Abstract
Background
Resting brain spontaneous neural activities across cortical regions have been correlated with specific functional properties in psychiatric groups. Individuals with Internet gaming disorder (IGD) demonstrate impaired executive control. Thus, it is important to examine executive control networks (ECNs) during resting states and their relationships to executive control during task performance.
Methods
Thirty-five IGD and 36 healthy control participants underwent a resting-state fMRI scan and performed a Stroop task inside and outside of the MRI scanner. Correlations between Stroop effect and functional connectivity among ECN regions of interest (ROIs) were calculated within and between groups.
Results
IGD subjects show lower functional connectivity in ECNs than do HC participants during resting state; functional-connectivity measures in ECNs were negatively correlated with Stroop effect and positively correlated with brain activations in executive-control regions across groups. Within groups, negative trends were found between Stroop effect and functional connectivity in ECNs in IGD and HC groups, separately; positive trends were found between functional connectivity in ECNs and brain activations in Stroop task in IGD and HC groups, separately.
Conclusions
Higher functional connectivity in ECNs may underlie better executive control and may provide resilience with respect to IGD. Lower functional connectivity in ECNs may represent an important feature in understanding and treating IGD.
Keywords: Executive control network, resting state fMRI, functional connectivity, Internet gaming disorder, behavioral addiction
1. Introduction
Internet addiction disorder (IAD) or problematic Internet use is defined as the excessive or uncontrolled use of the Internet with negative consequences to psychological, social, and/or work functioning aspects (Young, 1998, Dong and Potenza, 2014). It has been proposed as a diagnostic entity and studied for more than a decade; however, a standardized definition is only recently emerging, and this disorder was not included in the fourth edition of the Diagnostic and Statistical Manual (DSM) (Block, 2008, Shaw and Black, 2008, Liu et al., 2011). The DSM-5 committee considering substance-use disorders and addictions generated criteria for Internet gaming disorder (IGD, a subtype of IAD, refers to those who addicted to Internet online games), and this condition is included in the DSM's section 3, for disorders warranting additional study (American Psychiatric Association, 2013, Petry and O'Brien, 2013). People's online experience may change their cognitive function in a manner that may perpetuate Internet use, which may occur in the absence of drug-taking (Holden, 2001, Weinstein and Lejoyeux, 2010, Dong et al., 2011b), and individuals with certain brain and behavioral characteristics may have increased dispositions for developing IAD. However, the precise mechanisms underlying IAD are not well understood (Yau et al., 2012).
Internet addiction may consist of multiple subtypes; for example, subtypes relating to gaming, sexual preoccupations, and email/text messaging have been proposed (Block, 2008). In China, arguably the most important subtype of IAD may be IGD (Dong et al., 2011a, 2012, Dong et al., 2013c). One key feature of IGD is lost or diminished self-control over participation in Internet game-playing. Executive function enables individuals to inhibit their desires and limit engagement in hedonic behaviors under unfavorable circumstances (Everitt et al., 2007, Goldstein and Volkow, 2011, Dong et al., 2013a, Sofuoglu et al., 2013, Lin et al., 2014a). Behavioral and/or brain differences between IGD and healthy controls have been observed during task performance on go/no-go (Dong et al., 2010), attention bias (Ko et al., 2013), set-shifting (Zhou et al., 2012, Dong et al., 2014), Stroop (Dong et al., 2011c), and error-processing (Dong et al., 2013c) tasks. Together, these data provide a neurobiological explanation for behavioral control difficulties that individuals with IGD often exhibit.
Although executive control tasks performed during imaging are important for observing effects of IGD within circumscribed brain areas (Dong et al., 2010, 2011c, Zhou et al., 2012, Dong et al., 2014, Lin et al., 2014b), additional insight may be obtained by measuring alterations in interactions among brain regions (Chambers et al., 2003, Koob and Volkow, 2010, Soderpalm and Ericson, 2013). Neural activities in the human brain during rest, termed resting-state functional magnetic resonance imaging, have found that spontaneous neural activities are correlated across cortical regions in a non-random fashion (Fox and Raichle, 2007, Greicius et al., 2009, Zhu et al., 2011). These correlations may reflect functional connectivity (FC) among specific brain regions (Vincent et al., 2007, Honey et al., 2009). Studies on the functional connectivity in IAD subjects have already revealed some abnormal features. Hong et al., found a widespread and decrease of functional connectivity in the cortico-subcortical circuit (∼24% with prefrontal and ∼27% with parietal cortex) in 12 adolescents who were diagnosed with IAD (Hong et al., 2013); Li et al., found ineffective connectivity in the frontal-basal ganglia pathway in IAD adolescents, which was thought engaged in response inhibition (Li et al., 2014); Wee et al., found significant disruption in the functional connectome between regions located in the frontal, occipital, and parietal lobes in IAD subjects (Wee et al., 2014). Despite findings indicating FC across cortical regions, little is known about the psychological significance of these relationships, especially in clinical diagnoses like IGD.
In this study, we examine FC relationships in executive control networks (ECNs) during resting state and behavioral and neural measures of executive control (during Stroop performance) in 35 subjects with IGD and 36 healthy control (HC) subjects. The temporal binding model suggests that the synchronization of brain signals between neural systems is facilitates neural communications (Engel et al., 2001). Consistent with this model, resting brain activity can relate importantly to behavioral performance. For example, resting-state activity across face-selective cortical regions has been correlated with behavioral performance in a face performance task (Zhu et al., 2011). Individual differences in attitudes toward risk-taking have been related to the brain FC and may have implications for engaging in real-world risky behaviors (Cox et al., 2010). Thus, we hypothesized that executive control function might be indexed by resting-state brain activities in ECNs. To test this hypothesis, we examined correlations in spontaneous blood oxygen level dependence (BOLD) fluctuations among ECN brain regions during resting state and Stroop-related behavioral performance and brain activities.
