noticed a painless, nonpruriginous nodule on the internal edge of the right middle finger. This nodule did not change over the years. No significant skin trauma was mentioned by the patient.
In December 2012, the patient was hospitalized for renal graft rejection, despite treatment with cyclosporine, azathioprine, and prednisone. The patient received a double course of plasma exchange, rituximab, and intravenous immunoglobulins before switching to another immunosuppressive regimen that included 10 mg tacrolimus twice a day (b.i.d.), 500 mg mycophenolate-mofetil b.i.d., and 10 mg prednisone daily. The hospitalization was complicated by a blood collection around the renal graft with acute anemia, requiring blood transfusion. Nine days after the second immunosuppressive course, he developed cellulitis of the right third finger, with purulent ulceration on the internal edge from the previous nodule. He had neither fever nor signs of sepsis. Surgical excision was performed in January 2013, and empirical antibiotic treatment with amoxicillin-clavulanate was given for 7 days, leading to partial relief of his symptoms. Bacterial culture of the pus was negative, and the patient was discharged on no further antibiotherapy.
Three weeks later, the patient was readmitted with renal failure requiring hemodialysis, consistent with the extensive fibrosis and tubular atrophy noted on the transplant renal biopsy specimen. There was progression of the cellulitic lesion of his right hand, despite antibiotic treatment, with extension of the swelling and the persistence of purulent discharge. Another subcutaneous lesion subsequently appeared on the posterior part of his right elbow. The patient was afebrile. He was admitted in March 2013, 7 weeks after the development of the initial purulent lesion, to the Infectious Diseases Unit (Saint-Louis Hospital, Paris, France), where new microbiological swabs were collected from both lesions, including swabs for mycological analysis.
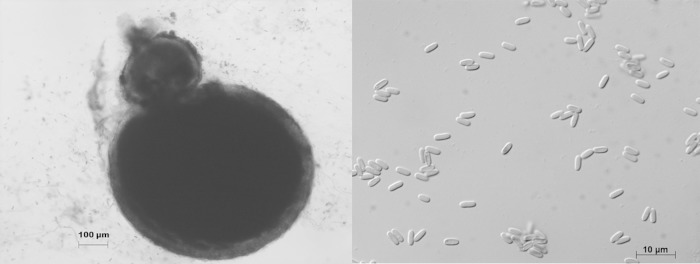
Microscopic examination of the pus collected from the phlegmon of the third finger of the right hand revealed 45° branched septate filaments. Primary culture on malt extract agar (MEA) medium with gentamicin and chloramphenicol recovered a filamentous fungus with woolly colonies that were initially white and evolved to gray with a beige reverse (Fig. 1). Microscopic examination of the colonies identified a Phaeoacremonium sp., based on the presence of long and branched pigmented conidiophores, mostly monophialidic phialides, and oblong conidia. Molecular identification based on the partial sequence of the β-tubulin target gene allowed the identification of Phaeoacremonium parasiticum (553/557 bp; 99.6% similarity to the type strain, CBS 860.73). The MICs determined using EUCAST methodology were as follows: amphotericin B (AMB), 0.25 mg/liter; itraconazole (ITC), 2 mg/liter; voriconazole (VRC), 0.25 mg/liter; and posaconazole (PSC), 0.125 mg/liter. The minimum effective concentration (MEC) for caspofungin (CAS) was 8 mg/liter, as determined upon microscopic observation and defined as the lowest concentration of caspofungin that led to changes in mycelium architecture.
FIG 1.
Microscopic features of Phaeoacremonium parasiticum upon culture on malt extract agar.
Direct microscopy of the right elbow pus revealed short, irregularly branched septate filaments harboring chlamydospores. Primary culture on MEA and Sabouraud dextrose agar (SDA) with gentamicin and chloramphenicol revealed a melanized filamentous fungus that was not microscopically identifiable due to a lack of fructification structures. Macroscopic examination after 20 days of incubation at 30°C showed the development of pycnidia (conidiomata) releasing small cylindrical conidia after 40 days of incubation (Fig. 2). Molecular identification based on the partial sequence of the β-tubulin target gene allowed the identification of Paraconiothyrium cyclothyrioides (463/469 bp; 98.7% similarity with the type strain, CBS 972.95). The MICs (EUCAST) were as follows: AMB, 0.25 mg/liter; ITC, 0.125 mg/liter; VRC, 0.25 mg/liter; and PSC, 0.03 mg/liter. The MEC for CAS was 4 mg/liter.
FIG 2.
Microscopic features of Paraconiothyrium cyclothyrioides upon culture on oatmeal agar. (Left) A conidioma. (Right) Cylindrical conidia.
Bacterial cultures were negative. From the sole plasma sample in which it was measured, plasma beta-(1-3)-d-glucan (Fungitell assay; Associates of Cape Cod Inc.) was detected at a level above 500 pg/ml (normal values, <80 pg/ml), whereas repeated serum galactomannan antigen testing (Platelia Aspergillus assay; Bio-Rad, Marnes-la-Coquette, France) was negative. Fungal cultures from bone marrow, blood, and sputum were negative after 3 weeks of incubation at 30°C.
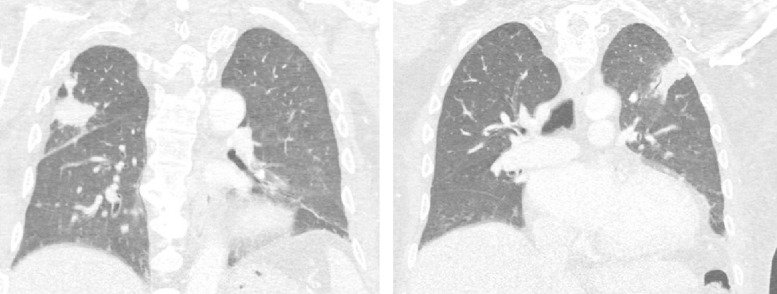
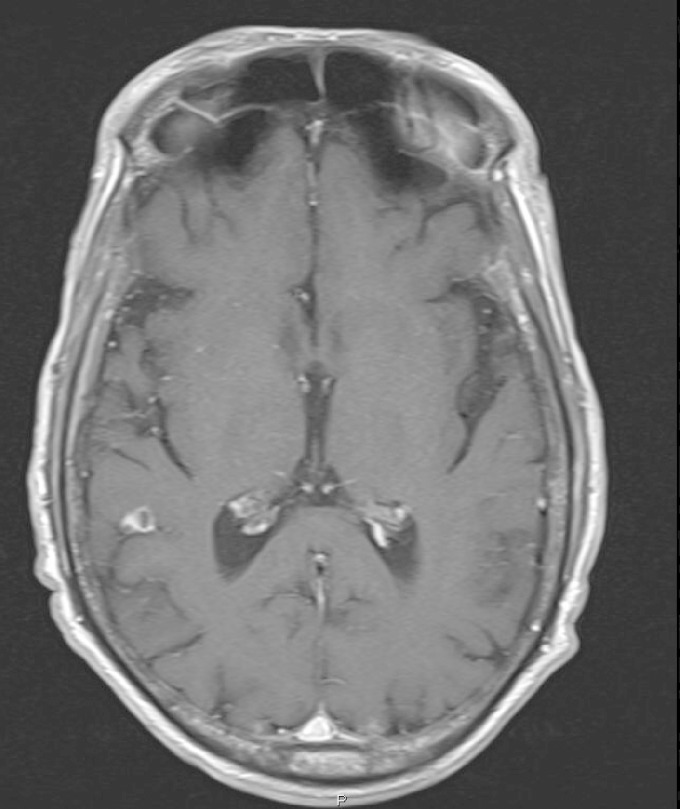
A systematic screen for secondary fungal localizations was performed. Chest X-ray and a computed tomography (CT) scan showed two bilateral consolidated opacities in the upper lung lobes (Fig. 3). Cranial magnetic resonance imaging (MRI) demonstrated a focal lesion in the right parietal lobe, measuring 0.4 by 0.4 cm, with focal vasogenic edema (Fig. 4). These lesions, though asymptomatic, were both considered likely to represent fungal metastases. X-rays of the upper limbs showed no bone involvement. Echocardiography and funduscopy did not show any sign of metastatic infection.
