Abstract
The galactomannan enzyme immunoassay (GM-EIA) is widely utilized for the diagnosis of invasive aspergillosis (IA). There is inconsistent reproducibility of results between centers when the assay is processed manually. Automation of EIAs can reduce variation. This study investigated the semiautomation of the GM-EIA on the DS2 (Dynex) platform in the following three stages: (i) DS2 GM-EIA method validation with experimental samples, (ii) DS2 retesting of case-defined clinical samples, and (iii) a 12-month audit of DS2 GM-EIA performance. In stage i, Bland-Altman analysis demonstrated a reduced variance between optical density index (ODI) values for samples processed on two DS2 platforms (mean difference, −0.02; limits of agreement [LOA], −0.19 to 0.14) compared with the variance between samples processed manually and on a DS2 platform (mean difference, 0.02; LOA, −0.25 to 0.3). In stage ii, 100% (14/14 samples) qualitative agreement was observed for serum samples from patients with IA, with no significant change in the ODI values when samples were processed on the DS2 platform. A significant decrease in ODI values was observed for control serum samples on the DS2 platform (difference, 0.01; P = 0.042). In stage iii, a significant reduction in the frequency of equivocal results, from 5.56% (136/2,443 samples) to 1.56% (15/961 samples), was observed after DS2 automation (difference, 4.0%; 95% confidence interval [CI], 2.7 to 5.2%; P < 0.01), with an equivalent increase in negative results. This study demonstrates that GM-EIA automation may reduce intersite variability. Automation does not have an impact on the repeatability of truly positive results but contributes to a reduction in false-positive (equivocal) GM-EIA results, reducing the need to retest a significant proportion of samples.
INTRODUCTION
The Bio-Rad Platelia Aspergillus galactomannan antigen sandwich enzyme immunoassay (GM-EIA) is widely used as a screening method for prospective surveillance of invasive aspergillosis (IA) in patients at high risk of disease. The assay is well established, as reflected by its recommendation in the European Organization for Research and Treatment of Cancer (EORTC) consensus criteria for defining invasive fungal disease (IFD) (1). Despite this fact, the diagnostic performance of the GM-EIA is variable, with meta-analyses showing combined sensitivities ranging from 0.71 to 0.78 and specificities ranging from 0.81 to 0.89 (2, 3). The false positivity experienced using the GM-EIA has been associated with antimicrobial treatment (e.g., piperacillin-tazobactam), other invasive fungal diseases (e.g., fusariosis), and even ingestion of an ice-pop (4–7). Consequently, the GM-EIA should not be used as a stand-alone diagnostic test but is an important component of the diagnostic strategy for managing IA (1).
Although initially the GM-EIA reproducibility was reported to be excellent between laboratories (8), recent reports documented a lack of reproducibility for repeat testing of positive samples (9, 10). In particular, samples with an optical density index (ODI) at or around the positivity threshold (≅0.5) of the assay were regularly found to be negative on repeat testing (8, 11). The storage conditions of specimens appear to have an impact on reproducibility, with a significant decline in the sample ODI reported after storage at −80°C (11). An IA diagnosis also appears to be important, with nonreproducibility observed more frequently for retesting of false-positive samples from patients without IA (11, 12). A significant correlation between the serum albumin concentration and the difference in ODI value on retesting was also recently reported. A larger reduction in indices was observed on retesting sera with increasing albumin concentrations (11).
While storage and disease status have been shown to affect the reproducibility of GM-EIA, other factors, such as human error, environmental contamination at the point of testing, and variability in local testing conditions between laboratories, may also have impacts on the assay's performance and reproducibility. The GM-EIA is performed manually by most laboratories and, consequently, is susceptible to fluctuations in the environmental temperature and to operator variability. Any measures taken to standardize the GM-EIA across laboratories would be advantageous, with the aim of minimizing operator and environmental influences. Automation of the GM-EIA on an enzyme-linked immunosorbent assay (ELISA) processing system will assist in standardizing the assay. There are several ELISA processing systems available, and although the data are unpublished, the GM-EIA has been automated using the Evolis ELISA processing system (Bio-Rad, written communication). The present study aimed to evaluate the automation of the GM-EIA on an alternative open platform, the DS2 (Dynex Technologies) ELISA processing system, to test the impact of the DS2 system in reducing test variability.
MATERIALS AND METHODS
Study design.
The study was conducted in the following three stages: (i) validation of the DS2 GM-EIA methodology by comparing results from experimental samples processed on two DS2 platforms against manual processing, (ii) DS2 retesting of positive/negative clinical samples based on the case definition of IA, and (iii) an audit of DS2 GM-EIA performance over a 12-month period of use in a routine diagnostic setting.
