Abstract
When mycobacteria are recovered in clinical specimens, timely species-level identification is required to establish the clinical significance of the isolate and facilitate optimization of antimicrobial therapy. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has recently been reported to be a reliable and expedited method for identification of mycobacteria, although various specimen preparation techniques and databases for analysis are reported across studies. Here we compared two MALDI-TOF MS instrumentation platforms and three databases: Bruker Biotyper Real Time Classification 3.1 (Biotyper), Vitek MS Plus Saramis Premium (Saramis), and Vitek MS v3.0. We evaluated two sample preparation techniques and demonstrate that extraction methods are not interchangeable across different platforms or databases. Once testing parameters were established, a panel of 157 mycobacterial isolates (including 16 Mycobacterium tuberculosis isolates) was evaluated, demonstrating that with the appropriate specimen preparation, all three methods provide reliable identification for most species. Using a score cutoff value of ≥1.8, the Biotyper correctly identified 133 (84.7%) isolates with no misidentifications. Using a confidence value of ≥90%, Saramis correctly identified 134 (85.4%) isolates with one misidentification and Vitek MS v3.0 correctly identified 140 (89.2%) isolates with one misidentification. The levels of accuracy were not significantly different across the three platforms (P = 0.14). In addition, we show that Vitek MS v3.0 requires modestly fewer repeat analyses than the Biotyper and Saramis methods (P = 0.04), which may have implications for laboratory workflow.
INTRODUCTION
The genus Mycobacterium has undergone tremendous taxonomic revision in recent decades. There are currently 170 recognized species (http://www.bacterio.net/mycobacterium.html; accessed 20 February 2014) with a wide range of pathogenic potential from benign environmental contaminants to the pathogenic Mycobacterium tuberculosis complex, which was estimated to be responsible for 9 million cases of disease and 1.5 million deaths worldwide in 2013 (1). While M. tuberculosis remains the most clinically significant species and public health threat within this genus, many non-tuberculosis mycobacteria (NTM) are well-established pathogens and may be increasing in prevalence in part due to increased numbers of immunocompromised individuals as well as to the increasing prevalence of medical hardware and indwelling devices (2).
When mycobacteria are recovered from clinical specimens, establishment of a species- or complex-level identification is critical to distinguish pathogenic species from common environmental contaminants (such as Mycobacterium gordonae) and to guide antimicrobial therapy, when indicated. Species-level identification has classically relied on a variety of characteristics and methodologies, including growth rate, pigmentation, enzymatic properties (3, 4), and high-performance liquid chromatography (HPLC), which generates species- or complex-specific mycolic acid profiles (5). In addition, molecular assays, including those using nucleic acid probes, have been used for identification of some common Mycobacterium spp., including M. tuberculosis and Mycobacterium avium complex (6). DNA sequencing of 16S rRNA, rpoB, and hsp65 genes is widely considered the gold standard for identification (7). However, these methods can be time-consuming and expensive, can require specific equipment and expertise, and often have limited availability outside reference laboratories.
Recently, matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) has been used to provide relatively rapid, inexpensive, and accurate identification of a variety of microorganisms, including Mycobacterium spp. (8–11). However, unlike most bacteria, which can be directly spotted onto a MALDI-TOF target plate, mycobacteria require inactivation and extraction steps prior to analysis, both for biosafety and for access to proteins in the cells. The inactivation step is commonly performed with heat-killing and/or ethanol (8). For optimal results, the mycobacterial protein must be extracted from the cells, most commonly by silica bead beating, given the poorly permeable and mycolic acid-rich cell wall. Each of these steps and protocols, in addition to the breadth and depth of the MALDI-TOF MS reference databases, may introduce assay variability and influence the analytical performance characteristics of the method.
There are two commercially available MALDI-TOF MS platforms: the Bruker Biotyper (Bruker, Billerica, MA) and the bioMérieux Vitek MS (bioMérieux, Durham, NC). Our objective was to compare the contemporary databases and specimen preparation methods recommended for the Bruker Biotyper to those recommended for the Vitek MS. We compared solid-medium types for organism cultivation prior to MS analysis, extraction methods, and the reliability and accuracy of Mycobacterium species identification.
MATERIALS AND METHODS
Clinical isolates and identification.
This was an analysis of 167 banked isolates of Mycobacterium spp. Ten isolates were used for an initial comparison of medium types and extraction methods, while 157 isolates were used to assess the analytical performance characteristics of each method. All isolates were recovered from clinical specimens submitted to the Barnes-Jewish Hospital microbiology laboratory between 2009 and 2014. M. tuberculosis complex, M. avium complex, Mycobacterium kansasii, and M. gordonae were identified by a chemiluminescent DNA probe hybridization assay (AccuProbe; Hologic, Inc., Bedford, MA). All other mycobacterial species were identified by the Wisconsin State Laboratory of Hygiene or the Oklahoma State Department of Public Health by mycolic acid profile analysis (using HPLC) and/or DNA sequencing of the 16S rRNA and rpoB genes.
Culture conditions.
Unless indicated otherwise, all isolates were cultured on Middlebrook 7H10 agar (7H10; Remel, Lenexa, KS) at 35°C without supplemental CO2. Mycobacterium marinum and Mycobacterium haemophilum were grown at 30°C. M. haemophilum was cultured on chocolate agar. To compare the effects of medium types, a subset of isolates were cultured on both 7H10 and Löwenstein-Jensen (LJ) agar at 35°C.
Experimental design.
To enable direct comparison of the Biotyper and Vitek MS mycobacterial identification methods, all extractions were performed for both assays from isolates grown on identical culture media at the same time. Similar biomasses, consisting of heaping inocula on a 1-μl disposable loop (Nunc, Roskilde, Denmark), were used for the two assays.
Comparison of solid media and extraction methods.
