Abstract
To clarify the factors causing an outbreak in 2014 of Getah virus infection among racehorses at the Miho training center, Japan, we isolated virus strains and performed an epizootiological investigation of affected horses and related horse populations. Three Getah virus isolates were recovered from clinical samples, and one of them (14-I-605) was used in a virus-neutralizing test. Of the affected horses (n = 33), 20 (60.6%) were 2-year-olds. We investigated the histories of Getah virus vaccination of the affected horses and the whole population at the Miho training center. Among the 2-year-old population, the prevalence of the disease in horses that had been vaccinated once was 14.1%. This was significantly higher than that in horses that had been vaccinated twice or more (1.3%; P < 0.01). Among horses that had entered the training center from farms in Ibaraki Prefecture surrounding the training center and from neighboring Chiba Prefecture, the rate of seropositivity for Getah virus was 13.0% in September 2014 and 42.9% in October 2014; that in the corresponding periods in 2010 and 2013 was 0%. In conclusion, we identified two possible causes of the outbreak of Getah virus infection in the training center in 2014: (i) the existence of susceptible horses that had received only one dose of vaccination before the outbreak and (ii) increased risk of exposure to the virus because of epizootic Getah virus infection among horses on surrounding farms in Ibaraki and Chiba prefectures.
INTRODUCTION
Getah virus is classified in the genus Alphavirus in the family Togaviridae (1). It is mosquito borne and is widespread from Eurasia to Australasia. This virus causes fever, body rash, and leg edema in horses (1), and it causes fetal death and reproduction disorders in pigs (2, 3). Both pigs and horses can play important roles in the amplification and circulation of Getah virus, because maximum virus titers in the blood of infected animals of these species are sufficient for the vector mosquitoes to be infected (1, 4–6).
Outbreaks of Getah virus infection in horses occurred in 1978, 1979, and 1983 in Japan and in 1990 in India (7–10). Although Japan has not experienced an outbreak of Getah virus infection for decades, the virus seems to be circulating among the horse population. Serological surveillance in the 1990s revealed that some of the horses at racetracks managed by prefectural or municipal governments had antibodies to Getah virus (11). Nevertheless, the epizootic status of the virus in the horses in recent years was not known. After the first outbreak of Getah virus infection at the Miho training center of the Japan Racing Association (JRA) in 1978, an inactivated whole-virus vaccine (Nisseiken Co. Ltd., Tokyo, Japan) was developed by using Getah virus strain MI-110, which was isolated from an affected horse during the outbreak. This vaccine has been used in the training centers of the JRA and in some other horse facilities in Japan since 1979, and up until 2014 no outbreaks of Getah virus infection had been reported among vaccinated horses at the training centers. The Getah virus vaccination program at the training centers is as follows: two vaccinations with an approximately 1-month interval in the first year (mainly 2-year-old horses) and then an annual booster before each mosquito season.
In a previous study, we reported that an outbreak of Getah virus infection occurred in racehorses at the Miho training center in autumn 2014 (12). It started in the middle of September and ended in late October. Among horses that were pyretic in this period, 33 were diagnosed with Getah virus infection through the detection of viral RNA by reverse transcription-PCR (RT-PCR) or by a significant increase in virus-neutralizing (VN) antibodies against Getah virus, or both (12). Because it had been believed that the vaccine was effectively protecting the horses at the training centers from the disease, the outbreak in 2014 was entirely unexpected to us. The aim of our current study was to clarify the factors causing the 2014 outbreak of Getah virus infection at the training center. To achieve this, we first isolated Getah virus strains from clinical samples taken from affected horses during the outbreak. We then performed a seroepizootiological investigation of the affected horses and of associated horse populations by using the isolated virus.
MATERIALS AND METHODS
Cell culture.
For virus isolation and VN testing we used Vero cells (Sumitomo Dainippon Pharma, Tokyo, Japan). Getah virus strain MI-110 isolated from a racehorse during the previous Getah virus outbreak in 1978 was used in the VN test. Cells were cultured in minimum essential medium (MEM; MP Biomedicals, Irvine, CA, USA) containing 10% fetal calf serum (Sigma-Aldrich Inc., St. Louis, MO, USA), 100 units/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich Inc.). MEM containing 2% fetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin was used as a maintenance medium for virus isolation and VN testing.
Virus isolation.
EDTA-treated blood samples were collected from 28 horses that had developed pyrexia during the period from 25 September to 10 October 2014. Buffy coat specimens containing leukocytes were frozen and thawed three times and then centrifuged at 800 × g for 20 min at 4°C. The supernatants were inoculated onto 1-day monolayer cultures of Vero cells or inoculated with the cells simultaneously. In some cases, the buffy coat was directly cultured with the cells without the freeze-thaw step described above. The next day, the cells were washed three times with phosphate-buffered saline and cultured in the maintenance medium. To identify Getah virus-specific nucleotide sequences, the supernatants of specimens that showed cytopathic effect (CPE) were tested by RT-PCR using primer sets M2W-S and M3W-S for the nsP1 gene and SagW8 and SagW9 for the capsid gene (13). The RT-PCR products were sequenced as described previously (12, 13).
