Abstract
Relatively little is known about the effects of hepatocytes on hepatic macrophages, particularly under the situation of endoplasmic reticulum (ER) stress. We examined the effects of hepatocytes conditioned media (CM) from HepG2 treated with ER stress inducers, Tunicamycin (TM) or Thapsigargin (TG), on the secretion of cytokines, expression of ER stress markers and polarization of PMA activated THP-1 cells (pTHP-1). We found that CM decreased the production of the pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and interleukin (IL)-1β as well as other cytokines and chemokines from pTHP-1 cells. These effects are mediated by the inhibition of TLR4 expression and NF-κB signaling pathway. In addition, hepatocytes CM increased the expression of binding immunoglobulin protein (BiP) and the transcription factor C/EBP homologous protein (CHOP) in pTHP-1 cells. Preconditioning with ER stress inhibitor, small molecular chaperone 4-phenylbutyrate (PBA) before addition of ER stressors, attenuated the ER stress in macrophages, the property of hepatocytes CM to alter TNF-α production and NF-κB expression by macrophages. Remarkably, treatment of macrophage with these CM leads to an alternative activation of macrophages mediated by peroxisome proliferator-activated receptor-γ (PPAR-γ) signaling pathway, which might be resulted from the secretion of IL-10 and IL-4 as well as releasing apoptotic bodies from hepatocytes under ER stress. Our results highlight a mechanism of ER stress transmission from hepatocytes to macrophage that drives an alternative activation of macrophages, which depends on the exposure of hepatocytes to severe and prolonged ER stress.
Keywords: Macrophages Polarization, Hepatocytes, Endoplasmic reticulum stress, Liver diseases, Cytokines
Introduction
It has been increasingly recognized that the interactions between hepatocytes and nonparenchymal cells, particularly, KCs, have implications in both homeostasic and pathological conditions [1]. KCs are a major player of the innate immune system in the liver. They play an important role in liver physiology and homeostasis by participating in the acute and chronic responses of the liver to toxic compounds during liver inflammation or metabolic stress or dysfunctions [2]. Whereas KCs protect hepatocytes from encountering large amounts of noxious material coming from the portal vein, they also represent a great potential damage to hepatocytes by secreting a large amount of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β [3, 4]. Many of these pro-inflammatory mediators can trigger hepatocytic cell death pathways, e.g., via caspase activation [5], therefore, KCs have been implicated in the pathogenesis of various liver diseases. For example, the effects of KCs on hepatic steatosis are partially mediated by their secretion of IL-1β [6]. In addition, KCs and infiltrating macrophages play an essential role in conferring insulin resistance of hepatocytes [7, 8]. Conversely, hepatocytes have a remarkable effect on macrophages as well. Regarding the large amount of cells, hepatocytes could be an important source of inflammatory mediators [9]. Recent study shows hepatocytes produce Th2 cytokines, such as IL-4 and IL-13, which control the transcriptional program of alternative activation in the macrophage [10]. The subsequent phenotypic switch to the M2 phenotype plays an important role in preventing uncontrolled inflammation.
The endoplasmic reticulum (ER) and related signaling networks are emerging as critical mediators at the intersection between inflammation and metabolic diseases [11]. ER stress and the activation of unfolded protein response (UPR) are of particular importance in cells rich in endoplasmic reticulum content and presenting high protein synthesis rates, such as lymphocytes, and hepatocytes [12]. ER stress is widely implicated in various pathological conditions such as diabetes and various liver diseases [12, 13]. As mentioned above, the interactions between hepatocytes and KCs play a big role in both liver homeostasis and diseases, but relatively little is known about the interaction between hepatic macrophages and hepatocytes under ER stress conditions. For instance, it is yet to be examined whether hepatocytes influence macrophage function or inflammatory cytokines production under conditions of ER stress.
In this study, we established an in vitro human macrophage differentiation model in which monocytic THP-1 cells were driven to macrophage-like cells by phorbol myristate acetate (PMA). Thapsigargin and Tunicamycin were used in this study to induce ER stress. TM, a nucleoside antibiotic, is a specific inhibitor of N-linked glycosylation that blocks the first step of glycoprotein synthesis thereby interrupts protein folding. TG specifically induces ER stress by inhibiting the endoplasmic reticulum Ca2+ ATPase. We isolated conditioned media from HepG2 cells treated with either TM or TG and applied these CM on differentiating THP-1 cells. We found that CM inhibited pro-inflammatory cytokines production from THP-1 cells. We also found that CM not only stimulated macrophage ER stress but also drove macrophage polarization towards a M2 phenotype.
Materials and Methods
Reagents and antibodies
Thapsigargin, PMA, Tunicamycin, FITC-Dextran (MW: 40K), Sodium Palmitate and LPS from Escherichia coli 0111:B4 were purchased from Sigma-Aldrich (Oakville, Ontario, Canada). PE-TLR4 was purchased from R&D (Minneapolis, MN, US). FITC-Annexin V, PE-Cy7-HLA-DR, APC-CD206, APC-CD209, PE-CY7-CD86, APC-CY7-CD16, PE-CD74, FITC-IL-1β and NF-κB p65 were purchased from Biolegend (San Diego, CA, USA). PE-CD14 was purchased from eBioscience Inc (San Diego, CA, USA). Human anti-Peroxisome proliferator-activated receptor (PPAR)-γ, STAT6, CHOP and GAPDH were purchased from Cell Signaling (Danvers, MA, USA). Anti-GRP78/BIP was purchased from Abcam (Cambridge, MA, USA).
Cell lines
The human monocytic cell line, THP-1 and HepG2, a human hepatocellular carcinoma cell line, were originally purchased from ATCC (Manassas, VA) and routinely kept in our lab.