We selected a panel of 10 commonly isolated NTM isolates to compare the Biotyper and Vitek MS protein extraction methods using both 7H10 and LJ solid media. This collection of isolates included four rapidly growing NTM species (two Mycobacterium abscessus, one Mycobacterium chelonae, and one Mycobacterium fortuitum) and six slowly growing NTM species (two each of M. avium complex, M. gordonae, and M. kansasii). For this pilot study, scores and confidence values of ≥2.0 and ≥90% were set as the diagnostic thresholds for the Biotyper and Vitek MS, respectively. Extractions were done in duplicate, with each extraction method applied once per target plate in each of two independent experiments, resulting in 40 total results per MALDI-TOF MS platform.
Comprehensive evaluation of diverse mycobacterial isolates.
For the comparisons among the 157 Mycobacterium spp., a single extraction was performed and the extracted material was spotted in duplicate in each of three independent experiments. Two independent operators tested each isolate.
Biotyper specimen preparation method.
Samples were extracted according to the Bruker MALDI Biotyper standard operating procedure (revision 2 January 2013). Briefly, mycobacterial isolates were suspended in 300 μl H2O and then heat inactivated for 30 min in a 95°C heat block. Samples were centrifuged, resuspended in 70% ethyl alcohol (EtOH), washed in H2O, and then resuspended in 50 μl H2O prior to incubation for 10 min at 95°C. Samples were then washed in 100% EtOH, dried, and then subjected to vortex mixing for 1 min with 0.5-mm-diameter glass beads (VWR, Radnor, PA) and 20 μl acetonitrile. After bead beating specimens horizontally by the use of a vortex adaptor, 20 μl of 70% formic acid was added. Samples were then centrifuged, and 1 μl of supernatant was spotted per target. After drying, 1 μl of HCCA (alpha-cyano-4-hydroxycinnamic acid; Bruker) matrix solution was overlaid. Bacterial Test Standard (Bruker) was direct spotted onto each target as a calibrant and control. All targets were analyzed within 3 h of the spotting procedure. Biotyper Real Time Classification software version 3.1 and Biotyper Mycobacteria Library 1.0 were used for analysis. All samples were analyzed exclusively in automatic mode. The Biotyper employs a laser frequency of 60 Hz and records mass spectra from 2,000 to 20,000 Da. Each spot was sampled in nine different areas with 40 shots per sampling area. The resulting spectra were flattened, compared to a database, and analyzed to produce a logarithmic score of from 0 to 3.0. Per the manufacturer, scores of ≥2.0 are considered reliable for species-level identification, scores of 1.7 to 1.9 are accurate for genus-level identification, and scores of <1.7 are unreliable (9).
Vitek MS extraction method.
Samples were extracted according to the Mycobacteria Test protocol (bioMérieux). Briefly, a suspension of mycobacteria was mixed with silica beads and 70% EtOH and then mechanically disrupted by vortex mixing for 10 to 15 min at 3,000 rpm (Vortex Genie 2 [Scientific Industries] with MoBio Vortex Adaptor). After bead beating was performed, samples were incubated for 10 min at room temperature and then the supernatant was transferred to a new tube. Samples were then pelleted, dried, and resuspended in 10 μl of 70% formic acid. The suspension was incubated for 2 to 5 min, and then 10 μl acetonitrile was added. The samples were then centrifuged, and 1 μl of supernatant was added per target spot. After the extract was dried, 1 μl of Vitek MS-CHCA Matrix (bioMérieux) was overlaid and allowed to dry completely. An Escherichia coli reference strain (ATCC 8739) was directly spotted on each acquisition group on each slide as a calibrant and control per manufacturer protocols. All slides were run within 3 h of organism application. All samples were run exclusively in automatic mode. Spectra were generated on the Vitek MS, and the resulting spectra were simultaneously analyzed using both the Saramis and v3.0 software. The v3.0 spectra were analyzed using SpectraIdentifier v2.1.0, a research and development tool. The v3.0 software is currently under development and is not yet commercially available.
The Vitek MS employs a laser frequency of 50 Hz and records mass spectra of from 2,000 to 20,000 Da. Each spot was pulsed 100 times, and each generated a unique mass spectrum, and the mass spectra were summed into a single spectrum that then underwent “mass binning” (12). This resulted in an isolate identification with a confidence value of from 0% to 99.99% for Saramis and 0% to 99.9% for v3.0. Results from low-quality or absent spectra receive a classification of “no identification” (12).
Discrepant resolution.
If the isolate identification obtained as part of routine testing (see the description of culture conditions and identification above) was identical to the identification generated by all three of the MALDI-TOF MS methods, the result was considered to be correct without the need for additional testing. For any isolates where discrepancies were observed, the isolate was identified by rpoB and/or 16S rRNA gene sequencing for definitive identification.
Assessment of organism inactivation following specimen preparation.
A M. tuberculosis control strain (ATCC 25177) was used to assess the ability of the Biotyper and Vitek MS specimen preparation protocols to inactivate this species. Each extraction method was performed in triplicate in each of two independent experiments. For the Bruker extraction, samples were inactivated for 30 min at 95°C followed by treatment with 70% EtOH. After the EtOH was decanted, the pellet was resuspended in 200 μl sterile H2O and the entire volume was used to inoculate a mycobacterial growth indicator tube (MGIT) with polymyxin B, amphotericin B, nalidixic acid, trimethoprim, and azlocillin (PANTA) antibiotic (BD, Franklin Lakes, NJ). For the Vitek MS extraction, M. tuberculosis was inactivated with 70% EtOH and the cell pellet was resuspended in 100 μl of sterile H2O and was used to inoculate a MGIT tube. For both methods, M. tuberculosis inactivation was confirmed by incubation of the MGIT for 6 weeks at 35°C in a Bactec MGIT mycobacterial detection system.
Statistical analysis.