Seroepizootiological investigation of horses infected with Getah virus and associated horse populations during the outbreak at the Miho training center.
Sera were collected from horses in the group of 33 that had been diagnosed as Getah virus infected during the outbreak at the Miho training center (12). Acute-phase sera were taken on the day the horses developed pyrexia, and convalescent-phase sera were taken at 20- to 30-day intervals. Sera were not obtained from some of the horses because they had already left the training centers by the time of sampling. In total, 25 paired sera and seven acute-phase sera were available.
Additionally, paired sera from 28 horses that had been kept together with the affected horses in the same barns were used. These horses were clinically healthy and had not developed pyrexia during the sampling period. The first collection of sera was on 8 and 9 October, about 1 week after we had first identified the cause of the disease as Getah virus infection. The second collection of sera was between 12 and 14 November. Paired sera from horses (n = 17) at the JRA's other training center, the Ritto training center in Shiga Prefecture in western Japan, that were observed between 3 and 23 October to be pyretic were also tested.
The Getah virus MI-110 strain and one strain isolated during the 2014 outbreak were used for VN testing. Sera were diluted at 1:4 with maintenance medium and inactivated for 30 min at 56°C. Fifty microliters of serial 2-fold dilutions of the 1:4 sera were prepared in duplicate on flat-bottomed 96-well plates (Asahi Glass Co. Ltd., Tokyo, Japan) and then mixed with each strain of Getah virus (100 50% tissue culture infective doses/50 μl/well). After incubation of the wells for 1 h at 37°C with 5% CO2, Vero cells were added at a concentration of 5 × 104 cells/50 μl/well. After incubation for 3 days at 37°C with 5% CO2, the cells were stained with crystal violet-formalin solution. The VN titer was defined as the reciprocal of the highest dilution that completely inhibited viral growth.
Vaccination histories of the whole horse population at the Miho training center and prevalence of Getah virus infection among populations stratified by number of vaccine doses received.
We investigated the vaccination histories of horses that were present at the Miho training center on 15 September 2014, i.e., the day on which the outbreak started (n = 1,950). On the basis of the number of vaccination doses they had received before the outbreak, horses in populations of each age were categorized into two groups, (i) those which had received one dose and (ii) those which had received two doses or more. Although some horses were replaced with new ones during the outbreak, we considered that the changes in age distribution and vaccination histories of the overall horse population were sufficiently small to be ignored. The prevalence of disease onset of Getah virus infection in each population during the period from 15 September to 25 October 2014 was calculated by dividing the number of affected horses in each category by the number of horses in the corresponding population. The statistical significance of differences in prevalence was evaluated by using Fisher's exact test.
Investigation of Getah virus epizootic infection among horses on surrounding farms in Ibaraki and Chiba prefectures.
Among horses introduced to the Miho training center between June and October 2010, 2013, and 2014, those that met all of the following criteria were randomly selected (50 horses/month, at maximum): (i) age of 2 years, (ii) history of being transferred from a farm in Ibaraki or Chiba prefecture, and (iii) no history of vaccination with inactivated Getah virus vaccine. Sera collected on the day each horse entered the Miho training center were subjected to VN testing for Getah virus by using the strain isolated during the 2014 outbreak.
We also tested the sera of the horses transferred from other areas of Japan (n = 170, randomly selected, from June to October 2014). These horses were also 2-year-olds and had no history of vaccination with Getah virus vaccine.
RESULTS
Virus isolation from blood samples of Getah virus-infected horses.
We initially tried to isolate Getah virus from blood samples from pyretic horses during the 2014 outbreak. We cultured buffy coat specimens of EDTA-treated blood with Vero cells. Among 28 samples, primary cocultivation resulted in the isolation of three strains with CPE. The CPE, which was characterized by the appearance of round cells, appeared 4 to 6 days after cocultivation. The culture supernatants were then tested by using RT-PCR, and these isolates were identified as Getah virus. Details of the horses from which the virus was isolated are given in Table 1. In our nucleic acid sequence analysis of the nsP1 and capsid genes, the sequences of the three isolates were identical to those of the RT-PCR products amplified from the clinical samples during the outbreak described in the previous study (12). In that study, the sequences were completely identical among the 10 specimens tested. The 10 horses from which the nucleotides were amplified are indicated in Table 1. From this result, one of the three isolates was chosen as representative of the strain circulating during the outbreak and was designated strain 14-I-605. This strain was passaged twice in the Vero cells, and the resulting 14-I-605 strain (V-3) was used for VN testing.