Conditioned media and THP-1 cell culture
When HepG2 cells were 70-80% confluent, 5μg/mL of TM or 100 nM of TG were added into the culture. Either 2 hours or 24 hours later, cells were gently washed three times with media and cultured further for another 24 hours. Culture media were harvested and carry over cells or large debris was removed by centrifuging at 800 g for 10 min. The remaining supernatants are referred as ER stress conditioned media (CM). TM-CM and TG-CM are derived from TM- or TG- treated HepG2 cells respectively. THP-1 cells were treated with 20 ng/mL of PMA for 24 hours. Then the supernatants were discarded and cultured with the CM for further 48 hours treatment.
Fractionation of the conditioned media
ER stress conditioned media were further fractionized by centrifuging at 10,000g for 30 mins. The supernatants collected as soluble part of the media was called SN in the following experiments. The pellet was resuspended in the same volume of media (called Pellet portion) and used as insoluble part of the conditioned media in the following experiments.
Cell staining and flow cytometry
All THP-1 cells treated with or without PMA or pTHP-1 cells treated with conditioned medium were stained with surface markers antibodies including TLR4, HLA-DR and CD86 in PBS containing 1% BSA. Labeled cells were all run on the BD LSR II Flow Cytometer and data were analyzed using FlowJo (v. 8.7) software.
Phagocytosis assay
Phagocytosis of FITC-Dextran by macrophages was measured as the cellular uptake of FITC-Dextran and quantified by Flow Cytometry as described in our previous publication [14].
Intracellular staining of THP-1 or pTHP-1
Intracellular cytokine staining (ICCS) of cytokines was performed according to our previous publication [14]. Cytokine-producing THP-1 cells were calculated by gating on HLA-DR+ THP-1 cells or on total THP-1 cells.
Cytokine profiling of pTHP-1 treated with CM and TM-treated HepG2 cells by Luminex assay
The cytokine profile of THP-1 cells treated with or without CM from hepatocytes was analyzed using the Luminex Multiplex system with the Milliplex (Millipore, Billerica, MA) human cytokine/chemokine 39-plex, following the manufacturer's protocol.
Western blotting
The protocol for Western blot is described in our previous publication [15]. Band intensities were detected, normalized and quantified with the Chemidoc and Image Lab 5.0 software (Bio-Rad Laboratories, Hercules, CA).
Statistical analyses
Data are presented as means ± SD. Statistical analyses were performed using Graphpad Prism 6.0. Statistical comparisons between 2-groups were calculated using a Student's t test. Differences between multiple groups were tested with ANOVA. A cutoff of 0.05 and lower was used to determine statistical significance.
Results
Conditioned media from ER stressed HepG2 cells regulate a variety of cytokines and chemokines secretion including TNF-α and IL-1 β from pTHP-1 cells
To investigate the interactions between hepatocytes and macrophage, we first established a human macrophage model of PMA activated THP-1 cells. An increasing number of investigators are now using lower doses and shorter activation periods [16, 17]. We induced THP-1 cells with 20 ng/mL of PMA for 24 hours followed by 48 hours resting. Compared to typical 72 hour activation, our protocol induced a comparable expression of cell surface markers associated with macrophage differentiation based on the expression of CD14, HLA-DR, CD86 and phagocytosis of high molecular weight Dextran (see Figure, Supplemental Digital Content 1A, 1B). Compared to wild type THP-1 cells, pTHP-1 cells showed to be more effective antigen presenting cells as reflected by the remarkably elevated capacity of phagocytosis with more FITC-Dextran and higher levels of HLA-DR and TLR4 as well as other markers (see Figure, Supplemental Digital Content 2). The most apparent morphologic change after PMA treatment is that the cells completely adhere to the bottom of the plastic culture flask within 24 hours (see Figure, Supplemental Digital Content 1C).
