Abstract
Scabies remains the most prevalent, endemic, and neglected ectoparasitic infestation globally and can cause institutional outbreaks. The sensitivity of routine microscopy for demonstration of Sarcoptes scabiei mites or eggs in skin scrapings is only about 50%. Except for three studies using conventional or two-tube nested PCR on a small number of cases, no systematic study has been performed to improve the laboratory diagnosis of this important infection. We developed a conventional and a real-time quantitative PCR (qPCR) assay based on the mitochondrial cytochrome c oxidase subunit 1 (cox1) gene of S. scabiei. The cox1 gene is relatively well conserved, with its sequence having no high levels of similarity to the sequences of other human skin mites, pathogenic zoonotic mites, or common house dust mite species. This mitochondrial gene is also present in large quantities in arthropod cells, potentially improving the sensitivity of a PCR-based assay. In our study, both assays were specific and were more sensitive than microscopy in diagnosing scabies, with positive and negative predictive values of 100%. The S. scabiei DNA copy number in the microscopy-positive specimens was significantly higher than that in the microscopy-negative specimens (median S. scabiei DNA copy number, 3.604 versus 2.457 log10 copies per reaction; P = 0.0213). In the patient with crusted scabies, the qPCR assay performed on lesional skin swabs instead of scrapings revealed that the parasite DNA load took about 2 weeks to become negative after treatment. The utility of using lesional skin swabs as an alternative sample for diagnosis of scabies by PCR should be further evaluated.
INTRODUCTION
Scabies remains a significant infectious problem worldwide and is a problem not merely in developing countries but also in the developed world. An estimated 300 million cases of scabies occur globally every year, with scabies having a particular association with young children, poor hygiene, homelessness, crowdedness, and poverty in the tropical and subtropical regions (1). The prevalence of scabies in some populations, such as Australian aborigines, can be as high as 25 to 50%, and the burden of disease and morbidity are further aggravated by complications with bacterial pyoderma, including that caused by methicillin-resistant Staphylococcus aureus (2, 3). In developed countries, scabies causes significant public health issues in vulnerable individuals, such as individuals at the extremes of ages and immunocompromised hosts. For example, an estimated incidence of 233 to 470 per 100,000 person-years was reported in a national study conducted from 1994 to 2003 in England (4). In addition, outbreaks readily occur in the community in schools, hospitals (including intensive care units), and other institutions (5–10). Among immunocompromised patients, such as HIV-infected individuals, organ transplant recipients, and patients on biologics or other immunosuppressive therapies, scabies can occur as an unrecognized complication with considerable delays in diagnosis and treatment or as a manifestation of the immune reconstitution inflammatory syndrome (11–15).
Scabies is a contagious skin infection characterized by intense itching due to a delayed type IV hypersensitivity reaction toward Sarcoptes scabiei antigens; some of these antigens also modulate the skin immune response to mites (16). Transmission is predominately person to person through close and prolonged contact, but fomites may also play a role in heavily infected patients, as in the case of crusted scabies (17). The clinical diagnosis of scabies remains problematic, especially in situations where the clinician's suspicion is low, especially in patients with atypical manifestations, or when clinical signs are modified by topical corticosteroids (18, 19). Laboratory confirmation traditionally involves the use of skin scrapings for microscopic examination. While microscopic examination of skin scrapings has a 100% positive predictive value and a short turnaround time, it has a low sensitivity, which further varies according to the quality and quantity of the skin scrapings received (19, 20). These difficulties result in delayed treatment and, more importantly, hinder the early initiation of infection control interventions to minimize the transmission of mites, resulting in outbreaks causing service interruptions with substantial cost implications (5, 8). Nucleic acid amplification tests (NAATs) have gained wider utility in diagnostic parasitology in recent years, offering higher sensitivities than conventional microscopy, and allow quantitation of the parasite load when quantitative assays are used (21). We therefore explored the role of PCR in the diagnosis and monitoring of scabies.
MATERIALS AND METHODS
We identified the baseline demographics of microscopy-confirmed scabies patients by reviewing the computer records of patients in the Hong Kong West Hospital Cluster, which comprises one 1,600-bed university hospital and three convalescence hospitals with a total of 3,200 beds. The data were retrieved for the period from January 2005 to December 2014 for comparison of the demographics of the study patients. The episodes in patients with multiple positive skin scrapings on microscopy within 4 weeks were counted as one episode. This study has been approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Hospital Cluster.
Collection of skin samples.
Skin scrapings were prospectively collected from patients with suspected scabies in the Hong Kong West Hospital Cluster and the United Christian Hospital, an acute care hospital with 1,174 beds, between 1 May 2014 and 9 August 2014. Scrapings were taken from clinically suspicious lesions. Each specimen was placed in a sterile plastic bottle and subjected to examination under a microscope and by PCR. For microscopic examination, the scrapings were digested with 2 drops of 10% potassium hydroxide and were observed for the presence of mites, nymphs, or eggs (22). Patients were considered to have definite scabies when mites or eggs were seen. Crusted scabies was diagnosed when scabietic patients presented with the typical picture of psoriasiform dermatitis with hyperkeratotic plaques (23).
For patients with crusted scabies, skin swab specimens were additionally taken at the same time that the skin scrapings were taken for PCR. Swab specimens were taken from the six body sites which are most often affected by scabies, viz., the scalp, finger web, wrist, elbow, popliteal fossa, and ankle. The skin of these sites was swabbed using separate rayon-tipped Copan swab applicators (Copa, Brescia, Italy) by applying firm rotatory rubbing motions over an area of 2 cm by 2 cm daily on day 1 to day 4 and then on days 14, 21, and 28 after the start of treatment with a scabicidal agent. The tip of the swabs was put into viral transport medium, which was then stored at 4°C for a maximum of 3 days before nucleic acid extraction. Viral transport medium was used for the convenience of storing skin scrapings in solution form, and it prevents sample deterioration due to overgrowth of bacteria or fungi. Environmental surveillance, performed by swabbing bedside tables, bedside lockers, bedside rails, curtains, and drip stands (as appropriate), was conducted for patients with crusted scabies.
DNA extraction from skin samples.