FIG 3.
CT scan showing two bilateral consolidated opacities in the right (left panel) and left (right panel) upper lobes.
FIG 4.
MRI T1 image with gadolinium showing a focal lesion in the right parietal lobe.
The patient started voriconazole at 400 mg b.i.d. orally on day 1 and then at 200 mg b.i.d., and based on antifungal susceptibility testing, this was combined with liposomal amphotericin B at 3 mg/kg of body weight/day.
Antirejection drugs (tacrolimus and mycophenolate-mofetil) were stopped, and steroids were rapidly tapered to 5 mg/day but then maintained to avoid graft necrosis.
Despite low MICs of voriconazole and amphotericin B and a second surgical debridement of the right hand cellulitis, the asymptomatic pulmonary and cerebral lesions enlarged by day 20 of antifungal treatment. The patient's trough plasma voriconazole level was 6.37 mg/liter (the recommended therapeutic range is 1 to 5.5 mg/liter according to Pascual et al. [5]), and thus the dose was subsequently lowered to 150 mg b.i.d. orally to avoid toxicity.
In addition, a urinary tract infection due to Citrobacter freundii was diagnosed when the patient became feverish. An abdominal CT scan showed a collected mass behind the renal graft, in the right iliac fossa, corresponding to the previously known hematoma. Purulent material collected by CT-guided drainage of this collection grew Citrobacter freundii, and cefepime (500 mg daily) was introduced. Fungal culture of the pus was sterile. Nevertheless, despite drainage of the infected hematoma and antifungal and antibiotic therapy, the patient died of bacterial sepsis and refractory septic shock in April 2013.
MATERIALS AND METHODS
Review of the literature.
We performed a systematic review of the literature by searching for articles using the following key words for the search in electronic databases: “Phaeoacremonium” and “phaeohyphomycosis” (MeSH key words). Relevant publications were selected using the PubMed, Embase, Google Scholar, and Cochrane databases through January 2015. The search was completed manually using the references of the most relevant studies. Reports about Phialophora parasitica, the name used for P. parasiticum before 1996, articles which did not concern human infections or Phaeoacremonium species or in which mycological identification of Phaeoacremonium was not ensured, and articles which lacked clinical data were excluded. Patients reported more than once are presented only once. A total of 36 articles fulfilled these criteria and were included in the review, corresponding to 41 cases. All are presented, along with our case, in Table 1 (42 total cases).
TABLE 1.
Reported cases of Phaeoacremonium infectiona
| Reference | Patient age (yr)/sex | Underlying disease | Type of infection | Distribution of lesions | Diagnostic specimen of procedure | Species (original identification) | Surgery | Antifungal treatmentb | Treatment duration (mo) | Evolution of disease or outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 6 | NA | Renal transplantation | SSTI | Arm | Cystic lesion | P. inflatipes | Yes | ITC | NA | Cured |
| 7 | 17/M | Chronic granulomatous disease | Pneumonia | Lung | Lung biopsy | P. parasiticum | Yes | CAS/ABLC/VRC/PSC | >19 | Cured |
| 7 | 9/M | Chronic granulomatous disease | Pneumonia | Lung | Lung biopsy | P. parasiticum | No | LAMB/ABLC/VRC/PSC | >4 | Cured |
| 8 | NA | Lung transplantation, rejection, diabetes | Osteomyelitis | Foot | MRI and biopsy | P. parasiticum | No | VRC | NA | Survived |
| 9 | 31/F | Aplastic anemia | Fungemia | Blood, skin lesions | Blood cultures, skin biopsy | P. parasiticum | No | ABLC | NA | Died (sepsis) |
| 9 | 40/M | Cardiac transplantation | SSTI | Right buttock, left forearm | Biopsy of nodular lesions | P. parasiticum | Yes | AMB/ITC/ABLC | 12 | Relapsed on ITC, died (heart disease) |
| 10 | 41/M | Renal transplantation | SSTI | Right forefinger | Biopsy of nodule | P. parasiticum | Yes | No | Relapsed and cured by surgery | |
| 11 | 76/F | Still's disease | SSTI | Right leg | Biopsy of abscess | P. rubrigenum | Yes | ITC | 3 | Cured |
| 12 | 26/M | Renal transplantation | Pulmonary nodules | Trachea, right lower lobe | Imaging, BAL, tracheal biopsy | P. parasiticum | No | ITC/VRC plus CAS | 2 | Partial response on VRC and CAS but then died (sepsis) |
| 12 | 69/M | Diabetes | Arthritis | Right knee | Arthroscopic biopsy | P. parasiticum | Yes | ITC | Prolonged course | Relapsed on ITC and cured by surgery |
| 13 | 49/M | Renal transplantation, diabetes | SSTI | Dorsum of left foot | Biopsy of cystic tumor | P. parasiticum | No | ITC plus local AMB | 2 | Cured |
| 18 | 49/M | Renal transplantation | Brain abscess | Disseminated brain lesions | Imaging, cerebral surgical biopsy | P. parasiticum and S. apiospermum | Yes | VRC | 8 | Cured |
| 19 | 28/M | Bone marrow transplantation | SSTI | Right arm and left knee | Biopsy of both sites | P. venezuelense and Plectophomella sp. | Yes | None | NA | LTFU |
| 20 | 49/F | Renal transplantation | Pulmonary nodules | Right upper lobe | Imaging, biopsy | Phaeoacremonium sp. | No | PSC | 4 | Cured |
| 28 | 56/M | Renal transplantation, diabetes | Myositis | Left thigh | Imaging | P. parasiticum | Yes | PSC | NA | Cured |
| 29 | 61/M | Renal transplantation, redialysis, diabetes | SSTI | Right forearm | Biopsy of abscess | Phaeoacremonium sp. | No | LAMB | 3 wk | Cured but died from pneumonia |
| 46 | 45/M | Liver transplantation | SSTI plus endocarditis | Interdigital space, blood | Blood cultures, valvular vegetations | P. parasiticum | No | AMB/FLC/AMB plus ITC | 3 | Died (disseminated infection) |
