Abstract
The recognition of carbapenemase-producing Enterobacteriaceae (CPE) isolates is a major laboratory challenge, and their inappropriate or delayed detection may have negative impacts on patient management and on the implementation of infection control measures. We describe here a matrix-assisted laser desorption ionization−time of flight (MALDI-TOF)-based method to detect carbapenemase activity in Enterobacteriaceae. After a 20-min incubation of the isolate with 0.5 mg/ml imipenem at 37°C, supernatants were analyzed by MALDI-TOF in order to identify peaks corresponding to imipenem (300 Da) and an imipenem metabolite (254 Da). A total of 223 strains, 77 CPE (OXA-48 variants, KPC, NDM, VIM, IMI, IMP, and NMC-A) and 146 non-CPE (cephalosporinases, extended-spectrum β-lactamases [ESBLs], and porin defects), were tested and used to calculate a ratio of imipenem hydrolysis: mass spectrometry [MS] ratio = metabolite/(imipenem + metabolite). An MS ratio cutoff was statistically determined to classify strains as carbapenemase producers (MS ratio of ≥0.82). We validated this method first by testing 30 of our 223 isolates (15 CPE and 15 non-CPE) 10 times to calculate an intraclass correlation coefficient (ICC of 0.98), showing the excellent repeatability of the method. Second, 43 strains (25 CPE and 18 non-CPE) different from the 223 strains used to calculate the ratio cutoff were used as external controls and blind tested. They yielded sensitivity and specificity of 100%. The total cost per test is <0.10 U.S. dollars (USD). This easy-to-perform assay is time-saving, cost-efficient, and highly reliable and might be used in any routine laboratory, given the availability of mass spectrometry, to detect CPE.
INTRODUCTION
Gram-negative bacteria (GNB) and especially Enterobacteriaceae, Pseudomonas aeruginosa. and Acinetobacter baumannii have reemerged as major bacterial pathogens in resistance (1, 2). In these species, resistance may affect all major classes of antimicrobial agents with activity against Gram-negative bacteria (e.g., β-lactams, fluoroquinolones, and aminoglycosides). Enterobacteriaceae account for the majority of antibiotic-resistant bacteria and are rapidly increasing (3–5). In some cases, resistance extends to the entire repertoire of available therapeutic agents (extremely drug-resistant phenotypes), posing a formidable challenge to antimicrobial therapy and turning back the clock to the preantibiotic era (6).
Currently, β-lactamase-mediated resistance does not spare even the newest and most powerful β-lactams (i.e., carbapenems), whose activity is challenged by the class B metallo-β-lactamases (MBLs) and by class A and D serine carbapenemases (5). The dissemination of these enzymes among Enterobacteriaceae is a matter of concern since these pathogens are a major cause of nosocomial and also community-acquired infections, and carbapenems are major antibiotics in the treatment of these infections (6).
Appropriate antimicrobial therapy is of paramount importance for decreasing the mortality of patients with bloodstream infections (7, 8). As the presence of carbapenemases has been associated with clinical failure in patients with severe infections caused by Enterobacteriaceae isolates with high carbapenem MICs, interpreted as resistant (9), screening of carbapenemases is a matter of importance for appropriate treatment and also for the implementation of infection control measures to prevent or control outbreaks.
Several phenotypic methods have been proposed for carbapenemase routine detection. The combination disk test with various carbapenemase inhibitors is commercially available and easy to implement. It achieves good performance for detection and presumptive identification of most carbapenemase-producing Enterobacteriaceae (CPE) (but not OXA-48) but is time-consuming (18 h) (10). More interestingly, the biochemical Carba NP test has recently been developed and confirms carbapenemase production within 2 h with excellent sensitivity and specificity (11). Molecular identification of carbapenemases may also be performed, but it remains costly and requires substantial expertise.
Recently, matrix-assisted laser desorption ionization−time of flight (MALDI-TOF) mass spectrometry (MS) has been introduced in bacteriology laboratories and has been proved to be efficient compared with other conventional methods for bacterial identification (12). It also had a positive impact on patient clinical management in guiding empirical treatment (13, 14). In the context of increasing carbapenemase producers, it is of the utmost importance to detect carbapenemases to guide the most appropriate treatment. MALDI-TOF has recently been used to determine β-lactamase activity (15–17) but, to our knowledge, has not been used in a routine setting to detect carbapenemase activity in clinical isolates. We describe here a method for carbapenemase detection in Enterobacteriaceae from primary culture plates in less than 30 min using MALDI-TOF.
MATERIALS AND METHODS
Bacterial strains and resistance detection.
A total of 266 clinical enterobacterial isolates originating from several countries worldwide were used. Species were identified using MALDI-TOF mass spectrometry (MALDI Biotyper; Bruker Daltonics, France). Imipenem, ertapenem, and meropenem MICs were determined by Etests (bioMérieux, La Balme-les-Grottes, France) and were interpreted using the EUCAST 2014 guidelines (18). CPE isolates (n = 102) were characterized by carbapenem hydrolysis (Carba NP test and/or UV spectrophotometry) (19) and by PCR and sequencing. For non-CPE strains (n = 164), the presence of extended-spectrum β-lactamases (ESBLs) was determined by a double-disk synergy test between an expanded-spectrum cephalosporin and clavulanic acid. For some isolates, the type of ESBL was determined by PCR and sequencing. The presence of cephalosporinases was shown by use of the ESBL + AmpC screen kit (ROSCO Diagnostica, Taastrup, Denmark) or cloxacillin-containing agar plates (bioMérieux).