DNA extraction was performed using a QIAamp DNA minikit (Qiagen, Hilden, Germany), as modified from a user-developed protocol (Qiagen, Hilden, Germany). Briefly, skin samples were digested using 40 μl of 1 M dithiothreitol, 40 μl of proteinase K, and 300 μl of ATL buffer (Qiagen, Hilden, Germany) at 56°C until the lysate was clear. The purification steps were done according to the manufacturer's instruction, and the purified DNA was eluted in 40 μl of AE buffer (Qiagen, Hilden, Germany). All specimens were spiked after extraction to identify any PCR inhibitors within the specimens. Since PCR inhibitors might not be completely removed by the purification steps described (results not shown), additional purification was performed using a QIAquick PCR purification kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions for PCR products when inhibitors are present. The purified DNA was eluted in 40 μl of EB buffer (10 mM Tris-HCl, pH 8.5).
Conventional PCR amplification.
A 250-bp fragment of the cytochrome c oxidase subunit 1 (cox1) gene of S. scabiei was amplified using specific forward primer scabF1 (5′-CTTATTATTCCTGGATTTGGRTA-3′) and specific reverse primer scabR2 (5′-CTAATTTTCCTCCTAATATTGTWGA-3′). No homologies between the primer sequences and the sequences of the cox1 genes of Demodex folliculorum, Demodex brevis, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Dermanyssus gallinae, Tyrophagus putrescentiae, and Cheyletus malaccensis available in the NCBI nucleotide sequence database were found. Each 25-μl PCR mix contained 1.0 μl of purified DNA extract, 0.5 μM each forward and reverse primers (IDT, Coralville, IA, USA), PCR buffer (10 mM Tris-HCl, pH 8.3, 50 nM KCl, 2 mM MgCl2, 0.01% gelatin), 200 μM each deoxynucleoside triphosphate (GeneAmp; Applied Biosystems, Foster City, CA, USA), and 1.0 U of Taq polymerase (AmpliTaq Gold; Applied Biosystems, Foster City, CA, USA). Thermocycling was performed in an automated thermocycler (Applied Biosystems, Foster City, CA, USA) with a hot start at 95°C for 10 min; 40 cycles of 95°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and a final extension at 72°C for 10 min. Five microliters of each amplified product was electrophoresed in a 2.0% (wt/vol) agarose gel with a molecular size marker (GeneRuler 50-bp DNA ladder; Fermentas, Ontario, Canada) in parallel. Electrophoresis in Tris-borate-EDTA buffer was performed at 120 V for 35 min. The gel was stained with ethidium bromide (0.5 mg/ml) for 25 min, rinsed, and photographed under UV light illumination. Positive and negative controls were included in each run. The positive control for S. scabiei was microscopy-positive skin scraping samples; the negative control consisted of all PCR reagents and PCR-grade water as well as a matrix-containing negative control (a skin scraping from normal skin). Standard precautions were taken to avoid PCR contamination, and no false-positive results were observed for the negative controls. Positive and negative controls were included in each run. The PCR products were gel purified using a QIAquick PCR gel extraction kit (Qiagen, Hilden, Germany). Both strands of the PCR products were sequenced with an ABI 3130xl genetic analyzer according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA) using PCR primers specific for each PCR product. The DNA sequences obtained were analyzed by a BLASTn search against the sequences in the online nonredundant nucleotide collection (nr/nt) database of NCBI to confirm their identity. DNA extracted from specimens with Demodex folliculorum, Demodex brevis, house dust mites (Dermatophagoides pteronyssinus, Dermatophagoides farinae), pure cultures of dermatophytes (Microsporum, Epidermophyton, and Trichophyton), and Candida was used to test the specificity of the PCR.
Real-time qPCR.
A 121-bp fragment of the cox1 gene of S. scabiei was amplified using specific forward primer Scab_cox1_120F (5′-TGCTATGATTTCTATTGCAACTTTAGG-3′), specific reverse primer Scab_cox1_241R (5′-GGGACAGCGATAATTATAGTAGCTGAA-3′), and TaqMan probe Scab_cox1_152P (6-FAM-TGTATGAGCTCATCATATATTTACTGTTG-3IABkFQ, where 6-FAM is 6-carboxyfluorescein fluorescent dye and 3IABkFQ is Iowa black quencher) in a real-time quantitative PCR (qPCR) using a QuantiFast multiple PCR kit (Qiagen, Hilden, Germany). The internal control was amplified using internal control forward primer ICF (5′-TGCTATGATTTCTATTGCAACTTTAGGCTGCTGCCCGACAACCA-3′), internal control reverse primer ICR (5′-TTCAGCTACTATAATTATCGCTGTCCCTGTGATCGCGCTTCTCGTT-3′), and internal control TaqMan probe ICP (Cyc5-TACCTGAGCACCCAGTCCGCCCT-3IABkFQ). The final product was inserted into the plasmid vector pPCR@II-Topo (Topo TA cloning kit, dual promoter; Invitrogen, Carlsbad, CA, USA). No homologies between the primer sequences and the sequences of the cox1 genes of Demodex folliculorum, Demodex brevis, Dermatophagoides pteronyssinus, Dermatophagoides farinae, Dermanyssus gallinae, Tyrophagus putrescentiae, and Cheyletus malaccensis in the NCBI nucleotide sequence database were found. Each 25-μl PCR mix contained 5.0 μl of purified DNA extract, 0.5 μM each forward and reverse primers (IDT, Coralville, IA, USA), 0.25 μl TaqMan probe (IDT, Coralville, IA, USA), 0.1 μM internal control TaqMan probe (IDT, Coralville, IA, USA), 0.1 μl plasmid suspension of internal control 1 × 104 copies/reaction, and 2× QuantiFast multiplex PCR master mix (without carboxy-X-rhodamine) (Qiagen, Hilden, Germany). Thermocycling was performed in an automated real-time LightCycler 96 apparatus (Roche Diagnostics, Switzerland) with a hot start at 95°C for 5 min and 55 cycles of denaturation at 95°C for 45 s and annealing and extension at 55°C for 45 s. Suspensions of plasmids carrying the cox1 gene were used as standards for quantification and positive controls. The plasmids were prepared by cloning the cox1 insert into the plasmid vector pPCR@II-Topo (Topo TA cloning kit, dual promoter; Invitrogen, Carlsbad, CA, USA). After propagation and purification of the plasmids, the concentration of the cox1 gene (number of copies per reaction) was derived from the A260 optical density measurement. Each PCR run comprised 5 serial 10-fold dilutions of the plasmid suspension, ranging from 105 to 101 copies of extracted DNA per reaction. The amount of S. scabiei in each specimen was estimated by comparing the cycle threshold (CT) number with that generated from a serial dilution of the plasmid suspension. The limit of detection was 10 copies per reaction mixture. Positive and negative controls were included in each run.