(i) DS2 method validation.
Prior to implementation in clinical practice, the DS2 system was evaluated using control serum samples and pooled sera spiked with increasing volumes (10, 20, 30, 40, and 50 μl) of a galactomannan positive control (supplied by Bio-Rad). Samples were processed on the day of spiking, manually and on two identical DS2 platforms, to allow analysis of interplatform reliability. Throughout the validation stage, we collected OD values for the GM-EIA threshold controls. The OD values were then used to define new threshold control characteristics for DS2 processing, with acceptable ODs being >0.3 and <1.2 and with a maximum OD difference between the two threshold controls of 0.5 (data not presented). Both OD and ODI values were analyzed in this stage of the study.
(ii) Reanalysis of samples defined by disease status.
To assess the clinical performance of DS2 processing, the ODI values obtained by manual and DS2 processing for case and control patient sera were compared.
(a) Positive sera.
Positive sera are not encountered frequently; therefore, an electronic search of samples processed through the year 2012 identified 17 positive serum samples (ODI > 0.5) for inclusion. Serum samples had been stored at −80°C and were tested manually and on the DS2 platform within 24 h of thawing. Samples were assigned to a case/control definition prior to retesting on the DS2 system. Twelve samples were obtained from six patients (range, 1 to 5 samples each) with IA. Five samples were from control patients with no evidence of IA.
(b) Negative sera.
Data were collected for serum samples tested prospectively manually and by the DS2 system over 1 month of testing. Only data for samples for which the disease status of the patient was known were included. In total, 55 negative sera were included; 53 samples came from control patients, and 2 samples came from patients with IA.
(iii) Postimplementation clinical evaluation.
The impact of DS2 automation was assessed through a 12-month audit of qualitative clinical results (positive, equivocal, and negative, interpreted from the ODI values) reported pre-DS2 implementation, from 1 January to 31 December 2009, and post-DS2 implementation, from 1 January to 31 December 2013. GM-EIA threshold control values were also collected to evaluate the performance of the adjusted DS2 threshold control parameters for DS2 processing. In the pre-DS2 time frame, samples initially were tested on the day of receipt or stored in the refrigerator until tested (January to March), and then the protocol was changed to storing samples in the freezer at −20°C (April to December) prior to testing when the test was not being performed on the day of receipt. In the post-DS2 implementation time frame, samples were either tested on the day of receipt or stored at −20°C until the day of testing.
GM-EIA processing.
GM-EIA kits with the same lot number were utilized during stage i, with the same operator performing all analyses. For stages ii and iii of the study, the operators varied and kits from different batches were used. Throughout all stages, serum was added to the treatment solution at a 3:1 ratio (either 360 μl of serum and 120 μl of treatment solution or 300 μl serum and 100 μl of treatment solution, for automated or manual processing, respectively). Sample pretreatment was performed per the manufacturer's instructions in a class 2 biological safety cabinet. Liquid handling processes were identical for manual and DS2 processing and were performed per the manufacturer's instructions. During stage i, all manually processed plates were washed manually (five washes) by the operator. During the second stage of the study, manually processed plates were washed using a benchtop plate washer (five stringent washes). The DS2 wash cycle program was as follows: a five-cycle supersweep wash stripwise with constant timing set to four dispenser loops, a bottom wash with 250 μl, and a final aspiration cycle with 380 μl of wash buffer.
GM-EIA result interpretation.
The ODs for samples and controls were measured at 450 nm/620 nm. The validity of each run was determined per the manufacturer's instructions. For manually processed plates, the threshold control had to be within the prerequisite OD limits of >0.3 and <0.8. For DS2 processing, the upper range of acceptance for threshold controls was increased to <1.2, with a control equation written into the DS2 program to ensure that the difference between the threshold control OD measurements was no greater than 0.5, as allowed by the manufacturer. ODI values were calculated per the manufacturer's instructions; any ODI value above 0.5 was considered positive.
Statistical analysis.