A total of 223 isolates of the 266 isolates (77 CPE and 146 non-CPE) were used to calculate a cutoff ratio. The 77 CPE strains included Klebsiella pneumoniae (n = 41), Escherichia coli (n = 18), Enterobacter cloacae (n = 9), Klebsiella oxytoca (n = 3), Citrobacter freundii (n = 3), Enterobacter asburiae (n = 2), and Raoultella planticola (n = 1). The resistance determinants present in these isolates were 48 OXA-48, 2 OXA-204, 1 OXA-181, 1 OXA-244, 8 KPC, 7 NDM, 4 VIM, 3 IMI, 2 IMP, and 1 NMC-A. The 146 non-CPE isolates comprised 66 E. coli, 34 E. cloacae, 14 K. pneumoniae, 9 C. freundii, 6 E. aerogenes, 4 Morganella morganii, 4 Proteus mirabilis, 3 K. oxytoca, 2 Citrobacter koseri, 1 E. asburiae, 1 Hafnia alvei, 1 Providencia stuartii, and 1 Serratia marcescens. Among these 146 non-CPE isolates, 103 produced a cephalosporinase, 24 produced an ESBL, 10 produced a cephalosporinase plus an ESBL, and 9 were porin deficient, as revealed by resistance to moxalactam, which is an excellent antibiotic marker for impermeability testing on a disk diffusion antibiogram, and by carbapenem resistance in the absence of carbapenem hydrolysis. The remaining 43 isolates (25 CPE and 18 non-CPE) were blind tested. They were 10 K. pneumoniae, 13 E. coli, 1 S. marcescens, and 1 E. cloacae. Resistance mechanisms consisted of 9 OXA-48, 9 KPC, 5 NDM, and 2 OXA-181. Noncarbapenemase producers were composed of 12 cephalosporinases, 4 cephalosporinases plus ESBLs, 1 porin defect, and 1 ESBL. There were 12 E. cloacae, 2 C. freundii, 1 E. aerogenes, 1 E. coli, 1 K. oxytoca, and 1 K. pneumoniae. K. pneumoniae YC-producing KPC-2 and K. pneumoniae CIP53153 (wild-type resistance phenotype) were used as positive and negative controls, respectively, to validate the MALDI-TOF assay (20).
MALDI-TOF mass spectrometry analysis of imipenem hydrolysis.
Commercially available imipenem containing cilastatin (Hospira, Meudon la Forêt, France) was diluted in molecular biology-grade water (Eurobio, Courtaboeuf, France). The imipenem solution (50 mg/ml) was stored at −80°C for 1 month in 100-μl aliquots. Imipenem was used at a final concentration of 0.5 mg/ml according to Sparbier et al. (21). For each experiment, two controls (CPE/non-CPE) were tested to validate the assay.
Cultures of the tested strains were incubated overnight on Mueller-Hinton (MH) agar (bioMérieux, La Balme-les-Grottes, France) at 37°C. A 1-μl loop-sized amount of bacteria was suspended in 20 μl of 0.5 mg/ml imipenem solution, incubated 20 min at 37°C, and centrifuged for 1 min at 14,000 × g. One microliter of the clear supernatant was spotted onto an MSP 96 target polished steel plate (Bruker Daltonics, Wissembourg, France) and allowed to dry at room temperature, before 1 μl of matrix (α-cyano-4-hydroxycinnamic acid [HCCA]; Bruker Daltonics) was overlaid onto each target spot. Mass spectra were acquired using a Microflex LT mass spectrometer and flexControl software v3.3 (Bruker Daltonics) operating in positive linear ion mode between 100 and 1,000 Da. The parameters were set as follows: ion source 1, 19 kV; ion source 2, 16.3 kV; lens, 7 kV; pulsed ion extraction, 0 ns; detector gain, 3.3×; electronic gain, enhanced; mode, low range; mass range selection, 80 to 1,120 Da; laser frequency, 60 Hz; digitizer trigger level, 2,500 mV; laser attenuator, 25%; and laser range, 30%. A total of 240 shots were acquired in one position for one spectrum.
Internal calibration of the MALDI-TOF mass spectrometer.
Peaks of HCCA, 2HCCA, bradykinin, and angiotensin II (peptide calibration standard II; Bruker Daltonics) were used as internal calibration of the mass spectrometer.
Spectrum analysis.
Spectra were analyzed by FlexAnalysis software v3.3 (Bruker Daltonics). They were smoothed, and the baseline was subtracted. Peaks of imipenem (C12H17N3O4S) (300 ± 0.2 Da m/z) and its only known metabolite (C11H17N3O2S) (254 ± 0.1 Da m/z) were manually labeled and their intensities noted. MS ratios of intensities, metabolite/(metabolite + imipenem) [M/(M + I)], were calculated for the 223 cutoff strains to establish a ratio threshold between CPE and non-CPE isolates. Strains were classified as carbapenemase producers when this ratio was superior or equal to the cutoff.
Statistical analysis of carbapenemase activity and interpretation.
SPSS v.20 (IBM Inc., New York, NY, USA) was used for all statistical analyses. Repeatability was evaluated using a subset of 30 isolates (15 CPE and 15 non CPE) that were tested 10 times each. All types of carbapenemases were tested: 3 OXA-48, 3 NDM, 3 KPC, 3 VIM, 2 IMP, and 1 OXA-181. Noncarbapenemase-producing strains were randomly chosen: 11 cephalosporinases, 3 ESBLs, and 1 cephalosporinase plus ESBL. First, among these 30 isolates, the mean and standard deviation (SD) were calculated (22). Second, the intraclass correlation coefficient (ICC) and its 95% confidence interval (95% CI), used as an absolute reliability index, were calculated by one-way random analysis of variance (ANOVA) (23).
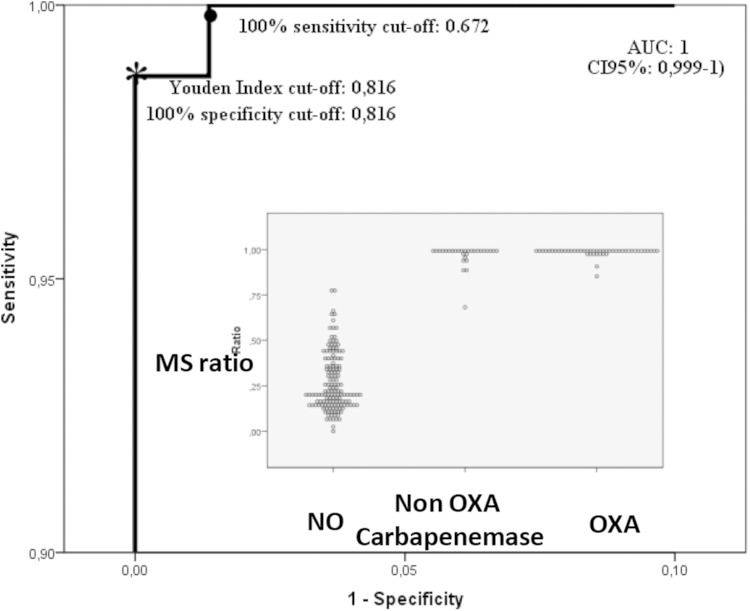
A receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff point (24). The area under the curve (AUC) and its 95% CI were estimated by a nonparametric method, and the optimal cutoff point was defined by the Youden index (25). Because two epidemiological patterns exist in the world, one with a higher incidence (India, United States, Greece, and others) and one with a lower incidence of carbapenemase (Northern Europe), secondary cutoffs in the ROC curve were defined: one with 100% sensitivity and one with 100% specificity.