TABLE 1.
Ages, vaccination histories, and VN antibody responses of pyretic horses with Getah virus infection during the outbreak at the Miho training center
| Horsea | Date of onset | Age (yr) | Vaccination with Getah virus vaccine |
Titer against: |
|||||
|---|---|---|---|---|---|---|---|---|---|
| MI-110 |
14-I-605 |
||||||||
| 2013 or before | 2014, 1st | 2014, 2nd | Acute | Convalescent | Acute | Convalescent | |||
| 1 | 15 Sept | 2 | − | 30 May | 28 Jun | <4 | 128 | <4 | 64 |
| 2 | 18 Sept | 5 | + | 14 May | <4 | ≥512 | <4 | 256 | |
| 3 | 20 Sept | 3 | + | 12 Jun | <4 | ≥512 | <4 | ≥512 | |
| 4 | 21 Sept | 3 | − | 14 May | 16 Jun | <4 | 256 | <4 | 256 |
| 5 | 23 Sept | 2 | − | 16 May | 13 Jun | <4 | 256 | <4 | 128 |
| 6 | 25 Sept | 4 | + | 21 May | <4 | ≥512 | <4 | ≥512 | |
| 7* | 25 Sept | 3 | + | 14 May | <4 | NDb | <4 | ND | |
| 8 | 26 Sept | 2 | − | 20 Jun | 20 Jul | <4 | 64 | <4 | 32 |
| 9* | 27 Sept | 2 | − | 24 Sept | ND | ND | ND | ND | |
| 10 | 28 Sept | 2 | − | 24 Sept | <4 | 256 | <4 | 128 | |
| 11# | 28 Sept | 4 | + | 14 May | <4 | 256 | <4 | 256 | |
| 12 | 30 Sept | 3 | + | 22 May | <4 | 128 | <4 | 128 | |
| 13* | 30 Sept | 5 | + | 9 Jun | 4 | ND | <4 | ND | |
| 14 | 1 Oct | 2 | − | 26 Sept | 4 | 256 | 4 | 128 | |
| 15 | 1 Oct | 2 | − | 26 Sept | 16 | 256 | 16 | 256 | |
| 16 | 1 Oct | 3 | − | 2 Jun | 18 Jun | 4 | ≥512 | 4 | ≥512 |
| 17* | 1 Oct | 4 | + | 16 Jul | <4 | 16 | <4 | 16 | |
| 18 | 1 Oct | 2 | − | 11 Jun | 19 Jul | 8 | 256 | 4 | 128 |
| 19 | 1 Oct | 2 | − | 26 Sept | 4 | 64 | <4 | 32 | |
| 20 | 4 Oct | 2 | − | 28 Aug | 1 Oct | <4 | 64 | <4 | 64 |
| 21 | 4 Oct | 7 | + | 2 Jun | 256 | ND | 128 | ND | |
| 22* | 5 Oct | 2 | − | 12 Jun | 3 Jul | <4 | ≥512 | <4 | 256 |
| 23# | 7 Oct | 2 | − | 5 Oct | <4 | ND | <4 | ND | |
| 24* | 8 Oct | 3 | + | 12 Jun | <4 | 128 | <4 | 64 | |
| 25* | 8 Oct | 4 | + | 20 May | <4 | ≥512 | <4 | 256 | |
| 26 | 9 Oct | 2 | − | 12 Jun | 10 Jul | <4 | 8 | <4 | 8 |
| 27 | 10 Oct | 2 | − | 9 Oct | <4 | 128 | <4 | 128 | |
| 28# | 10 Oct | 2 | − | 9 Oct | <4 | ND | <4 | ND | |
| 29* | 12 Oct | 2 | − | 31 May | 18 Jun | <4 | ND | <4 | ND |
| 30 | 15 Oct | 2 | − | 12 Oct | <4 | 256 | <4 | 128 | |
| 31 | 16 Oct | 2 | − | 20 Jun | 20 Jul | <4 | ND | <4 | ND |
| 32* | 16 Oct | 2 | − | 10 Sept | 15 Oct | <4 | 64 | <4 | 32 |
| 33* | 25 Oct | 2 | − | 18 Sept | <4 | 256 | <4 | 64 | |
#, horse from which Getah virus was isolated; *, horse whose blood samples were used for viral RNA amplification and sequencing in a previous study (12).
ND, no data.
Ages, VN antibody responses, and vaccination histories of pyretic horses with Getah virus infection during the outbreak at the Miho training center.