Data normality was tested using the Kolmogorov-Smirnov and Shapiro-Wilks tests. For stage i of the study, Bland-Altman plots (13) were used to demonstrate agreement between ODI results obtained by manual processing and the two DS2 platforms. Bland-Altman analysis calculates the mean difference between ODI values for the different processing methods (the “bias”) and the 95% limits of agreement (LOA) as the mean difference around the bias (2 standard deviations [SD]). It is expected that the 95% limits include 95% of differences between the two measurement methods. Spearman's rho method was used to test correlations, and the kappa statistic was calculated to test the interassay qualitative agreement between all samples and for cases and controls individually. In stage ii of the study, agreement between results was determined in both a qualitative manner (positive versus negative results) and a quantitative manner (comparison of median initial ODI versus retest ODI). The Wilcoxon signed-rank test was used to measure the differences across ODI values. Differences were determined at the 0.05 level of significance. In stage iii of the study, frequencies of negative, equivocal, and positive values were calculated for 1 year pre-DS2 processing and 1 year of DS2 use in a diagnostic setting. Analysis of differences between the frequencies was conducted using Fisher's exact test.
RESULTS
DS2 validation. (i) Threshold controls.
Data collected for 10 threshold controls were used to define the threshold control limits for DS2 testing (Table 1). A median OD of 0.48 (interquartile range [IQR], 0.42 to 0.53) was observed for GM-EIA plates processed manually. Median ODs of 0.88 (IQR, 0.76 to 1.0) and 0.79 (range, 0.68 to 1.1) were observed for GM-EIA plates processed on the two DS2 platforms (Table 1).
TABLE 1.
GM-EIA OD characteristics for testing of serum samples and threshold controls by manual and DS2 platform processing
| Parameter | Value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overalla (n = 121) |
Negative samples (n = 101) |
Spiked positive samples (n = 20) |
Threshold controls (n = 10) |
|||||||||
| Manual | DS2 platform 1 | DS2 platform 2 | Manual | DS2 platform 1 | DS2 platform 2 | Manual | DS2 platform 1 | DS2 platform 2 | Manual | DS2 platform 1 | DS2 platform 2 | |
| Range | 0.023–1.44 | 0.055–1.876 | 0.04–2.693 | 0.023–0.149 | 0.055–0.299 | 0.04–0.329 | 0.227–1.440 | 0.486–1.876 | 0.313–2.693 | 0.378–0.687 | 0.742–1.106 | 0.603–1.135 |
| 25th percentile | 0.04 | 0.076 | 0.079 | 0.037 | 0.073 | 0.075 | 0.442 | 0.807 | 0.688 | 0.42 | 0.88 | 0.68 |
| Median OD | 0.048 | 0.106 | 0.109 | 0.045 | 0.093 | 0.102 | 0.627 | 1.058 | 1.141 | 0.48 | 0.76 | 0.79 |
| 75th percentile | 0.078 | 0.151 | 0.159 | 0.057 | 0.127 | 0.127 | 0.912 | 1.341 | 1.580 | 0.53 | 1.0 | 1.1 |
Does not include threshold controls.
(ii) Sample population.
One hundred twenty-one samples were processed (20 GM-spiked serum samples and 101 blank serum samples). All 20 GM-spiked serum samples were positive (ODI ≥ 0.5) by manual and DS2 GM-EIA processing, and all 101 negative serum samples were confirmed as negative on repeat testing by manual processing and on the two DS2 platforms. The observed qualitative agreement was 100% (95% confidence interval [CI], 96.9 to 100%), with a kappa statistic of 1.0 between the manual results and those obtained on the two DS2 platforms.
The characteristics of the OD and ODI values for the 121 samples measured manually and on the DS2 platforms are presented in Tables 1 and 2. To assess the relationship between OD and ODI values, linear regression was performed and Spearman's rho correlation coefficients calculated. A strong, significant positive correlation was observed between the ODs for samples processed manually and those processed on the first (r = 0.67; P < 0.01) and second (r = 0.63; P < 0.01) DS2 platforms (see Fig. S1a and b in the supplemental material). A very strong positive correlation of OD measurements was observed between the two DS2 platforms (r = 0.78; P < 0.01) (see Fig. S1c).
TABLE 2.