Resting-state FC has been shown to differ between groups or as a function of clinically relevant measures. Performance deficits in cognitive control in drug-addicted individuals have been associated with reduced FC (Honey et al., 2009, Franken et al., 2010). Amygdala/medial-prefrontal-cortical connectivity at rest has been related to individual differences in anxiety (Kim et al., 2011). The aftermath of acute stress has been related to prolonged activation in an amygdala-related connectivity network (van Marle et al., 2010). Trauma victims (individuals exposed to earthquakes) show reduced temporal synchronization within the ‘default mode’ of resting-state brain function (Lui et al., 2009). Reduced FC between seeds within ECNs is consistent with behavioral and task-based imaging findings and self-reported cognitive deficits in drug-addicted populations (Ersche et al., 2006, Kelly et al., 2011). IGD subjects show impaired executive control ability relative to HC subjects (Dong et al., 2010, 2011c, Zhou et al., 2012, Dong et al., 2013b, Ko et al., 2013). Thus, we hypothesized that the impaired executive control in IGD would relate to decreased resting-state FC. To test this hypothesis, we examined between-group differences in FC in ECNs and examined whether FC related to brain activations and behavioral performance on the Stroop task.
2. Methods
Participant Selection
The experiment conforms to The Code of Ethics of the World Medical Association (Declaration of Helsinki). The Human Investigations Committee of Zhejiang Normal University approved this research. Participants were university students and were recruited through advertisements. Participants were right-handed males (35 IGD and 36 HC subjects). IGD and HC groups did not significantly differ in age (IGD mean (SD)=22.21(3.08) years; HC mean (SD)=22.81(2.36) years; t=0.69, p=0.49). All subjects participated in a resting-state fMRI scan. 31 (16 IGD, 15 HC) performed a Stroop task in the scanner. The other 40 subjects (19 IGD, 21HC) performed the same Stroop task outside of the scanner. Only males were included due to higher IGD prevalence in men than in women.
All participants provided written informed consent and underwent structured psychiatric interviews (MINI) (Lecrubier et al., 1997) performed by an experienced psychiatrist. The MINI was designed to meet the need for a short but accurate structured psychiatric interview for multicenter clinical trials and epidemiology studies. All participants were free of Axis-I psychiatric disorders assessed by the MINI (depression, anxiety disorder, schizophrenia, and substance dependence). Depression was further assessed with the Beck depression inventory (Beck et al., 1961) and only participants scoring less than 5 were included (IGD, 3.1±0.53; HC, 2.4±0.42). IGD and HC subjects did not fulfill DSM-IV criteria for abuse or dependence of any substances, although all IGD and HC participants reported some lifetime alcohol consumption. All participants were medication-free and were instructed not to use any substances, including coffee, on the day of scanning. IAD was determined based on Young's online internet addiction test (IAT) (Young, 2009) scores of 50 or higher (In this study, IAT score in IGD group:64.4±6.5; IAT score in HC: 22.8±4.7). Young's IAT consists of 20 items associated with online Internet use including psychological dependence, compulsive use, withdrawal, related problems in school or work, sleep, family or time management. The IAT has demonstrated validity and reliability and may be used in classifying IAD (Widyanto and McMurran, 2004, Widyanto et al., 2011). For each item, a graded response is selected from 1 = “Rarely” to 5 = “Always”, or “Does not Apply”. Scores over 50 indicate occasional or frequent Internet-related problems and scores over 80 indicate significant problems (www.netaddiction.com). To classify IGD, individuals with IAD also needed to respond positively to the following question: ‘you spend most of your online time playing games (>80%) (Yes, No)’.
Task and Scanning
Magnetic resonance imaging data were acquired using a Siemens Trio 3T scanner (Siemens, Erlangen, Germany) in East-China Normal University (Shanghai, China). The whole scanning process consisted of two parts: a resting state scan lasts for 7 minutes and a Stroop task lasts for 15 minutes.
Imaging Parameters
Structural images were collected using a T1-weighted three-dimensional spoiled gradient-recalled sequence covering the whole brain (176 slices, repetition time=1700 ms, echo time TE=3.93 ms, slice thickness=1.0 mm, skip=0 mm, flip angle=15, inversion time 1100ms, field of view=240*240mm, in-plane resolution=256* 256). Functional MRI was performed on a 3T scanner (Siemens Trio) with a gradient-echo EPI T2 sensitive pulse sequence in 33 slices (interleaved sequence, 3mm thickness, TR=2000ms, flip angle 90°, field of view 220 × 220 mm2, matrix 64 × 64). Stimuli were presented using Invivo synchronous system (Invivo Company, www.invivocorp.com/) through a screen in the head coil, enabling participants to view the stimuli.
Resting-state
The ‘resting-state’ was defined as no specific cognitive task during the fMRI scan. Participants were instructed to remain still, close their eyes and not to think of anything systematically (Zang et al., 2004, You et al., 2011). Participants were supine with their heads snugly fixed by a belt and foam pads to minimize head movement. Scanning lasted 7 minutes.
