Abstract
Neoscytalidium dimidiatum is a mold known to cause onychomycosis and dermatomycosis; however, it is an extremely rare cause of systemic infection. We report a case of pulmonary infection with Neoscytalidium dimidiatum in an immunocompromised patient and discuss in vitro susceptibility data from this case and previous literature.
CASE REPORT
A 50-year-old male was admitted to the hospital with a 2-day history of worsening alteration in mental status and abdominal distention after being discharged from a skilled nursing facility. His past medical history was significant for cirrhosis secondary to chronic hepatitis C viral infection. The patient had a previous hospital admission 1 month prior for altered mental status. He was found to have a frontal lobe mass during that admission, which was resected, and the patient was diagnosed with diffuse large B cell lymphoma (DLBCL). He was started on dexamethasone at 2 mg every 6 h for cerebral edema, which was continued on readmission.
Physical examination demonstrated pertinent findings of jaundice and a large, firm, distended abdomen, which was nontender to palpation. The patient's lungs were clear to auscultation bilaterally. Laboratory studies on readmission revealed the following clinical values: white blood cell count, 20,100 cells/mm3 (neutrophils, 84%); hemoglobin, 13.5 g/dl; hematocrit, 39%; platelets, 85,000/mm3; serum creatinine, 0.57 mg/dl; international normalized ratio, 1.62; total protein, 5.2 g/dl; albumin, 2.5 g/dl; aspartate aminotransferase, 52 U/liter; alanine aminotransferase, 130 U/liter; alkaline phosphatase, 138 U/liter; total bilirubin, 1.2 mg/dl (direct bilirubin, 0.5 mg/dl); ammonia, 131 μmol/liter; and lactate, 2.5 mmol/liter. Analysis of the peritoneal fluid showed 149 total nucleated cells/mm3, with 37% neutrophils and no bacterial growth on culture.
On hospital day 1, a computed tomography (CT) with contrast of the abdomen and pelvis to evaluate for ascites described new incidental findings of multiple bilateral pulmonary nodules, compared to a CT with contrast of the chest, abdomen, and pelvis from 27 days prior. There was concern for lymphomatous metastases of the lung. However, a noncontrast CT of the chest on hospital day 5 showed multiple lung nodules increasing in size, several cavitating, suggestive of infection. The patient remained afebrile, and his leukocytosis had improved to 16,200 cells/mm3 at the time of this finding. The infectious diseases team was consulted, and a bronchoalveolar lavage (BAL) was performed.
Cultures from sputum collected on hospital day 5 and BAL fluid collected on hospital day 6 were positive on hospital day 9 for growth of wooly, brown colonies on Sabouraud dextrose and inhibitory mold agars at 30°C. No growth was observed on brain heart infusion agar with cycloheximide and gentamicin or on Mycosel agar. Microscopically, the mold produced continuous, blocky, brown arthroconidia and septate hyphae, and a preliminary identification of Neoscytalidium sp. was made. As both the sputum and BAL fluid samples grew the same mold, no further cultures were collected. Additionally, no growth was observed in two sets of blood cultures from hospital days 1 and 7.
Urine Histoplasma antigen, serum Histoplasma antibody, serum Cryptococcus antigen, serum Coccidioides antigen, and serum Coccidioides IgG were negative. A serum Aspergillus galactomannan antigen and serum Coccidioides IgM were positive; however, the Coccidioides IgM was equivocal upon repeat testing 11 days later. The Platelia Aspergillus Ag test (Bio-Rad Laboratories, Redmond, WA) was completed twice on the same serum sample with galactomannan indices of 0.68 and 1.049 (positive cutoff of ≥0.5). The assay was not repeated later with the BAL fluid or a different serum sample.