The ratio-based model was validated: (i) for the internal validation, all of the 266 strains were used and the 0.632+ bootstrapping method of cross-validation enabled calculation of the coefficient of determination (R2) of the model (26); (ii) for the external validation, the results of 43 well-characterized isolates and blind tested using MS ratio interpretation were compared by the χ2 test (23) to molecular biology results. Identifications and resistance mechanisms were obtained only after MALDI-TOF hydrolysis testing was performed. The model was separately verified for the OXA-48-like carbapenemases and for the other carbapenemases.
Comparison between MS ratio model and meropenem MICs.
The meropenem MICs of the 266 isolates were determined using the EUCAST 2014 guidelines and interpretative criteria. Sensitivities and specificities were estimated for the screening cutoff MIC of >0.12 mg/liter, corresponding to the extended common object file format (ECOFF) (27), and for the optimal MS ratio cutoff.
RESULTS
Reproducibility and determination of the cutoff ratio.
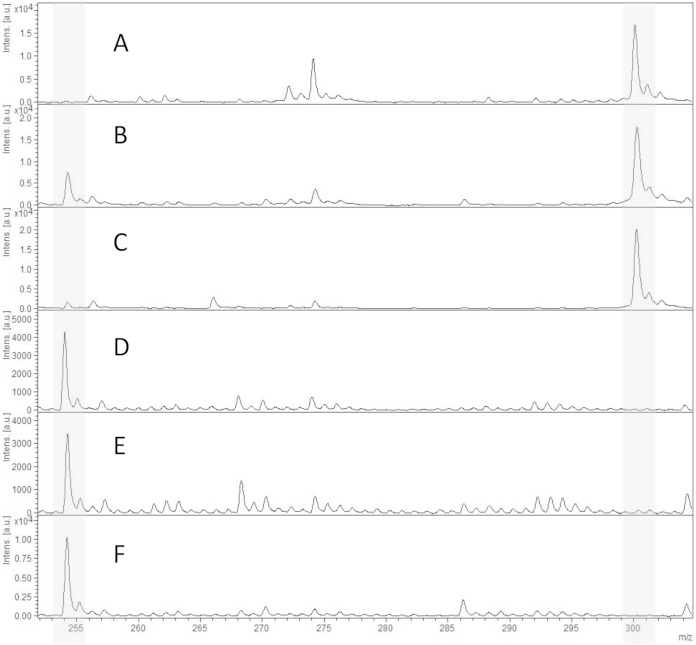
The 1-μl loop-full-of-bacteria technique has previously been used for other biochemical tests such as the Carba NP test (11). In order to test its reproducibility with the MALTI-TOF CPE detection assay, 30 bacteria were tested several times. No differences in the mass spectra were observed, suggesting that the amount of bacteria is similar for each test. In order to verify the stability of imipenem preparations, 20 isolates (10 CPE and 10 non-CPE) were tested with imipenem that was freshly prepared on the day of testing and with imipenem that was stored for 1 month at −80°C. No significant differences were observed between the spectra of freshly prepared imipenem and those of imipenem stored for 1 month at −80°C for all the 20 isolates tested (data not shown). These results suggest that imipenem preparations are stable at least for 1 month at −80°C. Finally, no alterations in the imipenem spectra were observed after the 20 min of incubation without a β-lactamase. For all of the non-CPE isolates, spectra contained the molecular peak of imipenem at 300 Da and a minor peak at 254 Da corresponding to the metabolite. For CPE isolates, spectra revealed the increase in the metabolite peak at 254 Da along with a disappearance or a decrease in the peak of imipenem (Fig. 1). The MS ratio M/(M + I) of CPE strains ranged from 0.68 to 1.00. The MS ratio of non-CPE strains ranged from 0 to 0.78. The Youden index provided an optimal cutoff at 0.82, which is identical to the maximal specificity cutoff. The maximal sensitivity cutoff is at 0.67. All non-CPE strains had a ratio of <0.82. All but one CPE isolate had a ratio of >0.82, and one OXA-204-producing isolate had an MS ratio (r = 0.68) less than the calculated cutoff.
FIG 1.
MALDI-TOF spectra of imipenem hydrolysis assays after a 20-min incubation at 37°C. Peaks of interest in gray represent the imipenem peak at 300 Da and its metabolite at 254 Da. (A) Imipenem alone; (B) cephalosporinase; (C) ESBL; (D) OXA-48; (E) KPC; (F) NDM. Units on the y axes represent relative intensity.
Validation of the model.
Forty-three isolates (Table 1) were blind tested using the calculated cutoff (r = 0.82) for external validation. All isolates were correctly classified. The 25 CPE isolates presented a ratio between 0.83 and 1.0 and the 18 non-CPE isolates had a ratio between 0.05 and 0.34. With use of external validation strains, the sensitivity and the specificity of this imipenem hydrolysis test were 100%. For the hydrolysis method with the whole 266 strains, sensitivity was 99% and specificity was 100%.
TABLE 1.