Comparison of GM-EIA ODI characteristics for testing of serum samples by manual and DS2 platform processing
| Parameter | Value |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 121) |
Negative samples (n = 101) |
Spiked positive samples (n = 20) |
|||||||
| Manual | DS2 platform 1 | DS2 platform 2 | Manual | DS2 platform 1 | DS2 platform 2 | Manual | DS2 platform 1 | DS2 platform 2 | |
| Range | 0.048–3.282 | 0.057–2.374 | 0.056–2.380 | 0.048–0.339 | 0.057–0.32 | 0.056–0.416 | 0.50–3.282 | 0.51–2.374 | 0.501–2.380 |
| 25th percentile | 0.085 | 0.09 | 0.097 | 0.078 | 0.088 | 0.093 | 0.894 | 0.887 | 0.868 |
| Median index | 0.101 | 0.115 | 0.132 | 0.093 | 0.105 | 0.12 | 1.337 | 1.209 | 1.317 |
| 75th percentile | 0.160 | 0.167 | 0.177 | 0.118 | 0.137 | 0.151 | 0.83 | 1.626 | 1.79 |
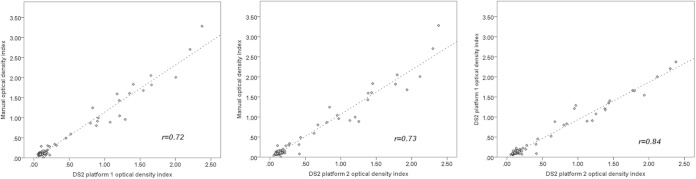
Figure 1 displays linear regression lines and Spearman's correlation coefficients for ODI values measured manually and on the two DS2 platforms. In applying Spearman's rho correlation to ODI values generated by manual and DS2 processing, again, a strong, significant positive correlation was observed between manual processing and the first (r = 0.72; P < 0.01) and second (r = 0.73; P < 0.01) DS2 platforms (Fig. 1, left and middle panels). A very strong positive correlation was observed between the indices generated on the two DS2 platforms (r = 0.84; P < 0.01) (Fig. 1, right panel).
FIG 1.
Linear regression lines and Spearman's correlation coefficients for optical density index values measured manually and on two DS2 platforms in stage i, validating the DS2 methods by using experimental samples.
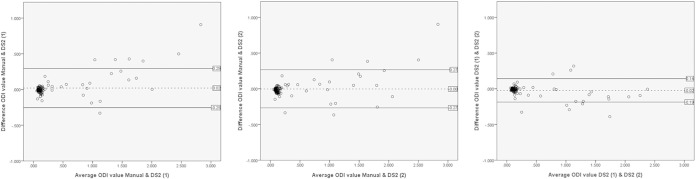
Given that Spearman's correlation measures the strength of a monotonic relationship between the data rather than agreement, Bland-Altman plots (13, 14) were generated for manual processing versus DS2 processing. In Bland-Altman analysis, the differences between ODI values are plotted against the average ODI values for the two processing methods being compared (Fig. 2).
FIG 2.
Bland-Altman plots of differences in optical density index (ODI) values against the means of the two measurements for GM-EIA ODI values measured manually and on two independent DS2 platforms.
In Fig. 2, the Bland-Altman plots demonstrate a greater variation between ODI values measured on the DS2 platforms and by manual processing than the variation between ODI values measured on the two DS2 platforms. The LOA (2 SD around the mean) was approximately halved for ODI values obtained from the two DS2 platforms (mean difference, −0.02; LOA, −0.19 to 0.14) compared with manual processing and the first DS2 platform (mean difference, 0.02; LOA, −0.25 to 0.3) or manual processing and the second DS2 platform (mean difference, −0.00; LOA, −0.27 to 0.26). The observed differences for the three methods fell within 2 SD of the mean for 94.2% (114/121 samples) of the samples between manual processing and the first DS2 platform, 95% (115/121 samples) of the samples between manual processing and the second DS2 platform, and 93.4% (113/121 samples) of the samples between the two DS2 platforms.
Reanalysis of samples defined by disease status.
In total, 72 samples were retested by the DS2 system, including 58 samples obtained from 42 control patients (range, 1 to 3 samples each) at high risk but with no evidence of fungal disease and 14 samples (range, 1 to 5 samples each) obtained from 6 patients defined as having cases of probable IA, with radiological evidence of sinusitis and/or pulmonary disease.
Of the 72 samples, 55 (53 from control patients and 2 from case patients) were initially negative by manual processing. Fifty-two samples remained negative by DS2 processing (94.5% observed agreement; 95% CI, 85.2 to 98.1%). Three samples were positive by DS2 processing on repeat testing; all were from control patients and had an initial ODI value in the range of 0.4 to 0.49, close to the positivity threshold of 0.5. Both of the negative samples from case patients were in the range of 0.4 to 0.49 when manually tested and on repeat testing by DS2 processing. Seventeen samples were initially positive when tested manually: 12 samples from cases of IA and 5 samples from control patients. On repeat testing, 94.1% (16/17 samples) of samples were positive when retested by the DS2 system (95% CI, 73.0 to 99.0%). Analysis by the disease status of samples initially testing positive (ODI > 0.5) by manual processing demonstrated that for case patients, 100% (12/12 samples) of the samples remained positive on repeat testing by DS2 processing. For controls, 80% (4/5 samples) of positive samples remained positive on repeat testing. One sample tested positive manually, with an ODI value of 0.51, but was negative by DS2 repeat testing, with an ODI value of 0.45.
