Abstract
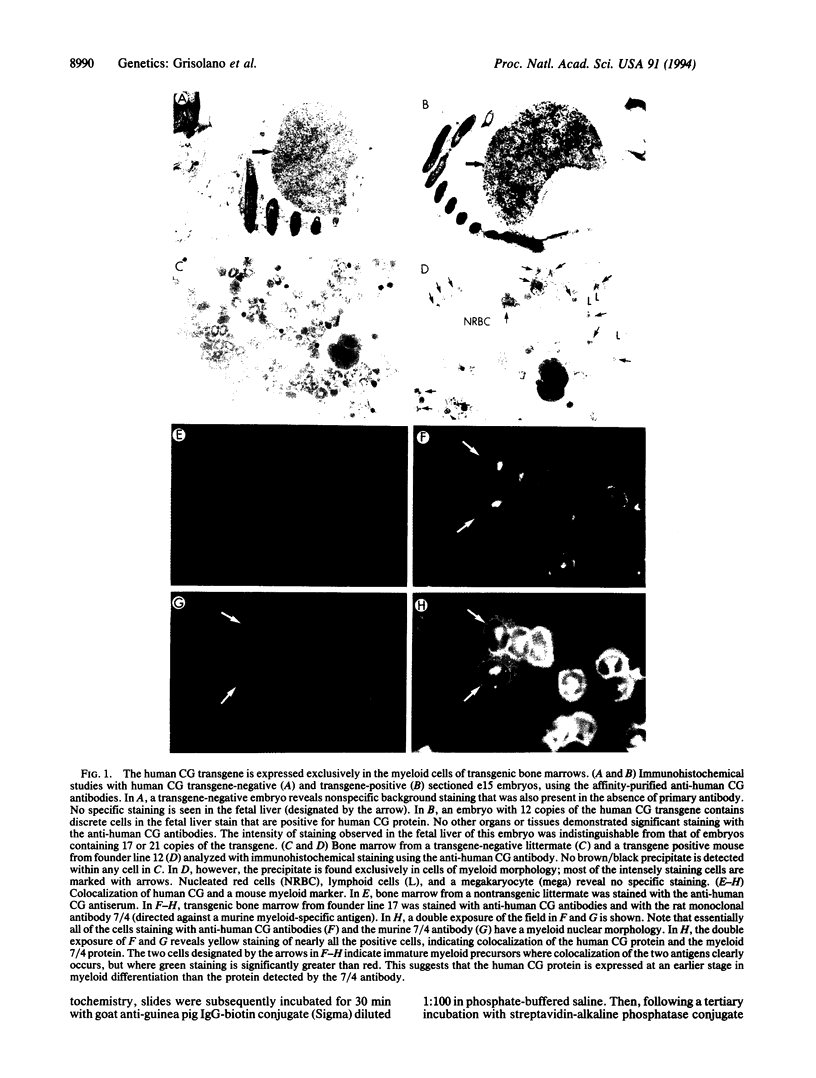
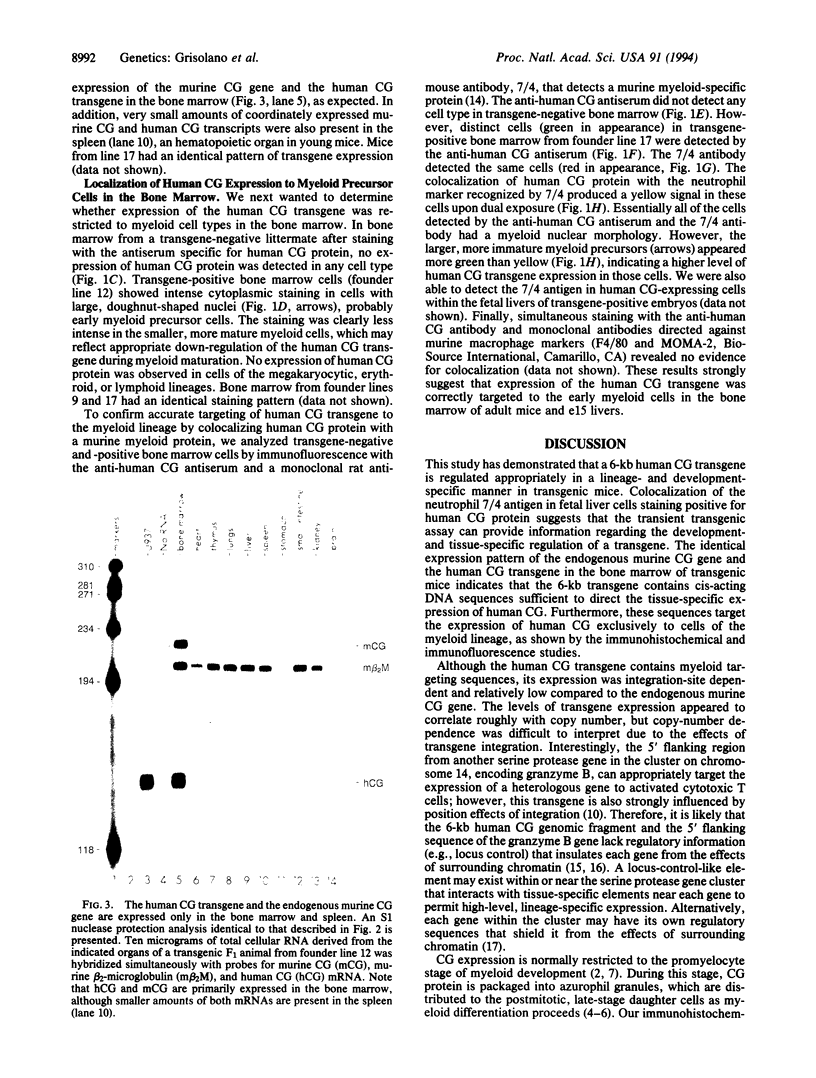
The human cathepsin G (CG) gene is expressed only in promyelocytes and encodes a neutral serine protease that is packaged in the azurophil (primary) granules of myeloid cells. To define the cis-acting DNA elements that are responsible for promyelocyte-specific "targeting," we injected a 6-kb transgene containing the entire human CG gene, including coding sequences contained in a 2.7-kb region, approximately 2.5 kb of 5' flanking sequence, and approximately 0.8 kb of 3' flanking sequence. Seven of seven "transient transgenic" murine embryos revealed human CG expression in the fetal livers at embryonic day 15. Stable transgenic founder lines were created with the same 6-kb fragment; four of five founder lines expressed human CG in the bone marrow. The level of human CG expression was relatively low per gene copy when compared with the endogenous murine CG gene, and expression was integration-site dependent; however, the level of gene expression correlated roughly with gene copy number. The human CG transgene and the endogenous murine CG gene were coordinately expressed in the bone marrow and the spleen. Immunohistochemical analysis of transgenic bone marrow revealed that the human CG protein was expressed exclusively in myeloid cells. Expression of human CG protein was highest in myeloid precursors and declined in mature myeloid cells. These data suggest that the human CG gene was appropriately targeted and developmentally regulated, demonstrating that the cis-acting DNA sequences required for the early myeloid cell-specific expression of human CG are present in this small genomic fragment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Hammer R. E., Brinster R. L., Palmiter R. D., Townes T. M. Two 3' sequences direct adult erythroid-specific expression of human beta-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7056–7060. doi: 10.1073/pnas.84.20.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk O., Mink S., Ringwald M., Klempnauer K. H. Synergistic activation of the chicken mim-1 gene by v-myb and C/EBP transcription factors. EMBO J. 1993 May;12(5):2027–2038. doi: 10.1002/j.1460-2075.1993.tb05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughey G. H., Schaumberg T. H., Zerweck E. H., Butterfield J. H., Hanson R. D., Silverman G. A., Ley T. J. The human mast cell chymase gene (CMA1): mapping to the cathepsin G/granzyme gene cluster and lineage-restricted expression. Genomics. 1993 Mar;15(3):614–620. doi: 10.1006/geno.1993.1115. [DOI] [PubMed] [Google Scholar]
