Abstract
Motor speech disorders, including apraxia of speech (AOS), account for over 50% of the communication disorders following stroke. Given its prevalence and impact, and the need to understand its neural mechanisms, we used resting state functional MRI to examine functional connectivity within a network of regions previously hypothesized as being associated with AOS (bilateral anterior insula (aINS), inferior frontal gyrus (IFG), and ventral premotor cortex (PM)) in a group of 32 left hemisphere stroke patients and 18 healthy, age-matched controls. Two expert clinicians rated severity of AOS, dysarthria and nonverbal oral apraxia of the patients. Fifteen individuals were categorized as AOS and 17 were AOS-absent. Comparison of connectivity in patients with and without AOS demonstrated that AOS patients had reduced connectivity between bilateral PM, and this reduction correlated with the severity of AOS impairment. In addition, AOS patients had negative connectivity between the left PM and right aINS and this effect decreased with increasing severity of non-verbal oral apraxia. These results highlight left PM involvement in AOS, begin to differentiate its neural mechanisms from those of other motor impairments following stroke, and help inform us of the neural mechanisms driving differences in speech motor planning and programming impairment following stroke.
Keywords: Apraxia of speech, Network connectivity, Resting-state fMRI, Stroke
Highlights
-
•
Resting state fMRI connectivity of a speech network in apraxia of speech (AOS)
-
•
AOS patients had reduced bilateral premotor (PM) connectivity relative to severity.
-
•
AOS patients also had reduced left PM–right anterior insula connectivity.
-
•
Left PM may drive disordered speech motor planning and programming post-stroke.
1. Introduction
Apraxia of speech (AOS), first delineated by Darley, Aronson, and Brown (e.g. 1975) as a speech disorder is associated with inefficiencies in the translation of speech sounds (phonemes) into the kinematic parameters associated with speech production (McNeil etal., 1997). While the diagnosis is made in the absence of fundamental (e.g. weakness or slowness) neuromuscular, cognitive, or linguistic impairments, it rarely occurs as an isolated entity (McNeil etal., 2011). Apraxia of speech is characterized by changes in speech rate (prolongation of speech sounds/segments and between sound or segment gaps), distorted sounds, consistency in error type, and abnormal prosody (de-stressing of typically stressed syllables and sounds).
Despite its prevalence and deleterious impact on communication (Duffy, 2013), there has been very little functional imaging work in the area. Hence the neural basis of the disorder remains elusive. To date there are few brain imaging studies on AOS, and most reports are case studies (Robin et al., 2008). The absence of imaging data limits our understanding of the exact nature and neurobiological mechanisms of AOS. Thus, there is a critical need for further functional imaging studies to identify neural mechanisms of action. Such information may increase diagnostic specificity and lead to mechanistically based treatments, not unlike the aphasia literature (e.g. Thompson etal., 2010).
Identification of a single brain region or site of lesion for AOS is a challenge and postulated regions are still debated. Early post-mortem studies of AOS first identified Broca's area (BA44) as a region associated with speech articulation difficulties (e.g. Nielsen, 1936; Wertz etal., 1984). Recently, Trupe etal. (2013) provided support for the role of BA44 in the disorder by using the volume of infarct as the key variable. Early lesion overlap studies identified the left anterior insula (aINS) as being associated with AOS (Dronkers, 1996). However, in Dronker's study over half of the patients diagnosed with AOS also presented with Broca's aphasia and 40% had dysarthria. More recently, behavioral measures of speech have been included in lesion overlap studies (Ogar etal., 2006) to examine how the extent of the lesioned region varies with performance. Ogar etal. identified the superior precentral gyrus of the insula as a region where all patients with AOS had lesions. With the introduction of lesion overlap and voxel-based lesion symptom mapping (VBLSM) methods, more precise lesion mapping is now possible. Other studies have argued against a relationship between AOS and the left insula. Hillis etal. (2004) performed diffusion and perfusion weighted imaging and found no relationship between AOS and diffusion or perfusion in the left insula in acute patients. Recently, Graff-Radford and colleagues (2014) identified the left premotor area (PM) and motor cortex as the most commonly affected anatomy in patients with pure AOS caused by stroke, AOS with aphasia, and neurodegenerative AOS. It is therefore unclear whether the anterior insula, BA44 or other regions are the primary regions responsible for AOS.
As noted above, understanding the pathophysiology of AOS can only be accomplished by studying a network of regions and their change following stroke. Hence, it is likely that the pathogenesis of AOS is associated with changes in a network of the debated regions related to AOS, rather than a single region. Therefore, this study sought to delineate functional network anomalies among the bilateral inferior frontal gyrus (IFG; BA44), PM, and aINS in AOS.