Statistical analysis.
Fisher's exact test, the Student t test, and the Mann-Whitney U test were used to compare patient data, as appropriate. The log-transformed parasitic loads between microscopy-negative and -positive specimens were compared using the Mann-Whitney U test. A P value of <0.05 was considered to represent statistical significance. All statistical analyses was performed using SPSS (version 18.0) software.
Nucleotide sequence accession numbers.
The sequence determined in this study was deposited in GenBank under accession no. KR477839 to KR477867.
RESULTS
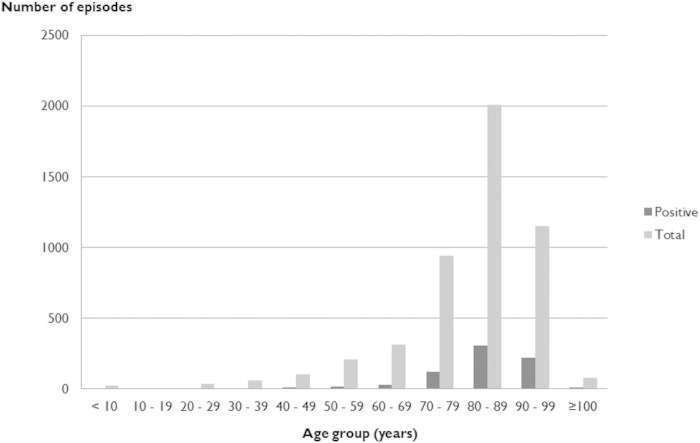
From January 2005 to December 2014 there were 863 episodes of scabies diagnosed in the Hong Kong West Hospital Cluster using microscopy. This corresponded to 763 individual patients with a male-to-female ratio of 1:1.6. The mean age of the patients was 83.1 years (range, 23 to 105 years). The highest incidence of disease occurred in patients over 70 years of age (Fig. 1). One hundred seventeen episodes (corresponding to 13.6% of all episodes) in 82 patients were considered to be treatment failures or reinfections with positive microscopy results more than 4 weeks apart.
FIG 1.
Age distribution of patients with scabies diagnosed by microscopy in the Hong Kong West Hospital Cluster from January 2005 to December 2014.
During the study period, a total of 100 skin scrapings were examined by microscopy and PCR for scabies. S. scabiei was detected in 29 skin scraping samples by PCR, while it was detected in only 17 of these by microscopy (Table 1). None of the specimens was microscopy positive and PCR negative. None of these patients had received antiscabietic treatment in the 4 weeks before the specimens were collected. Only one case of crusted scabies was diagnosed. The male-to-female ratio was 1:1.6, and the median age of these 29 patients was 87 years (range, 62 to 98 years). The mean age of the study patients was not significantly different from that of the historic cohort of patients in the previous 9 years (P = 0.349). Among the 29 patients, 24 (82.7%) resided in long-term-care facilities, 20 (69.0%) suffered from chronic neurological conditions, and 9 (31.0%) were found to have various immunocompromising conditions (Table 2). The skin lesions of all patients improved after the administration of antiscabietic treatment.
TABLE 1.
Performance of microscopy and cox1 PCR of skin scraping specimens for diagnosis of scabies
| cox1 PCR result | No. of samples with the following microscopy result: |
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 17 | 12 | 29 |
| Negative | 0 | 71 | 71 |
| Total | 17 | 83 | 100 |
TABLE 2.
Clinical characteristics of the 29 scabies patients in the current study
| Characteristic | Result for: |
P value | ||
|---|---|---|---|---|
| PCR-positive, microscopy-positive patients (n = 17) | PCR-positive, microscopy-negative patients (n = 12) | All patients tested (n = 29) | ||
| Median (range) age (yr) | 83 (75–92) | 87 (62–98) | 87 (62–98) | 0.610 |
| No. of M/no. of Fa (sex ratio) | 2/10 (1:5) | 8/9 (1:1.1) | 11/18 (1:1.6) | 0.126 |
| No. of patients with the following characteristics: | ||||
| Long-term-care facility resident | 15 | 9 | 24 | 0.622 |
| Neurological conditionb | 12 | 8 | 20 | 1.000 |
| Other immunocompromising conditionc | 7 | 2 | 9 | 0.234 |
| Missed or wrong diagnosis before scabies was diagnosed | 0 | 1 | 1 | 0.414 |
M, male; F, female.
Includes Alzheimer's disease and vascular dementia, previous cerebrovascular disease, noncommunicable state, and bed-bound or chair-bound condition.
Systemic lupus erythematosus (n = 1), end-stage renal disease (n = 1), diabetes mellitus (n = 6), and pemphigoid (n = 2).