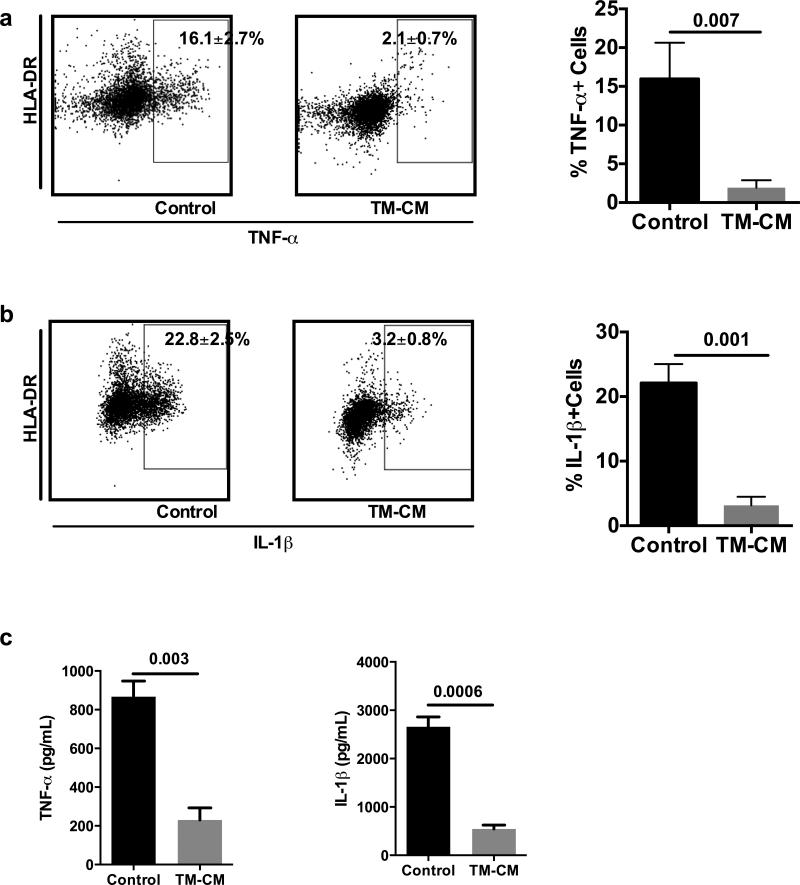
To examine whether conditioned media collected from ER stressed hepatocytes regulate macrophage cytokine secretion, we cultured pTHP-1 cells with the CM either collected from TM (5 μg/mL)- or TG (100 nM)- treated HepG2 cells. We treated these cells with CM at the cellular ratio of 3:1 for hepatocytes vs macrophages respectively. Our flow cytometry data showed that CM inhibited TNF-α and IL-1 β production by pTHP-1 cells as shown in Fig. 1. There were 16.1±2.7% of pTHP-1 cells with control media treatment expressing TNF-α, whereas only 2.1±0.7% of pTHP-1 cells expressed TNF-α upon exposure to TM-CM (p=0.007) (Fig. 1A). Additionally, TM-CM significantly decreased IL-1β expression in pTHP-1 cells exposed to LPS stimulation (p=0.001) (Fig. 1B). Results from a multi-analyte assay also showed a significant decrease of TNF-α and IL-1β secretion in the supernatants of TM-CM-treated pTHP-1 cells (p=0.003 and p=0.0006, respectively) (Fig. 1C). Furthermore, like TM, CM collected from TG (100 nM) exposed HepG2 cells also inhibited the production of pro-inflammatory cytokines TNF-α and IL-1β upon LPS stimulation (see Figure, Supplemental Digital Content 3).
Fig. 1. Conditioned media from TM-treated hepatocytes inhibited TNF-α and IL-1β secretion from pTHP-1 cells.
PMA-activated THP-1 cells were treated with CM isolated from HepG2 cells treated with TM or TG for 24 hours. 48 hours later, intracellular levels of TNF-α and IL-1β were determined by flow cytometry. TNF-α and IL-1β levels in the TM-CM were determined by Luminex assay. Data represent the intracellular staining of TNF-α (A) and IL-1β (B) in pTHP-1 cells treated with TM and TNF-α and IL-1β levels in the TM-CM (C). Values are means ± SD.
Using a multi-analyte assay, we further examined whether CM also regulates other cytokine and chemokine expression. As expected, treatment with CM for 48 hours inhibited a variety of pro-inflammatory cytokine and chemokine production from pTHP-1 cells (see Figure, Supplemental Digital Content 4). In summary, CM significantly inhibited TNF-α, IL-1β, IL-6 and IFN-γ, IP-10, MIP-1α, GM-CSF as well MCP-1. Interestingly, CM increased IL-10 production but decreased IL-12 as well, which indicated that macrophages were alternatively polarized (as shown below).
Based on previous reports showing that treatment of macrophages with CM collected from tumor cells shortly exposed to high dose of TM lead to increased pro-inflammatory cytokine secretion, we collected CM from HepG2 cells exposed to ER stress for a short duration [18]. The CM derived from short-term ER stress exposure in HepG2 led to a significant upregulation of pro-inflammatory cytokine secretion by pTHP-1 cells (see Figure, Supplemental Digital Content 5). Those cytokines are TNF-α, IP-10, IL-12 (p40 and p70), soluble (s) CD40L, IL-1β, IL-10 and GCSF. This result suggests that the properties of hepatocytes on influencing the pro-inflammatory cytokine secretion of macrophages are dependent on the duration of ER stress exposure.
Furthermore, to exclude the carryover of TM or TG in our CM, we determined TM and TG's direct effects on cytokine production from pTHP-1 cells. TM or TG could directly increase TNF-α production from pTHP-1 cells (see Figure, Supplemental Digital Content 6). In addition, by applying those CM to HepG2, we failed to detect visible ER stress (data not shown). This indicates that the inhibited cytokine secretion by CM should not result from any residue TM or TG in the CM after extensive washing.
Overall, our data indicate that the transition of acute to prolonged and severe ER stress leads to some extreme responses in hepatocytes and the resulting conditioned media from these hepatocytes may induce an anti-inflammatory response of macrophage.