There are various methods for studying network connectivity with fMRI data (Eickhoff and Grefkes, 2011). To study functional network connectivity in AOS, we used resting state fMRI, an efficient approach to studying brain function in health and disease. Examining functional network connections at rest allows for analysis of relations of brain regions independent of task performance and compliance, and thus provides information on more permanent temporal coherence between brain regions (van den Heuvel and Hulshoff, 2010). In turn, these stable network interactions are easily applied to clinical settings.
The use of functional imaging measures to understand AOS is very limited, and although debates exist as to the region(s)of damage associated with AOS, few data are available to test any neurobiological model of the disorder. There are currently no studies examining resting state functional connectivity (RSFC) following stroke in patients with AOS, though several studies have examined the effect of disrupted RSFC networks on limb motor impairment following stroke (Carter etal., 2009; Li etal., 2014; Park etal., 2011; Tuladhar etal., 2013). Thus, we chose to analyze connectivity of resting state data in an a priori network because it allows for the examination of specific regions rather than a whole brain network, such as the default mode network (DMN; cf. Schilbach etal., 2014).
Because AOS is considered a disorder of “motor planning and programming” (McNeil etal., 2011), it is important to consider current models of speech production. Directions into Velocities of Articulators (DIVA; Guenther etal., 2006) is a neurocomputational model devoted to identifying the neural regions and networks involved in healthy speech, accounting for the interactions among motor, somatosensory and auditory cortical areas. The premotor regions are commonly associated with the planning and programming of speech (see (Eickhoff etal., 2009)). Models of motor programing in AOS also suggest a deficit in the preprogramming (INT) stage of Klapp's (2003) model of speech motor programming (Maas etal., 2008). The INT stage of the model is designated for the organization of the structure of individual units of movement and reading them into a short-term memory store, or motor buffer (Klapp, 2003). During the second process in the model the motor programs are sequenced (SEQ) into the correct serial order for output. Compared to AOS-absent control subjects, patients with AOS have a longer INT time with normal SEQ and initiation times (Maas etal., 2008). Based on the DIVA model (Guenther etal., 2006), we can speculate that, in AOS, the INT process is implemented in the PM regions, and thus hypothesize that there would be reduced network connectivity in this seed in patients relative to controls and in patients diagnosed with AOS relative to those with no clinical signs of AOS. Differences between patients with AOS and those without AOS might also be reflected in a correlation analysis of connectivity with diagnostic ratings.
2. Materials and methods
2.1. Participants
Thirty-two, right-handed, chronic left-hemisphere stroke patients (27 males, 5 females; age = 62 ± 10 years; median months post-stroke onset (median MPO) = 27; range = 1–156 months; see Table 1 for clinical and demographic details) and 18 healthy volunteers (8 males, 10 females; age = 63 ± 9 years) without any record of neurological or psychiatric disorders were included in the analyses of resting state functional connectivity. Stroke patients were recruited from a larger sample of patients consecutively referred from local speech language pathologists and national and local support networks and were eligible if they had difficulty with speech or language. Healthy controls were recruited through advertising in the Neuroscience Research, Australia registry. Volunteers were tested in the order that they responded to the advertisement, regardless of gender. All participants were eligible if they were between the ages of 18 and 75; right handed, per participant report; native English speakers; had no contraindications for undergoing an MRI [materials that may distort signal, such as pacemaker, or other implant; claustrophobia (1 patient and 1 control)]; had no history of uncorrected hearing, vision, or other sensory impairment; cognitive impairment; premorbid speech, language, or reading impairment; or substance abuse. All subjects gave informed written consent to participate in the study. All procedures were approved by the human ethics committees of the Sydney Southwest Area Health Service and the University of Sydney.
Table 1.
Patient demographics. MPO = months post-onset of stroke; AOS = apraxia of speech; ExDx AOS = expert severity rating of apraxia of speech; ExDx Dys = expert severity rating of dysarthria; ExDx NVOA = expert severity rating of non-verbal orofacial apraxia; WAB AQ = Western Aphasia Battery— Revised (Kertesz, 2006) Aphasia Quotient (max score 100); RCPM = Raven's Colored Progressive Matrices (max score 36); PALPA (Kay, Lesser and Coltheart, 1992) = Auditory Discrimination subtest of the Psycholinguistic Assessments of Language Processing in Aphasia (max score 72); N/A = not administered.