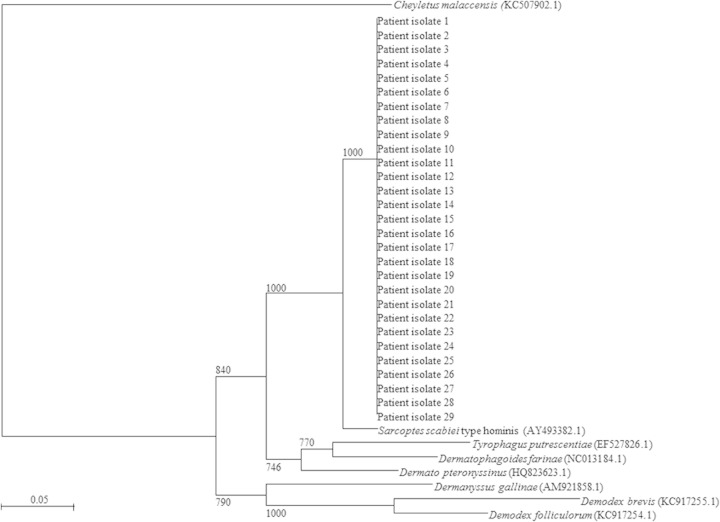
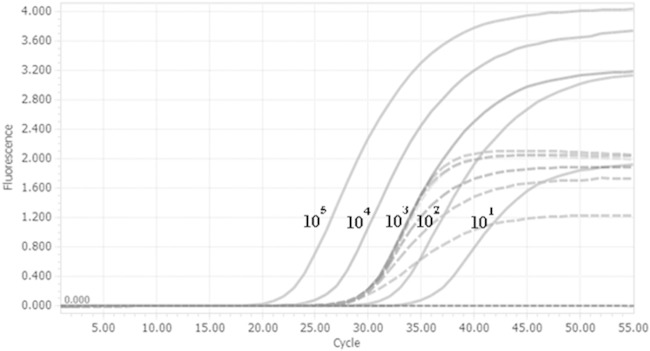
For the 29 specimens that were positive by the cox1 PCR, sequencing of the PCR product (GenBank accession no. KR477839 to KR477867) showed 98% nucleotide sequence identity with that of the S. scabiei type hominis cox1 gene (GenBank accession no. AY493388.1) (Fig. 2). In the study, 2% of the skin scraping specimens required additional purification for DNA extraction. S. scabiei DNA was not found in environmental samples taken from the immediate surroundings of the patient with crusted scabies, including bedside tables and lockers, bedside rail, curtains, and drip stands. The detection limit of the cox1 qPCR was 10 copies per reaction of extracted DNA, corresponding to a CT value of 36, while the validity of the test conditions and the absence of PCR inhibitors were safeguarded by the use of an internal control, which had a CT value of 28 (Fig. 3). The S. scabiei parasite DNA copy number was significantly higher for the microscopy-positive specimens than the microscopy-negative specimens (median parasite DNA copy number, 3.604 versus 2.457 log10 copies per reaction; P = 0.0213) (Fig. 4). All DNA samples extracted from specimens with Demodex folliculorum, Demodex brevis, Dermatophagoides pteronyssinus, and Dermatophagoides farinae and colonies of Candida, Microsporum, Epidermophyton, and Trichophyton were negative by the cox1 PCR. All negative controls, including skin scrapings from normal skin, were also negative by the cox1 PCR.
FIG 2.
Phylogenetic tree showing the relationships among patient isolates, human skin mites, pathogenic zoonotic mites, and common house dust mite species. A total of 193 nucleotide positions in each cox1 gene was included in the analysis. The tree was constructed using the neighbor-joining method and rooted using Cheyletus malaccensis (GenBank accession number KC507902.1). The bootstrap values calculated from 1,000 trees are shown when they are ≥70%. The scale bar indicates the estimated number of substitutions per 20 bases. The names and accession numbers (in parentheses) are presented as cited in the GenBank database.
FIG 3.
Amplification plots for a 10-fold dilution series of the S. scabiei cox1 gene (solid curves) and the signal produced by the internal control (dashed curves).
FIG 4.
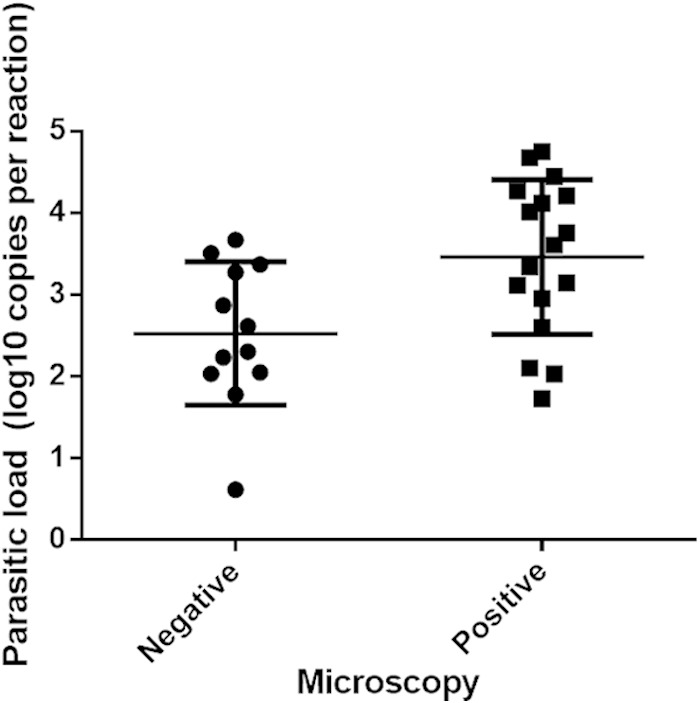
Comparison of Sarcoptes scabiei parasitic loads between microscopy-negative and -positive specimens (P = 0.0213).
Skin swab specimens were collected from the wrist, elbow, ankle, finger web, scalp, and popliteal fossa of the patient with crusted scabies at different points in time before and after initiation of treatment. All of these body sites were positive by conventional and real-time cox1 PCR. Before treatment (day 1), qPCR showed a higher S. scabiei DNA copy number in swab specimens taken from the wrist, finger web, and popliteal fossa than in those taken from the elbow, ankle, and scalp. As expected, the S. scabiei DNA copy number from all body sites began to decrease after initiation of treatment and became undetectable at day 14, day 21, and day 28 (Fig. 5). Interestingly, the S. scabiei DNA loads in the elbow and finger web showed an initial increase on day 1 to day 3 (range, 3.85 × 103 to 9.27 × 103 copies per reaction) and day 1 to day 2 (range, 1.46 × 104 to 3.88 × 104 copies per reaction), respectively (Fig. 5).
FIG 5.
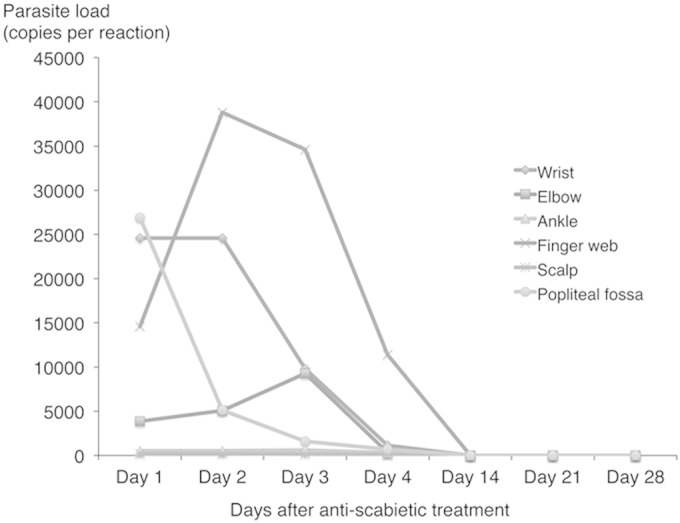
Quantitative cox1 PCR results with samples from different skin sites of a crusted scabies patient after commencement of treatment.