- Dudek H., Tantravahi R. V., Rao V. N., Reddy E. S., Reddy E. P. Myb and Ets proteins cooperate in transcriptional activation of the mim-1 promoter. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1291–1295. doi: 10.1073/pnas.89.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouret P., du Bois R. M., Bernaudin J. F., Takahashi H., Ferrans V. J., Crystal R. G. Expression of the neutrophil elastase gene during human bone marrow cell differentiation. J Exp Med. 1989 Mar 1;169(3):833–845. doi: 10.1084/jem.169.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. D., Krieder B. L., Venturelli D., Rovera G. Transcriptional regulation of two myeloid-specific genes, myeloperoxidase and lactoferrin, during differentiation of the murine cell line 32D C13. Blood. 1991 Nov 1;78(9):2426–2432. [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Han J., Unlap T., Rado T. A. Expression of the human neutrophil elastase gene: positive and negative transcriptional elements in the 5' flanking region. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1462–1468. doi: 10.1016/0006-291x(91)92104-r. [DOI] [PubMed] [Google Scholar]
- Hanson R. D., Connolly N. L., Burnett D., Campbell E. J., Senior R. M., Ley T. J. Developmental regulation of the human cathepsin G gene in myelomonocytic cells. J Biol Chem. 1990 Jan 25;265(3):1524–1530. [PubMed] [Google Scholar]
- Hanson R. D., Ley T. J. A-T-rich scaffold attachment regions flank the hematopoietic serine protease genes clustered on chromosome 14q11.2. Blood. 1992 Feb 1;79(3):610–618. [PubMed] [Google Scholar]
- Hanson R. D., Sclar G. M., Kanagawa O., Ley T. J. The 5'-flanking region of the human CGL-1/granzyme B gene targets expression of a reporter gene to activated T-lymphocytes in transgenic mice. J Biol Chem. 1991 Dec 25;266(36):24433–24438. [PubMed] [Google Scholar]
- Heck L. W., Rostand K. S., Hunter F. A., Bhown A. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil cathepsin G from normal donors. Anal Biochem. 1986 Oct;158(1):217–227. doi: 10.1016/0003-2697(86)90612-3. [DOI] [PubMed] [Google Scholar]