The Stroop Task
Inhibitory control is typically studied using paradigms that require overcoming conflict. In this study, we used the Stroop task, which consists of color words printed in colored ink and instructs participants to ignore the word and name its color. In congruent trials, the word and color match; in incongruent trials, the word and color do not match and the conflict between the word and color must be resolved before making a correct response. Overcoming this conflict needs longer time compared to congruent trials. The ‘Stroop effect’ is typically calculate time as incongruent trials minus congruent ones. The magnitudes of Stroop effects are used as an index of executive control abilities, reflecting the success in detecting, and resolving cognitive conflict.
An event-related Stroop color-word interference task was used. Three target color words (e.g. red, green, yellow) were presented in congruent (e.g., the word “RED” in red ink) or incongruent (e.g., the word “RED” in green ink) trials. The task was comprised of 2 sessions of 120 trials each. Each stimulus was presented for 2000ms. Participants were asked to press a button to indicate to the ink color of the word as soon as possible using three buttons (i.e., green=thumb, red=index finger, yellow=middle finger; counter-balanced between subjects) of a five-button response box (Invivo Corp.; http://www.invivocorp.com/). A black screen was presented for a random interval of 600–2400ms (average 1500ms) between trials. Stimuli were presented and behavioral data were collected using E-prime software (Psychology Software Tools, Inc.). Participants were told that they would be paid a guaranteed 50 Yuan (≈8 US$) for participation and, to encourage quick and accurate task performance, were told they would be rewarded with an additional 0–50 Yuan based on their task performance [1/(reaction time * error rate)]. Participants completed an out-of-scanner practice session which continued until they reached an accuracy rate of 90% or higher.
Data analysis in REST
Resting-state data were analyzed using REST and DPARSF (http://restfmri.org) (Yan and Zang, 2010). Preprocessing consisted of removal of the first 10 time points (due to signal equilibrium and to allow the participants to adapt to the scanning noise), physiological correction, slice timing, volume registration and head motion correction. Possible contamination from several nuisance signals, including the signal of white matter, cerebral spinal fluid, global signal, and six motion vectors, were regressed out. The time series of images of each subject were motion-corrected using a least squares approach and a six-parameter (rigid body) linear transformation (Friston et al., 1995). The individual structural image was co-registered to the mean functional image after motion correction using a linear transformation. The motion-corrected functional volumes were spatially normalized to the Montreal Neurological Institute (MNI) space and re-sampled to 3mm isotropic voxels using the normalization parameters estimated during unified segmentation. Further preprocessing included band-pass filtering between 0.01 and 0.08 Hz. To assess FC, we first calculated the Pearson's correlation coefficient between the mean signal intensity time courses of each region-of-interest (ROI) pair. A Fisher's r-to-z transformation was applied to each correlation map to obtain an approximately normal distribution of the FC values and to accordingly apply parametric statistics.
ROI selection
An ECN has been shown to have separable right and left hemisphere components (Damoiseaux et al., 2006, Habas et al., 2009, Shirer et al., 2012, Krmpotich et al., 2013). Thus, left executive control network (LECN) and right executive control network (RECN) templates were downloaded from Stanford's Functional Imaging in Neuropsychiatric Disorders lab (Shirer et al., 2012) (http://findlab.stanford.edu/functionalROIs.html). Seeds were chosen a priori based on published findings (Mansouri et al., 2009, Duncan, 2013) rather than deriving seed regions from the following Stroop task so as best to avoid bias and to increase the generalizability of findings. We selected ROIs from their templates: ventromedial prefrontal cortex (vmPFC), dorsolateral prefrontal cortex (dlPFC), and parietal cortex. Resting-state fMRI studies in addicted groups also found that FC among these regions was related to executive function (Ma et al., 2010, Yuan et al., 2010, Kelly et al., 2011). Functional connections among different ROIs were analyzed in the left and right ECNs, separately. The connections between left and right ECNs (hemispheric ECN - HECN) and the FC among all ROIs (total ECN - TECN) were also calculated. For each ROI, a representative BOLD time course was obtained by averaging the signal of all the voxels within the ROI.
Data analysis in Stroop task
The functional data were analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm), and Neuroelf (http://neuroelf.net) as described previously (DeVito et al., 2012, Krishnan-Sarin et al., 2013). Images were slice-timed, reoriented, and realigned to the first volume, with T1-co-registered volumes used to correct for head movements. Images were then normalized to MNI space and spatially smoothed using a 6mm FWHM Gaussian kernel. A general linear model (GLM) was applied to identify BOLD activation in relation to separate event types. Six head-movement parameters derived from the realignment stage were included to exclude motion related variances. A GLM approach was used to identify voxels that were significantly activated for the each event that was modeled.
Second level analysis treated inter-subject variability as a random effect. First, we determined voxels showing a main effect in incongruent and congruent conditions. Second, we tested for voxels that showed higher or lower activity in the contrasts of interest (incongruent-congruent). Third, we compared these two groups in the comparisons (IGD-HC). We first identified clusters of contiguously significant voxels at an uncorrected threshold p<0.05, as also used for display purposes in the figures. We then tested these clusters for cluster-level FWE correction p<0.05 and the AlphaSim estimation indicated that clusters with 102 contiguous voxels would achieve an effective FWE threshold p<0.05. The smoothing kernel used during simulating false-positive (noise) maps using AlphaSim was 6mm, and was estimated from the residual fields of the contrast maps being entered into the one-sample t-test. The formula used to compute the smoothness is that used in FSL (see http://www.fmrib.ox.ac.uk/analysis/techrep/tr00df1/tr00df1/node6.html for more information).