The patient was started on empirical liposomal amphotericin B at 5 mg/kg body weight/day on hospital day 9. On hospital day 12, the patient's serum creatinine increased to 0.97 mg/dl. Given the concern for nephrotoxicity, lack of identification of a confirmed fungal pathogen, and because the patient was not exhibiting clinical signs or symptoms of a pulmonary infection, liposomal amphotericin B was withheld. After 5 days without liposomal amphotericin B, the patient's serum creatinine remained stable at around 1.0 mg/dl, so liposomal amphotericin B was reinitiated. Two days later, the patient's serum creatinine began to increase again, peaking at 2.43 mg/dl on the third additional day of therapy. The patient also experienced some urinary retention, requiring Foley catheter placement. Amphotericin was discontinued, and posaconazole suspension at 200 mg four times daily was initiated.
The patient began whole-brain radiation therapy for treatment of DLBCL on hospital day 11. On hospital day 33, he experienced an acute cerebrovascular accident, and the decision was made to transition the patient to home hospice care. The patient was discharged on posaconazole suspension at 200 mg three times daily. On hospital day 13, the microbiology lab morphologically identified the mold as Neoscytalidium dimidiatum, and the isolate was then referred to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio (UTHSCSA) for confirmation of the identity and antifungal susceptibility testing.
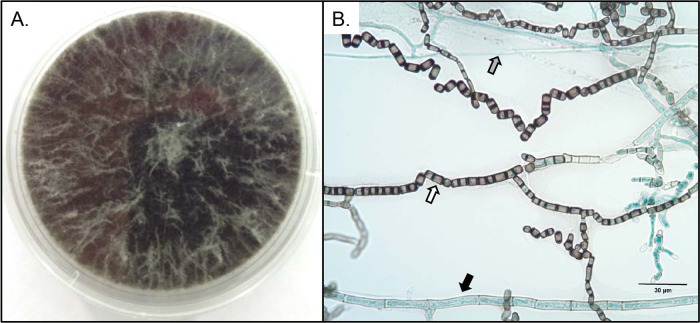
Colonies were black and wooly, growing rapidly on potato flakes agar (PFA) prepared in house and filling a 60-mm petri dish in 8 days (Fig. 1A). Microscopic features on a PFA slide culture incubated at 25°C for 12 days in ambient air with 24-h alternating light cycles revealed 1- to 2-cell (and occasionally 3-cell) dark, smooth, thick-walled arthroconidia in chains measuring 3.5 to 5.0 by 6.5 to 12.0 μm, thin hyaline hyphae, and broad phaeoid hyphae up to 10 μm in width (Fig. 1B). Temperature studies revealed growth at both 37°C and 40°C. The isolate also grew on media containing 10 μg/ml benomyl and 0.5 μg/ml cycloheximide prepared in house (1). Based on these features and the sequence results described below, the isolate was identified as Neoscytalidium dimidiatum.
FIG 1.
(A) Neoscytalidium dimidiatum after 8 days of incubation on potato flakes agar at 25°C. (B) Lactophenol cotton blue mount of a slide culture prepared on potato flakes agar after 12 days of incubation at 25°C. Microscopic features of Neoscytalidium dimidiatum include 1- to 3-cell dark, rectangular arthroconidia (white arrow), thin hyaline hyphae (gray arrow), and broad dark hyphae up to 10 μm in width (black arrow).
Template DNA was prepared by subculturing the isolate onto PFA and incubating at 30°C, and hyphal elements were scraped from the agar surface and suspended in CPL-100 buffer (VWR International, Inc., Radnor, PA). The specimen was then lysed by bead beating, and DNA was isolated manually using a chloroform extraction method. The extracted DNA was used for PCR amplification of the internal transcribed spacer (ITS) and D1/D2 regions as described with slight modification, and PCR products were sequenced using the ITS1 and ITS4 primers as well as the NL1 and NL4 primers in the UTHSCSA Molecular Diagnostics Laboratory (2, 3). Sequences were assembled and analyzed using DNASTAR software (DNASTAR, Inc., Madison, WI) and queried in GenBank using the BLASTn algorithm at the NCBI site (www.ncbi.nlm.nih.gov). Sequences were also compared to those available in the CBS-KNAW Fungal Biodiversity Centre database (www.cbs.knaw.nl). The ITS sequence demonstrated 100% identity to Neoscytalidium dimidiatum (GenBank accession no. GQ330903.1; base pair match, 569/569), and the D1/D2 sequence also showed 100% identity to Neoscytalidium dimidiatum (CBS accession no. 204.33; base pair match, 615/615). The isolate has been deposited into the University of Alberta Microfungus Collection & Herbarium under accession no. UAMH 11854 and in the UTHSCSA Fungus Testing Laboratory Collection as UTHSCSA DI14-340.