Both the insoluble and soluble portions of CM decreased TNF-α and IL-1 β
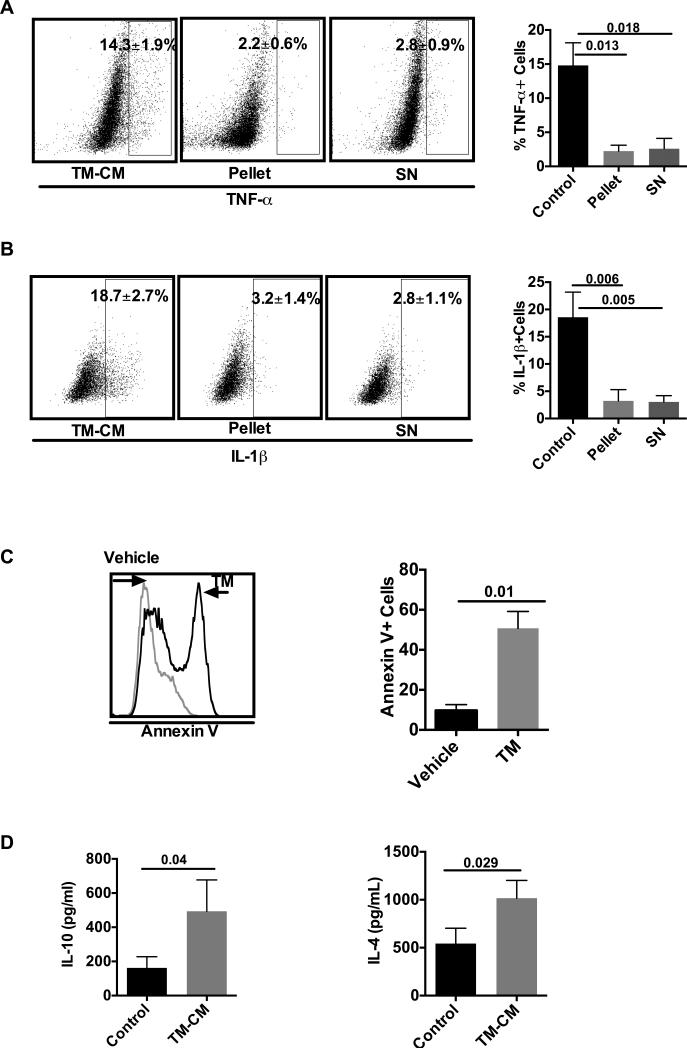
To further investigate the mechanisms involved in the decreased cytokine secretion, we fractionalized the CM into two portions, the pellet and the supernatants (SN). Surprisingly, both soluble and insoluble portions significantly inhibited TNF-α and IL-1β production by macrophages. Under control treatment, 14.3±1.9% of cells expressed TNF-α, whereas treatment with pellet and SN led to a significant decrease (2.2±0.6% and 2.8±0.9%, p=0.013 and p=0.018) respectively (Fig. 2A). Similar to TNF-α expression, subfractions of CM significantly inhibited IL-1β (Fig. 2B). There were 18.7±2.7% of IL-1β positive pTHP-1 cells with control treatment, the pellet and SN portions were 3.2±1.4%, 2.8±1.1% respectively. The significance of the differences between pellet or SN vs control are 0.006 and 0.005, respectively (Fig. 2B).
Fig. 2. Both the soluble and insoluble parts of CM inhibited pTHP-1 cell TNF-α secretion.
CM was further divided into pellet portion and supernatants portion. Their respective effects on TNF-α and IL-1β production by pTHP-1 cells were determined by ICS. Each portion of the CM inhibited TNF-α (A) and IL-1β (B). C) Annexin V expression of pTHP-1 cells treated with or without CM was determined by flow cytometry. D) IL-10 and IL-4 levels in the CM were determined by Luminex assay. Values are means ± SD.
To further investigate the underlying mechanism, we determined Annexin V expression in HepG2 cells and cytokine concentrations in the CM. Our data showed that TM treatment significantly increased Annexin V positive HepG2 cells (Fig. 2C). More than 50% of the total cells expressed Annexin V when treated with TM, whereas it was only 9.7±2.9% in control HepG2 cells. The difference between them is statistically significant (p=0.01). In addition, multiplex assay showed that there was a significant increase in the levels of IL-10 and IL-4 in the CM (p=0.04 and p=0.029 vs. control, respectively)), which may modulate the macrophage phenotype (Fig. 2D).
In conclusion, the conditioned media from hepatocytes dampens pro-inflammatory cytokine secretion by macrophages. The depressed cytokine production is the result of both insoluble mediators and soluble factors.
CM drives pTHP-1 cells to M2 polarization
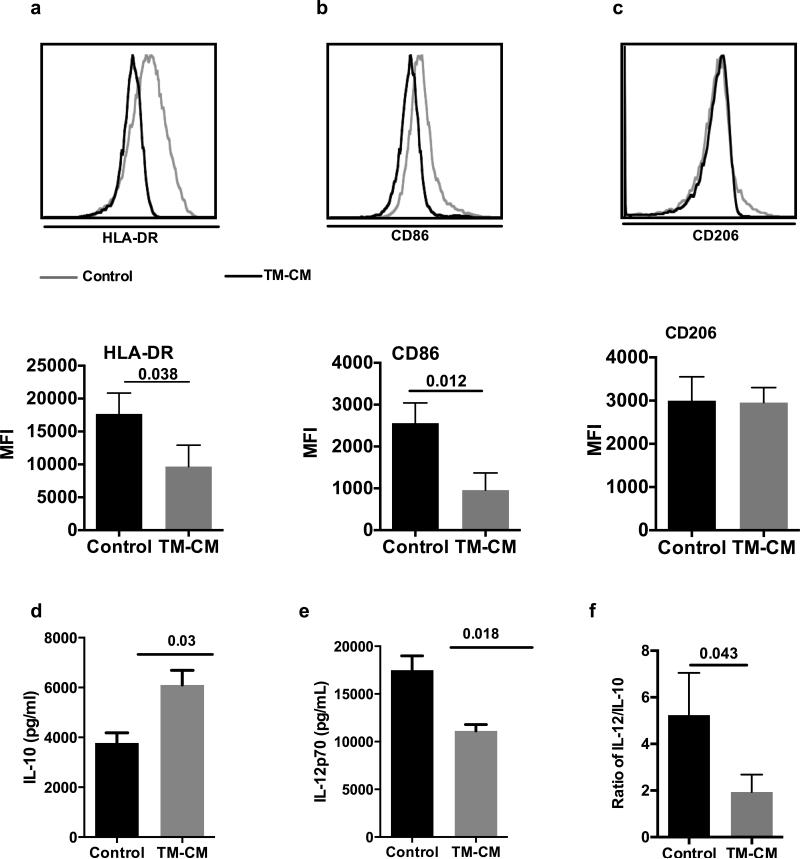
We further tested whether CM can also lead to an alternative activation of macrophages. To this end, we quantified characteristic surface markers for polarization and the ratio of IL-10 to IL-12, which is one of the most important criteria for the determination of M1 vs M2 [19]. As shown in Fig. 3, TM-CM significantly decreased the intensities of HLA-DR and CD86 expression on pTHP-1 cells compared to control (p=0.038 and p=0.012, respectively) (Fig. 3A, B). We failed to observe an increased CD206 expression on THP-1 cells (Fig.3C). In addition, CM enhanced IL-10 but inhibited IL-12 secretion as determined by multiplex assay (Fig. 3C) and consequently, the ratio of IL-12 to IL-10 was significantly decreased by about 2 folds in comparison with control (p=0.043) (Fig. 3D).