| ID | Age | Gender | MPO | Lesion volume (cm3) | AOS | ExDx AOS | ExDx Dys | ExDx NVOA | WAB AQ | RCPM | PALPA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DIS001 | 69 | M | 52 | 80.7 | N | 1 | 1 | 1 | 86.0 | 19 | 67 |
| DIS002 | 48 | M | 17 | 149.16 | Y | 7 | 2 | 7 | 11.3 | 21 | 72 |
| DIS003 | 72 | M | 156 | 34.3 | Y | 2.5 | 3 | 1 | 81.3 | 26 | 63 |
| DIS004 | 66 | M | 32 | 163.0 | Y | 3.5 | 1 | 5.5 | 83.7 | 34 | 70 |
| DIS005 | 56 | F | 44 | 66.2 | N | 1 | 1.5 | 1.5 | 93.0 | 32 | 72 |
| DIS006 | 54 | M | 36 | 59.2 | Y | 3.5 | 1 | 4.5 | 75.1 | 31 | 68 |
| DIS007 | 71 | M | 17 | 32.4 | N | 1.5 | 1.5 | 2 | 73.7 | 28 | 56 |
| DIS008 | 58 | M | 10 | 27.1 | N | 1 | 1 | 1 | 68.3 | 34 | 66 |
| DIS009 | 71 | M | 16 | 1.5 | N | 1 | 2.5 | 2 | 91.6 | 32 | 70 |
| DIS010 | 67 | M | 58 | 70.4 | N | 1 | 1 | 1.5 | 86.4 | 35 | 63 |
| DIS011 | 77 | M | 81 | 140.3 | Y | 6.5 | 3.5 | 5.5 | 60.5 | 30 | 70 |
| DIS012 | 69 | M | 27 | 55.3 | Y | 3 | 2.5 | 4.5 | 80.8 | 34 | 66 |
| DIS013 | 73 | M | 38 | 114.3 | N | 2 | 1.5 | 5 | 11.9 | 6 | N/A |
| DIS014 | 48 | M | 13 | 62.4 | Y | 4.5 | 1.5 | 4 | 41.6 | 36 | N/A |
| DIS015 | 66 | M | 84 | 171.1 | Y | 3 | 1.5 | 4 | 75.3 | 32 | 60 |
| DIS017 | 76 | M | 120 | 158.6 | Y | 6.5 | 3.5 | 6 | 39.6 | 22 | 63 |
| DIS018 | 66 | M | 21 | 21.0 | N | 1.5 | 2 | 2 | 97.3 | 27 | 71 |
| DIS022 | 64 | F | 121 | 222.4 | N | 2 | 1 | 5 | 54.5 | 29 | 63 |
| DIS023 | 49 | M | 14 | 49.5 | N | 1 | 1 | 1 | 72.5 | 32 | 69 |
| DIS024 | 59 | M | 69 | 132.9 | N | 1 | 1 | 1 | 80.7 | 27 | 66 |
| DIS025 | 55 | F | 92 | 17.7 | N | 1 | 1 | 1 | 98.7 | 35 | 71 |
| DIS026 | 71 | M | 11 | 217.4 | N | 1 | 1 | 1 | 66.6 | 25 | 72 |
| DIS027 | 73 | M | 26 | 298.5 | N | 1 | 1 | 4.5 | 50.0 | 31 | 72 |
| DIS028 | 50 | M | 9 | 62.2 | Y | 5 | 4 | 3 | 60.2 | N/A | 68 |
| DIS029 | 75 | M | 36 | 117.2 | Y | 6 | 3 | 4.5 | 17.8 | 15 | N/A |
| DIS030 | 61 | M | 3 | 1.1 | N | 1 | 1 | 1 | 88.9 | 35 | 67 |
| DIS031 | 63 | M | 23 | 56.2 | Y | 2.5 | 1 | 6 | 62.8 | 30 | 61 |
| DIS047 | 45 | F | 37 | 161.5 | N | 1 | 1 | 5.5 | 36.9 | 3 | 67 |
| DIS048 | 40 | M | 13 | 143.2 | Y | 5 | 1 | 6 | 23.9 | 21 | 66 |
| DIS050 | 51 | M | 6 | 37.4 | Y | 4 | 3 | 5 | 69.5 | 34 | 68 |
| DIS051 | 57 | M | 1 | 129.8 | Y | 4.5 | 1 | 5.5 | 64.8 | 28 | 68 |
| DIS052 | 74 | F | 5 | 1.3 | N | 1 | 1 | 2 | 96.0 | 35 | 68 |
2.2. AOS diagnosis and speech measures
All patients underwent a battery of speech and language tests to define their communication impairment(s), including: (1)a case history; (2)the Spontaneous Speech, Auditory Verbal Comprehension, Repetition, and Naming and Word Finding subtests from the Western Aphasia Battery— Revised (Kertesz, 2006) to determine aphasia severity andtype; (3)Raven's Progressive Colored Matrices (Raven, 1947) asscreen for nonverbal cognitive abilities; (4)the Motor Speech Examination (Duffy, 2005), the Apraxia Battery for Adults— 2 (Dabul, 2000) and connected speech samples generated from the Story Retell Procedure (McNeil etal., 1997) to generate speech samples for expert judgment of presence and severity of AOS, nonverbal apraxia and dysarthria; (5)the Assessment of Intelligibility of Dysarthric Speech (Yorkston etal., 1984); and (6)the Auditory Discrimination of Minimal Pairs subtest of the Psycholinguistic Assessments of Language Processing in Aphasia— 2 (Kay etal., 1992) to assess auditory perceptual impairment underlying any speech impairment.