Characteristics of CPE used in internal validation
| Carbapenemase type | Carbapenemase variant | Species | No. of strains | MIC range (mg/liter) |
MS ratio | ||
|---|---|---|---|---|---|---|---|
| Imipenem | Ertapenem | Meropenem | |||||
| IMI | IMI-1 | E. cloacae | 1 | >32 | >32 | >32 | 1.00 |
| IMI-2 | E. asburiae | 2 | >32 | 1.5–>32 | 1.5–24 | 0.99 | |
| IMP | IMP-1 | E. coli | 1 | 6 | 6 | 1.5 | 0.99 |
| IMP-8 | K. pneumoniae | 1 | 1 | 4 | 0.094 | 0.95 | |
| KPC | KPC-2 | E. coli | 2 | 4–6 | 3–>32 | 3–>32 | 0.93–0.99 |
| KPC-2 | K. pneumoniae | 4 | 24–>32 | 12–>32 | 6–>32 | 0.95–1.00 | |
| KPC-3 | K. pneumoniae | 2 | 0.25–>32 | 1.5–>32 | 1.5–>32 | 0.99 | |
| NDM | NDM-1 | E. coli | 2 | 6–>32 | 2–>32 | 2–>32 | 0.99–1.00 |
| NDM-1 | K. pneumoniae | 3 | 2–>32 | 8–>32 | 6–>32 | 0.88–0.99 | |
| NDM-2 | C. freundii | 1 | >32 | >32 | >32 | 1.00 | |
| NDM-4 | E. coli | 1 | >32 | >32 | >32 | 1.00 | |
| NMC A | NMC A | E. cloacae | 1 | >32 | 6 | 6 | 0.99 |
| OXA-48 like | OXA-181 | K. pneumoniae | 1 | >32 | >32 | >32 | 0.99 |
| OXA-204 | E. coli | 1 | 0.25 | 1 | 0.19 | 0.98 | |
| OXA-204 | K. pneumoniae | 1 | 0.25 | 1 | 0.125 | 0.68 | |
| OXA-244 | E. coli | 1 | 0.5 | 3 | 0.38 | 1.00 | |
| OXA-48 | C. freundii | 2 | 1–2 | 2–3 | 0.5 | 1.00 | |
| OXA-48 | E. cloacae | 7 | 0.75–3 | 2–>32 | 0.75–>32 | 0.97–1.00 | |
| OXA-48 | E. coli | 10 | 0.5–12 | 0.38–3 | 0.19–1 | 0.85–1.00 | |
| OXA-48 | K. oxytoca | 1 | 0.75 | 0.75 | 0.25 | 0.99 | |
| OXA-48 | K. pneumoniae | 27 | 0.25–>32 | 1–>32 | 0.19–>32 | 0.97–1.00 | |
| OXA-48 | R. planticola | 1 | 1 | 0.5 | 0.25 | 0.99 | |
| VIM | VIM-1 | K. oxytoca | 2 | 2 | 0.25 | 0.38 | 0.99–1.00 |
| VIM-2 | K. pneumoniae | 1 | >32 | 4 | 4 | 1.00 | |
| VIM-19 | K. pneumoniae | 1 | 6 | 8 | 3 | 0.99 | |
| Total | 77 | 0.68–1.00 | |||||
The model's ability to discriminate between the CPE and non-CPE isolates, using AUC values in the ROC curve analysis, showed an outstanding value of 1 (95% CI, 0.99 to 1) (Fig. 2). The internal and the external validations showed R2 values of 0.91 and 1, respectively. The repeatability also gave a consistent ICC of 0.98.
FIG 2.
ROC curve of MS ratio (scale, 0.0 to 0.1 and 0.9 to 1, respectively) with AUC estimation, optimal cutoff, and the maximal sensitivity cutoff. In the inset, the dot plot of the MS ratio in strains with or without OXA or non-OXA carbapenemase.
Susceptibility testing.
Carbapenem MICs are summarized in Tables 1, 2, and 3. With the recommended meropenem screening cutoffs in the EUCAST 2014 guidelines (MIC of >0.12 μg/ml) corresponding to the ECOFF (27), 99% (101/102) of the CPE and 30% (49/164) of the non-CPE were classified as potential CPE. One IMP-8-producing K. pneumoniae (meropenem MIC of 0.094 μg/ml) would have been considered a non-CPE with the EUCAST screening cutoff but had an MS ratio of 0.95. Among the 49 non-CPE isolates considered by the EUCAST screening cutoff to be possible carbapenemase producers, all isolates presented a negative MS ratio (r of <0.82). The only strain (OXA-204-producing K. pneumoniae) not detected as a CPE by the MS assay had a meropenem MIC of 0.125 μg/ml and was hence correctly suspected as a CPE using the EUCAST screening cutoff. The sensitivity and specificity of the EUCAST meropenem screening cutoff were 99% and 59%, respectively.
TABLE 2.
Characteristics of non CPE used in internal validation
| Resistance | Species | No. of strains | MIC range (mg/liter) |
MS ratio | ||
|---|---|---|---|---|---|---|
| Imipenem | Ertapenem | Meropenem | ||||
| ESBL | C. freundii | 1 | 0.25 | 1 | 0.38 | 0.47 |
| C. koseri | 2 | 0.25 | 0.012 | 0.032–0.047 | 0.14 | |
| E. aerogenes | 1 | 0.38 | 0.38 | 0.094 | 0.57 | |
| E. cloacae | 4 | 0.25–0.5 | 0.38–2 | 0.125–0.38 | 0.07–0.44 | |
| E. coli | 4 | 0.19–0.38 | 0.0160.5 | 0.023–0.094 | 0.19–0.46 | |
| K. oxytoca | 1 | 0.25 | 0.094 | 0.064 | 0.21 | |
| K. pneumoniae | 9 | 0.032–3 | 0.047–16 | 0.047–6 | 0.12–0.64 | |
| M. morganii | 1 | 2 | 0.016 | 0.125 | 0.07 | |
| P. mirabilis | 1 | 0.38 | 0.032 | 0.064 | 0.21 | |
| Cephalosporinase | C. freundii | 7 | 0.38–16 | 0.008–32 | 0.023–4 | 0.10–0.57 |
| E. aerogenes | 4 | 0.38–0.75 | 0.19–0.5 | 0.094–0.19 | 0.10–0.35 | |
| E. asburiae | 1 | 0.75 | 0.5 | 0.38 | 0.17 | |
| E. cloacae | 21 | 0.125–>32 | 0.094–>32 | 0.032–>32 | 0.11–0.66 | |
| E. coli | 62 | 0.125–0.75 | 0.004–0.25 | 0.12–0.094 | 0.02–0.77 | |
| H. alvei | 1 | 0.25 | 3 | 0.38 | 0.14 | |
| K. oxytoca | 2 | 0.25–0.38 | 0.064 | 0.064 | 0.19–0.78 | |
| K. pneumoniae | 3 | 0.25–0.38 | 0.047–8 | 0.047–1.5 | 0.41–0.47 | |
| P. mirabilis | 2 | 0.25–0.38 | 0.012 | 0.047 | 0.21–0.22 | |
| Cephalosporinase + ESBL | E. cloacae | 8 | 0.38–2 | 0.19–2 | 0.05–0.25 | 0.08–0.42 |
| K. pneumoniae | 1 | 2 | 16 | 2 | 0.17 | |
| Impermeability | E. aerogenes | 1 | 0.25 | 0.19 | 0.064 | 0.13 |
| K. pneumoniae | 1 | 1 | 0.032 | 0.125 | 0.45 | |
| M. morganii | 3 | 2–4 | 0.016–0.047 | 0.094–0.125 | 0.07–0.22 | |
| P. mirabilis | 1 | 0.75 | 0.012 | 0.064 | 0.20 | |
| P. stuartii | 1 | 1.5 | 0.016 | 0.064 | 0.15 | |
| E. cloacae | 1 | 3 | 4 | 1 | 0 | |
| C. freundii | 1 | 0.75 | 0.5 | 0.25 | 0.11 | |
| S. marcescens | 1 | 1 | 1 | 0.25 | 0.61 | |
| Total | 164 | 0–0.78 | ||||
TABLE 3.