Fig. 3. CM drive pTHP-1 cells M2 polarization.
Both flow cytometric and graph format data of HLA-DR, CD86 and CD206 expression on pTHP-1 cells after 48 hours treatment with CM are shown in (A), (B) and (C) respectively. The levels of IL-12/IL-10 in the CM and the ratio of IL-12/IL-10 are shown in (D), (E) and (D) respectively. Values are means ± SD.
Overall, our data showed that treatment of pTHP-1 cells with conditioned media from ER stressed hepatocytes led to an alternative activation of macrophages.
Conditioned media inhibit cytokine production and drive M2 polarization by down-regulating NF-κB and up-regulating PPAR-γ signaling respectively
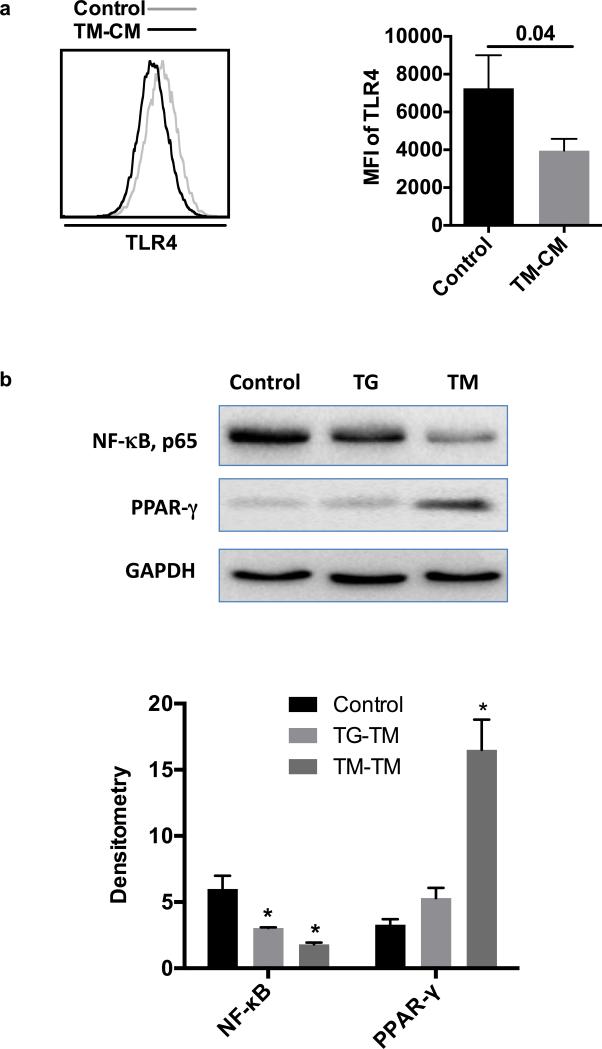
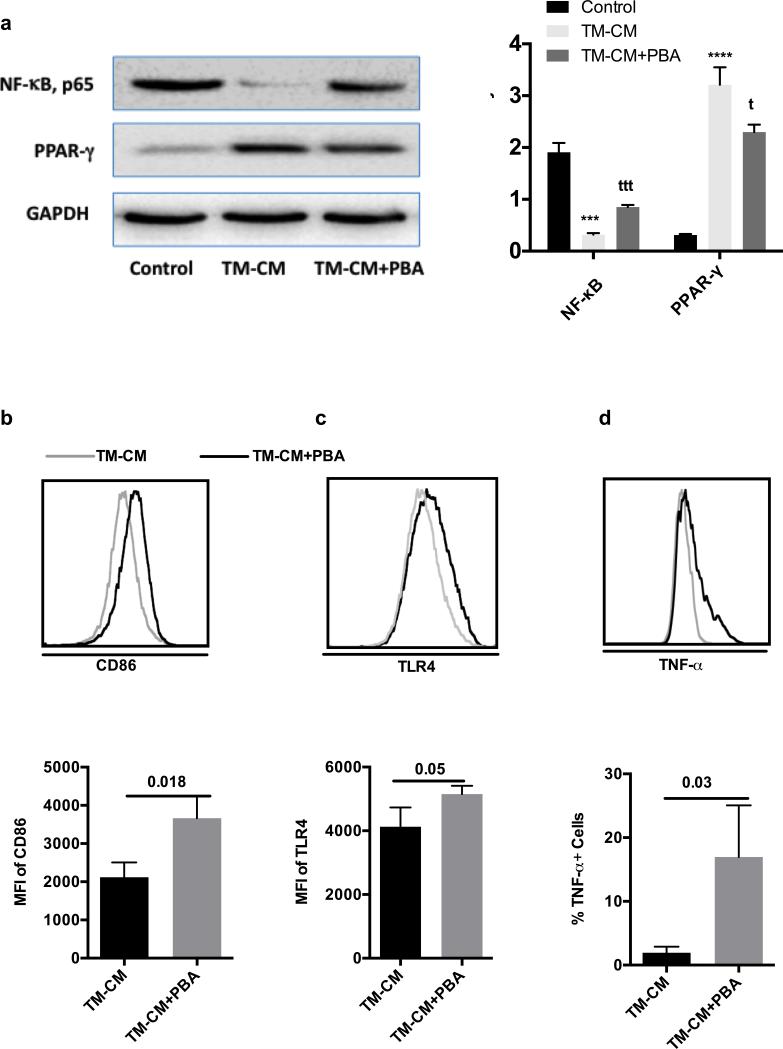
In order to investigate the underlying mechanisms involved in the inhibition of cytokines secretion by THP-1 treated with CM, we determined the expression of TLR4 and evaluated the NF-κB signaling pathway in pTHP-1 cells. As illustrated in Fig. 4A, TM-CM significantly inhibited TLR4 expression based on the MFI of TLR4 expression on pTHP-1 cells that determined by flow cytometry (p=0.04). In addition, CM inhibited NF-κB expression (Fig. 4B). Compared to control treatment, TM-CM and TG-CM had approximately 3 and 2-fold decrease in NF-κB expression determined by Western blot, respectively. To explore the signaling pathway of M2 polarization influenced by TM-CM, we determined PPAR-γ, which is an essential transcriptional factor for M2 polarization. Compared to control treatment, TM-CM significantly increased PPAR-γ expression by almost 5 folds (p<0.05). However, TG only slightly increased PPAR-γ expression without statistical significance (Fig. 4B).