| 47 | 1.5/M | Aplastic anemia | Fungemia | Blood, spleen, liver | Blood and bone marrow cultures | P. inflatipes | No | AMB | 1 | Died (septic shock) |
| 48 | 24/M | Chronic granulomatous disease | Disseminated | Upper lung, temporal lobe | Imaging, cerebral biopsy | P. parasiticum | No | CAS plus LAMB/VRC plus TER | 1.5 | Died (fungal ventriculitis) |
| 49 | 74/M | Lung transplantation | Pulmonary nodules | Bilateral lungs | Imaging, no differential diagnosis | P. parasiticum | No | VRC | 3 | Cured |
| 50 | 52/M | Glucose intolerance | Spondylodiscitis | Cervical spine | Imaging | P. venezuelense | No | AMB/VRC | 6 | Cured |
| 51 | 59/F | None | SSTI | Right knee | Biopsy of cystic lesion | P. parasiticum | Yes | None | Cured | |
| 52 | 26/F | None | SSTI | Left forearm | Biopsy of lesion | P. parasiticum | Yes | AMB/ITC | 2 | Cured |
| 53 | 30/M | NA | SSTI | Left foot | Biopsy of foot mass | P. inflatipes | Yes | AMB/ITC | 2.5 | Cured |
| 54 | 61/F | Rheumatoid arthritis, hemodialysis | SSTI | Left foot | Biopsy of subcutaneous tumor | P. griseorubrum (P. rubrigenum) | Yes (3) | ITC/FLC | 5 | Relapsed on ITC and then on FLC |
| 55 | 55/M | Renal transplantation | SSTI | Left foot and ankle | Biopsy of nodules | P. parasiticum (P. rubrigenum) | Yes (2) | ITC/ITC plus TER/FLC | >11 | Relapsed on ITC and then on ITC plus TER, failure on FLC |
| 55 | 19/M | None | SSTI | Left ankle | Biopsy of nodule | P. alvesii (P. aleophilum) | Yes | ITC | 2 | Relapsed 6 times with surgery alone, cured with ITC and surgery |
| 56 | 61/M | Renal transplantation, diabetes | SSTI | Right middle finger | Extended swelling | P. aleophilum | No | ABLC/VRC | 1.5 | Cured |
| 57 | 54/M | Renal transplantation, diabetes | SSTI | Right middle finger | Biopsy of large mass | P. aleophilum | Yes | FLC plus AMB | 2 days | Cured |
| 58 | 74/M | Rheumatoid arthritis | SSTI | Left leg | Biopsy of a crusted nodule | Phaeoacremonium sp. | Yes | ITC | 23 | Cured |
| 59 | NA/M | Rheumatoid arthritis and vasculitis | SSTI | Dorsum of hand | Biopsy of nodule | P. parasiticum | Yes | None/VRC | 6 | Relapsed after surgery alone and cured with VRC and surgery |
| 59 | 57/M | Diabetes | Arthritis | Left knee | X-ray and MRI | P. parasiticum | No | FLC/ITC/AMB/LAMB/VRC | More than 7 | Cured |
| 60 | 66/M | None | SSTI | Ankle | Needle puncture | P. inflatipes | Yes | None | NA | Cured |
| 61 | 83/F | None | SSTI and arthritis | Left foot and knee | Biopsy of foot mass | P. alvesii (P. inflatipes) | Yes | None | NA | Cured |
| 62 | 14/F | None | Arthritis | Right knee | Imaging | Phaeoacremonium sp. | Yes | VRC | 12 | Cured |
| 63 | 66/M | Coronary disease | Tenosynovitis | Left middle finger | Imaging | Phaeoacremonium sp. | Yes | ITC | 3 | Relapse after surgery on ITC consistent with tendon rupture, cured by another surgery |
| 64 | 54/F | Myelodysplasia and IgA deficiency | Osteomyelitis and bursitis | Right olecranon | X-ray, bone erosion, puncture | Phaeoacremonium sp. | Yes | ITC | Quickly stopped because of side effects | Cured |
| 65 | 52/F | Diabetes | Mycetoma | Right foot | MRI, biopsy of nodules | P. parasiticum | Yes | ITC/AMB/ITC | >24 | Partial response after five surgical procedures and prolonged course of ITC |
| 66 | 41/M | NA | Mycetoma | Right foot | Biopsy of sinuses | P. krajdenii | No | ITC | 4 | LTFU |
| 67 | 55/M | None | Onychomycosis | Right big toenail | Nail biopsy | P. parasiticum | Yes | Topical sulconazole | 2 | Cured |
| 68 | 19/M | None | Endophthalmitis | Left eye | Vitreous biopsy | P. parasiticum | Yes | VRC plus local AMB | 2 | Partial response on VRC, LTFU |
| Our case | 70/M | Renal transplantation | SSTI and then disseminated | Pulmonary upper lobes, parietal lobe | Imaging, subcutaneous biopsy | P. parasiticum and P. cyclothyrioides | Yes | VRC/VRC plus LAMB | 1 | Died (sepsis) |
Abbreviations: ABLC, amphotericin B lipid complex; AMB, amphotericin B; BAL, bronchoalveolar lavage; CAS, caspofungin; F, female; FLC, fluconazole; ITC, itraconazole; LAMB, liposomal amphotericin B; LTFU, lost to follow-up; M, male; NA, not available; PSC, posaconazole; SSTI, skin and soft tissue infection; TER, terbinafine; VRC, voriconazole.
Successive courses are indicated by slashes.
RESULTS
Types of articles.
Articles identified in this literature review were mostly one- or two-patient case reports. We found one series of cases of phaeohyphomycosis (6), a series of fungal lung infections in patients with chronic granulomatous disease (7), and a series of invasive fungal infections in lung transplant patients (8), as well as four brief literature reviews, each reporting a few cases of Phaeoacremonium infections (9–12).
Patient characteristics.
More than two-thirds of patients were male (30/40 cases [75%]), with a median age of 52 years (range, 1.5 to 83 years). Only 28% of patients were over 60 years of age (11/39 patients), and most (31/42 cases [74%]) had underlying immunosuppressive medical conditions at the time when the Phaeoacremonium infection was diagnosed. Causes of immunosuppression were organ or bone marrow transplantation (17/31 cases [55%]), including 12 renal transplant recipients; chronic hematological diseases, such as aplastic anemia (2/31 cases [6%]) and myelodysplastic syndrome (1/31 cases [3%]); rheumatoid arthritis or Still's disease (3/31 cases [10%]); diabetes mellitus or glucose intolerance (10/31 cases [32%]); and chronic granulomatous disease (3/31 cases [10%]).
For 10 patients (10/42 patients [24%]), an initial cutaneous trauma was reported, usually several months or years earlier. Potential environmental exposure because of working or leisure activities was noted in 8 cases, including for our patient. In some cases, no trauma was noted (9).
Types of infection.