The Wilcoxon matched-pair signed-rank test was used to analyze the impact of the extraction method on scores. Fisher's exact test was used to compare the effects of the extraction methods on the frequencies of samples without identification. To compare the numbers of repeats needed to obtain an identification, a chi-square test comparing the three methods with the four outcomes (identification by the first, second, or third experiment or no identification) was performed. A chi-square test for trend (Cochran-Armitage test for trend) was then used for pairwise analysis. A P value of less than 0.05 was considered significant. Data were analyzed with Prism 6 (GraphPad Software Inc., La Jolla, CA) and SPSS Version 22 (IBM, Armonk, NY).
RESULTS
Comparison of solid media.
We compared NTM identification using isolates obtained from 7H10 and LJ solid media and the Biotyper, Saramis, and Vitek MS v3.0 methods on a panel of 10 NTM isolates. All 10 isolates were correctly identified in at least one of two replicates in at least one of two independent experiments, while no isolates were misidentified above the given thresholds (score of ≥2.0 for Biotyper and confidence value of ≥90% for Saramis and v3.0). Using the Biotyper, 37/40 samples from 7H10 and 33/40 from LJ agar were correctly identified (Table 1). Using the Saramis database, 37/40 and 31/40 were correctly identified from 7H10 and LJ agar, respectively. Finally, using the Vitek MS v3.0 database, 39/40 and 35/40 samples were correctly identified from 7H10 and LJ, respectively. The differences in the frequencies of correct identifications between 7H10 and LJ for the Biotyper, Saramis, and v3.0 methods individually were not significantly different, but the cumulative identification rate from 7H10 (113/120; 94.2%) was significantly better than that seen with LJ (99/120; 82.5%) overall (P = 0.008).
TABLE 1.
Equivalence of 7H10 and LJ solid media
| Species | No. (%) of samples tested by database and extraction method combination with score ≥2.0 (Bruker) or ≥90% confidence value (Vitek) in indicated medium/no. of samples tested |
|||||
|---|---|---|---|---|---|---|
| Bruker and Bruker |
Saramis and Vitek MS |
v3.0 and Vitek MS |
||||
| 7H10 | LJ | 7H10 | LJ | 7H10 | LJ | |
| M. abscessus | 4/4 | 3/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| M. abscessus | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| M. chelonae | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| M. avium complex | 4/4 | 4/4 | 4/4 | 1/4 | 4/4 | 2/4 |
| M. avium complex | 2/4 | 4/4 | 3/4 | 4/4 | 3/4 | 4/4 |
| M. fortuitum complex | 4/4 | 4/4 | 4/4 | 3/4 | 4/4 | 3/4 |
| M. gordonae | 3/4 | 2/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| M. gordonae | 4/4 | 4/4 | 4/4 | 3/4 | 4/4 | 3/4 |
| M. kansasii | 4/4 | 2/4 | 2/4 | 2/4 | 4/4 | 3/4 |
| M. kansasii | 4/4 | 2/4 | 4/4 | 2/4 | 4/4 | 4/4 |
| Total | 37/40 (92.5) | 33/40 (82.5) | 37/40 (92.5) | 31/40 (78) | 39/40 (97.5) | 35/40 (87.5) |
Comparison of extraction methods.
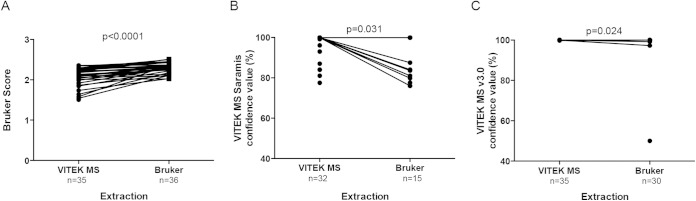
Next, we evaluated whether the method of extraction of mycobacteria grown on 7H10 medium impacted the reliability of identification. For samples analyzed by the Biotyper, 35/40 isolates extracted with the Vitek MS method and 36/40 extracted with the Biotyper method yielded an identification (P = 0.99). Application of the Vitek MS extraction method resulted in Biotyper scores significantly lower than those obtained with the same samples extracted with the Biotyper extraction method (median difference, 0.16; P < 0.0001) (Fig. 1A). For samples analyzed with Saramis, there were significantly more results with an identification when extraction was performed with the Vitek MS (32/40) procedure than with the Biotyper method (15/40) extraction procedure (P < 0.0002). Application of the Biotyper extraction method led to less-reliable scores for samples analyzed with the Saramis (median difference, 6.2%; P < 0.031) (Fig. 1B). For samples analyzed by Vitek MS v3.0, 35/40 spots generated an identification with the Vitek MS extraction and 30/40 gave an identification with the Biotyper extraction (P < 0.25). The Vitek MS v3.0 was relatively insensitive to the extraction method (median difference, 0.01%; P < 0.024) (Fig. 1C).
FIG 1.
The extraction method impacts the MALDI-TOF quality score. Ten NTM isolates were extracted in parallel with either the Vitek extraction method or the Bruker extraction method and then analyzed. (A) The Biotyper generated higher scores for samples extracted with the Bruker method. (B) The Saramis generated higher confidence values and was more likely to identify samples extracted with the Vitek method. (C) Vitek MS v3.0 was relatively insensitive to the extraction method.
Comparison of the Biotyper, Saramis, and Vitek MS v3.0 methods for mycobacterial identification.
We assessed the performance of the Biotyper, Saramis, and Vitek MS v3.0 for the identification of 157 mycobacterial isolates cultivated on 7H10 (Table 2). The isolates comprise 22 different mycobacterial complexes or species, including 9 complexes or species of rapid-growing NTM (n = 72), 12 complexes or species of slow-growing NTM (n = 69), and M. tuberculosis (n = 16).
TABLE 2.