The observed qualitative agreement between manual processing and DS2 processing was 94.4% (68/72 samples; 95% CI, 86.6 to 97.8%), with a kappa statistic of 0.64. The observed agreement for controls was 93.1% (54/58 samples; 95% CI, 83.6 to 97.3%), with a kappa statistic of 0.63. For case patients, a 100% observed agreement (14/14 samples; 95% CI, 78.5 to 100%) was achieved between manual and DS2 processing, but it was not possible to determine the kappa statistic due to the small sample number. The median GM ODI values (for samples tested manually and on the DS2 platform) for the overall population, cases, and controls are shown in Table 3. On the DS2 platform, the GM ODI for the overall population was not significantly different (P = 0.116) from the initial ODI obtained by manual testing. Analysis by disease status showed that there was no significant difference between ODI values for testing of samples from case patients by DS2 processing. For testing of samples from control patients with no evidence of IFD, a significant reduction in ODI values (P = 0.042) was observed. Spearman's correlation coefficient between the original and retest ODI values overall was positive (r = 0.59), representing a strong positive correlation between manual and DS2 testing (P < 0.001).
TABLE 3.
GM-EIA ODI reproducibility for testing of case-defined clinical serum samples manually and on the DS2 platform
| Parameter | Value |
|||||
|---|---|---|---|---|---|---|
| Overall (n = 72)a |
Case samples (n = 14) |
Control samples (n = 58) |
||||
| Initial ODI | Retest ODI | Initial ODI | Retest ODI | Initial ODI | Retest ODI | |
| Range | 0.05–1.59 | 0.04–1.71 | 0.45–1.59 | 0.46–1.71 | 0.05–0.51 | 0.04–0.56 |
| 25th percentile | 0.08 | 0.06 | 0.5 | 0.57 | 0.07 | 0.05 |
| Median | 0.11 | 0.10 | 0.85 | 0.88 | 0.10 | 0.09 |
| 75th percentile | 0.48 | 0.5 | 1.1 | 1.15 | 0.21 | 0.11 |
| Wilcoxon P value | 0.116 | 0.972 | 0.042 | |||
For the overall results, Spearman's rho correlation coefficient was 0.593, and the P value was <0.01.
Postimplementation clinical evaluation. (i) Threshold control analysis.
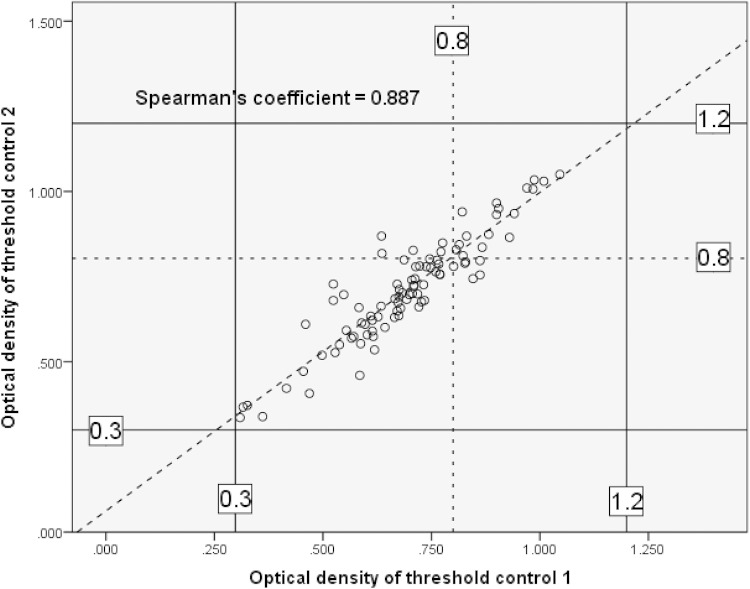
A strong, significant positive Spearman's correlation of 0.89 (P < 0.01) was achieved between threshold control limit 1 and threshold control limit 2. The median OD value was 0.72 (IQR, 0.62 to 0.8) for threshold control limit 1 and 0.7 (IQR, 0.61 to 0.79) for threshold control limit 2. In Fig. 3, the manufacturer's recommended optical density threshold controls (>0.3 and <0.8) are indicated by dotted lines. The optical density thresholds for DS2 automation in this study were >0.3 and <1.2, as shown by solid lines in Fig. 3.
FIG 3.
Linear regression analysis of OD values for threshold controls collected over a 1-year period of DS2 GM-EIA processing. The dotted lines indicate the manufacturer's recommended upper threshold limit, and the solid lines indicate the adjusted upper threshold limit for DS2 processing.