Correlation analysis between FC during rest and behavioral/brain Stroop task performance
We first compared the brain activation between IGD and HC groups and then took the clusters that survived as ROIs for further analysis. For each ROI, a representative BOLD beta value was obtained by averaging the signal of all the voxels within the ROI. Correlation analyses were calculated between FC in ECNs (identified using REST (http://restfmri.org)) and brain/behavioral Stroop task performance.
3. Results
FC differences in ECNs in IGD and HC subjects
FC was calculated among ECN ROIs in different networks (Figure 1a). A two-way ANOVA of FC (Hemisphere, ECN ROIs) shows significant hemisphere effect (F(1,71)=11.32, p<0.01). This finding is consistent with previous data indicating that the ECN has separable right and left hemisphere components (Damoiseaux et al., 2006, Habas et al., 2009, Shirer et al., 2012). Thus, we separated the whole ECN into left (LECN) and right ECN (RECN). Additionally, we calculated the FC between left and right hemisphere ECN ROIs (HECN); and the total value of all these ECNs (TECN) (mean value of LECN, RECN, and HECN). To simplify the analyses, a representative FC value was obtained by averaging the different FCs between ECN ROIs. For example, in the left ECN, there are three ROIs; thus, there should be three FC among these ROIs. We used the mean value of these three FCs for further analysis.
Figure 1. The brain regions in ECNs and the FC between IGD and HC groups.
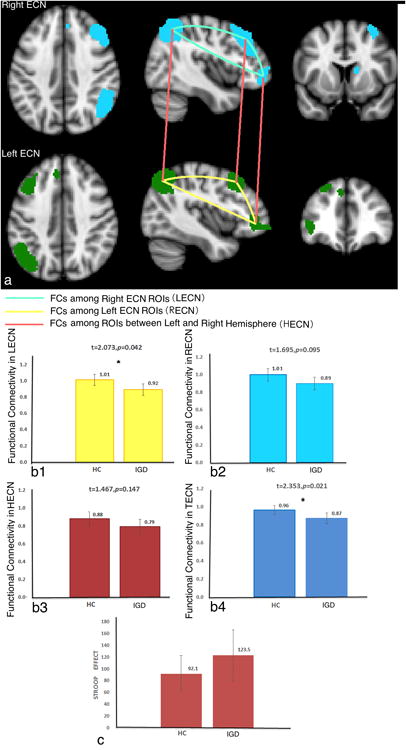
a, The ROIs that were used in left and right ECNs and the FC calculated among ROIs are displayed. For each ROI, a representative BOLD time course was obtained by averaging the signal of all the voxels within the ROI. The ECN templates were downloaded from Stanford's Functional Imaging in Neuropsychiatric Disorders lab (http://findlab.stanford.edu/functionalROIs.html).
b1-b4, The FC values between IGD and HC subjects are displayed in different ECNs. We used four ECNs in our study: LECN, RECN, HECN, and TECN.
c, The Stroop effect between IGD and HC groups is displayed.
Figure 1b shows the values of FCs in different ECNs in IGD and HC subjects. From this figure, we can find that IGD relative to HC subjects show decreased FC in TECN, with LECN seemingly contributing most to this effect and RECN more marginally.
Correlations between FC in ECNs and Stroop effect
Figure 1c shows the Stroop effect (RT in incongruent-congruent) in IGD and HC groups. The left row of Figure 2 shows the correlations between FC in different networks and the Stroop effect in all subjects. Marginally significant negative correlations were found between Stroop effect RTs and TECN (p=0.063), with marginal contributions from both LECN (p=0.056) and RECN (p=0.090). Largely similar patterns were observed in IGD and HC subjects with respect to correlational patterns in TECN, as well as in LECN and RECN (right column in Figure 2).
Figure 2. Correlations between FC in different ECNs and Stroop effect.
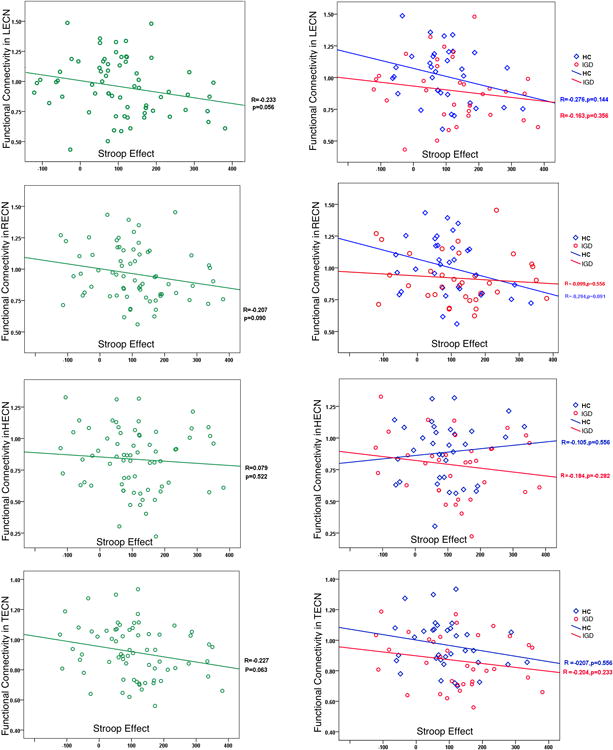
Left row, correlations between FC in different ECNs and Stroop effect in the total sample are displayed.
Right row, correlations between FC in different ECNs and Stroop effect in IGD and HC subjects, separately, are displayed.