Isolates tested and classification scheme
| Classification | Species |
|---|---|
| Rapid growers | |
| M. abscessus complex | M. abscessus |
| M. bolletii | |
| M. massiliense | |
| M. chelonae | M. chelonae |
| M. fortuitum complex | M. fortuitum |
| M. porcinum | |
| M. septicum | |
| M. senegalense | |
| M. conceptionense | |
| M. houstonense | |
| M. neworleansense | |
| M. immunogenum | M. immunogenum |
| M. moriokaense | M. moriokaense |
| M. mucogenicum complex | M. mucogenicum |
| M. phocaicum | |
| M. neoaurum | M. neoaurum |
| M. phlei | M. phlei |
| M. smegmatis complex | M. smegmatis |
| M. goodii | |
| M. wolinskyi | |
| M. mageritense | |
| Slow growers | |
| M. avium complex | M. avium |
| M. intracellulare | |
| M. chimaera | |
| M. gordonae | M. gordonae |
| M. haemophilum | M. haemophilum |
| M. kansasii | M. kansasii |
| M. kubicae | M. kubicae |
| M. malmoense | M. malmoense |
| M. marinum complex | M. marinum |
| M. shottsii | |
| M. parascrofulaceum | M. parascrofulaceum |
| M. scrofulaceum | M. scrofulaceum |
| M. seoulense | |
| M. simiae complex | M. simiae |
| M. lentiflavum | |
| M. szulgai | M. szulgai |
| M. terrae complex | M. terrae |
| M. arupense | |
| M. nonchromogenicum | |
| M. tuberculosis complex | M. tuberculosis |
| M. bovis | |
| M. vaccae | M. vaccae |
| M. xenopi | M. xenopi |
For the Biotyper, 2/157 (1.3%) isolates were not identified at any score in any of the three independent extractions (Table 3). Using a modified score threshold of ≥1.5, 147/157 (93.6%) were correctly identified, 3/157 (1.9%) were incorrectly identified, and 7/157 (4.5%) had a score below the threshold. At a score threshold of ≥1.7, 133/157 (84.7%) isolates were correctly identified, 2/157 (1.3%) isolates were incorrectly identified, and 22/157 (14.0%) isolates were not identified above the threshold. At a score threshold of ≥1.8, 128/157 (81.5%) isolates were correctly identified, no isolates were misidentified, and 29/157 (18.5%) isolates had a score below the threshold. At a score threshold of ≥2.0, 111/157 (70.7%) isolates were correctly identified, no isolates were misidentified, and 46/157 (29.3%) isolates had scores below 2.0.
TABLE 3.
Isolates correctly identified after three independent extractionsa
| Strain | No. of isolates (n = 157) | No. (%) of isolates with indicated score or confidence value (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruker Biotyper |
Vitek MS Saramis |
Vitek MS v3.0 |
||||||||||||
| No ID | ≥1.5 | ≥1.7 | ≥1.8 | ≥2.0 | No ID | ≥80 | ≥90 | ≥95 | No ID | ≥80 | ≥90 | ≥95 | ||
| Rapid growers | ||||||||||||||
| M. abscessus | 19 | 1 | 18 | 17 | 17 | 14 | 1 | 18 | 16 | 16 | 2 | 16 | 16 | 15 |
| M. chelonae | 13 | 0 | 10b | 5c | 2 | 1 | 0 | 13 | 13 | 8 | 0 | 13 | 13 | 13 |
| M. fortuitum complex | 16 | 0 | 16 | 14 | 14 | 14 | 1 | 14d | 14 | 14 | 1 | 15 | 13 | 13 |
| M. immunogenum | 4 | 0 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | 4 | 0 | 4 | 4 | 4 |
| M. moriokaense | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. mucogenicum complex | 13 | 1 | 12 | 10 | 10 | 5 | 0 | 13 | 13 | 12 | 0 | 13 | 12 | 12 |
| M. neoaurum | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| M. phlei | 2 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| M. smegmatis complex | 3 | 0 | 3 | 3 | 2 | 1 | 0 | 3 | 2 | 1 | 1 | 2 | 2 | 2 |
| Slow growers | ||||||||||||||
| M. avium complex | 13 | 0 | 13 | 13 | 13 | 13 | 0 | 13 | 12 | 12 | 2 | 11 | 11 | 11 |
| M. gordonae | 12 | 0 | 12 | 11 | 11 | 10 | 1 | 9 | 9 | 9 | 0 | 12 | 12 | 12 |
| M. haemophilum | 3 | 0 | 3 | 3 | 3 | 3 | 0 | 3 | 3 | 3 | 0 | 3 | 3 | 3 |
| M. kansasii | 12 | 0 | 12 | 12 | 12 | 12 | 0 | 12 | 12 | 12 | 0 | 12 | 12 | 12 |
| M. kubicae | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| M. marinum complex | 3 | 0 | 3 | 3 | 3 | 3 | 0 | 3 | 3 | 3 | 0 | 3 | 3 | 3 |
| M. parascrofulaceum | 1 | 0 | 0g | 0g | 0g | 0g | 0 | 0g | 0g | 0g | 0 | 0g | 0g | 0g |
| M. scrofulaceum complex | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| M. simiae complex | 5 | 0 | 5 | 4 | 4 | 2 | 0 | 3e | 3 | 3 | 0 | 4 | 3 | 3 |
| M. szulgai | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 |
| M. tuberculosis complex | 16 | 0 | 16 | 16 | 16 | 16 | 0 | 16 | 16 | 16 | 0 | 16 | 16 | 16 |
| M. terrae complex | 13 | 0 | 12 | 11 | 11 | 9 | 4 | 7f | 7 | 7 | 2 | 11 | 11 | 11 |
| M. xenopi | 4 | 0 | 4 | 4 | 4 | 4 | 0 | 4 | 4 | 4 | 0 | 4 | 4 | 4 |
| Total no. correctly identified | NA | 147 (93.6) | 133 (84.7) | 128 (81.5) | 111 (70.7) | NA | 139 (88.5) | 134 (85.4) | 127 (80.9) | NA | 144 (91.7) | 140 (89.2) | 139 (88.5) | |
| Total no. with incorrect (or no) identification | 2 (1.3) | 3 (1.9) | 2 (1.3) | 0 | 0 | 10 (6.4) | 5 (3.2) | 1 (0.6) | 1 (0.6) | 10 (6.4) | 1 (0.6) | 1 (0.6) | 1 (0.6) | |
| Total no. below score threshold | NA | 7 (4.5) | 22 (14.0) | 29 (18.5) | 46 (29.3) | NA | 13 (8.3) | 22 (14.0) | 29 (18.5) | NA | 12 (7.6) | 16 (10.2) | 17 (10.8) | |
ID, identification; NA, not applicable.