(ii) GM-EIA audit conducted pre- and post-DS2 automation.
Analysis of the results reported from the GM-EIA over two 12-month periods, pre- and post-DS2 automation, showed that the number of positive results decreased slightly, from 4.01% (98/2,443 samples) in 2009 to 3.54% (34/961 samples) in 2013, but that this decline was not statistically significant (difference, 0.47%; 95% CI, −1.06 to 1.78%; P = 0.555). The number of equivocal reports (for samples that tested positive initially and negative on repeat testing) decreased almost 5-fold, from 5.56% (136/2,443 samples) in 2009 to 1.56% (15/961 samples) in 2013 (difference, 4.0%; 95% CI, 2.7 to 5.2%; P < 0.01). The number of negative reports increased from 90.42% (2,209/2,443 samples) in 2009 to 94.9% (912/961 samples) in 2013 (95% CI, 2.6 to 6.2%; P < 0.001).
DISCUSSION
This study has demonstrated that the use of an automated liquid handling platform, such as the DS2 platform, can replace manual processing of the GM-EIA. Almost complete agreement (97.9% [189/193 samples]) was observed between qualitative results (negative/positive) across simulated and clinical samples combined when the samples were processed manually and on the DS2 platform. Manual processing is responsible for variation within the GM-EIA. In stage i, where GM-EIA was performed by a single operator, OD and ODI values measured manually were compared against those measured on the two DS2 platforms. A stronger positive correlation was observed between OD and ODI values measured on the two DS2 platforms. This indicates that manual processing was responsible for the reduced correlation in comparing manual and DS2 OD and ODI values. Bland-Altman plots demonstrated that the bias across all three comparisons was minimal (0.02). However, the limits of agreement around the bias were almost halved in comparing ODI values measured on the two DS2 platforms. This supports the view that automation of the GM-EIA across laboratories will contribute to a reduction in variance, an issue that has been reported in several other studies (8, 9, 15). This is further supported by other studies reporting a decrease in variance within EIAs after automation (16, 17).
On testing of samples defined by disease status in stage ii of the study, there was no significant difference in ODI values for cases tested by the DS2 platform (difference, 0; P = 0.082), but for samples from patients with no evidence of IFD, a significant reduction of ODI values was observed when samples were tested on the DS2 platform. Qualitative results (positive/negative) differed between manual and DS2 processing for four samples, all of which were from control patients (changing from negative to positive for three samples and from positive to negative for one sample). Importantly, all four samples had ODI values of 0.4 to 0.49, close to the positivity threshold of the assay. As these patients had no other evidence of invasive fungal disease, these samples represent false-positive results. The change in qualitative results for these four samples was not likely a result of DS2 automation but a result of the variability in measured ODIs seen for false-positive samples, which was reported in a similar study (11). A lack of repeatability for samples with ODI values close to the positivity threshold of the assay was also reported by Marr et al., as 10.2% of positive serum samples included in their study, all with ODIs of 0.5 to 0.7, tested negative on repeat testing (8).
False positivity of the GM assay has been reported in several case reports, in association with factors such as the use of piperacillin-tazobactam, which is an antimicrobial frequently used to treat neutropenic sepsis (4, 6, 18). Weaker nonspecific binding of cross-reacting epitopes in false-positive samples could cause increased variation in ODI values upon repeat testing and may explain the lack of repeatability observed for control samples. In a recent report, Kimpton et al. suggested that molecules causing false-positive results bind with less affinity to the EB-A2 antibody after freezing and storage, which leads to them being negative on repeat testing (11). Conversely, the target molecule galactomannan binds with a high affinity, which leads to the repeatability of results for truly positive samples. The complete qualitative agreement seen for results from positive serum samples spiked with control GM in stage i of the study and for positive samples from case patients with IA in stage ii of the study supports this hypothesis. Furthermore, two “negative” samples from cases of IA with ODI values close to the positivity threshold (0.45 and 0.46 by manual testing and 0.46 and 0.47 by DS2 testing) likely represented low-level positive samples as measured by both processing methods. To control for this possibility, the manufacturer's recommendations for GM-EIA testing are that all positive GM samples be retested. In our center, we adhere to these recommendations and account for positive GM samples that test negative on repeat testing as equivocal results.