As the fungus is usually acquired by traumatic inoculation from soil or by contamination with material through unnoticed skin fissures (13), infections due to Phaeoacremonium were mostly localized to the skin and the soft tissues (24/42 cases [57%]) and were usually only subcutaneous (21/42 cases [50%]), including 2 mycetomas and 1 onychomycosis. In three patients, skin infection secondarily extended to joints or became disseminated. In eight patients (19%), isolated osteoarticular involvement was reported (arthritis, tenosynovitis, myositis, osteomyelitis, and spondylodiscitis). In five patients (12%), isolated pulmonary infections with an appearance consistent with pulmonary nodules were reported. Airway colonization has been described (12), and a pseudo-outbreak caused by a contaminated hospital ice dispenser was reported, with P. parasiticum recovered from bronchoscopy specimens from 31 patients, but with no infection (14). One patient had an endophthalmitis secondary to a penetrating globe injury a few years prior.
Six patients, including ours (14%), had invasive disseminated infections associated with fungemia (n = 3), brain abscesses (n = 3), endocarditis (n = 1), liver and spleen abscesses (n = 1), and skin infectious metastasis (n = 1). All were immunocompromised.
Diagnosis of Phaeoacremonium infection.
Using the European Organisation for Research and Treatment of Cancer (EORTC) criteria developed to define opportunistic invasive fungal infections in immunocompromised patients, positive cultures of Phaeoacremonium from sterile body sites are assumed to represent infection (15). Invasive fungal infection due to Phaeoacremonium is consistent on histopathological examination with the presence of hyphal elements with hematoxylin and eosin or periodic acid-Schiff stain and is more visible with Gomori methenamine silver staining, accompanied by associated inflammation and tissue damage. The combination of histopathological examination and tissue cultures is needed to establish a definitive diagnosis. A proven diagnosis of phaeohyphomycosis infection, with or without molecular identification of the Phaeoacremonium species, was available for 27 patients (67%), which is significantly more than the case for other invasive fungal infections, such as aspergillosis, in immunocompromised subjects, for whom possible and probable diagnoses represent almost all diagnoses (16).
Microscopic findings consist of branched, septate hyphae, simple hyphae, or hyphae occurring in strands that are smooth or verruculose to tuberculate and medium brown, becoming lighter brown to hyaline toward the conidiogenous region (2). Conidiophores are simple or branched and mostly pigmented, particularly the basal cells; phialides are aculeate, with a narrow collarette; conidia are generally allantoid, at least partly, and hyaline. Colonies on MEA are usually buff to gray-olivaceous, green-brown, or honey, rarely red, and moderately spreading and have gray to brown aerial mycelia.
The optimum temperature varies according to the Phaeoacremonium species, from 25 to 30°C, but Phaeoacremonium is also able to grow at temperatures of up to 40°C (1).
Identification of Phaeoacremonium species is difficult because the cultural and microscopic distinguishing characteristics are relatively minor (2). Also, some of the most common species causing human disease were not described before 2005 (or taxonomically redressed since then) and therefore were probably misidentified in earlier publications (9). Molecular methods have been developed to confirm species identifications (17); these are best supported by phylogenetic analyses of actin, β-tubulin, and calmodulin gene sequences. Mostert et al. developed a multiple-character electronic identification key to facilitate routine species identification based on micromorphological characters, such as conidiophore morphology, phialide type and morphology, conidial size and shape, and cultural characters, such as colony color on MEA, yellow pigment production on potato dextrose agar (PDA), growth rate at 25°C, and maximal growth temperature, in addition to phylogenetic analyses of selected regions of the β-tubulin gene (2). Using this identification key, four strains of Phaeoacremonium in our review were reidentified a posteriori (2). In our case, the culture sample was sent to the National Reference Laboratory (Institut Pasteur, Paris, France) to confirm the species identification by polyphasic identification.
Eight different Phaeoacremonium species causing human infections were recorded. The most frequent species identified in human infections were P. parasiticum, P. inflatipes, and P. rubrigenum, but emerging species, such as P. aleophilum and P. venezuelense, have been described since 2005 (1, 2). Phaeoacremonium parasiticum was the most prevalent species, causing 23 cases of infection (55%). Other species identified in this review were three cases of P. inflatipes, two cases each of P. alvesii and P. venezuelense, and one case each of P. aleophilum, P. griseorubrum, P. krajdenii, and P. rubrigenum. In six cases, species identification was not available. However, misidentification of Phaeoacremonium species could have occurred, as molecular diagnosis was available for only 38% of cases (16/42 cases), in addition to standard microscopic identification from fungal cultures. In four cases, the Phaeoacremonium species identification was retrospectively modified using molecular diagnosis after the initial microscopic diagnosis—former descriptions of P. rubrigenum, P. aleophilum, and P. inflatipes were changed to P. griseorubrum, P. parasiticum, and P. alvesii, respectively (2).
For three patients, including our patient, a second fungus was identified contemporaneously. One patient had a brain abscess due to P. parasiticum and Scedosporium apiospermum (18). The second had a subcutaneous infection, with different samples growing P. venezuelense and Plectophomella sp. (19). In our patient, P. cyclothyrioides was isolated in addition to P. parasiticum. All three patients were immunocompromised. In one case of Phaeoacremonium lung infection, another dematiaceous fungus (Dactylaria constricta) was isolated from bronchial fluid but not from the lung biopsy specimen and was assumed to be a colonizer of the respiratory tract rather than a pathogen (20).
Treatment.
There is no standard treatment for phaeohyphomycosis (21). The reported systemic antifungal agents used to treat Phaeoacremonium phaeohyphomycosis included amphotericin B in deoxycholate or lipid form, azoles (voriconazole, itraconazole, fluconazole, and posaconazole), terbinafine, and caspofungin, but the optimal antifungal treatment for invasive disease is uncertain. In vitro, Phaeoacremonium is usually susceptible to azoles, particularly voriconazole, and to amphotericin B (9, 22), but reduced amphotericin B and itraconazole sensitivities have been described (23, 24). In a large literature review of in vitro antifungal activities for reference antifungal agents on various fungi (23), MIC ranges for 18 isolates of P. parasiticum were 0.125 to 2 mg/liter for voriconazole, 0.125 to 16 mg/liter for amphotericin B, and 0.25 to 32 mg/liter for itraconazole. These are consistent with previous data (24). However, Phaeoacremonium sp. in vitro susceptibility testing against antifungal drugs is not standardized, and methods for MIC determination and breakpoints still need to be established (9).
Recent European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and European Confederation of Medical Mycology (ECMM) guidelines concerning phaeohyphomycosis proposed voriconazole for the treatment of central nervous system phaeohyphomycosis because of its ability to achieve adequate cerebrospinal fluid levels (25). Combination antifungal therapy is recommended in general for systemic phaeohyphomycosis, for cerebral abscesses when surgery is not possible, and for disseminated infections in immunocompromised patients (25), but there are neither data nor specific recommendations for Phaeoacremonium infections in these settings. There are also no data about the optimal dosing, treatment duration, and resistance management. Experts recommend a prolonged duration of therapy, ranging from weeks to months (25).