The horses (n = 33) that developed fever and were confirmed to be infected with Getah virus during the outbreak in the previous study (12) are listed in Table 1. The affected horses were 2 to 7 years old; 20 of them (60.6%) were 2-year-olds. Although we had performed VN testing using MI-110 for the diagnosis of Getah virus infection in the previous study, the titers for each horse were not indicated in that study (12). In order to compare the cross-reactivity of VN antibodies against the vaccine strain MI-110 and the currently isolated 14-I-605 strain, the titers are shown in Table 1. Because the titers against the two strains were almost equal, and no samples showed titer differences of more than 4-fold between the two strains, we describe the results of our further analysis in terms of titers against the 14-I-605 strain. All of the 25 paired sera that were available for the analysis showed a significant increase in VN titer (more than 4-fold). Among the affected horses, 27 (81.8%) had titers of less than 4 in the acute-phase sera. According to the vaccination histories of the affected horses, 21 had already received Getah virus vaccine at least twice and at least 1 month before the onset of pyrexia. Ten horses had received only one dose of vaccine, and the remaining two horses had been vaccinated twice but the second dose had been given just before the onset of pyrexia.
Subclinical infection in horses reared with Getah virus-infected horses during the outbreak at the Miho training center.
In order to survey for subclinical Getah virus infection at the Miho training center, we tested the paired sera from clinically healthy horses kept together with the affected horses in the same barns (n = 28) for Getah virus VN antibodies. The ages, VN antibody responses, and vaccination histories of these horses are shown in Table 2. VN testing using the 14-I-605 strain revealed seroconversion in four horses, although two of these might have been due to the fact that the horses were vaccinated just before the sampling period. Therefore, at least two (horses 41 and 48) out of 28 horses (7.1%) were judged to have been infected but without clinical symptoms in this period. Among the 28 horses, 18 had VN titers of less than 4 in their sera from the first collection. The vaccination histories of the 28 horses revealed that 24 horses had already received Getah virus vaccine at least twice and at least 1 month before the first sampling; the remaining four horses had received only one dose of vaccine. Both the horses with subclinical infection had VN titers of less than 4 in their sera from the first collection, although they had been vaccinated at least twice before the outbreak (Table 2).
TABLE 2.
Ages, vaccination histories, and VN antibody responses of clinically healthy horses kept together with the affected horses during the outbreak at the Miho training center
| Horsea | Age (yr) | Vaccination with Getah virus vaccine |
Titer against 14-I-605 |
|||
|---|---|---|---|---|---|---|
| 2013 or before | 2014, 1st | 2014, 2nd | Sera from 1st collection | Sera from 2nd collection | ||
| 34 | 2 | − | 6 Sept | <4 | 8 | |
| 35 | 3 | + | 9 Jul | <4 | <4 | |
| 36 | 4 | + | 15 May | 32 | 32 | |
| 37 | 2 | − | 14 Jun | 24 Jul | <4 | <4 |
| 38 | 7 | + | 30 Sept | 16 | 8 | |
| 39 | 4 | + | 25 Jun | 16 | 16 | |
| 40 | 4 | + | 15 May | <4 | <4 | |
| 41# | 2 | − | 21 Jul | 30 Jul | <4 | 8 |
| 42 | 4 | + | 18 Jun | <4 | <4 | |
| 43 | 5 | + | 15 May | 4 | 4 | |
| 44 | 4 | + | 20 May | 128 | 128 | |
| 45 | 4 | + | 12 Jun | <4 | <4 | |
| 46 | 4 | + | 15 May | 19 Sept | <4 | <4 |
| 47 | 2 | − | 8 Oct | <4 | <4 | |
| 48# | 3 | + | 9 Jun | <4 | ≥512 | |
| 49 | 6 | + | 6 Sept | <4 | <4 | |
| 50 | 3 | + | 27 Aug | 16 | 8 | |
| 51 | 2 | − | 5 Jul | 2 Aug | 8 | <4 |
| 52 | 4 | + | 15 May | 11 Jun | 4 | <4 |
| 53 | 2 | − | 10 Jun | 11 Jul | <4 | <4 |
| 54 | 2 | − | 23 May | 12 Jun | 16 | 16 |
| 55 | 2 | − | 14 May | 21 Aug | <4 | <4 |
| 56 | 2 | − | 15 May | 12 Jun | <4 | <4 |
| 57 | 2 | − | 20 Sept | <4 | 16 | |
| 58 | 3 | + | 13 May | 4 | 4 | |
| 59 | 4 | + | 20 May | <4 | <4 | |
| 60 | 2 | − | 17 Jul | 5 Oct | <4 | <4 |
| 61 | 4 | + | 14 May | <4 | <4 | |
#, horse with subclinical infection with Getah virus.
Vaccination histories of all horse population at the Miho training center and prevalence of Getah virus infection among populations stratified by number of vaccine doses received.