Imaging results in Stroop task when comparing IGD to HC
As compared to HC subjects, IGD subjects show relatively increased brain activations in bilateral superior frontal gyrus (BA 8) and decreased activations in dorsolateral prefrontal cortex (BA 9), ventral anterior cingulate cortex (BA 24), and left orbitofrontal cortex (BA 11) (Figure 3a; Table 1). The majority of regions have all been implicated with executive control to varying degrees (see reviews (Mansouri et al., 2009, Duncan, 2013)). We select the identified clusters as ROIs to index brain activations of executive control, with the exception of the superior frontal gyrus given its potential contribution to self-awareness and sensory system more so than executive control (Goldberg et al., 2006) and no significant correlations were found between the activation of this cluster and ECN or behavioral performances. The differences in IGD and HC subjects were related to lower brain activation during incongruent trials in IGD subjects (Figure 3b). Figure 3c shows that the brain activation in Stroop task is negatively correlated with Stroop effect, suggesting that greater regional brain activation is associated with better executive control performance.
Figure 3. Clusters surviving after comparing IGD to HC subjects on Stroop task performance.
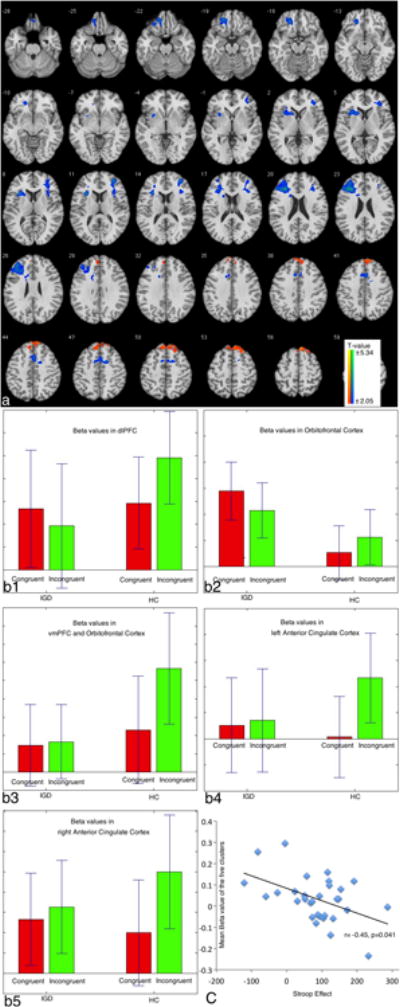
a, The brain map of surviving clusters when comparing IGD to HC subjects is shown.
b1-b5, Beta values of the surviving clusters in incongruent and congruent trials in IGD and HC groups.
c, Correlation between Stroop effect and beta values in the five surviving clusters.
Table 1.
Regional brain activity changes in different comparisons.
| x, y, za | Hemisphere | Peak intensity | Number of voxelsb | Regionc | Brodmann's area |
|---|---|---|---|---|---|
| IGD–HC (incongruent–congruent) (lower activated) | |||||
| −30, 32, 31 | L | −4.575 | 383 | Dorsolateral prefrontal cortex | 9 |
| −18, 45, -21 | L | −3.710 | 121 | Orbitofrontal cortex | 11 |
| 33, 48, 12 | R | −3.605 | 146 | Orbitofrontal cortex, ventro-lateral prefrontal cortex | 10, 45 |
| −10, 8, 48 | L | −2.911 | 108 | Anterior cingulate cortex | 33 |
| 15, 6, 48 | R | −3.374 | 113 | Anterior cingulate cortex | 32, 33 |
| IGD–HC (incongruent–congruent) (higher activated) | |||||
| 6, 36, 57 | R | 4.193 | 216 | Superior frontal cortex | 8 |
Peak MNI coordinates.
We first identified clusters of contiguously significant voxels at an uncorrected threshold p < 0.05, as also used for display purposes in the figures. We then tested these clusters for cluster-level FWE correction p < 0.05 and the AlphaSim estimation indicated that clusters with 102 contiguous voxels would achieve an effective FWE threshold p < 0.05. Voxel size = 3 * 3 * 3.
The brain regions were referenced to the software Xjview (http://www.alivelearn.net/xjview8) and double checked with atlas.
Correlations between FC during rest and brain activations during Stroop task performance
To examine whether the FC during resting state is related to task-related brain activations, we calculated correlations between FC and the beta values in Stroop task. We examined the five surviving clusters as task-related ROIs and used their mean value as a brain activation index in these analyses.
Figure 4 (left row) shows the correlations between FC and mean beta values of the 5 task-related ROIs in all subjects. From this figure, we observed a significant correlation between FC in TECN, LECN, and HECN and beta values in the task-related ROIs. When examining the IGD and HC groups (right column in Figure 4), similar patterns were observed for the two groups in the TECN, LECN and HECN.
Figure 4. Correlations between FC in different ECNs and brain activations in Stroop task.