Antifungal susceptibility testing was performed using the Clinical and Laboratory Standards Institute M38-A2 reference methodology for broth microdilution (4), and the MICs were read using the 100% growth inhibition endpoint after 48 h of incubation at 35°C. Amphotericin B and voriconazole demonstrated in vitro activity against this isolate as the MIC of both agents was 1 mg/liter (Table 1). However, the MIC for posaconazole was >16 mg/liter. As these results were returned after the patient's cerebrovascular accident and discharge with home hospice care, no further changes were made in the patient's antifungal therapy. The patient died less than a month after discharge.
TABLE 1.
In vitro susceptibilities of isolates from case reports of invasive infections caused by Neoscytalidium dimidiatum
| Case report (reference no.) | Infection site | Methoda | MIC (mg/liter)b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AMB | VRC | POS | KTC | ITC | FLC | 5FC | CSP | |||
| Elinav et al. (11) | Pulmonary | Etest | 0.032 | 0.032 | 0.75 | >32 | >256 | >32 | ||
| Sigler et al. (12) | Subcutaneous | Macrodilution | ≤0.29 | 0.3 | ||||||
| Dunn et al. (13) | Sinusitis | Microdilution | 0.5 | 1 | 4 | >8 | >64 | 16 | 16 | |
| Willinger et al. (14) | Disseminated | Etest | 0.032 | 0.012 | 1 | 0.5 | 24 | >32 | ||
| Present case | Pulmonary | Microdilution | 1 | 1 | >16 | |||||
Included are the Etest (AB Biodisk, Solna, Sweden), macrodilution, and broth microdilution (Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio).
Abbreviations: AMB, amphotericin B; VRC, voriconazole; POS, posaconazole; KTC, ketoconazole; ITC, itraconazole; FLC, fluconazole; 5FC, flucytosine; CSP, caspofungin.
Neoscytalidium dimidiatum is a predominately phaeoid mold known to cause chronic dermatomycosis and onychomycosis in humans, mainly involving the feet (5). The nomenclature of N. dimidiatum has been controversial due to the production of both arthroconidia and pycnidial synanamorphs and also hyaline as well as phaeoid colonies. Other names by which this organism has been classified include Hendersonula toruloidea, Scytalidium dimidiatum, Nattrassia mangiferae, Fusicoccum dimidiatum, and Neoscytalidium hyalinum (6–10). In this article, we use the name N. dimidiatum, as “dimidiata” is the oldest species epithet used (8).
Infections caused by this species generally occur in endemic tropical and subtropical areas, such as Africa, South America, the Caribbean, India, and Asia, through direct or indirect exposure to contaminated soil (5). While N. dimidiatum is known to cause dermatomycosis and onychomycosis, there are limited case reports in the literature describing invasive infections. Risk factors for invasive infections caused by Neoscytalidium dimidiatum appear to be similar to those of other invasive fungal infections, such as immunosuppression, diabetes mellitus, solid organ transplant, and trauma (11–14). A variety of sites of infection have been reported, including central nervous system (CNS) abscesses, endophthalmitis, sinusitis, fungemia, and osteomyelitis (11). To our knowledge, this is the first reported case of isolated pulmonary infection caused by N. dimidiatum without evidence of penetrating trauma.