Overall, 86% of patients (36/42 patients) received systemic antifungal therapy. Different classes of antifungal drugs were used for the treatment of Phaeoacremonium phaeohyphomycosis. Azoles (41 prescriptions) were usually prescribed, including itraconazole (n = 18), voriconazole (n = 14), fluconazole (n = 5), and posaconazole (n = 4). Amphotericin B was used in 16 patients (38%), including liposomal amphotericin B (n = 5) and amphotericin B lipid complex (n = 5). Other antifungal systemic therapies were caspofungin (n = 3) and terbinafine (n = 2). Combinations of antifungal drugs and sequential therapies because of side effects, failure, or relapse were prescribed for 17% (6/36 patients) and 39% (14/36 patients) of treated patients, respectively.
Antifungal susceptibility testing was mentioned for nine patients, and MICs (or MECs) were specified in eight cases (Table 2). The lowest MICs were seen with the extended-spectrum triazoles voriconazole and posaconazole. In contrast, MICs of fluconazole and flucytosine were high, ranging from 8 to 64 mg/liter.
TABLE 2.
Antifungal susceptibilities of reported isolates of Phaeoacremonium spp.
| Reference | MIC or MEC (mg/liter) at indicated time(s) (h)a |
|||||||
|---|---|---|---|---|---|---|---|---|
| AMB | ITC | VRC | PSC | FLC | CAS | TER | 5FC | |
| 9 | 0.5/2 (48/72) | 1/8 (48/72) | 0.125/0.125 (48/72) | 0.25/0.25 (48/72) | NA | NA | NA | NA |
| 9 | 1/2 (48/72) | 0.03/0.125 (48/72) | 0.03/0.06 (48/72) | 0.03/0.03 (48/72) | NA | NA | NA | NA |
| 47 | 0.75 | NA | NA | NA | NA | NA | NA | NA |
| 48 | 2 (24) | NA | 0.25 (24) | 0.5 (24) | NA | >8 (24) | 0.5 (24) | NA |
| 55 | 2 (72) | >16 (72) | 1 (72) | NA | 8 (72) | NA | 2 (72) | NA |
| 55 | 2 (72) | 8 (72) | 1 (72) | NA | 8 (72) | NA | 2 (72) | NA |
| 68 | 0.25 | 1 | 0.06 | NA | 64 | NA | NA | 64 |
| This study | 0.25 | 2 | 0.25 | 0.125 | NA | 8 | NA | NA |
All data except those for CAS are MICs. Data for CAS are MECs. AMB, amphotericin B; ITC, itraconazole; VRC, voriconazole; PSC, posaconazole; FLC, fluconazole; CAS, caspofungin; TER, terbinafine; 5FC, 5-fluorocytosine; NA, not available.
The mean duration of systemic antifungal treatment was 3 months (range, 0.06 to 24 months) in the evaluable reports. Topical antifungal agents were used on three patients, in addition to surgical treatment and/or systemic antifungal drugs.
In addition, similarly to other invasive fungal infections (26), surgical resection should be considered for Phaeoacremonium phaeohyphomycosis to reduce the infectious inoculum size, particularly in brain abscesses. Resection of infected lesions permits the elimination of areas containing viable fungi in necrotic tissue or sites where antifungal drug penetration is low. Moreover, for subcutaneous and localized Phaeoacremonium infections, wide-excision surgery appears to be the main treatment option to avoid relapses and may be used alone to cure localized infections in immunocompetent subjects (9, 10).
Twenty-seven patients (64%) underwent surgical excision or drainage of the lesions, among whom 26 had localized infections. Among them, 14% of patients (6/42 patients) received no systemic antifungal therapy and were successfully cured by surgery alone. Ten patients underwent multiple surgical interventions (range, 2 to 7), and one underwent drainage of brain abscesses.
Among the patients who were immunocompromised, a reduction of the immunosuppressive therapy in order to improve the immune response against infection and/or to prevent drug-induced toxicity was mentioned in five cases, including our patient, for whom surgical removal of the transplanted kidney was planned to allow the cessation of prednisone, but unfortunately, he died before surgery.
Outcomes.
The follow-up duration was variable, with a median time of 12 months (range, 2 to 72 months), and it was unavailable for many (21/42 patients [50%]) patients. Overall, 28 patients (67%) were completely cured after treatment. Partial responses or failures were described for four patients (10%), and relapses for eight (19%). The number of relapses was variable (range, 1 to 6). Three patients (7%) were lost to follow-up.
Eight patients (19%) died, either related directly to the fungal infection (n = 2), related to preexisting medical conditions (n = 1), or related to another sepsis complication (n = 5). The median time between diagnosis and death due to infection was 60 days (range, 30 to 386 days). All of these patients were immunocompromised.
No death related to fungal infection was reported for those with localized infections.
DISCUSSION
Among the 42 different species of Phaeoacremonium already described (27), less than a fourth were involved in human infections, and the most frequent species identified in human infections was P. parasiticum, as in our report patient.
As environmental exposition and/or trauma was often reported prior to the infection, these infections were mainly subcutaneous and predominated in lower limbs. In solid organ transplant recipients, similarly to our report, infections due to dematiaceous fungi occur late and present most frequently as skin or soft tissue infections with an indolent course and delayed diagnosis (28), varying from 2 months to 14 years after transplantation, with the fungal lesion evolving over weeks to years before the microbiological diagnosis is made. Our patient developed a subcutaneous nodule of the finger many years ago that remained stable over time. We assumed that this nodule was a chronic localization of P. parasiticum probably contracted during his gardening activity, from which an invasive extension to soft tissue occurred following an increase in immunosuppressive therapy, a time when disseminated infections mainly concern immunocompromised subjects, such as organ transplant patients, and are associated with a poor prognosis (9).
We observed a high plasma level of (1-3)-β-d-glucan in our patient, whereas he had no clinical signs of disseminated infection. The (1-3)-β-d-glucan is a polysaccharide component of the cell walls of most fungi, and its measurement in plasma offers a noninvasive method for the surveillance and diagnosis of invasive fungal infections. To our knowledge, elevated levels of (1-3)-β-d-glucan have been reported only once for Phaeoacremonium infections (29), but with a low level compared to our case, and have never been described for Paraconiothyrium infections. We suggest that detection of serum (1-3)-β-d-glucan could be added to the management of Phaeoacremonium phaeohyphomycosis if a disseminated infection is suspected and/or for immunocompromised subjects. Ben-Ami et al. suggested a possible cross-reactivity of galactomannan-directed antibodies with dematiaceous molds, but there are no data concerning Phaeoacremonium (30). It should be noted that, in our case, the serum was negative for the galactomannan antigen.
Interestingly, in three patients, including ours, concomitant dual fungal infections were reported, involving Scedosporium apiospermum, Plectophomella sp., and Paraconiothyrium cyclothyrioides. Although infections by more than one fungus are not rare in immunocompromised patients (31–33), even by two species of the same genus (34) or by different serotypes of the same species (35), it is not easy to identify mixtures (19). Moreover, reports of mixed dematiaceous infections are scarce (36, 37). These data underline the need to ideally obtain tissue samples from involved organs to improve diagnosis and treatment. In our case, it was difficult to assess which fungus caused disseminated disease, in the absence of cerebral or pulmonary specimens and because of the adverse evolution of our patient's illness. We believe that Paraconiothyrium cyclothyrioides, which was also isolated from the pus of the left elbow lesion, may have been an aggravating factor. Nevertheless, because the optimum growth temperature for P. cyclothyrioides ranges from 27°C to 33°C (38), it is less likely that this fungus was involved in the disseminated lesions. Paraconiothyrium cyclothyrioides is a coelomycete found in the soil and has been reported in only two human infections (39, 40). In these reports, both patients were organ transplant recipients (kidney and liver) and had chronic skin lesions of the lower extremities. In one for whom the information was provided, posaconazole led to a complete resolution of the lesions (39). Other reports of Paraconiothyrium infection in humans involved P. maculicutis (41) and a Paraconiothyrium sp. (42), also in immunocompromised individuals with chronic skin lesions, while coelomycetes in general have been associated with subcutaneous infection (43), deep-seated infection (44), and disseminated infection (45) in immunocompromised patients.