After implementation of DS2 GM-EIA processing, we observed a significant decrease in equivocal results (initially positive samples that tested negative on repeat testing) reported from our laboratory, from 5.56% to 1.56%, with an equivalent increase in negative results reported in this period. This suggests that automation contributed to a reduction in false positivity. The reduction in equivocal results using the DS2 platform may be a result of an increased stringency of the washing program. Weakly bound cross-reacting molecules may be washed away more effectively. However, the observed reduction in equivocal results could also be due to an effect of sample storage at −20°C prior to testing, which was introduced for all samples not being tested on the day of receipt in our center around the time of DS2 automation. The low reproducibility of GM-positive results after storage at −20°C has been highlighted in two studies (10, 19); however, the long-term stability of galactomannan in serum at −20°C has been demonstrated to be up to 5 years (20). This suggests that freezing may lead to a reduction in false positivity but not affect genuinely positive samples. In this study, there was no significant difference in the frequency of positive samples (those testing positive twice) after DS2 automation and freezing measures.
Finally, this is the first study to describe a protocol for GM-EIA automation, and it highlights an important technical issue. During the validation stage of this study, the measured optical densities on both DS2 platforms were significantly higher across the entire GM-EIA plate, for samples and controls, than those obtained by manual processing (difference in median, 0.058 [P < 0.01]; difference, 0.061 [P < 0.01]). Using the optical density threshold control limits defined by the manufacturer on the DS2 platform consequently resulted in a failure of the assay's quality control, so the limits for threshold controls required adjustment. The threshold control limits (OD values of >0.3 and <0.8) control for intra-assay variance by ensuring that the difference in OD between the two threshold controls does not exceed 0.5. The manufacturer's recommended upper limit of <0.8 was frequently exceeded during the validation stage of this study, but crucially, the differences between the ODs of the two cutoff controls were negligible (range, 0.002 to 0.088) (data not presented). To enable quality control of the GM-EIA on the DS2 platform, the threshold control upper limit was increased to <1.2, with an additional measure written into the DS2 program to reject an OD difference of >0.5 between the two threshold controls. Increasing the threshold control upper limit did not have an impact on the final qualitative results obtained by DS2 processing. The ODI value is calculated by division of the sample OD by the mean OD value for the two threshold controls. Since the OD increases were proportional across samples and controls, the effect was normalized by this calculation. This is supported by the absolute agreement of results for samples processed manually and by the DS2 platform. Threshold control data obtained from a 12-month audit of 92 GM-EIA runs also demonstrated that the OD did not exceed 1.2, as demonstrated in Fig. 2, and that the largest difference between the two threshold controls was again negligible (range, 0.002 to 0.112). There is strong evidence from this study that the allowed OD variation of 0.5 between threshold controls can be reduced significantly.
In conclusion, this study has demonstrated that the GM-EIA can be automated on an ELISA processing system, such as the DS2 platform, without affecting the assay's performance and that this will contribute to a reduction in variance of results obtained from the assay. Automation of EIAs leads to increased standardization and time-saving benefits for the laboratory and allows for more robust comparisons of results across centers that employ automated platforms for ELISA processing.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute for Health Research (NIHR) as part of the Chief Scientific Officers NIHR Ph.D.-funded fellowship scheme.
C.C.K. has received honoraria from Astellas, Gilead, MSD, and Pfizer. P.L.W. is a founding member of the EAPCRI, received project funding from Myconostica, Luminex, and Renishaw Diagnostics, was sponsored by Myconostica, MSD, and Gilead Sciences to attend international meetings, and was a consultant for Renishaw Diagnostics Limited.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.00157-15.