We investigated the vaccination histories of the whole horse population at the Miho training center in mid-September, focusing on how many doses of Getah virus vaccine they had received before the outbreak (Table 3). All horses had been vaccinated at least once before the outbreak. Among the 2-year-olds (n = 858), 787 (91.7%) had been vaccinated twice or more, and the remaining 71 (8.3%) had been vaccinated only once. Among the 3-year-olds (n = 550), 544 (98.9%) had been vaccinated twice or more, and six (1.1%) had been vaccinated only once. All horses in the categories of 4 years old (n = 245) and 5 years old or older (n = 297) had been vaccinated twice or more. In the whole population, 3.9% of horses had been vaccinated only once.
TABLE 3.
Numbers horses that had received Getah virus vaccine and were being kept at the Miho training center on 15 September 2014
| Age (yr) | No. (%) of horses |
||
|---|---|---|---|
| Receiving no. of vaccination doses |
Total | ||
| One | Two or more | ||
| 2 | 71 (8.3) | 787 (91.7) | 858 |
| 3 | 6 (1.1) | 544 (98.9) | 550 |
| 4 | 0 (0.0) | 245 (100.0) | 245 |
| 5 or older | 0 (0.0) | 297 (100.0) | 297 |
| Total | 77 (3.9) | 1,873 (96.1) | 1,950 |
On the basis of the vaccination histories of the affected horses and of the whole population, we calculated the prevalence of Getah virus infection during the outbreak. The prevalence in 2-year-olds (20/858 [2.3%]) was higher than that in horses aged 3 years or older (13/1,092 [1.2%]), although this difference was not statistically significant (P = 0.053). Among the 2-year-olds, the prevalence in horses that had been vaccinated once was 10/71 (14.1%); this was significantly higher than that in horses vaccinated twice or more (10/787 [1.3%]; P < 0.01).
Seroepizootiological investigation of pyretic horses at the Ritto training center.
To investigate whether Getah virus was epizootic at the other JRA training center, namely, the Ritto training center in Shiga Prefecture in western Japan (approximately 375 km from Miho), we tested the sera from horses there that were observed between 3 and 23 October to be pyretic. Out of 17 horses, one showed seroconversion to Getah virus; RT-PCR revealed that this horse was infected with the virus. The nucleotide sequence of the RT-PCR product was identical to that of the product amplified from the clinical samples at the Miho training center (12). We investigated the vaccination history and movements of the horse, because this single case at such a distance from the Miho training center was unexpected. The horse had been transferred from a surrounding farm in Ibaraki Prefecture on 2 October and had received the first dose of Getah virus vaccine the same day. Pyrexia began on 5 October, 3 days after the horse's introduction to the Ritto training center.
Epizootic Getah virus infection on surrounding farms in Ibaraki and Chiba prefectures.
Next, we estimated the magnitude of Getah virus epizootic infection on farms surrounding the Miho training center. In each month of summer and autumn, we measured VN antibodies to Getah virus in the sera of horses at the time of their transfer from surrounding farms in Ibaraki and Chiba prefectures to the Miho training center. We then compared the seropositivities in 2014 with those in 2013 and 2010 (Fig. 1). The horses were 2-year-olds with no history of Getah virus vaccination. From June to August 2014, the rate of seropositivity each month was less than 4.0%. It started to increase in September (13.0%) and reached 42.9% in October. In contrast, only one horse entering the facility in summer 2013 and no horses entering in 2010 had VN antibodies to Getah virus. Thus, the magnitude of Getah virus epizootic infection in 2014 in Ibaraki and Chiba prefectures was much larger than that in 2010 or 2013. We also tested the sera of horses transferred from other areas of Japan (n = 170, randomly selected, from June to October 2014). Only one horse, from Miyazaki Prefecture in southwestern Japan (approximately 933 km from Miho), had antibodies against Getah virus (data not shown).
FIG 1.