In conclusion, we present a case of a fatal disseminated infection due to Phaeoacremonium parasiticum and Paraconiothyrium cyclothyrioides in a renal transplant recipient. A literature review emphasizes the fact that environmental exposure and comorbidities are important in Phaeoacremonium infection physiopathology, that appropriate identification may be difficult, and that optimal treatment should combine surgery and antifungal agents.
ACKNOWLEDGMENTS
We are greatly thankful to Dominic Dwyer and Jean-Michel Molina for their critical reviews of the manuscript and for English editing.
We have no transparency declarations to declare.
S.G. and M.-A.C. were responsible for the study concept and design; M.-A.C., G.M., A.A., B.D., D.G.-H., B.L., S.B., M.-N.P., and D.G. were responsible for the acquisition of data; M.-A.C., A.A., B.D., G.M., and S.G. were responsible for the analysis and interpretation of the data; M.-A.C., A.A., G.M., and S.G. drafted the manuscript; M.-A.C., G.M., A.A., B.D., B.L., S.B., M.-N.P., D.G., S.B., and S.G. performed critical revisions of the manuscript for important intellectual content; and S.G. was responsible for study supervision.
REFERENCES
- 1.Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW, Samson RA. 2006. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Stud Mycol 54:1–115. doi: 10.3114/sim.54.1.1. [DOI] [Google Scholar]
- 2.Mostert L, Groenewald JZ, Summerbell RC, Robert V, Sutton DA, Padhye AA, Crous PW. 2005. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J Clin Microbiol 43:1752–1767. doi: 10.1128/JCM.43.4.1752-1767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajello L, Georg LK, Steigbigel RT, Wang CJ. 1974. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia 66:490–498. doi: 10.2307/3758492. [DOI] [PubMed] [Google Scholar]
- 4.Crous PW, Gams W, Wingfield MJ, van Wyk PS. 1996. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88:786–796. doi: 10.2307/3760973. [DOI] [Google Scholar]
- 5.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis 46:201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa MM, Galante NZ, Godoy P, Fischman-Gompertz O, Martelli F, Colombo AL, Tomimori J, Medina-Pestana JO. 2009. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J Am Acad Dermatol 61:977–985. doi: 10.1016/j.jaad.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Segal BH, Barnhart LA, Anderson VL, Walsh TJ, Malech HL, Holland SM. 2005. Posaconazole as salvage therapy in patients with chronic granulomatous disease and invasive filamentous fungal infection. Clin Infect Dis 40:1684–1688. doi: 10.1086/430068. [DOI] [PubMed] [Google Scholar]
- 8.Pinney MF, Rosenberg AF, Hampp C, Schain D, Akindipe O, Baz M. 2011. Invasive fungal infections in lung transplant recipients not receiving routine systemic antifungal prophylaxis: 12-year experience at a university lung transplant center. Pharmacotherapy 31:537–545. doi: 10.1592/phco.31.6.537. [DOI] [PubMed] [Google Scholar]
- 9.Baddley JW, Mostert L, Summerbell RC, Moser SA. 2006. Phaeoacremonium parasiticum infections confirmed by beta-tubulin sequence analysis of case isolates. J Clin Microbiol 44:2207–2211. doi: 10.1128/JCM.00205-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farina C, Gotti E, Mouniée D, Boiron P, Goglio A. 2007. Phaeoacremonium parasiticum subcutaneous infection in a kidney-transplanted patient successfully treated by surgery. Transpl Infect Dis 9:253–255. doi: 10.1111/j.1399-3062.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 11.Furudate S, Sasai S, Numata Y, Fujimura T, Aiba S. 2012. Phaeohyphomycosis caused by Phaeoacremonium rubrigenum in an immunosuppressive patient: a case report and review of the literature. Case Rep Dermatol 4:119–124. doi: 10.1159/000339622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To KK, Lau SK, Wu AK, Lee RA, Ngan AH, Tsang CC, Ling IW, Yuen KY, Woo PC. 2012. Phaeoacremonium parasiticum invasive infections and airway colonization characterized by agar block smear and ITS and β-tubulin gene sequencing. Diagn Microbiol Infect Dis 74:190–197. doi: 10.1016/j.diagmicrobio.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Marques SA, Camargo RMP, Summerbell RC, De Hoog GS, Ishioka P, Chambô-Cordaro LM, Marques ME. 2006. Subcutaneous phaeohyphomycosis caused by Phaeoacremonium parasiticum in a renal transplant patient. Med Mycol 44:671–676. doi: 10.1080/13693780600895181. [DOI] [PubMed] [Google Scholar]
- 14.Blake M, Embil JM, Trepman E, Adam H, Myers R, Mutcher P. 2014. Pseudo-outbreak of Phaeoacremonium parasiticum from a hospital ice dispenser. Infect Control Hosp Epidemiol 35:1063–1065. doi: 10.1086/677150. [DOI] [PubMed] [Google Scholar]
- 15.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrissey CO, Chen SC, Sorrell TC, Milliken S, Bardy PG, Bradstock KF, Szer J, Halliday CL, Gilroy NM, Moore J, Schwarer AP, Guy S, Bajel A, Tramontana AR, Spelman T, Slavin MA, Australasian Leukaemia Lymphoma Group and the Australia and New Zealand Mycology Interest Group. 2013. Galactomannan and PCR versus culture and histology for directing use of antifungal treatment for invasive aspergillosis in high-risk haematology patients: a randomised controlled trial. Lancet Infect Dis 13:519–528. doi: 10.1016/S1473-3099(13)70076-8. [DOI] [PubMed] [Google Scholar]
- 17.Aroca A, Raposo R, Lunello P. 2008. A biomarker for the identification of four Phaeoacremonium species using the beta-tubulin gene as the target sequence. Appl Microbiol Biotechnol 80:1131–1140. doi: 10.1007/s00253-008-1647-3. [DOI] [PubMed] [Google Scholar]
- 18.Larbcharoensub N, Chongtrakool P, Wirojtananugoon C, Watcharananan SP, Sumethkul V, Boongird A, Jirasiritham S. 2013. Treatment of a brain abscess caused by Scedosporium apiospermum and Phaeoacremonium parasiticum in a renal transplant recipient. Southeast Asian J Trop Med Public Health 44:484–489. [PubMed] [Google Scholar]