Characteristics of isolates used for external validation
| Resistance mechanism and variant | Strain | Species | MIC range (mg/liter) |
MS ratio | ||
|---|---|---|---|---|---|---|
| Imipenem | Ertapenem | Meropenem | ||||
| ESBL | VT43 | K. pneumoniae | 0.5 | 16 | 2 | 0.20 |
| Porin defect | VT12 | E. coli | 0.25 | 1.5 | 0.125 | 0.32 |
| Cephalosporinase | VT28 | C. freundii | 0.25 | 0.5 | 0.125 | 0.20 |
| VT31 | C. freundii | >32 | >32 | 12 | 0.11 | |
| VT10 | E. aerogenes | 0.19 | 0.19 | 0.064 | 0.22 | |
| VT24 | E. cloacae | 0.19 | 0.19 | 0.032 | 0.18 | |
| VT32 | E. cloacae | 0.125 | 0.38 | 0.047 | 0.25 | |
| VT37 | E. cloacae | 0.25 | 0.25 | 0.094 | 0.32 | |
| VT40 | E. cloacae | 0.25 | 0.5 | 0.047 | 0.11 | |
| VT4 | E. cloacae | 0.19 | 0.38 | 0.064 | 0.27 | |
| VT20 | E. cloacae | 0.25 | 0.5 | 0.125 | 0.17 | |
| VT22 | E. cloacae | 1.5 | 1.5 | 0.5 | 0.17 | |
| VT11 | E. cloacae | 4 | >32 | 6 | 0.20 | |
| VT14 | K. oxytoca | 0.19 | 0.064 | 0.047 | 0.34 | |
| VT1 | E. cloacae | 0.25 | 0.75 | 0.125 | 0.25 | |
| Cephalosporinase + ESBL | VT26 | E. cloacae | 0.38 | 1 | 0.125 | 0.13 |
| VT7 | E. cloacae | 1.5 | 0.5 | 0.19 | 0.38 | |
| VT42 | E. cloacae | 3 | 2 | 0.5 | 0.15 | |
| KPC | ||||||
| KPC-2 | VT23 | E. cloacae | 3 | 6 | 2 | 0.98 |
| KPC-2 | VT29 | E. coli | 0.5 | 8 | 1 | 0.97 |
| KPC-2 | VT30 | K. pneumoniae | 1 | 8 | 1 | 0.99 |
| KPC-2 | VT17 | K. pneumoniae | 6 | >32 | 4 | 1.00 |
| KPC-2 | VT25 | K. pneumoniae | 1.5 | 4 | 1.5 | 0.98 |
| KPC-2 | VT38 | K. pneumoniae | 2 | 24 | 2 | 1.00 |
| KPC-2 | VT35 | K. pneumoniae | 12 | 12 | 16 | 1.00 |
| KPC-2 | VT41 | K. pneumoniae | >32 | >32 | >32 | 1.00 |
| KPC-2 | VT36 | S. marcescens | >32 | >32 | >32 | 0.99 |
| NDM | ||||||
| NDM-1 | VT6 | E. coli | 0.75 | 2 | 0.75 | 1.00 |
| NDM-1 | VT27 | E. coli | 8 | 8 | 32 | 0.98 |
| NDM-4 | VT3 | E. coli | 0.15 | >32 | 8 | 0.99 |
| NDM-5 | VT15 | E. coli | 2 | >32 | 4 | 1.00 |
| NDM-7 | VT9 | E. coli | 2 | >32 | 6 | 1.00 |
| OXA-48 group | ||||||
| OXA-181 | VT13 | E. coli | 0.25 | 1 | 0.125 | 0.98 |
| OXA-181 | VT44 | E. coli | 0.38 | 1.5 | 0.38 | 1.00 |
| OXA-48 | VT2 | E. coli | 0.38 | 0.5 | 0.19 | 1.00 |
| OXA-48 | VT5 | E. coli | 0.25 | 0.5 | 0.19 | 1.00 |
| OXA-48 | VT18 | E. coli | 0.25 | 0.5 | 0.25 | 0.98 |
| OXA-48 | VT33 | E. coli | 0.38 | 0.5 | 0.25 | 0.95 |
| OXA-48 | VT34 | E. coli | 0.38 | 1 | 0.25 | 0.99 |
| OXA-48 | VT16 | K. pneumoniae | 0.25 | 1 | 0.25 | 1.00 |
| OXA-48 | VT8 | K. pneumoniae | 0.38 | 1.5 | 0.38 | 1.00 |
| OXA-48 | VT39 | K. pneumoniae | 1 | 1.5 | 0.5 | 1.00 |
| OXA-48 | VT21 | K. pneumoniae | 1.5 | >32 | 4 | 1.00 |
DISCUSSION
As dissemination of CPE is drastically increasing worldwide, an urgent need for rapid identification methods has emerged. The MALDI-TOF technique is becoming the method of choice for bacterial identification due to its rapid and reliable results, and, as a result, MALDI-TOF spectrometers are rapidly deployed in microbiology laboratories worldwide. In our study, we have successfully used the MALDI-TOF technique to rapidly detect carbapenemase activity in enterobacterial isolates, irrespective of the carbapenemase gene present. We tested >250 isolates, corresponding to a large variety of resistance mechanisms (different carbapenemases, ESBLs, cephalosporinases, porin defects, and combinations of mechanisms) among enterobacterial species. Using a ratio cutoff of 0.82, we found this assay reliable (99% sensitivity with only one false-negative result [one OXA-204] and 100% specificity) and fast and easy to set up on a daily basis in a laboratory routine.