One isolate was identified as M. immunogenum, and one was identified as M. abscessus.
One isolate was identified as M. abscessus.
One isolate was identified as Streptococcus anginosus.
One isolate was identified as Hanseniaspora guilliermondii.
One isolate was identified as Staphylococcus epidermidis, and one was identified as Clostridium cadaveris.
One isolate was identified as M. scrofulaceum.
For the Saramis, 10/157 (6.4%) isolates were not identified at any score in any of the three independent extractions (Table 3). At a confidence value threshold of ≥80%, 139/157 (88.5%) isolates had a correct identification, 5/157 (3.2%) had an incorrect identification, and 13/157 (8.3%) had an identification below the threshold. At a confidence value cutoff of ≥90%, 134/157 (85.4%) of isolates were correctly identified, 1/157 (0.6%) was incorrectly identified, and 22/157 (14.0%) isolates had a score below the threshold. At a confidence value threshold of ≥95%, 127/157 (80.9%) isolates were correctly identified, 1/157 (0.6%) was incorrectly identified, and 29/157 (18.5%) isolates had a score below the threshold.
For the Vitek MS v3.0, 10/157 (6.4%) isolates were not identified at any score in any of the three independent extractions (Table 3). At a confidence value threshold of ≥80%, 144/157 (91.7%) isolates had a correct identification, 1/157 (0.6%) isolates had an incorrect identification, and 12/157 (7.6%) isolates had an identification below the threshold. At a confidence value cutoff of ≥90%, 140/157 (89.2%) isolates were correctly identified, 1/157 (0.6%) isolates was incorrectly identified, and 16/157 (10.2%) isolates had a score below the threshold. At a confidence value threshold of 95%, 139/157 (88.5%) of isolates were correctly identified, 1/157 (0.6%) was incorrectly identified, and 17/157 (10.8%) isolates had a score below the threshold. Among the three platforms, there was trend in the frequency of isolates that did not generate an identification after three attempts (P < 0.11). In a pairwise comparison, there was a trend toward fewer unidentified isolates with Vitek MS v3.0 than with the Biotyper (P < 0.052) and, to a lesser extent, Saramis (P < 0.39).
Of the 157 isolates, 15 had a discrepancy between the original identification and the identification obtained on one or more of the three MALDI-TOF MS methods. Definitive identification of these isolates was confirmed by DNA sequencing (Table 4).
TABLE 4.
Analysis of discrepant identificationsa
| Original ID | Biotyper | Best score | Vitek MS Saramis | Best confidence value (%) | Vitek MS v3.0 | Best confidence value (%) | Sequencing result | Target sequenced |
|---|---|---|---|---|---|---|---|---|
| M. terrae complex | M. goodii | 1.10 | No ID | 0 | No ID | 0 | M. moriokaense | 16S rRNA gene |
| M. smegmatis | M. phlei | 1.99 | M. phlei | 88.4 | M. phlei | 96 | M. phlei | 16S rRNA gene and rpoB |
| M. scrofulaceum | M. seoulense | 1.52 | M. scrofulaceum | 99.99 | M. scrofulaceum | 99.99 | M. scrofulaceum | 16S rRNA gene |
| M. mucogenicum | M. chelonae | 2.14 | M. chelonae | 99.99 | M. chelonae | 99.99 | M. chelonae | rpoB |
| M. smegmatis | M. wolinskyi | 2.14 | M. goodii | 96.2 | M. goodii | 99.99 | M. smegmatis groupb | 16S rRNA gene and rpoB |
| M. smegmatis | M. wolinskyi | 1.95 | M. goodii | 92.8 | M. goodii | 100 | M. smegmatis groupb | 16S rRNA gene and rpoB |
| M. chelonae | M. immunogenum | 1.61 | M. chelonae | 90 | M. chelonae | 99.99 | M. chelonae | rpoB |
| M. chelonae | M. abscessus | 1.71 | M. chelonae | 99.99 | M. chelonae | 99.99 | M. chelonae | rpoB |
| M. parascrofulaceum | M. scrofulaceum | 1.73 | M. scrofulaceum | 99.9 | M. scrofulaceum | 99.99 | M. parascrofulaceum | 16S rRNA gene and rpoB |
| M. mucogenicum | M. arupense | 1.86 | M. arupense | 0 | M. arupense | 100 | M. terrae complexc | 16S rRNA gene |
| M. marinum | M. shottsii | 2.14 | M. marinum | 99.9 | M. marinum | 99.99 | M. marinum | 16S rRNA gene |
| M. terrae complex | M. arupense | 1.51 | M. fortuitum | 85.8 | No ID | 0 | M. terrae complexc | 16S rRNA gene |
| M. vaccae | M. intracellulare | 1.18 | No ID | 0 | No ID | 0 | M. phlei | 16S rRNA gene |
| M. septicum | M. conceptionense | 1.69 | No ID | 0 | M. fortuitum | 80.5 | M. septicum | rpoB |
| M. conceptionense | M. senegalense | 2.13 | M. fortuitum | 99.89 | M. fortuitum | 99.99 | M. conceptionense | rpoB |
ID, identification.
The M. smegmatis group includes M. smegmatis, M. goodii, and M. wolinskyi.
The M. terrae complex includes M. terrae, M. nonchromogenicum, and M. arupense.
Necessity of repeat testing.