DISCUSSION
Scabies is the latest addition to the World Health Organization list of neglected tropical diseases (24). The disease is generally considered to be the most prevalent in resource-poor communities and is associated with overcrowding and poverty. However, high-income countries are not immune to scabies, and outbreaks in institutions and health care facilities are not infrequent (5–10, 25). Nosocomial outbreaks can sometimes be extremely protracted, with huge numbers of secondary cases being found in patients and health care workers and tertiary cases being found in family members of affected health care workers (26, 27). One of the most important risk factors for nosocomial outbreaks is failure to recognize, isolate, and treat scabietic patients, especially those with crusted scabies (5, 28). Misdiagnosis of scabies is not uncommon, owing partly to the multifarious manifestations of scabies but also the lack of clinical awareness by attending clinicians (19, 29, 30). The classical burrows, though present, are often not readily visible and may require dermoscopy and the burrow ink test to facilitate their detection (31, 32).
In contrast to the epidemiology cited in the literature, a 10-year review of the demographics of scabietic patients in our cluster of hospitals showed that scabies is rare among children and adolescents in our patient populations (33). Scabies is, in our setting, predominantly a disease of the elderly, especially in those residing in long-term-care facilities (Table 2). This pattern is echoed in some developed countries, where a high incidence of scabies among the elderly population in seen (34, 35). Nevertheless, we are uncertain whether this is a genuine situation territory-wide or whether pediatric scabies patients were being managed in the primary care and outpatient settings and, hence, scabies may not have been diagnosed by our hospital-based laboratories. As noted previously in the literature, we also found that dementia and other debilitating neurological conditions (resulting in a bedridden status) were prevalent among our scabietic patients, with a substantial proportion (13.6% of all episodes) of recurrent infections being detected (36, 37). Most of these recurrent episodes probably represented reinfections rather than treatment failure with permethrin or benzoyl benzoate emulsion, because the majority of these episodes occurred months to years apart (data not shown).
We developed and evaluated a PCR test to explore alternative means of diagnosis to minimize the delayed and missed diagnosis of scabies. NAATs have been shown to offer substantial improvements in sensitivity compared to that of conventional microscopy for the diagnosis of various parasitic infections. Demonstration of S. scabiei in skin scrapings from crusted scabies patients is relatively simple because of the huge number of mites present. The problem with the diagnosis of ordinary scabies lies not only in the difficulty with the identification of the burrows but also in the relatively small number of mites present. It is estimated that only 10 to 15 mites are present in a patient with ordinary scabies, in contrast to the 1,000 to over a million present in patients with crusted scabies (38, 39). This partly explains the poor sensitivity of skin scraping microscopy, which is only about 50% (20, 40). We have shown that our PCR assay not only detected scabies in all 17 microscopy-positive patients but also confirmed the diagnosis in an additional 12 patients. Although these 12 patients were negative by microscopy, the PCR results most likely represented genuine infections because of the clinically compatible skin lesions, the resolution of symptoms after initiation of antiscabietic treatment, and the specificity of the PCR, as confirmed by sequencing of the product. Using PCR as the diagnostic “gold standard,” the sensitivity of conventional microscopy was 58.6% in our series. Using response to antiparasitic treatment as the gold standard, the sensitivity, specificity, and positive and negative predictive values were 100%, 100%, 100%, and 100%, respectively, with PCR and 58.6%, 100%, 100%, and 85.6%, respectively, with microscopy.
Previous molecular studies of S. scabiei in animals and humans have utilized various gene targets, such as microsatellites, ITS-2 ribosomal DNA (rDNA), mitochondrial 12S/16S rRNA, and S. scabiei myosin heavy chain genes (41–48).
In the three studies evaluating the role of PCR in the diagnosis of scabies, S. scabiei microsatellite 15 (Sarms 15), S. scabiei myosin heavy chain genes, or ITS-2 rDNA was used, but relatively few sequence data for these genes are available in GenBank (41–43). Moreover, two studies tested the PCR assay with mite samples or skin biopsy specimens, but relatively few clinical specimens, like skin scrapings, were tested (41, 43). Additionally, the 2-tube nested PCR used by Fukuyama et al. is prone to cross contamination (42). Therefore, we developed a conventional and real-time PCR assay targeting the cox1 gene for scabies. To our best knowledge, no real-time PCR for the diagnosis of scabies has yet been reported in the literature. We chose the cox1 gene as the PCR target in our study because it is relatively conserved and is widely separated from the cox1 genes of other common human ectoparasites on the phylogenetic tree (Fig. 2) and a large amount of sequence data is available. The cox1 gene appears to be a good target for the molecular detection of S. scabiei. There are no high sequence similarities between the S. scabiei primer sequences used in this study and the cox1 sequences of other human skin mites, pathogenic zoonotic mites, and common house dust mite species. The use of a mitochondrial gene target can potentially improve the sensitivity of the PCR because of the large number of mitochondria and, hence, the large number of gene copies present in each arthropod cell (49). In addition, the use of skin swabs may be a potential alternative to the use of skin scrapings for S. scabiei PCR, but this method will require further evaluation in future studies. Using a skin swab for PCR rather than the conventional scraping technique for microscopy could be advantageous, as it circumvents the need for good-quality skin scrapings, the collection of which depends on the expertise of the health care workers in identifying the most likely sites of infection and in obtaining sufficient skin samples. While microscopy relies on the visualization of mites and/or eggs within the skin scrapings, PCR does not rely on demonstration of these arthropod structures. We postulate that mite excreta and its cellular DNA are incorporated into the cells of the stratum corneum, which then appear in the squames and are detectable by PCR. On the basis of this postulation, the increased sensitivity of PCR over microscopy, as demonstrated in this study, can therefore be explained by the fact that mites do not have to be physically present in the scrapings or swabs for the detection of their DNA. If lesional skin swabs are indeed a viable alternative to scrapings due to the higher sensitivity offered by the PCR assay, the process of sampling of patients for scabies could potentially be simplified, especially during outbreak investigations, where a large number of patients may have to be screened. It also mitigates the difficulties with the detection of the burrows, which is necessary for the conventional method of clinical diagnosis.