MALDI-TOF has recently been used to detect β-lactamases, ESBLs, and particularly carbapenemases (15–17, 28, 29). In these approaches, the action of the β-lactamases was monitored by the analysis of antibiotics and their degradation products. We have observed in CPE a peak of 254 Da corresponding to an imipenem metabolite, described by Kempf et al. (30) and associated with a strong reduction and even absence of the imipenem peak of 300 Da (Fig. 1D, E, and F). For non-CPE, the imipenem peak (300 Da) was always present, but for some isolates, the 254-Da metabolite peak could also be observed (Fig. 1B and C), suggesting that the presence of this peak (254 Da) itself was not sufficient to categorize a strain as a carbapenemase producer. This leads to use of the MS ratio to reproducibly discriminate carbapenemase from noncarbapenemase producers.
The repeatability of this approach yielded an ICC value of 0.98, which is high enough to allow single testing of the strains. On the other hand, the validity of the MS ratio model is confirmed by the sensitivity and specificity of 100% for the blind-tested isolates and the robustness of the test as assessed by the R2 of the internal validation that is >0.90. These performances are consistent, considering that it is a blind test of a panel of clinical isolates compared with the gold standard that is PCR and sequencing. The only misclassified isolate was an OXA-204-producing K. pneumoniae. OXA-204 is known to be a weak carbapenem-hydrolyzing enzyme, and its expression is likely at the limit of detection (11).
MALDI-TOF MS has recently been shown to be a reliable method for KPC and NDM detection in Enterobacteriaceae with high sensitivity and specificity (15, 16, 30–35). Only one study investigated 6 OXA-48 producers (33). OXA-48, the most prevalent carbapenemase found in Enterobacteriaceae in France (75.4%) (36), is one of the most difficult to detect in routine settings using phenotypic methods. Our assay classified correctly all of the 57 OXA-48 producers tested. Furthermore, despite low carbapenem MICs, hydrolysis by OXA-48 was important: 43% of our strains had an MS ratio of 1.00, corresponding to a total degradation of imipenem in 20 min and suggesting that our test can be used for early detection of the most important carbapenemases in various epidemiological situations. Carbapenemase detection was, until recently, based on the analysis of susceptibility testing results. According to the EUCAST 2014 guidelines, the meropenem MIC seems to have the best sensitivity and specificity to detect carbapenemases compared to imipenem and ertapenem (37, 38). Interestingly, one CPE (IMP-8-producing K. pneumoniae) which had a meropenem MIC under the EUCAST screening cutoff was correctly identified as a carbapenemase producer with a positive MS ratio (r = 0.95). In parallel, none of the non-CPE with meropenem MICs higher than the EUCAST screening cutoff had a positive MS ratio (r = 0 to 0.66). Our assay, combining the 223 strains used for cutoff determination and the 43 external validation strains (sensitivity of 99%; specificity of 100%), performed better than the EUCAST screening cutoff (sensitivity of 99%; specificity of 59%). Unlike EUCAST, for which the meropenem screening cutoffs may vary depending on the prevalence of OXA-48 producers in the country, here only one MS cutoff was used.
As detection of CPE has become a major health issue, numerous diagnostic confirmation tests have been developed. Phenotypic methods such as the modified Hodge test (MHT) is not recommended anymore by the EUCAST 2014 guidelines (27) due to frequent false-positive and false-negative results (37, 39), and the combined disk test method, which is well validated and commercially available, is still lacking an inhibitor for class D enzymes (40, 41).
A rapid biochemical test, the Carba NP test, gave excellent results for a large variety of carbapenemases, including many OXA-48 (11, 42). In one publication, the Carba NP test experienced lower sensitivity with mucoid isolates and for some Enterobacteriaceae producing OXA-48 (43), but others showed 100% sensitivity and specificity (11, 42, 44). Among the 23 OXA-48-like isolates tested for the internal validation, 16 were negative or invalid with the Carba NP test (P. Bogaerts, personal communication). These 16 isolates (except one OXA-204 producer) were correctly classified by our test. UV spectrophotometry, which is considered to be the reference method for carbapenemase detection, has been evaluated and yielded a sensitivity of 100% and specificity ranging from 98.5% to 100% (11, 19), but this technique requires technical expertise, is time-consuming, and thus is not suitable for routine use.
Our MALDI-TOF CPE detection assay was performed in less than 30 min, including solution preparation, incubation, centrifugation, spotting, MALDI-TOF analysis, and interpretation. The 1-min centrifugation step is initiated just before spotting on the steel plate and thus does not delay significantly the workflow. To our knowledge, it is the fastest assay to detect carbapenemase activity. This speed is a critical point for patient management at the hospital to prevent CPE spread. Phenotypic detection methods (modified Hodge test, combined disk tests, selective agar, etc.) are based on cultures that require at least 18 to 24 h of incubation. In the literature, most assays based on mass spectrometry need 1 h to 4 h of incubation (15, 16, 21, 30–35, 45). The Carba NP test requires 2 h, especially for OXA-48 producers (42) and visual interpretation, which can be subjective. With the MALDI-TOF CPE detection assay, interpretation is computer assisted, thus allowing very little error in interpretation. Finally, this assay is easily scaled up in the sense that up to 48 or 96 samples can be processed at once on the mass spectrophotometer.
The choice of imipenem was dictated by the fact that masses (m/z) of the nondegraded form (300 Da) and its metabolite (254 Da) were distinct from that of the matrix HCCA (380 Da) (21, 30, 31). Hence, meropenem had an overlapping peak between the meropenem molecular peak (384 Da) and the HCCA peak (380 Da) (16, 30). Furthermore, there are only two peaks of interest for imipenem compared to meropenem and ertapenem (2 to 6 molecular peaks and 1 to 7 metabolite peaks) (15, 16, 31–35). This enables an easy MS ratio [M/(M + I)] interpretation. Furthermore, in the very near future, it will be possible to automatically calculate the ratio with the development of a software tool. Wang et al. used ClinProTools software to automatically label peaks and classify strains as CPE or non-CPE (35), while Hrabák et al. and Hoyos-Mallecot et al. used a special Flex analysis 3.0 software to detect peaks (16, 32). These software programs allow nonsubjective peak labeling. It is then possible to improve our method by developing a software tool for an automated interpretation of data.