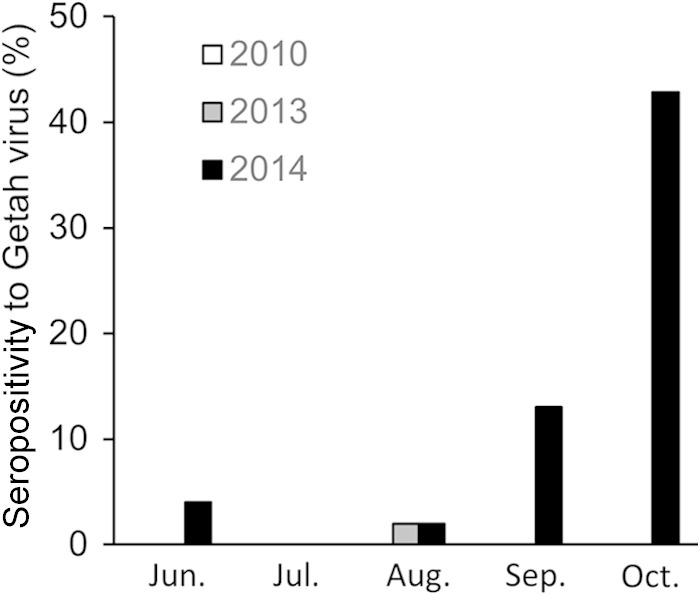
Seropositivities to Getah virus in horses transferred from farms in Ibaraki and Chiba prefectures to the Miho training center. Sera were collected from horses introduced to the Miho training center between June and October 2010, 2013, or 2014. The horses (n = 50/month, at maximum) were 2-year-olds that were transferred from Ibaraki or Chiba Prefecture and had no history of Getah virus vaccination. Sera were subjected to VN testing using the 14-I-605 strain.
DISCUSSION
To clarify the causes of the 2014 outbreak of Getah virus infection at the Miho training center in Japan, we first investigated the relationship between the antibody titers of the affected horses and their Getah virus vaccination histories. The VN titers of the affected horses at the time they developed fever were below the detection limit in many cases, even in horses that had been vaccinated properly (Table 1). This was consistent with a previous report showing that VN antibodies in horses vaccinated with Getah virus vaccine quickly disappeared (14). In that study, two doses of vaccine were administered experimentally to horses; their VN titers 4 weeks after the second dose were in the range of 8 to 32. However, the titers 8 weeks after the second dose were less than 4 in all horses tested. Interestingly, these horses were completely protected after viral challenge, even though they did not have detectable VN antibodies (14). Therefore, the protective effect of the vaccine seemed not to be related to the VN titer, although the mechanism by which the horses were protected was unclear. In support of this finding, detectable VN antibodies were also absent in many clinically healthy horses that had been vaccinated twice or more and had been kept together with the affected horses at the Miho training center (Table 2).
Most horses belonging to the JRA are introduced to the training centers at the age of 2 years. Most of them receive Getah virus vaccine only after entering the facility, because vaccination in other farms is not common. In the training centers, the Getah virus vaccination period generally starts in May and finishes in October to cover the mosquito season. The horses that are present at the training centers in spring receive the first dose in May and the second dose in June. For the horses that enter the facility after the mosquito season has started, the first dose is administered when they enter, and the second dose is given about 1 month after the first. From the second season onward, the horses are vaccinated annually before each mosquito season. Under this program, some of the 2-year-olds are likely reared with relatively naive immune status, as they do not finish the two-dose priming course (8.3% of the 2-year-old population) (Table 3). This is because many horses enter the facility after the mosquito season has started. As we expected, horses that had been vaccinated only once were more likely to be infected with Getah virus than those that had received two or more doses. In addition to disease progression in affected individuals, the existence of these susceptible horses might increase the speed of spread of the virus in the population, thus contributing to epizootic infection. In this regard, the populations in the training centers have been at risk of epizootic Getah virus infection for decades.
Next, we tried to clarify the causative factors that were specific to 2014 and had not been present in the preceding years. JRA horses are repeatedly transferred between the training centers and the surrounding farms for training, conditioning, and rest. In Ibaraki Prefecture and neighboring Chiba Prefecture, there are more than 30 farms surrounding the Miho training center. Unlike in the training center, on the surrounding farms Getah virus vaccination is not common. Only about 20% of horses entering the Miho training center for the first time between June and October have already been vaccinated (regardless of repeated vaccination doses) (data not shown). The populations on the surrounding farms are therefore more susceptible to Getah virus infection than the populations in the training centers. We therefore focused on epizootic infection on farms in the surrounding area, because infected horses from these farms could have carried the virus into the Miho training center. As shown in Fig. 1, in autumn 2014, Getah virus was epizootic not only in the Miho training center but also on the surrounding farms in Ibaraki and Chiba prefectures.
Among the affected horses at the Miho training center, eight became sick within 3 days after entering the facility; seven of these had come from Ibaraki Prefecture. In these cases, the horses might have been infected with the virus on the surrounding farms in Ibaraki where they had stayed before coming to Miho. Likewise, the Getah virus-positive horse found at the Ritto training center also seemed to have been infected with the virus at a farm in Ibaraki Prefecture before coming to the facility. At the Ritto training center, because no other positive cases were found, the virus might not have been transmitted to other horses, or transmission—if it occurred—may have been limited. The absence of an outbreak at the Ritto training center may be explained by the fact that introduction of horses from the surrounding farms in Ibaraki and Chiba prefectures was less common than in the case of Miho training center.