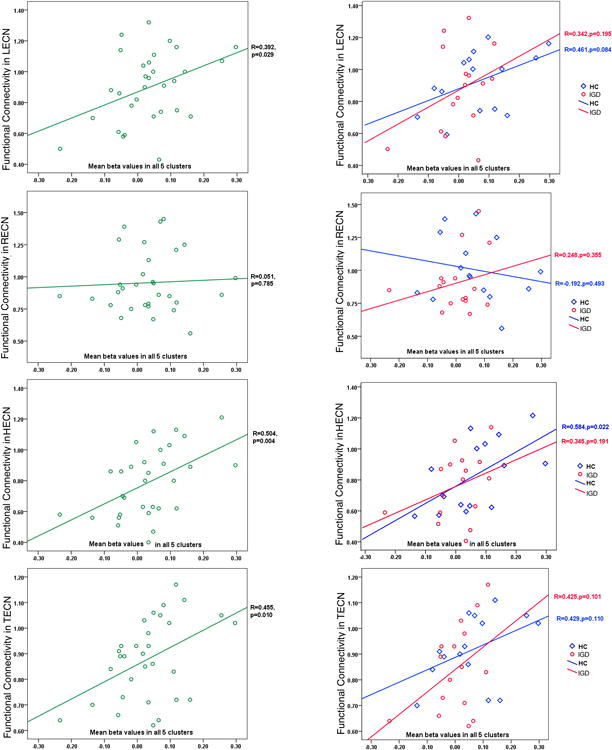
For each ROI, a representative BOLD beta value was obtained by averaging the signal of all the voxels within the ROI.
Left row, Correlations between FC and beta values in all decreased ROIs in the total sample are displayed.
Right row, Correlations between FC and beta values in all decreased ROIs in IGD and HC subjects, separately, are displayed.
4. Discussion
This study investigated whether synchronized spontaneous neural activity across ECNs is related to the cognitive control executive functioning and may relate to poor executive control in IGD. The results showed that the FC in different ECN ROIs are correlated with individuals' performance in a variety of ways, suggesting that synchronized spontaneous neural activities in ECN is behaviorally relevant.
FC in ECNs during resting state is related to Stroop task performance
When observing the correlation between FC and behavioral performance in all subjects, we found the FC is negatively correlated with the Stroop effect, with a higher Stroop effect suggesting lower executive control. In addition, FC is positively correlated with the brain activations during Stroop task performance. Previous studies have found that the FC in specific networks relates to relevant behavioral performance (Seeley et al., 2007, Spreng et al., 2010, Krmpotich et al., 2013). In the current study, the correlations between FC during resting state and task performance (both behaviorally and neurally) suggest that FC in ECNs is relevant to executive functioning: people who show higher FC among ECN ROIs at rest show better executive control functioning during task performance.
Prior fMRI studies have identified multiple executive control regions in the brain (see reviews (Mansouri et al., 2009, Duncan, 2013)). Our study suggests that coordinating multiple regions relies on synchronized neural activity (Fries, 2009), and relative deficiencies in synchronized connectivity among ECN regions parallels impaired executive control functioning (Sutherland et al., 2012). The result suggests that typical executive process might depend on the interactions among ECN regions (Liang et al., 2013).
We were intent to find the correlations between ECN in rest and their real performance in task in IGD subjects, however, from these figures, we can find the similar correlations were also existed in HC groups, which might suggest the ECN in rest and their correlation with executive control ability in task is a common feature in all subjects.
Decreased FC in ECN at rest: an important feature for IGD
Although individual differences in FC appeared to relate largely similarly to Stroop performance and Stroop-related activations, between-group differences were also observed between individuals with and without IGD. Specifically, IGD relative to HC subjects show decreased FC in ECNs. As correlation analyses indicate that when the FC increases, Stroop-related brain activations and executive control function increase, the results together raise the possibility that FC in ECNs during resting state may represent an important feature in indexing impaired executive control in IGD.
The result that IGD subjects show relatively decreased FC is consistent with findings in substance addictions. Difficulties in inhibiting drug-related behaviors may represent a failure of executive control (Barros-Loscertales et al., 2011, Volkow et al., 2011, Krmpotich et al., 2013). Data suggest that performance decrements on cognitive-control tasks in drug-addicted individuals are associated with reduced functional engagement (Li and Sinha, 2008, Hester et al., 2009, Franken et al., 2010). Neuroimaging studies have demonstrated that FC in ECNs is associated with approaching behaviors in substance dependence (Krmpotich et al., 2013), and abnormal connectivity between ECN seeds were observed in both heroin- and cocaine-addicted samples (Ma et al., 2010, Yuan et al., 2010, Kelly et al., 2011). Taken together with the current findings, that the data suggest that decreased FC in ECNs may represent an important feature in indexing impaired executive control across substance and behavioral addictions.
Limitations
Several limitations should be noted. First, as few women exhibit IGD, only selected male subjects were included in this study. Given gender-related differences in behavioral and substance addictions, future studies should examine the extent to which findings might generalize to women with IGD. Second, due to funding limitations, some but not all participants performed the Stroop task in the scanner. Third, in calculating correlations between FC and behavioral performance or task-related brain activations, some correlations approached but did not reach statistical significance. Future studies involving larger samples are needed. Nonetheless, the current findings represent an important step forward in understanding the neurobiological processes underlying IGD and are consistent with recently proposed neurocognitive models of IGD (Brand et al., 2014, Dong and Potenza, 2014, King and Delfabbro, 2014).
Conclusions
In summary, the current study found that IGD subjects show lower functional connectivity in ECNs than do HC participants during resting state. In addition, positive trends were found between functional connectivity in ECNs and brain activations in Stroop task in IGD and HC groups, separately. All these may represent important features in understanding and treating IGD.
Acknowledgments
This research was supported by National Science Foundation of China (31371023). National Institute of Health (R01 DA035058, P50 DA09241, P20 DA027844), the Connecticut Department of Mental Health and Addictive Services, and the National Center for Responsible Gaming.
Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Lundbeck, Ironwood ad Shire; has received research support from the National Institutes of Health, Mohegan Sun Casino and the National Center for Responsible Gaming; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for gambling entities and law offices on issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Role of the funding Source: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Contributors: Guangheng Dong designed the experiment and wrote the first draft of the manuscript. Xiao Lin collected and analyzed the data, prepared the figures. Marc Potenza discussed the results, advised on interpretation and contributed to the final draft of the manuscript. All authors contributed to and have approved the final manuscript.
Competing Interests: The authors declared that no competing interests exist.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry research. 2011;194:111–118. doi: 10.1016/j.pscychresns.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Block JJ. Issues for DSM-V: internet addiction. The American journal of psychiatry. 2008;165:306–307. doi: 10.1176/appi.ajp.2007.07101556. [DOI] [PubMed] [Google Scholar]
- Brand M, Young KS, Laier C. Prefrontal control and internet addiction: a theoretical model and review of neuropsychological and neuroimaging findings. Frontiers in human neuroscience. 2014;8:375. doi: 10.3389/fnhum.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. The American journal of psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Gotimer K, Roy AK, Castellanos FX, Milham MP, Kelly C. Your Resting Brain CAREs about Your Risky Behavior. PloS one. 2010;5 doi: 10.1371/journal.pone.0012296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and alcohol dependence. 2012;122:228–235. doi: 10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Hu Y, Lin X. Reward/punishment sensitivities among internet addicts: Implications for their addictive behaviors. Progress in neuro-psychopharmacology & biological psychiatry. 2013a;46:139–145. doi: 10.1016/j.pnpbp.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Dong G, Hu Y, Lin X, Lu Q. What makes Internet addicts continue playing online even when faced by severe negative consequences? Possible explanations from an fMRI study. Biological psychology. 2013b;94:282–289. doi: 10.1016/j.biopsycho.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Dong G, Huang J, Du X. Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: an fMRI study during a guessing task. Journal of psychiatric research. 2011a;45:1525–1529. doi: 10.1016/j.jpsychires.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Dong G, Huang J, Du X. Alterations in regional homogeneity of resting-state brain activity in internet gaming addicts. Behavioral and brain functions: BBF. 2012;8:41. doi: 10.1186/1744-9081-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Lin X, Zhou H, Lu Q. Cognitive flexibility in internet addicts: fMRI evidence from difficult-to-easy and easy-to-difficult switching situations. Addict Behav. 2014;39:677–683. doi: 10.1016/j.addbeh.2013.11.028. [DOI] [PubMed] [Google Scholar]
- Dong G, Lu Q, Zhou H, Zhao X. Precursor or sequela: pathological disorders in people with Internet addiction disorder. PloS one. 2011b;6:e14703. doi: 10.1371/journal.pone.0014703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Potenza MN. A cognitive-behavioral model of Internet gaming disorder: Theoretical underpinnings and clinical implications. Journal of psychiatric research. 2014;58(1):7–11. doi: 10.1016/j.jpsychires.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Shen Y, Huang J, Du X. Impaired error-monitoring function in people with internet addiction disorder: an event-related FMRI study. European addiction research. 2013c;19:269–275. doi: 10.1159/000346783. [DOI] [PubMed] [Google Scholar]
- Dong G, Zhou H, Zhao X. Impulse inhibition in people with Internet addiction disorder: electrophysiological evidence from a Go/NoGo study. Neuroscience letters. 2010;485:138–142. doi: 10.1016/j.neulet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Dong G, Zhou H, Zhao X. Male Internet addicts show impaired executive control ability: evidence from a color-word Stroop task. Neuroscience letters. 2011c;499:114–118. doi: 10.1016/j.neulet.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Duncan J. The structure of cognition: attentional episodes in mind and brain. Neuron. 2013;80:35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nature reviews Neuroscience. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW. The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Annals of the New York Academy of Sciences. 2007;1121:576–597. doi: 10.1196/annals.1401.022. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Kuijpers I. Evidence for a deficit in the salience attribution to errors in smokers. Drug and alcohol dependence. 2010;106:181–185. doi: 10.1016/j.drugalcdep.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu Rev Neurosci. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. NeuroImage. 1995;2:166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Goldberg II, Harel M, Malach R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron. 2006;50:329–339. doi: 10.1016/j.neuron.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden C. ‘Behavioral’ Addictions: Do They Exist? Science. 2001;294:980–982. doi: 10.1126/science.294.5544.980. [DOI] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SB, Zalesky A, Cocchi L, Fornito A, Choi EJ, Kim HH, Suh JE, Kim CD, Kim JW, Yi SH. Decreased functional brain connectivity in adolescents with internet addiction. PloS one. 2013;8:e57831. doi: 10.1371/journal.pone.0057831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cerebral cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DL, Delfabbro PH. The cognitive psychology of Internet gaming disorder. Clinical psychology review. 2014;34:298–308. doi: 10.1016/j.cpr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Ko CH, Liu GC, Yen JY, Yen CF, Chen CS, Lin WC. The brain activations for both cue-induced gaming urge and smoking craving among subjects comorbid with Internet gaming addiction and nicotine dependence. Journal of psychiatric research. 2013;47:486–493. doi: 10.1016/j.jpsychires.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Balodis IM, Kober H, Worhunsky PD, Liss T, Xu JS, Potenza MN. An Exploratory Pilot Study of the Relationship Between Neural Correlates of Cognitive Control and Reduction in Cigarette Use Among Treatment-Seeking Adolescent Smokers. Psychology of Addictive Behaviors. 2013;27:526–532. doi: 10.1037/a0032479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotich TD, Tregellas JR, Thompson LL, Banich MT, Klenk AM, Tanabe JL. Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug and alcohol dependence. 2013;129:1–7. doi: 10.1016/j.drugalcdep.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, Janavs J, Dunbar GC. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–231. [Google Scholar]