If molecular methods are still the gold standard for accurate identification of carbapenemases, most of the time they require two steps: PCR for detection and sequencing for variant determination. They have excellent specificities and sensitivities and require <6 h to get a result but are expensive and require a trained microbiologist for interpretation. They can detect not only the presence of known carbapenemase genes but also whether they are expressed, unlike phenotypic assays (46), and even when the gene is detected, like OXA-48 variants such as OXA-163, this does not necessarily mean the presence of a carbapenemase. Compared to the molecular method, MS assays save costs, provided that laboratories have a mass spectrometer, which is very likely to occur in the near future, since MALDI-TOF is becoming the gold standard method for bacterial identification. The cost per test of our assay (including standard, matrix, and imipenem) is <0.10 U.S. dollars (USD).
Our protocol and its reproducibility need to be evaluated in other laboratories and also with different culture media since here only MH medium was evaluated. Other media such as chromogenic selective or Drigalski plates need to be evaluated. Finally, a future evaluation with β-lactamase inhibitors may directly indicate the carbapenemase type present.
Our study shows CPE detection in a routine setting in less than 30 min using MALDI-TOF technology. In consideration of our 266 tested isolates, our assay resulted in 99% sensitivity and 100% specificity on a large variety of Enterobacteriaceae expressing the most clinically relevant carbapenemases such as OXA-48, NDM, and KPC. It is an easy and cost-effective assay, which could be used in real time in the flow of routine lab work, which is of the utmost importance to prevent outbreaks and their consequences and to treat patients appropriately.
ACKNOWLEDGMENTS
We gratefully acknowledge Katrin Sparbier and Peio Mogabure for their help and also Quentin Lasserre, who helped us to correct the language.
This work was partially funded by grants from the Université Paris Sud, France, and by a grant from the European Community (MAGIC-BULLET contract, FP7/HEALTH-F3-2012-278232). T.N. and G.C. are members of the Laboratory of Excellence LERMIT supported by a grant from ANR (ANR-10-LABX-33).
REFERENCES
- 1.World Health Organization. 2014. Antimicrobial resistance: global report on surveillance. http://www.who.int/drugresistance/documents/surveillancereport/en/.
- 2.ECDC/Joint Technical Report EMEA. 2009. The bacterial challenge: time to react. http://www.ecdc.europa.eu/en/publications/Publications/Forms/ECDC_DispForm.aspx?ID=444.
- 3.Cantón R, Akova M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen O, Seifert H, Woodford N, Nordmann P. 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin Microbiol Infect 18:413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 4.Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9:228–236. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 5.Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. 2011. Carbapenems: past, present, and future. Antimicrob Agents Chemother 55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micek ST, Welch EC, Khan J, Pervez M, Doherty JA, Reichley RM, Kollef MH. 2010. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother 54:1742–1748. doi: 10.1128/AAC.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seifert H. 2009. The clinical importance of microbiological findings in the diagnosis and management of bloodstream infections. Clin Infect Dis 48(Suppl 4):S238–S245. doi: 10.1086/598188. [DOI] [PubMed] [Google Scholar]
- 9.Weisenberg SA, Morgan DJ, Espinal-Witter R, Larone DH. 2009. Clinical outcomes of patients with Klebsiella pneumoniae carbapenemase-producing K. pneumoniae after treatment with imipenem or meropenem. Diagn Microbiol Infect Dis 64:233–235. doi: 10.1016/j.diagmicrobio.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pournaras S, Zarkotou O, Poulou A, Kristo I, Vrioni G, Themeli-Digalaki K, Tsakrisa A. 2013. A combined disk test for direct differentiation of carbapenemase-producing Enterobacteriaceae in surveillance rectal swabs. J Clin Microbiol 51:2986–2990. doi: 10.1128/JCM.00901-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dortet L, Brechard L, Cuzon G, Poirel L, Nordmann P. 2014. Strategy for rapid detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 58:2441–2445. doi: 10.1128/AAC.01239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bizzini A, Durussel C, Bille J, Greub G, Prod'hom G. 2010. Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol 48:1549–1554. doi: 10.1128/JCM.01794-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clerc O, Prod'hom G, Vogne C, Bizzini A, Calandra T, Greub G. 2013. Impact of matrix-assisted laser desorption ionization time-of-flight mass spectrometry on the clinical management of patients with Gram-negative bacteremia: a prospective observational study. Clin Infect Dis 56:1101–1107. doi: 10.1093/cid/cis1204. [DOI] [PubMed] [Google Scholar]
- 14.Vlek AL, Bonten MJ, Boel CH. 2012. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One 7:e32589. doi: 10.1371/journal.pone.0032589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burckhardt I, Zimmermann S. 2011. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J Clin Microbiol 49:3321–3324. doi: 10.1128/JCM.00287-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrabák J, Walkova R, Studentova V, Chudackova E, Bergerova T. 2011. Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 49:3222–3227. doi: 10.1128/JCM.00984-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledeboer N, Hodinka R. 2011. Molecular detection of resistance determinants. J Clin Microbiol 49:S20–S24. doi: 10.1128/JCM.00771-11. [DOI] [Google Scholar]
- 18.The European Committee on Antimicrobial Susceptibility Testing. 2014. Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0. http://www.eucast.org.