Ibaraki and Chiba prefectures are major pig production areas. According to the statistics of the Ministry of Agriculture, Forestry, and Fisheries of Japan, in 2013 there were 721 pig farms in these two prefectures raising more than 1.2 million pigs (15). In contrast, in Shiga Prefecture, the location of the Ritto training center, there were only seven farms in 2013, raising 6550 pigs. The pigs are reported to be involved in the circulation of Getah virus and play an important role as amplifiers or natural hosts of the virus (4, 16, 17). Serological surveillance in 1980 revealed that seropositivity against Getah virus in pigs in the Kanto area (which includes Ibaraki and Chiba prefectures) was the highest (19.1%) in Japan (17). In 2011 and 2012, 30% to 50% of pig farms in the two prefectures were Getah virus positive, and the epizootic infection in pigs was also confirmed in 2014 (Reproduction Disorders Caused by Getah Virus Infection [Kyoto Biken Information for Swine Veterinarians, 2013, no. 5] and Prevention of Pigs from Getah Virus Infection [Kyoto Biken Information for Swine Veterinarians, 2015, no. 10], in Japanese; http://kyotobiken.sakura.ne.jp/security/index.php), although it was not clear whether the magnitude in 2014 was larger than in previous years. Further investigation in pigs might reveal the relationship between disease outbreaks in pigs and horses in 2014.
A seroepizootiological study of horses at racecourses in the 1990s showed that there might be sporadic cases of Getah virus infection or minor disease outbreaks in horse populations in Japan (11). These findings, taken together with the recent epizootic situation in pigs, suggest that the risk of outbreaks at training centers has existed for decades. The herd immunity created by vaccination might be the reason why only sporadic infections occurred in the training centers before 2014. In the 2014 outbreak, the magnitude of epizootic infection in Ibaraki and Chiba prefectures was much larger than in previous years. The amount of virus circulating might thus have been beyond the capacity of the herd immunity, allowing the virus to spread in the horse population at the training center. The outbreak in 2014 highlighted the problems with our current preventive strategy for Getah virus infection, in which only horses at the training center are targeted in the vaccination program. We need to place more emphasis on the fact that the training center and the surrounding farms are located close to each other and that movement of horses between the facilities is common. To use herd immunity for advanced control of Getah virus outbreaks, it would be practical to include the horse population on surrounding farms in the vaccination target. However, the current vaccination program at the training center also partly helped to reduce the magnitude of the outbreak. During the 2014 outbreak, 64 (3.3%) of 1,950 horses were pyretic. In contrast, during the previous outbreak at the Miho training center in 1978, at which time the horses had not been vaccinated at all, 571 (30.0%) out of 1,903 horses developed fevers (7). As we demonstrated, the occurrence of subclinical infection among clinically healthy horses during the 2014 outbreak (7.1%) was also much less than that in 1978 (34.2%) (18).
We identified two possible causes of the 2014 outbreak of Getah virus infection at the Miho training center. One was that some horses at the training center had not finished their two-dose priming regimen before the outbreak. Because the existence of these susceptible horses is an inevitable outcome of the current vaccination program, the program may need to be improved for better control of Getah virus outbreaks. Another factor was the fact that Getah virus was highly prevalent among the horses in Ibaraki and Chiba prefectures in 2014. The increased amount of circulating virus probably exceeded the protective effect of the vaccine, leading to the outbreak in vaccinated horses at the training center. The precise factors causing the outbreak among the horses in Ibaraki and Chiba prefectures in 2014 are still unclear. In further studies, we will need to examine whether the antibodies raised after vaccination with the MI-110 strain can react to the circulating 14-I-605 strain. Furthermore, pathogenic differences in experimental horses between the two strains should be addressed.
ACKNOWLEDGMENTS
We thank the veterinarians at the Miho training center and the Ritto training center for collecting the clinical samples. We also thank Akira Kokubun, Akiko Suganuma, and Kazue Arakawa of the Epizootic Research Center of the Equine Research Institute for their technical assistance.