As in the case of the molecular detection of other pathogens, DNA may persist in the clinical specimens for some time after successful treatment. Treatment failure cannot be confirmed solely by the presence of detectable DNA. In the only crusted scabies patient evaluated in the present study, we noticed that the PCR result turned negative by day 14 after antiscabietic treatment. In the analysis of the historical cohort of patients with scabies in our cluster hospitals, we empirically used persistently positive microscopy for over 4 weeks as a cutoff. This is based on the epidermal transit time of normal skin. S. scabiei normally inhabits the stratum granulosum layer of the skin (31). In normal skin, it takes about 14 days for the cells to progress from the stratum basale to the stratum corneum and another 14 to 20 days before they are desquamated (50, 51). The time for renewal of the stratum corneum is prolonged in the elderly, with renewal often requiring more than 30 days (51). Since the acquisition of skin specimens for scabies PCR, be it skin scrapings or swabs, generally involves sampling of the stratum corneum, we can expect the persistence of mite DNA for at least 2 weeks and possibly longer in the elderly, who predominate among our hospitalized scabies patients.
We encountered only one crusted scabies patient in our series. Serial monitoring by qPCR assay showed that mite DNA disappeared by 2 weeks after treatment (Fig. 5). Interestingly, swabs from the finger webs and elbow showed an increase in the DNA load on days 2 and 3 after treatment, respectively. The increase was only modest (less than 1 log unit) and could have been due to either sampling variation or release of the DNA from the mites after they were killed by permethrin. The temporal persistence of mite DNA in cases of ordinary scabies should be studied in the future to better understand the kinetics and aid with the interpretation of PCR results. Although we failed to find any mite DNA in the swab samples taken from the immediate surroundings of this patient, we cannot exclude the possibility that the environment and fomites play roles in the transmission of scabies. Previous studies have demonstrated the presence of mites in dust samples in the vicinity of scabies patients, and our sampling method may not have been sensitive enough to detect the small amount of mite DNA in the environment (31, 52). Further improvements in environmental sampling techniques that increase the quantity of desquamated skin in these samples may increase the sensitivity of the PCR assays for environmental surveillance. If the PCR assay can be adopted for screening of fomites and samples from the environment, it may prove valuable for surveillance in the nosocomial and long-term-care settings for the early identification of scabietic patients, where the implementation of timely treatment and control measures is essential for the prevention of outbreaks.
A small number of reports in the literature also demonstrated the usefulness of PCR for the diagnosis of scabies (41, 42). In particular, PCR was positive for some patients who had conditions clinically compatible with scabies but who were microscopy negative, as shown in our study (42). This technique can therefore be considered an adjunct method for the diagnosis of scabies, especially in microscopy-negative suspected cases, outbreak investigations, or environmental sampling when large quantities of specimens are sampled.
Each test of our conventional PCR assay and qPCR was estimated to cost approximately $6.70 and $11.0, respectively, whereas the cost of microscopy is $3.90. Although PCR assays are more expensive, the higher sensitivity facilitates identification of even microscopy-negative scabietic patients. This means that infested patients can be identified at an early stage, when their parasitic load is still low, and infection control measures and treatment can be initiated before significant transmission occurs, minimizing the risk of outbreaks. This advantage may outweigh the associated cost of the assay in developed countries, where outbreaks have been associated with significant cost and service disruptions (5, 8). Furthermore, if NAATs are proven to be of value for the diagnosis of scabies, modification of PCR to other formats, such as loop-mediated isothermal amplification, may potentially improve its accessibility in resource-limited settings. In most clinical scenarios, the conventional PCR assay will suffice as a diagnostic tool and the extra cost of qPCR would not be justified, as the addition of a quantitative result is unlikely to influence management.
The potential limitations of our study include the relatively small number of patients studied and the lack of sequential studies in ordinary scabies patients. The kinetics of PCR positivity in both ordinary and crusted scabies patients must be further studied. We have tested the utility of lesional skin swab specimens for PCR from only one crusted scabies patient. The potential role of swabs in addition to scrapings should be examined with a larger number of patients in the setting of both ordinary scabies and crusted scabies.
Conclusion.
Our study showed that cox1 PCR of skin swab samples has a better sensitivity for the diagnosis of scabies than microscopic examination. Although PCR may not replace microscopy as the diagnostic test of choice, it may have a role in aiding the diagnosis of scabies in patients with atypical presentations, outbreak investigations, and environmental studies.
ACKNOWLEDGMENTS
We are grateful to the Hui Hoy & Chow Sin Lan Charity Fund Limited, K. M. Dunphy, S. Narain, V. F. S. Leung, and M. Leung for their generous support for infectious disease research.
We have no conflicts of interest to declare.