Fig. 4. Conditioned media inhibit cytokine secretion and M2 polarization by inhibiting NF-κB and PPAR-γ signaling respectively.
(A) TLR4 expression in pTHP-1 cells treated with TM-CM and (B) NF-κB and PPAR-γ expression with TM-CM or control CM treatment. Band intensities were detected, normalized and quantified with the Chemidoc and Image Lab 5.0 software. Bars represent means ± SD. * p<0.05 vs control CM.
Taken together, TM-CM significantly down-regulates NF-κB and up-regulates PPAR-γexpression and consequently, leads to inhibited cytokine secretion and M2 polarization of pTHP-1 cells.
ER stress transfer from hepatocytes to macrophage
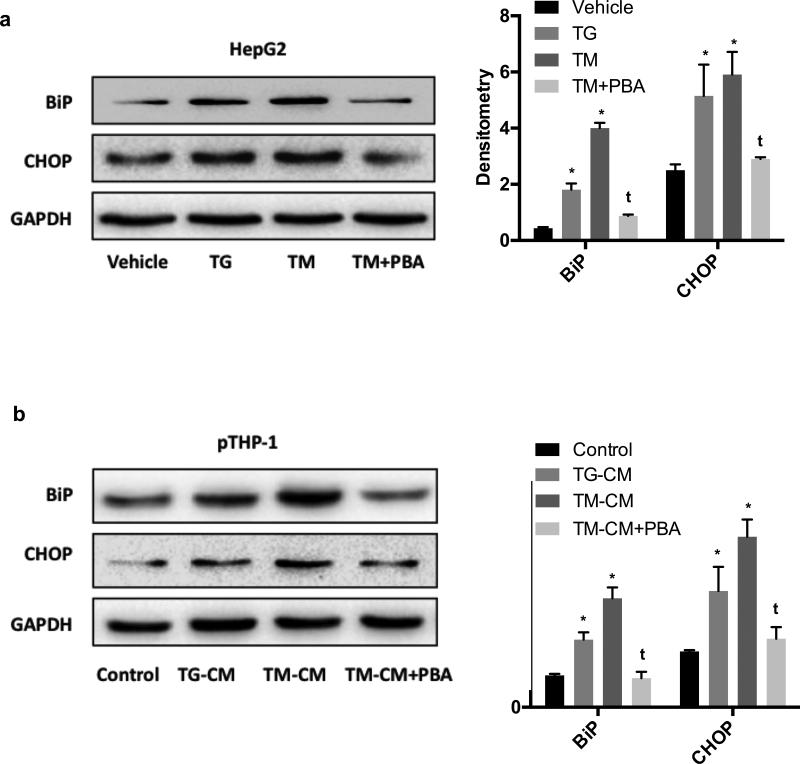
Instead of acute exposure to ER stressor [18], the CM was collected from HepG2 that had been exposed to lethal doses of TM for prolonged (24 hours) period of time. ER stress of hepatocyte was confirmed by increased BiP and CHOP expressions (Fig. 5A). Compared to vehicle control, both TM and TG induced a significant expression of BiP and CHOP (p<0.05 for all). TM appeared to be stronger than TG in inducing CHOP induction at the concentrations used in this study. Interestingly, pretreatment of HepG2 cells with PBA significantly reversed those effects (p<0.05 as compared to TM). Extending this analysis to examine whether pTHP-1 cells could have ER stress after being treated with CM, our results showed that CM led to ER stress of pTHP-1 cells as indicated by elevated expression of BiP and CHOP compared to control CM (p<0.05) (Fig. 5B). Furthermore, CM of HepG2 cells pretreated with PBA led to ER stress level comparable to control pTHP-1 cells. The differences between TM-CM and TM-CM plus PBA are significant in expression of both BiP and CHOP (p<0.05).
Fig. 5. Transmission of ER stress from HepG2 cells to PMA-THP-1 cells.
(A) BiP and CHOP expression in HepG2 cells treated with TM for 24 hours. (B) BiP and CHOP expression in pTHP-1 cells after treatment with CM for 48 hours. Values are means ± SD. *p<0.05 vs vehicle (5A) or control (5B). t <0.05 vs TM (5A) or TM-CM (5B).