REFERENCES
- 1.Fukunaga Y, Kumanomido T, Kamada M. 2000. Getah virus as an equine pathogen. Vet Clin North Am Pract 16:605–617. [DOI] [PubMed] [Google Scholar]
- 2.Yago K, Hagiwara S, Kawamura H, Narita M. 1987. A fatal case in newborn piglets with Getah virus infection: isolation of the virus. Jpn J Vet Sci 49:989–994. doi: 10.1292/jvms1939.49.989. [DOI] [PubMed] [Google Scholar]
- 3.Izumida A, Takuma H, Inagaki S, Kubota M, Hirahara T, Kodama K, Sasaki N. 1988. Experimental infection of Getah virus in swine. Jpn J Vet Sci 50:679–684. doi: 10.1292/jvms1939.50.679. [DOI] [PubMed] [Google Scholar]
- 4.Kumanomido T, Wada R, Kanemaru T, Kamada M, Hirasawa K, Akiyama Y. 1988. Clinical and virological observations on swine experimentally infected with Getah virus. Vet Microbiol 16:295–301. doi: 10.1016/0378-1135(88)90033-8. [DOI] [PubMed] [Google Scholar]
- 5.Kamada M, Wada R, Kumanomido T, Imagawa H, Sugiura T, Fukunaga Y. 1991. Effect of viral inoculum size on appearance of clinical signs in equine Getah virus infection. J Vet Med Sci 53:803–806. doi: 10.1292/jvms.53.803. [DOI] [PubMed] [Google Scholar]
- 6.Sentsui H, Kono Y. 1980. An epidemic of Getah virus infection among racehorses: isolation of the virus. Res Vet Sci 29:157–161. [PubMed] [Google Scholar]
- 7.Kamada M, Ando Y, Fukunaga Y, Kumanomido T, Imagawa H, Wada R, Akiyama Y. 1980. Equine Getah virus infection: isolation of the virus from racehorses during an enzootic in Japan. Am J Trop Med Hyg 29:984–988. [DOI] [PubMed] [Google Scholar]
- 8.Sugiura T, Ando Y, Imagawa H, Kumanomido T, Fukunaga Y, Kamada M, Wada R, Hirasawa K, Akiyama Y. 1981. An epizootiological study of Getah virus among light horses in Japan in 1979. Bull Equine Res Inst 18:103–109. [Google Scholar]
- 9.Sentsui H, Kono Y. 1985. Reappearance of Getah virus infection among horses in Japan. Jpn J Vet Sci 47:333–335. doi: 10.1292/jvms1939.47.333. [DOI] [PubMed] [Google Scholar]
- 10.Brown CM, Timoney PJ. 1998. Getah virus infection of Indian horses. Trop Anim Health Prod 30:241–252. doi: 10.1023/A:1005079229232. [DOI] [PubMed] [Google Scholar]
- 11.Sugiura T, Shimada K. 1999. Seroepizootiological survey of Japanese encephalitis virus and Getah virus in regional horse race tracks from 1991 to 1997 in Japan. J Vet Med Sci 61:877–881. doi: 10.1292/jvms.61.877. [DOI] [PubMed] [Google Scholar]
- 12.Nemoto M, Bannai H, Tsujimura K, Kobayashi M, Kikuchi T, Yamanaka T, Kondo T. 2015. Outbreak of Getah virus infection among racehorses in Japan in 2014. Emerg Infect Dis 21:883–885. doi: 10.3201/eid2105.141975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wekesa SN, Inoshima Y, Murakami K, Sentsui H. 2001. Genomic analysis of some Japanese isolates of Getah virus. Vet Microbiol 83:137–146. doi: 10.1016/S0378-1135(01)00417-5. [DOI] [PubMed] [Google Scholar]
- 14.Imagawa H, Sugiura T, Matsumura T, Kamada M, Wada R, Akiyama Y, Tanaka Y, Samejima T. 2003. In-field evaluation of efficacy of an inactivated Getah virus vaccine. Umanokagaku 40:24–32. (In Japanese.) [Google Scholar]
- 15.Department of Ministry of Agriculture, Forestry, and Fisheries. 2013. Number of farm households feeding livestock and number of livestock fed etc., p 213–245. In The 88th statistical yearbook of Ministry of Agriculture, Forestry and Fisheries (2012–2013). Statistics Department of Ministry of Agriculture, Forestry and Fisheries, Tokyo, Japan. [Google Scholar]
- 16.Kumanomido T, Fukunaga Y, Ando Y, Kamada M, Imagawa H, Wada R, Akiyama Y, Tanaka Y. 1982. Ecological survey on Getah virus among swine in Japan. Bull Equine Res Inst 19:89–92. [Google Scholar]
- 17.Hohdatsu T, Ide S, Yamagishi H, Eiguchi Y, Nagano H, Maehara N, Tanaka Y, Fujisaki Y, Yago K, Taguchi K, Inoue Y, Matumoto M. 1990. Enzyme-linked immunosorbent assay for the serological survey of Getah virus in pigs. Jpn J Vet Sci 52:835–837. doi: 10.1292/jvms1939.52.835. [DOI] [PubMed] [Google Scholar]
- 18.Imagawa H, Ando Y, Kamada M, Sugiura T, Kumanomido T, Fukunaga Y, Wada R, Hirasawa K, Akiyama Y. 1981. Sero-epizootiological survey on Getah virus infection in light horses in Japan. Jpn J Vet Sci 43:797–802. doi: 10.1292/jvms1939.43.797. [DOI] [PubMed] [Google Scholar]