REFERENCES
- 1.Hengge UR, Currie BJ, Jäger G, Lupi O, Schwartz RA. 2006. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis 6:769–779. doi: 10.1016/S1473-3099(06)70654-5. [DOI] [PubMed] [Google Scholar]
- 2.Currie BJ, Carapetis JR. 2000. Skin infections and infestations in aboriginal communities in northern Australia. Australas J Dermatol 41:139–143. doi: 10.1046/j.1440-0960.2000.00417.x. [DOI] [PubMed] [Google Scholar]
- 3.Whitehall J, Kuzulugil D, Sheldrick K, Wood A. 2013. Burden of paediatric pyoderma and scabies in north west Queensland. J Paediatr Child Health 49:141–143. doi: 10.1111/jpc.12095. [DOI] [PubMed] [Google Scholar]
- 4.Pannell RS, Fleming DM, Cross KW. 2005. The incidence of molluscum contagiosum, scabies and lichen planus. Epidemiol Infect 133:985–991. doi: 10.1017/S0950268805004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buehlmann M, Beltraminelli H, Strub C, Bircher A, Jordan X, Battegay M, Itin P, Widmer AF. 2009. Scabies outbreak in an intensive care unit with 1,659 exposed individuals—key factors for controlling the outbreak. Infect Control Hosp Epidemiol 30:354–360. doi: 10.1086/596113. [DOI] [PubMed] [Google Scholar]
- 6.Ejidokun OO, Aruna OS, O'Neill B. 2007. A scabies outbreak in a further education college in Gloucestershire. Epidemiol Infect 135:455–457. doi: 10.1017/S0950268806007072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjioe M, Vissers WH. 2008. Scabies outbreaks in nursing homes for the elderly: recognition, treatment options and control of reinfestation. Drugs Aging 25:299–306. doi: 10.2165/00002512-200825040-00003. [DOI] [PubMed] [Google Scholar]
- 8.Stoevesandt J, Carlé L, Leverkus M, Hamm H. 2012. Control of large institutional scabies outbreaks. J Dtsch Dermatol Ges 10:637–647. doi: 10.1111/j.1610-0387.2012.07892.x. [DOI] [PubMed] [Google Scholar]
- 9.Capobussi M, Sabatino G, Donadini A, Tersalvi CA, Castaldi S. 2014. Control of scabies outbreaks in an Italian hospital: an information-centered management strategy. Am J Infect Control 42:316–320. doi: 10.1016/j.ajic.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Bouvresse S, Chosidow O. 2010. Scabies in healthcare settings. Curr Opin Infect Dis 23:111–118. doi: 10.1097/QCO.0b013e328336821b. [DOI] [PubMed] [Google Scholar]
- 11.Guldbakke KK, Khachemoune A. 2006. Crusted scabies: a clinical review. J Drugs Dermatol 5:221–227. [PubMed] [Google Scholar]
- 12.Boureau AS, Cozic C, Poiraud C, Varin S, Chaillous B, Cormier G. 2014. Does immunodepression induced by TNF antagonists promote atypical scabies? Joint Bone Spine 81:186–187. doi: 10.1016/j.jbspin.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Baccouche K, Sellam J, Guegan S, Aractingi S, Berenbaum F. 2011. Crusted Norwegian scabies, an opportunistic infection, with tocilizumab in rheumatoid arthritis. Joint Bone Spine 78:402–404. doi: 10.1016/j.jbspin.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Pipitone MA, Adams B, Sheth A, Graham TB. 2005. Crusted scabies in a patient being treated with infliximab for juvenile rheumatoid arthritis. J Am Acad Dermatol 52:719–720. doi: 10.1016/j.jaad.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Cheng VC, Yuen KY, Wong SS, Woo PC, Ho PL, Lee R, Chan RM. 2001. Immunorestitution diseases in patients not infected with HIV. Eur J Clin Microbiol Infect Dis 20:402–406. doi: 10.1007/s100960100507. [DOI] [PubMed] [Google Scholar]
- 16.Walton SF. 2010. The immunology of susceptibility and resistance to scabies. Parasite Immunol 32:532–540. doi: 10.1111/j.1365-3024.2010.01218.x. [DOI] [PubMed] [Google Scholar]
- 17.Heukelbach J, Feldmeier H. 2006. Scabies. Lancet 367:1767–1774. doi: 10.1016/S0140-6736(06)68772-2. [DOI] [PubMed] [Google Scholar]
- 18.Lay CJ, Wang CL, Chuang HY, Chen YL, Chen HL, Tsai SJ, Tsai CC. 2011. Risk factors for delayed diagnosis of scabies in hospitalized patients from long-term care facilities. J Clin Med Res 3:72–77. doi: 10.4021/jocmr520w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong MY, Lee CC, Chuang MC, Chao SC, Tsai MC, Chi CH. 2010. Factors related to missed diagnosis of incidental scabies infestations in patients admitted through the emergency department to inpatient services. Acad Emerg Med 17:958–964. doi: 10.1111/j.1553-2712.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 20.Walton SF, Currie BJ. 2007. Problems in diagnosing scabies, a global disease in human and animal populations. Clin Microbiol Rev 20:268–279. doi: 10.1128/CMR.00042-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SS, Fung KS, Chau S, Poon RW, Wong SC, Yuen KY. 2014. Molecular diagnosis in clinical parasitology: when and why? Exp Biol Med (Maywood) 239:1443–1460. doi: 10.1177/1535370214523880. [DOI] [PubMed] [Google Scholar]
- 22.Alasaad S, Rossi L, Soriguer RC, Rambozzi L, Soglia D, Pérez JM, Zhu XQ. 2009. Sarcoptes mite from collection to DNA extraction: the lost realm of the neglected parasite. Parasitol Res 104:723–732. doi: 10.1007/s00436-009-1333-0. [DOI] [PubMed] [Google Scholar]
- 23.Stone SP, Goldfarb JN, Bacelieri R. 2008. Scabies, other mites, and pediculosis, p 2029–2037. In Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ (ed), Fitzpatrick's dermatology in general medicine, 7th ed, vol 2 McGraw-Hill, New York, NY. [Google Scholar]
- 24.Steer A. 2014. Scabies joins the list of WHO neglected tropical diseases. Lancet Global Health Blog; http://globalhealth.thelancet.com/2014/07/07/scabies-joins-list-who-neglected-tropical-diseases Accessed 14 December 2014. [Google Scholar]
- 25.Fuller LC. 2013. Epidemiology of scabies. Curr Opin Infect Dis 26:123–126. doi: 10.1097/QCO.0b013e32835eb851. [DOI] [PubMed] [Google Scholar]
- 26.Lettau LA. 1991. Nosocomial transmission and infection control aspects of parasitic and ectoparasitic diseases. Part III. Ectoparasites/summary and conclusions. Infect Control Hosp Epidemiol 12:179–185. doi: 10.1086/646313. [DOI] [PubMed] [Google Scholar]
- 27.Pasternak J, Richtmann R, Ganme AP, Rodrigues EA, Silva FB, Hirata ML, Ciosak S. 1994. Scabies epidemic: price and prejudice. Infect Control Hosp Epidemiol 15:540–542. doi: 10.1086/646974. [DOI] [PubMed] [Google Scholar]