In general, conditioned media of hepatocytes with prolonged exposure to ER stress drive macrophage ER stress.
PBA preconditioning attenuates the effects of Tunicamycin
PBA is one of a group of low molecular weight compounds known to stabilize proteins conformation, improve ER folding capacity, and facilitate the trafficking of mutant proteins [20]. Therefore, in order to examine the possible actions of PBA on TM, we preconditioned HepG2 cells with PBA (20 ng/mL) for overnight prior to the addition of TM. PBA preconditioning significantly attenuated TM’s effects on NF-κB expression (Fig. 6A). There was a significant difference in NF-κB expression between TM-CM and TM-CM plus PBA (p<0.001). Once again, PBA preconditioning significantly attenuated the effects of Tunicamycin on PPAR-γ expression (p<0.05). To determine whether PBA pretreatment can lead to functional changes, we determined surface markers of pTHP-1 cells and their cytokine secretion. Expectedly, PBA attenuated TM’s effects on CD86, TLR4 expression and TNF-α secretion (Fig. 6B-D). The differences between TM-CM vs TM-CM plus PBA in CD86 and TLR4 are significant (p=0.018 and p=0.05 respectively). Without PBA preconditioning, only about 2.0±0.5% of pTHP-1 cells produced TNF-α, on the contrary, there was a 19.5 ± 4.5% of pTHP-1 cells producing TNF-α, with significant difference (p=0.03) (Fig. 6D). We also find a similar effect on IL-1β (data not shown). However, we only observed a slight recovery of HLA-DR expression in pTHP-1 cells treated with CM derived from PBA pretreated HepG2 cells compared to TM-CM treatment (data not shown).
Fig. 6. Molecular chaperones decreases ER stress and attenuates TM effects.
PMA-activated THP-1 cells were treated with CM that was collected from TM-treated HepG2 either with or without pretreatment with PBA (CM or PBA-CM). (A) NF-κB and PPAR-γ expression and their corresponding densitometry; (B) CD86 expression and (C) TLR4 expression and (D) TNF-α production and their corresponding graphic format data. For WB, bars represent the densities of protein bands as the as the mean of 3 independent experiments ± SD. Values are means ± SD. ** p<0.05 vs TM-CM; *** p<0.001, ****p<0.0001 vs control CM. T <0.05, ttt<0.001 vs TM-CM.
In general, we found that PBA pretreatment led to significant modification of some markers of pTHP-1 cells and ER stress markers on both HepG2 cells and pTHP-1 cells.
Consequently, it seems that PBA was only partly effective in reversing the effects of TM.
Discussion
Little is known about the influence of hepatocytes on macrophages phenotypes under ER stress. In this study, our results show that hepatocytes influence macrophages by releasing soluble and insoluble factors acting in a paracrine manner during chronic ER stress (Fig. 7). Hepatocytes not only drive macrophages polarization to an alternative phenotype, they also transfer ER stress to macrophage upon sensing the stress. Consequently, this drives an alternative polarization of macrophages. The conditioned media from ER stressed hepatocytes inhibit the secretion of pro-inflammatory cytokines including IL-6, TNF-α, MCP-1 and IL-1β production from macrophages, which is mediated by inhibiting TLR4 expression and NF-κB signaling. This effect could be partially reversed by the ER stress inhibitor, PBA. To our knowledge, this is the first report demonstrating that hepatocytes under prolonged ER stress induce an anti-inflammatory response on macrophage in response to LPS stimulation.
There are inherent challenges when studying the interactions between human primary hepatocytes and liver macrophages. Therefore, using indirect co-culture assays with conditioned media collected from one cell line to another cell line is the commonly used protocol to study these interactions [1]. Most of the protocols currently used for THP-1 macrophages differentiation consist in either chronic activation with PMA for up to 72 hours or short term activation combining longer period of resting [17]. PMA concentration used by various laboratory ranged anywhere from 10 ng/ml to 400 ng/ml [21]. For our purposes, we chose a protocol combining a 24-hour activation with a low dose of PMA (20 ng/mL) and 48-h resting. This protocol leads to a reliable macrophage-like differentiation of THP-1 cells based on cell morphology, cell adhesion, expression of surface markers and phagocytic capacity (Supplementary Fig. 1).
One of the most important features of macrophages is their plasticity. Based on their functions and their secretion of cytokines, they can be generally divided into two types: classically activated macrophage or M1 or alternatively activated macrophage or M2, which represent extremes of a continuum of activation states [2]. The imbalance of M1 and M2 Kupffer cells occurs in vivo under physiological conditions (e.g., ontogenesis and pregnancy) and in pathology (allergic and chronic inflammation, tissue repair, infection, and cancer) [22]. It has been known that IL-10, glucocorticoid, hormones, apoptotic cells, immune complex also polarize macrophages to an M2-like phenotype [7]. The macrophages activation state and functional properties are largely influenced by tissue microenvironment. Recently, a growing number of studies have implicated metabolic signals in the modulation of KCs activation status [23]. Macrophages in the liver are exposed to high levels of fatty acids, a ligand for PPAR-γ, which drives them in an alternative activation state. These alternatively activated KCs play a beneficial role in oxidative metabolism and lipid homeostasis in peripheral tissues [24]. Our results indicate that hepatocytes influence macrophage polarization based on HLA-DR and CD86 expression as well as the ratio of IL-10 and IL-12 production. In the situations of prolonged potentially lethal ER stress, hepatocytes secrete soluble factors, such as IL-10 and IL-4, and possibly, apoptotic bodies, which contribute to the alternative macrophage activation. Innate immune responses, especially those involving resident KCs and infiltrating macrophages are of greater significance in the liver because the liver's adaptive immune system is maintained in a baseline state of active tolerance in the liver. Kupffer cells act as the first responders to pathogens, toxins and particles released from damaged tissues and react in producing pro-inflammatory cytokines, such as TNF-α, IFN-γ, and IL-1β as well as growth control mediators that influence neighboring hepatocytes [25]. However, our results indicate that the activation status of macrophages during ER stress is modified by hepatocytes, a major cell population in liver. Under conditions of ER stress, hepatocytes remarkably influence resident KCs and infiltrating macrophages by polarizing them into an M2 state by inducing PPAR-γ signaling pathway.