Next, we compared the numbers of independent extractions, performed on different days, required to obtain a correct identification with score or confidence value cutoffs of ≥1.8, ≥90%, and ≥90% for the Biotyper, Saramis, and Vitek MS v3.0 methods, respectively. For the Biotyper at a score cutoff of ≥1.8, 99/157 (63.1%) isolates were correctly identified to the complex level after a single extraction, 19/157 (12.1%) isolates were identified after two independent extractions, 10/157 (6.4%) were identified after three independent extractions, and 29/157 (18.5%) isolates were never correctly identified (Table 5). For the Saramis at a confidence value cutoff of ≥90%, 106/157 (67.5%), 11/157 (7.0%), and 18/157 (11.5%) isolates were first correctly identified in the first, second, and third independent experiments, respectively, while 22/157 (14.0%) isolates were never identified. For the Vitek MS v3.0 at a cutoff of ≥90%, 121/157 (77.1%), 11/157 (7.0%), and 9/157 (5.7%) of the mycobacterial isolates were first correctly identified in the first, second, and third independent experiments, respectively, while 16/157 (10.2%) isolates were never correctly identified. Among the three methods, there was a significant difference in the number of independent experiments needed for identification at the given threshold (P = 0.040). In a pairwise comparison, the Biotyper performed similarly to Saramis (P < 0.53) whereas the Biotyper required more runs for identification than Vitek MS v3.0 (P < 0.012). Saramis showed a trend toward increased numbers of runs needed for identification relative to Vitek MS v3.0 (P < 0.057).
TABLE 5.
Number of repeats needed for correct identificationa
| Species | Total no. of isolates | No. of isolates identified at indicated score or confidence value and no. of runs needed for correct ID |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bruker Biotyper ≥ 1.8 |
Vitek MS Saramis ≥ 90% |
Vitek MS v3.0 ≥ 90% |
|||||||||||
| No ID | 1 | 2 | 3 | No ID | 1 | 2 | 3 | No ID | 1 | 2 | 3 | ||
| Rapid growers | |||||||||||||
| M. abscessus | 19 | 2 | 10 | 4 | 3 | 3 | 13 | 2 | 1 | 3 | 12 | 1 | 3 |
| M. chelonae | 13 | 11 | 2 | 0 | 0 | 0 | 11 | 2 | 0 | 0 | 12 | 1 | 0 |
| M. fortuitum complex | 16 | 2 | 14 | 0 | 0 | 2 | 13 | 0 | 1 | 3 | 9 | 3 | 1 |
| M. immunogenum | 4 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 |
| M. moriokaense | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. mucogenicum complex | 13 | 3 | 8 | 1 | 1 | 0 | 11 | 1 | 1 | 1 | 12 | 0 | 0 |
| M. neoaurum | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| M. phlei | 2 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| M. smegmatis complex | 3 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0 |
| Slow growers | |||||||||||||
| M. gordonae | 12 | 1 | 8 | 0 | 3 | 3 | 7 | 0 | 2 | 0 | 12 | 0 | 0 |
| M. haemophilum | 3 | 0 | 3 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 3 | 0 | 0 |
| M. kansasii | 12 | 0 | 11 | 0 | 1 | 0 | 6 | 2 | 4 | 0 | 7 | 4 | 1 |
| M. kubicae | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| M. avium complex | 13 | 0 | 7 | 6 | 0 | 1 | 11 | 1 | 0 | 2 | 9 | 0 | 2 |
| M. marinum complex | 3 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 0 |
| M. scrofulaceum complex | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| M. simiae complex | 5 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 0 | 2 | 2 | 0 | 1 |
| M. szulgai | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| M. tuberculosis complex | 16 | 0 | 13 | 3 | 0 | 0 | 15 | 0 | 1 | 0 | 16 | 0 | 0 |
| M. terrae complex | 13 | 2 | 9 | 2 | 0 | 6 | 0 | 0 | 7 | 2 | 11 | 0 | 0 |
| M. xenopi | 4 | 0 | 3 | 1 | 0 | 0 | 4 | 0 | 0 | 0 | 4 | 0 | 0 |
| M. parascrofulaceum | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Total | 157 | 29 | 99 | 19 | 10 | 22 | 106 | 11 | 18 | 16 | 121 | 11 | 9 |
ID, identification.
DISCUSSION
Arguably the most important role of a clinical mycobacteriology laboratory, from both a patient care perspective and a public health perspective, is to differentiate M. tuberculosis from NTM. In addition, current American Thoracic Society/Infectious Diseases Society of America guidelines recommend that “clinically significant NTM isolates should be routinely identified to the species level” (13). In this study, we compared the Biotyper, Saramis, and Vitek MS v3.0 MALDI-TOF MS methods for the identification of mycobacteria cultivated on solid media. We demonstrated that all three methods provide accurate and reliable identification to the complex and/or species level for the vast majority of mycobacterial clinical isolates. Importantly, all three MALDI-TOF MS methods had 100% accuracy for the identification of the 16 M. tuberculosis complex isolates analyzed in this study. This suggests that MALDI-TOF MS may substitute for current M. tuberculosis identification methods, including those using DNA probes.
MALDI-TOF MS is commonly used for routine bacterial identification in clinical laboratories. However, mycobacteria require a protein extraction procedure for organism inactivation and optimal identification. The Biotyper extraction method requires approximately 90 min for preparation of 18 samples compared to approximately 45 min for a similar number of samples using the Vitek MS protocol. Therefore, we assessed whether the extraction methods were interchangeable on the respective platforms. While deviation from each manufacturer's recommended extraction method did not result in misidentifications, it had a negative impact on the frequency of identification and score/confidence values for the Biotyper and Saramis. The Vitek MS v3.0 platform appeared relatively unaffected by the extraction method, at least for the 10 isolates assessed. This emphasizes that clinical samples should be processed using the method used to generate the reference database used for analysis (8).