Due to the rarity of invasive infections caused by this species, there is limited information available regarding clinical progression or treatment. Delay in identification of the causative pathogen is the main obstacle when treating these patients, and rates of false-negative cultures can be as high as 30% (5). The prognosis for invasive N. dimidiatum infection is typically poor: in a review of 10 cases by Elinav et al., 50% of the patients died, and one case of endophthalmitis required enucleation (11). Most patients in these reports received amphotericin B or voriconazole therapy in conjunction with surgical debridement (11).
In vitro susceptibility testing performed by Madrid et al. on isolates of N. dimidiatum obtained from primarily noninvasive human infections and some plant infections demonstrated that amphotericin B and terbinafine had the greatest potency of the antifungal agents tested, with geometric mean (GM) MICs of 0.32 and 0.70 mg/liter, respectively (15). Voriconazole (GM MIC, 1.37 mg/liter; range, 0.06 to 4 mg/liter) also appeared to have greater in vitro potency than posaconazole (GM MIC, 6.15 mg/liter; range, 0.06 to 32 mg/liter) (15). Micafungin appeared to have little activity, while anidulafungin had activity against some isolates (15). In a study by Lacroix et al., amphotericin B and voriconazole again exhibited the lowest MICs of systemic antifungals against Scytalidium clinical isolates obtained from patients with dermatomycoses and onychomycoses (16). S. hyalinum isolates appeared to be more susceptible to antifungal therapy than S. dimidiatum isolates in vivo (16). However, data to correlate in vitro susceptibilities to clinical outcomes are lacking.
Based on our case and previous reports in the literature, amphotericin B appears to be an appropriate empirical option against N. dimidiatum infections (Table 1). However, it carries the risk of nephrotoxicity, which increases with a longer duration of use (17). Many patients that develop these infections are already at high risk for nephrotoxicity and will require long-term antifungal treatment. Use of amphotericin B in our patient was limited by the side effects of the drug. Due to the lower mean MICs and availability as both an intravenous and oral formulation, voriconazole appears to be the best empirical alternative to amphotericin B. Posaconazole and anidulafungin may also be considered if activity has been confirmed through antifungal susceptibility testing.
If N. dimidiatum is suspected based on morphology, confirmatory genetic identification and antifungal susceptibility testing should be performed to determine long-term therapeutic options. There is no established duration of therapy, but patients who survive generally receive surgical intervention along with a minimum of 6 weeks of antifungal therapy (11–14, 17–20).
Nucleotide sequence accession numbers.
The ITS and D1/D2 nucleotide data have been deposited into GenBank under accession no. KM357894 and KM357895, respectively.
ACKNOWLEDGMENTS
We thank Karissa Culbreath, Antonio Gallegos, and Ellen Richards at Tricore Reference Laboratories for their contributions to this case report.
There was no funding for this study.
B.D., L.N., S.A.L., D.A.S., N.P.W., J.L., H.F., and B.J. report no conflicts of interest.