- Heusel J. W., Hanson R. D., Silverman G. A., Ley T. J. Structure and expression of a cluster of human hematopoietic serine protease genes found on chromosome 14q11.2. J Biol Chem. 1991 Apr 5;266(10):6152–6158. [PubMed] [Google Scholar]
- Heusel J. W., Scarpati E. M., Jenkins N. A., Gilbert D. J., Copeland N. G., Shapiro S. D., Ley T. J. Molecular cloning, chromosomal location, and tissue-specific expression of the murine cathepsin G gene. Blood. 1993 Mar 15;81(6):1614–1623. [PubMed] [Google Scholar]
- Hohn P. A., Popescu N. C., Hanson R. D., Salvesen G., Ley T. J. Genomic organization and chromosomal localization of the human cathepsin G gene. J Biol Chem. 1989 Aug 15;264(23):13412–13419. [PubMed] [Google Scholar]
- Kargi H. A., Campbell E. J., Kuhn C., 3rd Elastase and cathepsin G of human monocytes: heterogeneity and subcellular localization to peroxidase-positive granules. J Histochem Cytochem. 1990 Aug;38(8):1179–1186. doi: 10.1177/38.8.2164060. [DOI] [PubMed] [Google Scholar]
- Ley T. J. The pharmacology of hemoglobin switching: of mice and men. Blood. 1991 Mar 15;77(6):1146–1152. [PubMed] [Google Scholar]
- Lübbert M., Miller C. W., Koeffler H. P. Changes of DNA methylation and chromatin structure in the human myeloperoxidase gene during myeloid differentiation. Blood. 1991 Jul 15;78(2):345–356. [PubMed] [Google Scholar]
- Ness S. A., Marknell A., Graf T. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell. 1989 Dec 22;59(6):1115–1125. doi: 10.1016/0092-8674(89)90767-8. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzow J., Friedman A. D. The murine myeloperoxidase promoter contains several functional elements, one of which binds a cell type-restricted transcription factor, myeloid nuclear factor 1 (MyNF1). Mol Cell Biol. 1993 Apr;13(4):2141–2151. doi: 10.1128/mcb.13.4.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M., Medcalf R. L., Fink T. M., Mattmann C., Lichter P., Jenne D. E. Three human elastase-like genes coordinately expressed in the myelomonocyte lineage are organized as a single genetic locus on 19pter. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8215–8219. doi: 10.1073/pnas.89.17.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. W., van de Gevel J. S., Beelen R. H., Fluitsma D., Hoefsmit E. C., van Furth R. Culture of human bone marrow in the teflon culture bag: identification of the human monoblast. J Reticuloendothel Soc. 1982 Nov;32(5):355–369. [PubMed] [Google Scholar]