Despite the reliability of all three methods to distinguish M. tuberculosis from NTM, a small number of sporadic errors were noted with the various MS methods. All three methods misclassified the one M. parascrofulaceum isolate as M. scrofulaceum. The maximal score for this isolate was 1.7 on Biotyper, while Saramis and Vitek MS v3.0 both reported a maximum confidence value of 99.9%. This isolate was confirmed by 16S rRNA and rpoB gene sequencing (14), suggesting that current MALDI-TOF MS methods may have difficulty distinguishing M. scrofulaceum and M. parascrofulaceum. Testing of additional related organisms is needed to further characterize this observation.
The Biotyper reproducibly identified two M. smegmatis isolates as M. wolinskyi. However, due to the phylogenetic relationship, all three isolates were classified as belonging to the M. smegmatis group in this study (15). In addition, the Biotyper reproducibly classified one M. marinum isolate as M. shottsii, which has been reported as a pathogen of striped bass but not as a human pathogen (16). M. shottsii is nonpigmented, while the isolate tested was pigmented, as is M. marinum, suggesting the possibility of an error in the classification of this entry in the Biotyper reference database. An additional limitation of the Biotyper method was the suboptimal scores for M. chelonae isolates, with only 2/13 identified above a score of ≥1.8. Lowering the score threshold of M. chelonae would have resulted in the clinically significant misclassification of two isolates as M. abscessus and M. immunogenum. Mather et al. previously reported suboptimal identification of M. chelonae by the Biotyper, with only 7/14 isolates correctly identified at a score threshold of ≥2.0 (8). The frequency of correct identification was not affected by the length of cultivation prior to testing (8). The extraction method and Biotyper RTC software version were the same as those used here, while the mycobacterial reference database differed (8). In contrast, Balada-Llasat et al. reported correct identification of 23/24 M. chelonae isolates at a score threshold of ≥2.0 (9). The extraction method and mycobacterial database were the same as used here, while the Biotyper RTC software version differed. Therefore, the discrepancies among the three studies may have been due to different software versions, reference databases, or isolate-specific variations or to a combination of these factors.
The Saramis method adequately identified the vast majority of isolates, including all 13 M. chelonae isolates. However, unlike the Biotyper, the Saramis method resulted in no identification for 6/13 M. terrae isolates. Four of these M. terrae isolates were completely unidentified. One of the six isolates was identified as M. fortuitum at a confidence value of 86%. The sixth isolate was identified as two different Gram-positive bacterial species in two independent experiments at scores between 80% and 90%, which would have resulted in the clinical laboratory questioning the identification and commencing troubleshooting steps. Furthermore, at a score cutoff of ≥80%, Saramis misidentified an M. fortuitum isolate and an M. simiae isolate as Streptococcus anginosus and Hanseniaspora guilliermondii, respectively.
Other than the misidentified M. parascrofulaceum isolate, the Vitek MS v3.0 method did not misclassify any isolate at above a score of ≥80%. In addition, while the identification of M. terrae isolates by Vitek MS v3.0 was still suboptimal relative to the Biotyper method, it was improved relative to Saramis. Our panel contained one isolate previously identified as M. mageritense (grouped in the M. smegmatis group in this study) (17). This isolate was correctly identified by both Biotyper and Saramis, while no identification was provided by Vitek MS v3.0. Testing of other M. mageritense isolates on the Vitek MS v3.0 platform is warranted. Finally, Vitek MS v3.0 was the only method to identify the single M. szulgai isolate examined in this investigation.
This study had a number of strengths. First, we evaluated a diverse and large collection of contemporary clinical isolates, including rare NTM, many of which have not been included in any previously published MALDI-TOF MS identification studies (8, 9, 18). Second, we directly compared the performances of the Biotyper and Vitek MS instruments. Third, this report represents the first description of the Vitek MS v3.0 platform for identification of Mycobacterium spp. and suggests that the Vitek MS v3.0 may offer modest advantages over the Biotyper and Saramis, especially by reducing the necessity of repeat identification attempts. Finally, we compared commercial mycobacterial reference databases, rather than laboratory-developed databases, which enables generalizability of our findings to other clinical laboratories.
This study had several limitations. First, we assayed only isolates cultivated on solid medium. Second, we did not directly study the impact of organism age on identification. In general, isolates were tested at least once during both “early” and “late” growth. We performed the Biotyper and Vitek MS extractions in parallel, so all isolates were assayed at the same stage of growth on both instrumentation platforms. Third, despite our using a large panel of clinically relevant NTM isolates, rare NTM spp. had different levels of representation based on availability. Further work evaluating a larger number of these rare isolates is needed to definitively assess the reliability of identification by MALDI MS. And finally, multidrug-resistant and extensively drug-resistant M. tuberculosis isolates were not evaluated in this study.
In summary, this study demonstrated that the Biotyper, Saramis, and Vitek MS v3.0 systems can all readily and reliably identify mycobacterial species and that commonly used extraction protocols are adequate for organism inactivation. Implementation of MALDI-TOF MS in the clinical laboratory may decrease turnaround time, labor, and marginal cost per identification relative to current methods.
ACKNOWLEDGMENTS
C.-A.D.B. has received research support and speaker honoraria from bioMérieux. bioMérieux provided some of the supplies used in this investigation.
We thank Meghan Wallace, Bobbi Heeren, Fran Wilkinson-Spizzo, and Joan Hoppe-Bauer for their technical assistance and helpful discussions. We acknowledge David Pincus (bioMérieux) for his assistance with analysis of the Vitek-generated spectra on the Vitek MS v3.0 platform and for helpful technical discussions. A subset of supplies to perform the study was provided by bioMérieux as part of a collaborative research agreement.