REFERENCES
- 1.Summerbell RC. 1993. The benomyl test as a fundamental diagnostic method for medical mycology. J Clin Microbiol 31:572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J Clin Microbiol 48:741–752. doi: 10.1128/JCM.01948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White TJ, Bruns TD, Lee SB, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, Inc, New York, NY. [Google Scholar]
- 4.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard— 2nd ed Document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 5.Machouart M, Menir P, Helenon R, Quist D, Desbois N. 2013. Scytalidium and scytalidiosis: what's new in 2012? J Mycol Med 23:40–46. doi: 10.1016/j.mycmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Nattrass RM. 1933. A new species of Hendersonula (H. toruloidea) on deciduous trees in Egypt. Trans Br Mycol Soc 18:189–198. doi: 10.1016/S0007-1536(33)80030-1. [DOI] [Google Scholar]
- 7.Sutton BC, Dyko BJ. 1989. Revision of Hendersonula. Mycol Res 93:466–488. doi: 10.1016/S0953-7562(89)80040-1. [DOI] [Google Scholar]
- 8.Farr DF, Elliot M, Rossman AY, Edmonds RL. 2005. Fusicoccum arbuti sp. nov. causing cankers on Pacific madrone in western North America with notes on Fusicoccum dimidiatum, the correct name for Scytalidium dimidiatum and Nattrassia mangiferae. Mycologia 97:730–741. doi: 10.3852/mycologia.97.3.730. [DOI] [PubMed] [Google Scholar]
- 9.Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJ, Alves A, Burgess T, Barber P, Groenewald JZ. 2006. Phylogenetic lineages in the Botryosphaeriaceae. Stud Mycol 55:235–253. doi: 10.3114/sim.55.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW. 2013. The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167. doi: 10.3114/sim0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elinav H, Izhar U, Benenson S, Admon D, Hidalgo-Grass C, Polacheck I, Korem M. 2009. Invasive Scytalidium dimidiatum infection in an immunocompetent adult. J Clin Microbiol 47:1259–1263. doi: 10.1128/JCM.01874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigler L, Summerbell RC, Poole L, Wieden M, Sutton DA, Rinaldi MG, Aguirre M, Estes GW, Galgiani JN. 1997. Invasive Nattrassia mangiferae infections: case report, literature review, and therapeutic and taxonomic appraisal. J Clin Microbiol 35:433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn JJ, Wolfe MJ, Trachtenberg J, Kriesel JD, Orlandi RR, Carroll KC. 2003. Invasive fungal sinusitis caused by Scytalidium dimidiatum in a lung transplant recipient. J Clin Microbiol 41:5817–5819. doi: 10.1128/JCM.41.12.5817-5819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willinger B, Kopetzky G, Harm F, Apfalter P, Makristathis A, Berer A, Bankier A, Winkler S. 2004. Disseminated infection with Nattrassia mangiferae in an immunosuppressed patient. J Clin Microbiol 42:478–480. doi: 10.1128/JCM.42.1.478-480.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madrid H, Ruiz-Cendoya M, Cano J, Stchigel A, Orofino R, Guarro J. 2009. Genotyping and in vitro antifungal susceptibility of Neoscytalidium dimidiatum isolates from different origins. Int J Antimicrob Agents 34:351–354. doi: 10.1016/j.ijantimicag.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Lacroix C, de Chauvin FM. 2008. In vitro activity of amphotericin B, itraconazole, voriconazole, posaconazole, caspofungin and terbinafine against Scytalidium dimidiatum and Scytalidium hyalinum clinical isolates. J Antimicrob Chemother 61:835–837. doi: 10.1093/jac/dkn011. [DOI] [PubMed] [Google Scholar]
- 17.Tan DH, Sigler L, Gibas CF, Fong IW. 2008. Disseminated fungal infection in a renal transplant recipient involving Macrophomina phaseolina and Scytalidium dimidiatum: case report and review of taxonomic changes among medically important members of the Botryosphaeriaceae. Med Mycol 46:285–292. doi: 10.1080/13693780701759658. [DOI] [PubMed] [Google Scholar]
- 18.Gumbo T, Mkanganwi N, Robertson VJ, Masvaire P. 2002. Case report. Nattrassia mangiferae endophthalmitis. Mycoses 45:118–119. doi: 10.1046/j.1439-0507.2002.00723.x. [DOI] [PubMed] [Google Scholar]
- 19.al-Rajhi AA, Awad AH, al-Hedaithy SS, Forster RK, Caldwell KC. 1993. Scytalidium dimidiatum fungal endophthalmitis. Br J Ophthalmol 77:388–390. doi: 10.1136/bjo.77.6.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benne CA, Neeleman C, Bruin M, de Hoog GS, Fleer A. 1993. Disseminating infection with Scytalidium dimidiatum in a granulocytopenic child. Eur J Clin Microbiol Infect Dis 12:118–121. doi: 10.1007/BF01967587. [DOI] [PubMed] [Google Scholar]