REFERENCES
- 1.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leeflang MM, Debets-Ossenkopp YJ, Visser CE, Scholten RJ, Hooft L, Bijlmer HA, Reitsma JB, Bossuyt PM, Vandenbroucke-Grauls CM. 2008. Galactomannan detection for invasive aspergillosis in immunocompromized patients. Cochrane Database Syst Rev 2008:CD007394. doi: 10.1002/14651858.CD007394. [DOI] [PubMed] [Google Scholar]
- 3.Pfeiffer CD, Fine JP, Safdar N. 2006. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 4.Gerlinger MP, Rousselot P, Rigaudeau S, Billon C, Touratier S, Castaigne S, Eloy O. 2012. False positive galactomannan Platelia due to piperacillin-tazobactam. Med Mal Infect 42:10–14. doi: 10.1016/j.medmal.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Guigue N, Menotti J, Ribaud P. 2013. False positive galactomannan test after ice-pop ingestion. N Engl J Med 369:97–98. doi: 10.1056/NEJMc1210430. [DOI] [PubMed] [Google Scholar]
- 6.Tortorano AM, Esposto MC, Prigitano A, Grancini A, Ossi C, Cavanna C, Cascio GL. 2012. Cross-reactivity of Fusarium spp. in the Aspergillus galactomannan enzyme-linked immunosorbent assay. J Clin Microbiol 50:1051–1053. doi: 10.1128/JCM.05946-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergidis P, Walker RC, Kaul DR, Kauffman CA, Freifeld AG, Slagle DC, Kressel AK, Wheat LJ. 2012. False-positive Aspergillus galactomannan assay in solid organ transplant recipients with histoplasmosis. Transpl Infect Dis 14:213–217. doi: 10.1111/j.1399-3062.2011.00675.x. [DOI] [PubMed] [Google Scholar]
- 8.Upton A, Gugel A, Leisenring W, Limaye A, Alexander B, Hayden R, Marr KA. 2005. Reproducibility of low galactomannan enzyme immunoassay index values tested in multiple laboratories. J Clin Microbiol 43:4796–4800. doi: 10.1128/JCM.43.9.4796-4800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bizzini A, Marchetti O, Meylan P. 2012. Response to: lack of intra-laboratory reproducibility in using Platelia Aspergillus enzyme immunoassay test for detection of Aspergillus galactomannan antigen. Transpl Infect Dis 14:218–219. doi: 10.1111/j.1399-3062.2011.00707.x. [DOI] [PubMed] [Google Scholar]
- 10.Pedroza KC, de Matos SB, de Moura DL, Oliveira MB, Araujo MA, Nascimento RJ, Lima FW. 2013. Reproducibility of positive results for the detection of serum galactomannan by Platelia Aspergillus EIA. Mycopathologia 176:295–297. doi: 10.1007/s11046-013-9670-z. [DOI] [PubMed] [Google Scholar]
- 11.Kimpton G, Lewis WP, Barnes RA. 2014. The effect of sample storage on the performance and reproducibility of the galactomannan EIA test. Med Mycol 52:618–626. doi: 10.1093/mmy/myu014. [DOI] [PubMed] [Google Scholar]
- 12.Furfaro E, Mikulska M, Miletich F, Viscoli C. 2012. Galactomannan: testing the same sample twice? Transpl Infect Dis 14:E38–E39. doi: 10.1111/j.1399-3062.2012.00733.x. [DOI] [PubMed] [Google Scholar]
- 13.Altman DG, Bland JM. 1983. Measurement in medicine; the analysis of method comparison studies. Statistician 32:307–317. doi: 10.2307/2987937. [DOI] [Google Scholar]
- 14.Bland JM, Altman DG. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet i:307–310. [PubMed] [Google Scholar]
- 15.Oren I, Avidor I, Sprecher H. 2012. Lack of intra-laboratory reproducibility in using Platelia Aspergillus enzyme immunoassay test for detection of Aspergillus galactomannan antigen. Transpl Infect Dis 14:107–109. doi: 10.1111/j.1399-3062.2011.00663.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Campenhout CM, Van Cotthem KA, Stevens WJ, De Clerck LS. 2007. Performance of automated measurement of antibodies to cyclic citrullinated peptide in the routine clinical laboratory. Scand J Clin Lab Invest 67:859–867. doi: 10.1080/00365510701408582. [DOI] [PubMed] [Google Scholar]
- 17.Whitworth WC, Goodwin DJ, Racster L, West KB, Chuke SO, Daniels LJ, Campbell BH, Bohanon J, Jaffar AT, Drane W, Sjoberg PA, Mazurek GH. 2014. Variability of the QuantiFERON(R)-TB gold in-tube test using automated and manual methods. PLoS One 9:e86721. doi: 10.1371/journal.pone.0086721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blijlevens NM, Donnelly JP, Meis JF, Verweij PE, de Pauw BE. 2002. Aspergillus galactomannan antigen levels in allogeneic haematopoietic stem cell transplant recipients given total parenteral nutrition. Transpl Infect Dis 4:64–65. doi: 10.1034/j.1399-3062.2002.00015.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson GL, Sarker SJ, Hill K, Tsitsikas DA, Morin A, Bustin SA, Agrawal SG. 2013. Significant decline in galactomannan signal during storage of clinical serum samples. Int J Mol Sci 14:12970–12977. doi: 10.3390/ijms140712970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheat LJ, Nguyen MH, Alexander BD, Denning D, Caliendo AM, Lyon GM, Baden LR, Marty FM, Clancy C, Kirsch E, Noth P, Witt J, Sugrue M, Wingard JR. 2014. Long-term stability at −20 degrees C of Aspergillus galactomannan in serum and bronchoalveolar lavage specimens. J Clin Microbiol 52:2108–2111. doi: 10.1128/JCM.03500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.