- 19.Guarro J, Silvestre AM, Verkley G, Cano J, Gompertz OF, Gené J, Ogawa MM, Tomimori-Yamashita J, Teixeira SP, de Almeida FA. 2006. Limitations of DNA sequencing for diagnosis of a mixed infection by two fungi, Phaeoacremonium venezuelense and a Plectophomella sp., in a transplant recipient. J Clin Microbiol 44:4279–4282. doi: 10.1128/JCM.00496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monaganti S, Santos CAQ, Markwardt A, Pence MA, Brennan DC. 2014. Pulmonary phaeohyphomycosis caused by Phaeoacremonium in a kidney transplant recipient: successful treatment with posaconazole. Case Rep Med 2014:902818. doi: 10.1155/2014/902818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chabasse D. 2002. Phaeohyphomycetes agents of phaeohyphomycosis: emerging fungus. J Mycol Med 12:65–85. [Google Scholar]
- 22.De Hoog GS, Guarro J, Gené J, Figueras MJ. 2000. Atlas of clinical fungi. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- 23.Espinel-Ingroff A, Boyle K, Sheehan DJ. 2001. In vitro antifungal activities of voriconazole and reference agents as determined by NCCLS methods: review of the literature. Mycopathologia 150:101–115. doi: 10.1023/A:1010954803886. [DOI] [PubMed] [Google Scholar]
- 24.McGinnis MR, Pasarell L. 1998. In vitro testing of susceptibilities of filamentous ascomycetes to voriconazole, itraconazole, and amphotericin B, with consideration of phylogenetic implications. J Clin Microbiol 36:2353–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chowdhary A, Meis JF, Guarro J, de Hoog GS, Kathuria S, Arendrup MC, Arikan-Akdagli S, Akova M, Boekhout T, Caira M, Guinea J, Chakrabarti A, Dannaoui E, van Diepeningen A, Freiberger T, Groll AH, Hope WW, Johnson E, Lackner M, Lagrou K, Lanternier F, Lass-Flörl C, Lortholary O, Meletiadis J, Muñoz P, Pagano L, Petrikkos G, Richardson MD, Roilides E, Skiada A, Tortorano AM, Ullmann AJ, Verweij PE, Cornely OA, Cuenca-Estrella M, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of systemic phaeohyphomycosis: diseases caused by black fungi. Clin Microbiol Infect 20(Suppl 3):S47–S75. doi: 10.1111/1469-0691.12515. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, Schuler U, Lutsar I, Troke P, Thiel E. 2005. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 106:2641–2645. doi: 10.1182/blood-2005-02-0733. [DOI] [PubMed] [Google Scholar]
- 27.Raimondo ML, Lops F, Carlucci A. 2014. Phaeoacremonium italicum sp. nov., associated with esca of grapevine in southern Italy. Mycologia 106:1119–1126. doi: 10.3852/14-080. [DOI] [PubMed] [Google Scholar]
- 28.Mulcahy H, Chew FS. 2011. Phaeoacremonium parasiticum myositis: a case report with imaging findings. Radiol Case Rep 6:485. doi: 10.2484/rcr.v6i2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakata Y, Kitayama A, Yoshimura R, Anzawa K, Fujii T, Fujimoto K, Yokoyama H, Mochizuki T. 2015. Case of cutaneous phaeohyphomycosis caused by Phaeoacremonium sp. in a renal transplant recipient. J Dermatol 42:263–266. doi: 10.1111/1346-8138.12719. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Ami R, Lewis RE, Raad II, Kontoyiannis DP. 2009. Phaeohyphomycosis in a tertiary care cancer center. Clin Infect Dis 48:1033–1041. doi: 10.1086/597400. [DOI] [PubMed] [Google Scholar]
- 31.Lopes JO, de Mello ES, Klock C. 1995. Mixed intranasal infection caused by Fusarium solani and a zygomycete in a leukaemic patient. Mycoses 38:281–284. doi: 10.1111/j.1439-0507.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 32.Vasiloudes P, Morelli JG, Weston WL. 1997. Painful skin papules caused by concomitant Acremonium and Fusarium infection in a neutropenic child. J Am Acad Dermatol 37:1006–1008. doi: 10.1016/S0190-9622(97)70087-2. [DOI] [PubMed] [Google Scholar]
- 33.Jaya S, Vipparti H. 2014. Mixed fungal lung infection with Aspergillus fumigatus and Candida albicans in an immunocompromised patient: case report. J Clin Diagn Res 8:DD08–DD10. doi: 10.7860/JCDR/2014/8048.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guarro J, Nucci M, Akiti T, Gené J. 2000. Mixed infection caused by two species of Fusarium in a human immunodeficiency virus-positive patient. J Clin Microbiol 38:3460–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desnos-Ollivier M, Patel S, Spaulding AR, Charlier C, Garcia-Hermoso D, Nielsen K, Dromer F. 2010. Mixed infections and in vivo evolution in the human fungal pathogen Cryptococcus neoformans. mBio 1:e00091-10. doi: 10.1128/mBio.00091-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marriott DJ, Wong KH, Aznar E, Harkness JL, Cooper DA, Muir D. 1997. Scytalidium dimidiatum and Lecythophora hoffmannii: unusual causes of fungal infections in a patient with AIDS. J Clin Microbiol 35:2949–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavoie SR, Espinel-Ingroff A, Kerkering T. 1993. Mixed cutaneous phaeohyphomycosis in a cocaine user. Clin Infect Dis 17:114–116. doi: 10.1093/clinids/17.1.114. [DOI] [PubMed] [Google Scholar]
- 38.Verkley G, da Silva M, Wicklow D, Crous P. 2004. Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Stud Mycol 50:323–335. [Google Scholar]
- 39.Gordon RA, Sutton DA, Thompson EH, Shrikanth V, Verkley GJ, Stielow JB, Mays R, Oleske D, Morrison LK, Lapolla WJ, Galfione S, Tyring S, Samathanam CA, Fu J, Wickes BL, Mulanovich V, Wanger A, Arias CA. 2012. Cutaneous phaeohyphomycosis caused by Paraconiothyrium cyclothyrioides. J Clin Microbiol 50:3795–3798. doi: 10.1128/JCM.01943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balajee SA, Sigler L, Brandt ME. 2007. DNA and the classical way: identification of medically important molds in the 21st century. Med Mycol 45:475–490. doi: 10.1080/13693780701449425. [DOI] [PubMed] [Google Scholar]
- 41.De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW. 2013. Redisposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36. doi: 10.3114/sim0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quillet-Dye C, Meniane J-C, Quist D, Desbois N. 2012. Triple cutaneous mycosis (Cunninghamella bertholletiae, Phomopsis spp and Paraconiothyrium spp) in an immunocompromised patient: a Martinican case report. J Mycol Med 22:357–361. doi: 10.1016/j.mycmed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Everett JE, Busick NP, Sielaff T, Wahoff DC, Dunn DL. 2003. A deeply invasive Phoma species infection in a renal transplant recipient. Transplant Proc 35:1387–1389. doi: 10.1016/S0041-1345(03)00440-8. [DOI] [PubMed] [Google Scholar]