- Li B, Friston KJ, Liu J, Liu Y, Zhang G, Cao F, Su L, Yao S, Lu H, Hu D. Impaired frontal-basal ganglia connectivity in adolescents with internet addiction. Scientific reports. 2014;4:5027. doi: 10.1038/srep05027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neuroscience and biobehavioral reviews. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zou Q, He Y, Yang Y. Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:1929–1934. doi: 10.1073/pnas.1214900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Zhou H, Dong G, Du X. Impaired risk evaluation in people with Internet gaming disorder: fMRI evidence from a probability discounting task. Progress in neuro-psychopharmacology & biological psychiatry. 2014a;56C:142–148. doi: 10.1016/j.pnpbp.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Lin X, Zhou H, Dong G, Du X. Progress in neuro-psychopharmacology & biological psychiatry in press. 2014b. Impaired risk evaluation in people with Internet gaming disorder: fMRI evidence from a probability discounting tasks. [DOI] [PubMed] [Google Scholar]
- Liu TC, Desai RA, Krishnan-Sarin S, Cavallo DA, Potenza MN. Problematic Internet use and health in adolescents: data from a high school survey in Connecticut. The Journal of clinical psychiatry. 2011;72:836–845. doi: 10.4088/JCP.10m06057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Li D, Kuang W, Chan RC, Mechelli A, Sweeney JA, Gong Q. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15412–15417. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. NeuroImage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nature reviews Neuroscience. 2009;10:141–152. doi: 10.1038/nrn2538. [DOI] [PubMed] [Google Scholar]
- Petry NM, O'Brien CP. Internet gaming disorder and the DSM-5. Addiction. 2013;108:1186–1187. doi: 10.1111/add.12162. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M, Black DW. Internet addiction: definition, assessment, epidemiology and clinical management. CNS drugs. 2008;22:353–365. doi: 10.2165/00023210-200822050-00001. [DOI] [PubMed] [Google Scholar]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral cortex. 2012;22:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderpalm B, Ericson M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Current topics in behavioral neurosciences. 2013;13:127–161. doi: 10.1007/7854_2011_170. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernandez G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage. 2010;53:348–354. doi: 10.1016/j.neuroimage.2010.05.070. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee CY, Zhao Z, Yap PT, Wu G, Shi F, Price T, Du Y, Xu J, Zhou Y, Shen D. Disrupted brain functional network in internet addiction disorder: a resting-state functional magnetic resonance imaging study. PloS one. 2014;9:e107306. doi: 10.1371/journal.pone.0107306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein A, Lejoyeux M. Internet Addiction or Excessive Internet Use. Am J Drug Alcohol Ab. 2010;36:277–283. doi: 10.3109/00952990.2010.491880. [DOI] [PubMed] [Google Scholar]
- Widyanto L, Griffiths MD, Brunsden V. A psychometric comparison of the Internet Addiction Test, the Internet-Related Problem Scale, and self-diagnosis. Cyberpsychology, behavior and social networking. 2011;14:141–149. doi: 10.1089/cyber.2010.0151. [DOI] [PubMed] [Google Scholar]
- Widyanto L, McMurran M. The psychometric properties of the internet addiction test. Cyberpsychology & behavior: the impact of the Internet, multimedia and virtual reality on behavior and society. 2004;7:443–450. doi: 10.1089/cpb.2004.7.443. [DOI] [PubMed] [Google Scholar]
- Yan CG, Zang YF. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau YH, Crowley MJ, Mayes LC, Potenza MN. Are Internet use and videogame-playing addictive behaviors? Biological, clinical and public health implications for youths and adults. Minerva psichiatrica. 2012;53:153–170. [PMC free article] [PubMed] [Google Scholar]
- You H, Wang J, Wang H, Zang YF, Zheng FL, Meng CL, Feng F. Altered regional homogeneity in motor cortices in patients with multiple system atrophy. Neurosci Lett. 2011;502:18–23. doi: 10.1016/j.neulet.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Young Internet Addiction: The Emergence of a New Clinical Disorder. CyberPsychology & Behavior. 1998;1:237–244. %U http://online.liebertpub.com/doi/abs/210.1089/cpb.1998.1081.1237. [Google Scholar]
- Young KS. Internet Addiction Test (IAT) 2009 [Google Scholar]
- Yuan K, Qin W, Dong MH, Liu JX, Sun JB, Liu P, Zhang Y, Wang W, Wang YR, Li QA, Zhao LY, von Deneen KM, Liu YJ, Gold MS, Tian J. Gray matter deficits and resting-state abnormalities in abstinent heroin-dependent individuals. Neuroscience letters. 2010;482:101–105. doi: 10.1016/j.neulet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Yuan G, Yao J. Cognitive biases toward Internet game-related pictures and executive deficits in individuals with an Internet game addiction. PloS one. 2012;7:e48961. doi: 10.1371/journal.pone.0048961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zhang JD, Luo YLL, Dilks DD, Liu J. Resting-State Neural Activity across Face-Selective Cortical Regions Is Behaviorally Relevant. J Neurosci. 2011;31:10323–10330. doi: 10.1523/JNEUROSCI.0873-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