- 28.Ladbury G, Morroy G, van Hoeven-Dekkers S, Botermans C, Veelenturf C, Bastiaens M, van Abeelen C, Wijkmans C. 2012. An outbreak of scabies in multiple linked healthcare settings in The Netherlands. Infect Control Hosp Epidemiol 33:1047–1050. doi: 10.1086/667736. [DOI] [PubMed] [Google Scholar]
- 29.Wong SS, Woo PC, Yuen KY. 2005. Unusual laboratory findings in a case of Norwegian scabies provided a clue to diagnosis. J Clin Microbiol 43:2542–2544. doi: 10.1128/JCM.43.5.2542-2544.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Beer G, Miller MA, Tremblay L, Monette J. 2006. An outbreak of scabies in a long-term care facility: the role of misdiagnosis and the costs associated with control. Infect Control Hosp Epidemiol 27:517–518. doi: 10.1086/504365. [DOI] [PubMed] [Google Scholar]
- 31.Arlian LG. 1989. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annu Rev Entomol 34:139–161. doi: 10.1146/annurev.en.34.010189.001035. [DOI] [PubMed] [Google Scholar]
- 32.Golant AK, Levitt JO. 2012. Scabies: a review of diagnosis and management based on mite biology. Pediatr Rev 33:e1–e12. doi: 10.1542/pir.33-1-e1. [DOI] [PubMed] [Google Scholar]
- 33.Hay RJ, Steer AC, Engelman D, Walton S. 2012. Scabies in the developing world—its prevalence, complications, and management. Clin Microbiol Infect 18:313–323. doi: 10.1111/j.1469-0691.2012.03798.x. [DOI] [PubMed] [Google Scholar]
- 34.Lapeere H, Naeyaert JM, De Weert J, De Maeseneer J, Brochez L. 2008. Incidence of scabies in Belgium. Epidemiol Infect 136:395–398. doi: 10.1017/S0950268807008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassa S, Campbell MJ, Bennett CE. 2011. Epidemiology of scabies prevalence in the U.K. from general practice records. Br J Dermatol 164:1329–1334. doi: 10.1111/j.1365-2133.2011.10264.x. [DOI] [PubMed] [Google Scholar]
- 36.Tsutsumi M, Nishiura H, Kobayashi T. 2005. Dementia-specific risks of scabies: retrospective epidemiologic analysis of an unveiled nosocomial outbreak in Japan from 1989-90. BMC Infect Dis 5:85. doi: 10.1186/1471-2334-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang CH, Lee SC, Huang SS, Kao YC, See LC, Yang SH. 2012. Risk factors for scabies in Taiwan. J Microbiol Immunol Infect 45:276–280. doi: 10.1016/j.jmii.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy JS, Kemp DJ, Walton SF, Currie BJ. 2004. Scabies: more than just an irritation. Postgrad Med J 80:382–387. doi: 10.1136/pgmj.2003.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts LJ, Huffam SE, Walton SF, Currie BJ. 2005. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect 50:375–381. doi: 10.1016/j.jinf.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Walter B, Heukelbach J, Fengler G, Worth C, Hengge U, Feldmeier H. 2011. Comparison of dermoscopy, skin scraping, and the adhesive tape test for the diagnosis of scabies in a resource-poor setting. Arch Dermatol 147:468–473. doi: 10.1001/archdermatol.2011.51. [DOI] [PubMed] [Google Scholar]
- 41.Bezold G, Lange M, Schiener R, Palmedo G, Sander CA, Kerscher M, Peter RU. 2001. Hidden scabies: diagnosis by polymerase chain reaction. Br J Dermatol 144:614–618. doi: 10.1046/j.1365-2133.2001.04096.x. [DOI] [PubMed] [Google Scholar]
- 42.Fukuyama S, Nishimura T, Yotsumoto H, Gushi A, Tsuji M, Kanekura T, Matsuyama T. 2010. Diagnostic usefulness of a nested polymerase chain reaction assay for detecting Sarcoptes scabiei DNA in skin scrapings from clinically suspected scabies. Br J Dermatol 163:892–894. doi: 10.1111/j.1365-2133.2010.09913.x. [DOI] [PubMed] [Google Scholar]
- 43.Naz S, Rizvi DA, Javaid A, Ismail M, Chaudhry FR. 2013. Validation of PCR assay for identification of Sarcoptes scabiei var. hominis. Iran J Parasitol 8:437–440. [PMC free article] [PubMed] [Google Scholar]
- 44.Berrilli F, D'Amelio S, Rossi L. 2002. Ribosomal and mitochondrial DNA sequence variation in Sarcoptes mites from different hosts and geographical regions. Parasitol Res 88:772–777. doi: 10.1007/s00436-002-0655-y. [DOI] [PubMed] [Google Scholar]
- 45.Skerratt LF, Campbell NJ, Murrell A, Walton S, Kemp D, Barker SC. 2002. The mitochondrial 12S gene is a suitable marker of populations of Sarcoptes scabiei from wombats, dogs and humans in Australia. Parasitol Res 88:376–379. doi: 10.1007/s00436-001-0556-5. [DOI] [PubMed] [Google Scholar]
- 46.Walton SF, Dougall A, Pizzutto S, Holt D, Taplin D, Arlian LG, Morgan M, Currie BJ, Kemp DJ. 2004. Genetic epidemiology of Sarcoptes scabiei (Acari: Sarcoptidae) in northern Australia. Int J Parasitol 34:839–849. doi: 10.1016/j.ijpara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Alasaad S, Soglia D, Spalenza V, Maione S, Soriguer RC, Pérez JM, Rasero R, Degiorgis MP, Nimmervoll H, Zhu XQ, Rossi L. 2009. Is ITS-2 rDNA suitable marker for genetic characterization of Sarcoptes mites from different wild animals in different geographic areas? Vet Parasitol 159:181–185. doi: 10.1016/j.vetpar.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Oleaga A, Alasaad S, Rossi L, Casais R, Vicente J, Maione S, Soriguer RC, Gortázar C. 2013. Genetic epidemiology of Sarcoptes scabiei in the Iberian wolf in Asturias, Spain. Vet Parasitol 96:453–459. doi: 10.1016/j.vetpar.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 49.Pisani D, Carton R, Campbell LI, Akanni WA, Mulville E, Rota-Stabelli O. 2013. An overview of arthropod genomics, mitogenomics, and the evolutionary origins of the arthropod proteome, p 41–61. In Minelli A, Boxshall G, Fusco G (ed), Arthropod biology and evolution. Springer, Heidelberg, Germany. [Google Scholar]
- 50.Somller BR, Hiatt KM. 2009. Dermatopathology: the basics. Springer, Heidelberg, Germany. [Google Scholar]
- 51.Grove GL, Kligman AM. 1983. Age-associated changes in human epidermal cell renewal. J Gerontol 38:137–142. doi: 10.1093/geronj/38.2.137. [DOI] [PubMed] [Google Scholar]
- 52.Arlian LG, Estes SA, Vyszenski-Moher DL. 1988. Prevalence of Sarcoptes scabiei in the homes and nursing homes of scabietic patients. J Am Acad Dermatol 19:806–811. doi: 10.1016/S0190-9622(88)70237-6. [DOI] [PubMed] [Google Scholar]