REFERENCES
- 1.WHO. 2014. Global Tuberculosis Report 2014. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Joint Tuberculosis Committee of the British Thoracic Society. 2000. Management of opportunist mycobacterial infections: Joint Tuberculosis Committee Guidelines 1999. Subcommittee of the Joint Tuberculosis Committee of the British Thoracic Society. Thorax 55:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wayne LG, Engbaek HC, Engel HWB, Froman S, Gross W, Hawkins J, Käppler W, Karlson AG, Kleeberg HH, Krasnow I, Kubica GP, McDurmont C, Nel EE, Pattyn SR, Schröder KH, Showalter S, Tárnok I, Tsukamura M, Vergmann B, Wolinsky E. 1974. Highly reproducible techniques for use in systematic bacteriology in the genus Mycobacterium: tests for pigment, urease, resistance to sodium chloride, hydrolysis of Tween 80, and β-galactosidase. Int J Syst Evol Microbiol 24:412–419. doi: 10.1099/00207713-24-4-412. [DOI] [Google Scholar]
- 4.Wayne LG, Engel HWB, Grassi C, Gross W, Hawkins J, Jenkins PA, Käppler W, Kleeberg WH, Krasnow I, Nel EE, Pattyn SR, Richards PA, Showalter S, Slosarek M, Szabo I, Tárnok I, Tsukamura M, Vergmann B, Wolinsky E. 1976. Highly reproducible techniques for use in systematic bacteriology in the genus Mycobacterium: tests for niacin and catalase and for resistance to isoniazid, thiophene 2-carboxylic acid hydrazide, hydroxylamine, and p-nitrobenzoate. Int J Syst Evol Microbiol 26:311–318. doi: 10.1099/00207713-26-3-311. [DOI] [Google Scholar]
- 5.Butler WR, Guthertz LS. 2001. Mycolic acid analysis by high-performance liquid chromatography for identification of Mycobacterium species. Clin Microbiol Rev 14:704–726, table of contents. doi: 10.1128/CMR.14.4.704-726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lumb R, Lanser JA, Lim IS. 1993. Rapid identification of mycobacteria by the Gen-Probe Accuprobe system. Pathology 25:313–315. doi: 10.3109/00313029309066597. [DOI] [PubMed] [Google Scholar]
- 7.Yam WC, Yuen KY, Kam SY, Yiu LS, Chan KS, Leung CC, Tam CM, Ho PO, Yew WW, Seto WH, Ho PL. 2006. Diagnostic application of genotypic identification of mycobacteria. J Med Microbiol 55:529–536. doi: 10.1099/jmm.0.46298-0. [DOI] [PubMed] [Google Scholar]
- 8.Mather CA, Rivera SF, Butler-Wu SM. 2014. Comparison of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of mycobacteria using simplified protein extraction protocols. J Clin Microbiol 52:130–138. doi: 10.1128/JCM.01996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balada-Llasat JM, Kamboj K, Pancholi P. 2013. Identification of mycobacteria from solid and liquid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry in the clinical laboratory. J Clin Microbiol 51:2875–2879. doi: 10.1128/JCM.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JH, Yam WC, Ngan AH, Fung AM, Woo WL, Yan MK, Choi GK, Ho PL, Cheng VC, Yuen KY. 2013. Advantages of using matrix-assisted laser desorption ionization-time of flight mass spectrometry as a rapid diagnostic tool for identification of yeasts and mycobacteria in the clinical microbiological laboratory. J Clin Microbiol 51:3981–3987. doi: 10.1128/JCM.01437-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pence MA, McElvania TeKippe E, Wallace MA, Burnham CA. 2014. Comparison and optimization of two MALDI-TOF MS platforms for the identification of medically relevant yeast species. Eur J Clin Microbiol Infect Dis 33:1703–1712. doi: 10.1007/s10096-014-2115-x. [DOI] [PubMed] [Google Scholar]
- 12.Rychert J, Burnham CA, Bythrow M, Garner OB, Ginocchio CC, Jennemann R, Lewinski MA, Manji R, Mochon AB, Procop GW, Richter SS, Sercia L, Westblade LF, Ferraro MJ, Branda JA. 2013. Multicenter evaluation of the Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of Gram-positive aerobic bacteria. J Clin Microbiol 51:2225–2231. doi: 10.1128/JCM.00682-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 14.Turenne CY, Cook VJ, Burdz TV, Pauls RJ, Thibert L, Wolfe JN, Kabani A. 2004. Mycobacterium parascrofulaceum sp. nov., novel slowly growing, scotochromogenic clinical isolates related to Mycobacterium simiae. Int J Syst Evol Microbiol 54:1543–1551. doi: 10.1099/ijs.0.02940-0. [DOI] [PubMed] [Google Scholar]
- 15.Adékambi T, Drancourt M. 2004. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int J Syst Evol Microbiol 54:2095–2105. doi: 10.1099/ijs.0.63094-0. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes MW, Kator H, Kotob S, van Berkum P, Kaattari I, Vogelbein W, Quinn F, Floyd MM, Butler WR, Ottinger CA. 2003. Mycobacterium shottsii sp. nov., a slowly growing species isolated from Chesapeake Bay striped bass (Morone saxatilis). Int J Syst Evol Microbiol 53:421–424. doi: 10.1099/ijs.0.02299-0. [DOI] [PubMed] [Google Scholar]
- 17.Brown BA, Springer B, Steingrube VA, Wilson RW, Pfyffer GE, Garcia MJ, Carmen Menendez M, Rodriguez-Salgado B, Jost KC Jr, Chiu SH, Onyi GO, Böttger EC, Wallace RJ Jr. 1999. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int J Syst Evol Microbiol 49(Pt 4):1493–1511. doi: 10.1099/00207713-49-4-1493. [DOI] [PubMed] [Google Scholar]
- 18.Buchan BW, Riebe KM, Timke M, Kostrzewa M, Ledeboer NA. 2014. Comparison of MALDI-TOF MS with HPLC and nucleic acid sequencing for the identification of Mycobacterium species in cultures using solid medium and broth. Am J Clin Pathol 141:25–34. doi: 10.1309/AJCPBPUBUDEW2OAG. [DOI] [PubMed] [Google Scholar]