- 19.Bernabeu S, Poirel L, Nordmann P. 2012. Spectrophotometry-based detection of carbapenemase producers among Enterobacteriaceae. Diagn Microbiol Infect Dis 74:88–90. doi: 10.1016/j.diagmicrobio.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Naas T, Nordmann P, Vedel G, Poyart C. 2005. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 49:4423–4424. doi: 10.1128/AAC.49.10.4423-4424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparbier K, Schubert S, Weller U, Boogen C, Kostrzewa M. 2012. Matrix-assisted laser desorption ionization−time of flight mass spectrometry-based functional assay for rapid detection of resistance against beta-lactam antibiotics. J Clin Microbiol 50:927–937. doi: 10.1128/JCM.05737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310. [PubMed] [Google Scholar]
- 23.Vaz S, Falkmer T, Passmore AE, Parsons R, Andreou P. 2013. The case for using the repeatability coefficient when calculating test-retest reliability. PLoS One 8:e73990. doi: 10.1371/journal.pone.0073990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. 1982. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 25.Youden WJ. 1950. Index for rating diagnostic tests. Cancer 3:32–35. doi:. [DOI] [PubMed] [Google Scholar]
- 26.Efron B, Tibshirani R. 1997. Improvements on cross-validation: The .632+ bootstrap method. J Am Stat Assoc 92:548–560. doi: 10.1080/01621459.1997.10474007. [DOI] [Google Scholar]
- 27.The European Committee on Antimicrobial Susceptibility Testing. 2013. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 1.0. EUCAST, Basel, Switzerland. [Google Scholar]
- 28.Nagy E, Becker S, Soki J, Urban E, Kostrzewa M. 2011. Differentiation of division I (cfiA-negative) and division II (cfiA-positive) Bacteroides fragilis strains by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Med Microbiol 60:1584–1590. doi: 10.1099/jmm.0.031336-0. [DOI] [PubMed] [Google Scholar]
- 29.Wybo I, De Bel A, Soetens O, Echahidi F, Vandoorslaer K, Van Cauwenbergh M, Pierard D. 2011. Differentiation of cfiA-negative and cfiA-positive Bacteroides fragilis isolates by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 49:1961–1964. doi: 10.1128/JCM.02321-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempf M, Bakour S, Flaudrops C, Berrazeg M, Brunel JM, Drissi M, Mesli E, Touati A, Rolain JM. 2012. Rapid detection of carbapenem resistance in Acinetobacter baumannii using matrix-assisted laser desorption ionization-time of flight mass spectrometry. PLoS One 7:e31676. doi: 10.1371/journal.pone.0031676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carvalhaes CG, Cayo R, Assis DM, Martins ER, Juliano L, Juliano MA, Gales AC. 2013. Detection of SPM-1-producing Pseudomonas aeruginosa and class D beta-lactamase-producing Acinetobacter baumannii isolates by use of liquid chromatography-mass spectrometry and matrix-assisted laser desorption ionization−time of flight mass spectrometry. J Clin Microbiol 51:287–290. doi: 10.1128/JCM.02365-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyos-Mallecot Y, Cabrera-Alvargonzalez JJ, Miranda-Casas C, Rojo-Martin MD, Liebana-Martos C, Navarro-Mari JM. 2014. MALDI-TOF MS, a useful instrument for differentiating metallo-beta-lactamases in Enterobacteriaceae and Pseudomonas spp. Lett Appl Microbiol 58:325–329. doi: 10.1111/lam.12203. [DOI] [PubMed] [Google Scholar]
- 33.Hrabák J, Studentova V, Walkova R, Zemlickova H, Jakubu V, Chudackova E, Gniadkowski M, Pfeifer Y, Perry JD, Wilkinson K, Bergerova T. 2012. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization−time of flight mass spectrometry. J Clin Microbiol 50:2441–2443. doi: 10.1128/JCM.01002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee W, Chung HS, Lee Y, Yong D, Jeong SH, Lee K, Chong Y. 2013. Comparison of matrix-assisted laser desorption ionization−time-of-flight mass spectrometry assay with conventional methods for detection of IMP-6, VIM-2, NDM-1, SIM-1, KPC-1, OXA-23, and OXA-51 carbapenemase-producing Acinetobacter spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae. Diagn Microbiol Infect Dis 77:227–230. doi: 10.1016/j.diagmicrobio.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Han C, Sui W, Wang M, Lu X. 2013. MALDI-TOF MS applied to indirect carbapenemase detection: a validated procedure to clearly distinguish between carbapenemase-positive and carbapenemase-negative bacterial strains. Anal Bioanal Chem 405:5259–5266. doi: 10.1007/s00216-013-6913-2. [DOI] [PubMed] [Google Scholar]
- 36.Dortet L, Cuzon G, Nordmann P. 2014. Dissemination of carbapenemase-producing Enterobacteriaceae in France, 2012. J Antimicrob Chemother 69:623–627. doi: 10.1093/jac/dkt433. [DOI] [PubMed] [Google Scholar]
- 37.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V. 2012. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 18:432–438. doi: 10.1111/j.1469-0691.2012.03815.x. [DOI] [PubMed] [Google Scholar]
- 38.Vading M, Samuelsen O, Haldorsen B, Sundsfjord AS, Giske CG. 2011. Comparison of disk diffusion, Etest and VITEK2 for detection of carbapenemase-producing Klebsiella pneumoniae with the EUCAST and CLSI breakpoint systems. Clin Microbiol Infect 17:668–674. doi: 10.1111/j.1469-0691.2010.03299.x. [DOI] [PubMed] [Google Scholar]
- 39.Pasteran F, Mendez T, Guerriero L, Rapoport M, Corso A. 2009. Sensitive screening tests for suspected class A carbapenemase production in species of Enterobacteriaceae. J Clin Microbiol 47:1631–1639. doi: 10.1128/JCM.00130-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. 2012. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol 50:3877–3880. doi: 10.1128/JCM.02117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giske CG, Gezelius L, Samuelsen O, Warner M, Sundsfjord A, Woodford N. 2011. A sensitive and specific phenotypic assay for detection of metallo-beta-lactamases and KPC in Klebsiella pneumoniae with the use of meropenem disks supplemented with aminophenylboronic acid, dipicolinic acid and cloxacillin. Clin Microbiol Infect 17:552–556. doi: 10.1111/j.1469-0691.2010.03294.x. [DOI] [PubMed] [Google Scholar]
- 42.Nordmann P, Poirel L, Dortet L. 2012. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 18:1503–1507. doi: 10.3201/eid1809.120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tijet N, Boyd D, Patel SN, Mulvey MR, Melano RG. 2013. Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:4578–4580. doi: 10.1128/AAC.00878-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasoo S, Cunningham SA, Kohner PC, Simner PJ, Mandrekar JN, Lolans K, Hayden MK, Patel R. 2013. Comparison of a novel, rapid chromogenic biochemical assay, the Carba NP test, with the modified Hodge test for detection of carbapenemase-producing Gram-negative bacilli. J Clin Microbiol 51:3097–3101. doi: 10.1128/JCM.00965-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Álvarez-Buylla A, Picazo JJ, Culebras E. 2013. Optimized method for Acinetobacter species carbapenemase detection and identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 51:1589–1592. doi: 10.1128/JCM.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordmann P, Poirel L. 2013. Strategies for identification of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 68:487–489. doi: 10.1093/jac/dks426. [DOI] [PubMed] [Google Scholar]