M2 dominance in metabolic tissues including liver is featured in many critical illnesses [26]. It has shown that adipose tissue protein levels of PPAR-γ are increased in prolonged critically ill patients. But the trigger of M2 dominance in liver in critical illness is not yet identified. Our results suggest that the ER stress might be the cause of M2 dominance. In addition, in consistent with many studies that show PPAR-γ is important for M2 polarization in many metabolic diseases [27] as well critical illness [26], we found a significantly increased expression of PPAR-γ in pTHP-1 cells treated with conditioned media. It suggests that hepatocytes under the condition of ER stress in critical illness might drive liver macrophages and polarization by inducing PPAR-γ activation.
Overall, unlike the CM isolated from short-term ER stressed hepatocytes, which induces a pro-inflammatory response of macrophage, prolonged ER stress induces hepatocytes secretion of IL-10 and IL-4 and apoptotic bodies as well, which possibly drive an alternative macrophage polarization. Regulation of ER stress consequences by pretreatment with PBA implicates a new therapeutic target for liver diseases in the situation of ER stress.
Supplementary Material
Acknowledgements
The authors thank Dr. David Patsouris for his critical reviewing of the manuscripts.
Funding resources: This work is supported by grants from the National Institutes of Health (R01 GM087285-01); CIHR Funds (123336), CFI Leader's Opportunity Fund (Project #25407); Physician's Services Incorporated Foundation: Health Research Grant Program.
Footnotes
Disclosure: The authors declare they have no competing interests
References
- 1.Kmiec Z. Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol. 2001;161(III-XIII):1–151. doi: 10.1007/978-3-642-56553-3. [DOI] [PubMed] [Google Scholar]
- 2.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 4.Helk E, Bernin H, Ernst T, Ittrich H, Jacobs T, Heeren J, Tacke F, Tannich E, Lotter H. TNFalpha-mediated liver destruction by Kupffer cells and Ly6Chi monocytes during Entamoeba histolytica infection. PLoS Pathog. 2013;9:e1003096. doi: 10.1371/journal.ppat.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang K. Molecular mechanisms of hepatic apoptosis. Cell Death Dis. 2014;5:e996. doi: 10.1038/cddis.2013.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Müller M. Kupffer cells promote hepatic steatosis via interleukin-1betadependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 7.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, Ju C, Aouadi M, Czech MP, Kunos G. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat Med. 2013;19:1132–1140. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowell DL, Eckmann L, Dwinell MB, Carpenter SP, Raucy JL, Yang SK, Kagnoff MF. Human hepatocytes express an array of proinflammatory cytokines after agonist stimulation or bacterial invasion. Am J Physiol. 1997;273:G322–332. doi: 10.1152/ajpgi.1997.273.2.G322. [DOI] [PubMed] [Google Scholar]
- 10.Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dara L, Ji C, Kaplowitz N. The contribution of endoplasmic reticulum stress to liver diseases. Hepatology. 2011;53:1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 14.Xiu F, Stanojcic M, Jeschke MG. Norepinephrine inhibits macrophage migration by decreasing CCR2 expression. PLoS One. 2013;8:e69167. doi: 10.1371/journal.pone.0069167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diao L, Marshall AH, Dai X, Bogdanovic E, Abdullahi A, Amini-Nik S, Jeschke MG. Burn plus lipopolysaccharide augments endoplasmic reticulum stress and NLRP3 inflammasome activation and reduces PGC-1alpha in liver. Shock. 2014;41:138–144. doi: 10.1097/SHK.0000000000000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takashiba S, Van Dyke TE, Amar S, Murayama Y, Soskolne AW, Shapira L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor kappaB. Infect Immun. 1999;67:5573–5578. doi: 10.1128/iai.67.11.5573-5578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocytederived macrophages. PLoS One. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108:6561–6566. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoger JL, Goossens P, de Winther MP. Macrophage heterogeneity: relevance and functional implications in atherosclerosis. Curr Vasc Pharmacol. 2010;8:233–248. doi: 10.2174/157016110790886983. [DOI] [PubMed] [Google Scholar]
- 20.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res. 2007;56:45–50. doi: 10.1007/s00011-007-6115-5. [DOI] [PubMed] [Google Scholar]
- 22.Sica A, Invernizzi P, Mantovani A. Macrophage plasticity and polarization in liver homeostasis and pathology. Hepatology. 2014;59:2034–2042. doi: 10.1002/hep.26754. [DOI] [PubMed] [Google Scholar]
- 23.Chinetti-Gbaguidi G, Staels B. Macrophage polarization in metabolic disorders: functions and regulation. Curr Opin Lipidol. 2011;22:365–372. doi: 10.1097/MOL.0b013e32834a77b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 26.Langouche L, Marques MB, Ingels C, Gunst J, Derde S, Vander Perre S, D'Hoore A, Van den Berghe G. Critical illness induces alternative activation of M2 macrophages in adipose tissue. Crit Care. 2011;15:R245. doi: 10.1186/cc10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.