- 44.Kiehn TE, Polsky B, Punithalingam E, Edwards FF, Brown AE, Armstrong D. 1987. Liver infection caused by Coniothyrium fuckelii in a patient with acute myelogenous leukemia. J Clin Microbiol 25:2410–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutton DA. 1999. Coelomycetous fungi in human disease. A review: clinical entities, pathogenesis, identification and therapy. Rev Iberoam Micol 16:171–179. [PubMed] [Google Scholar]
- 46.Heath CH, Lendrum JL, Wetherall BL, Wesselingh SL, Gordon DL. 1997. Phaeoacremonium parasiticum infective endocarditis following liver transplantation. Clin Infect Dis 25:1251–1252. doi: 10.1086/516963. [DOI] [PubMed] [Google Scholar]
- 47.Wang SC, Hsueh PR, Liaw SJ, Chang LY, Lu CY, Jou ST, Huang LM. 2005. Fatal fungemia due to Phaeoacremonium inflatipes in a child with severe aplastic anemia. Clin Infect Dis 40:1067–1068. doi: 10.1086/428358. [DOI] [PubMed] [Google Scholar]
- 48.McNeil CJ, Luo RF, Vogel H, Banaei N, Ho DY. 2011. Brain abscess caused by Phaeoacremonium parasiticum in an immunocompromised patient. J Clin Microbiol 49:1171–1174. doi: 10.1128/JCM.00830-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shah SK, Parto P, Lombard GA, James MA, Beckles DL, Lick S, Valentine VG. 2013. Probable Phaeoacremonium parasiticum as a cause of cavitary native lung nodules after single lung transplantation. Transplant Infect Dis 15:E9–E13. doi: 10.1111/tid.12040. [DOI] [PubMed] [Google Scholar]
- 50.Mostofi K, Jeanbourquin D, Charles JI. 2012. Cervical spondylitis due to Phaeoacremonium venezuelense in an immunocompetent patient. A first case report. J Mycol Med 22:197–200. doi: 10.1016/j.mycmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Kitamura K, Mochizuki T, Ishizaki H, Fukushiro R. 2000. Phaeomycotic cyst caused by Phaeoacremonium parasiticum. Nihon Ishinkin Gakkai Zasshi 41:89–95. doi: 10.3314/jjmm.41.89. [DOI] [PubMed] [Google Scholar]
- 52.Baradkar VP, Mathur M, Kumar S. 2009. Phaeohyphomycosis of subcutaneous tissue caused by Phaeoacremonium parasiticum. Indian J Med Microbiol 27:66–69. [PubMed] [Google Scholar]
- 53.Baradkar VP, Kumar S. 2011. Subcutaneous granulomatous infection caused by Phaeoacremonium inflatipes on foot. Indian J Dermatol 56:244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsui T, Nishimoto K, Udagawa S, Ishihara H, Ono T. 1999. Subcutaneous phaeohyphomycosis caused by Phaeoacremonium rubrigenum in an immunosuppressed patient. Nihon Ishinkin Gakkai Zasshi 40:99–102. doi: 10.3314/jjmm.40.99. [DOI] [PubMed] [Google Scholar]
- 55.Guarro J, Alves SH, Gené J, Grazziotin NA, Mazzuco R, Dalmagro C, Capilla J, Zaror L, Mayayo E. 2003. Two cases of subcutaneous infection due to Phaeoacremonium spp. J Clin Microbiol 41:1332–1336. doi: 10.1128/JCM.41.3.1332-1336.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harigopal P, Ciancio G, Dowdy L. Phaeoacremonium aleophilum skin and soft tissue infection in a renal transplant patient, abstr 22. Abstr Focus Fungal Infect 15th Annu Conf. [Google Scholar]
- 57.Choi J, Lee Y, Chung H-S, Koo J-S, Yong D, Kim YS, Lee K, Chong Y. 2011. Subcutaneous phaeohyphomycosis caused by Phaeoacremonium species in a kidney transplant patient: the first case in Korea. Korean J Lab Med 31:201–204. doi: 10.3343/kjlm.2011.31.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazzurco JD, Ramirez J, Fivenson DP. 2012. Phaeohyphomycosis caused by Phaeoacremonium species in a patient taking infliximab. J Am Acad Dermatol 66:333–335. doi: 10.1016/j.jaad.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 59.Deligny C, Arfi S, Dehlinger V, Dubreuil F, Jean-Baptiste G, Dueymes M, Desbois-Nogard N. 2010. Proceeding of Congress of the French Medical Mycology Society. J Mycol Med 20:136–163. doi: 10.1016/j.mycmed.2010.04.002. [DOI] [Google Scholar]
- 60.Merlo C, Merlo P, Holzinger F, Pranghofer S, Pfeiffer D, Nüesch R. 2014. A very slow growing ankle swelling in a healthy male. Praxis 103:1023–1026. doi: 10.1024/1661-8157/a001757. [DOI] [PubMed] [Google Scholar]
- 61.Padhye AA, Davis MS, Baer D, Reddick A, Sinha KK, Ott J. 1998. Phaeohyphomycosis caused by Phaeoacremonium inflatipes. J Clin Microbiol 36:2763–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Warrier KC, Friswell M. 2012. Fungal septic arthritis in an immunocompetent girl. Pediatr Rheumatol 10(Suppl 1):A39. doi: 10.1186/1546-0096-10-S1-A39. [DOI] [Google Scholar]
- 63.Chahal J, Dhotar HS, Anastakis DJ. 2009. Phaeohyphomycosis infection leading to flexor tendon rupture: a case report. Hand (NY) 4:335–338. doi: 10.1007/s11552-009-9178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Llinas L, Olenginski TP, Bush D, Gotoff R, Weber V. 2005. Osteomyelitis resulting from chronic filamentous fungus olecranon bursitis. J Clin Rheumatol 11:280–282. doi: 10.1097/01.rhu.0000182197.73637.96. [DOI] [PubMed] [Google Scholar]
- 65.Aguilar-Donis A, Torres-Guerrero E, Arenas-Guzmán R, Hernández-Hernández F, López-García L, Criales-Vera S, Teliz-Meneses MA. 2011. Mycetoma caused by Phaeoacremonium parasiticum—a case confirmed with B-tubulin sequence analysis. Mycoses 54:e615–e618. doi: 10.1111/j.1439-0507.2010.01929.x. [DOI] [PubMed] [Google Scholar]
- 66.Hemashettar BM, Siddaramappa B, Munjunathaswamy BS, Pangi AS, Pattan J, Andrade AT, Padhye AA, Mostert L, Summerbell RC. 2006. Phaeoacremonium krajdenii, a cause of white grain eumycetoma. J Clin Microbiol 44:4619–4622. doi: 10.1128/JCM.01019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun P-L, Ju Y-M. 2011. Onychomycosis caused by Phaeoacremonium parasiticum: first case report. Mycoses 54:172–174. doi: 10.1111/j.1439-0507.2009.01789.x. [DOI] [PubMed] [Google Scholar]
- 68.Huynh TK, Lee LR, Ellis D. 2007. Late-onset post-traumatic Phaeoacremonium parasiticum endophthalmitis. Clin Exp Ophthalmol 35:366–368. doi: 10.1111/j.1442-9071.2007.01486.x. [DOI] [PubMed] [Google Scholar]