From the assessment battery, a 15–20 minute video was generated for each patient, capturing their responses to the speech and oral-nonspeech tasks in the Motor Speech Examination, the Apraxia Battery for Adults— 2, and the Story Retell Procedure. Two expert clinicians, blinded to patients' speech and language test scores, independently viewed each video and judged presence and severity of AOS, dysarthria, nonverbal oral apraxia, and phonologically-based sound errors (i.e.,paraphasias) by selecting from dropdown menus on a web-based survey. Severity was rated on a 7 point Likert-type scale (1 = normal, 2 = minimal, 3 = mild, 4 = mild-moderate, 5 = moderate, 6 = moderate–severe, 7 = severe). Patients were rated in the order they entered the study. For disagreements on the presence/absence of greater than one point on the severity scale a third expert judged the samples and the majority rating was used. All raters had more than 25 years of clinical experience with AOS. The diagnostic criteria for AOS were articulatory distortions, slow speech rate, prolonged inter-word intervals, syllable segregation, and equal stress across words and syllables (McNeil etal., 2011).
JD rated all cases and MM, DR, or KB served as second or third rater for 28 speech samples. Agreement for the presence/absence of AOS was 24/28 (86%) and agreement on the severity rating scale for AOS (within 1 point) was also 24/28 (86%). Agreement for the presence/absence of dysarthria was 21/28 (75%; in four of these cases, the disagreement was between 1 = normal and 2 = minimal) and severity rating within one point was 26/28 (93%). Agreement between raters on nonverbal oral apraxia presence was 24/28 (86%) and severity rating within one point was 23/28 (82%). Severity ratings for AOS and those for NVOA were moderately correlated (r = .718). A third rater resolved disagreements over presence/absence. Average severity over the two most closely agreeing raters was entered in analyses, with the exception of severity of phonological paraphasias due to low rater agreement. Due to most disagreements being between ratings of “1” (normal) and “2” (minimal), we conservatively assigned patients with scores of 2.5 or higher on the severity rating scale to the AOS group for connectivity analyses. Fifteen individuals were identified as having AOS (15 males; age = 61 ± 12 years; lesion volume 102.6 ± 50.8 cm3; median MPO = 27; range = 1–156 months) and 17 were without AOS (12 males and 5 females; ages 64 ± 9 years; lesion volume 89.2 ± 89.5 cm3; median MPO = 26; range = 3–121 months).
2.3. MRI data acquisition
All structural and functional fMRI data were acquired on a Philips 3T TX MRI scanner. Ahigh-resolution 1 mm3T1-weighted structural image was collected. Two hundred sixteen resting state EPI images were acquired using blood-oxygen-level-dependent (BOLD) contrast [gradient-echo EPI pulse sequence, TR = 2.2 s, TE = 30 ms, flip angle = 90°, in-plane resolution = 3.1 × 3.1 mm2, 36 axial slices (3.1 mm thickness) covering the entire brain]. Immediately before the session, participants were asked to lie still and stay awake with their eyes open.
2.4. Data analysis
2.4.1. Structural image analysis and lesion classification
SPM8 software (Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm) was used to spatially normalize the structural T1 scan to standard MNI space using the “unified segmentation” algorithm (Ashburner and Friston, 2005). An additional step was added to optimize the solution for the stroke patients. This step included an extra empirically derived tissue class (“lesion”) being added to the segmentation priors to allow the lesion to be represented in a tissue class other than gray/white/CSF (Seghier etal., 2008). All segmentation output images were then smoothed with an isotropic kernel of 8 mm at full-width at half maximum. After smoothing, the value of each voxel in the image represented the probability that the tissue belongs to one class and not to one of the others (gray matter, white matter, non-brain, or lesion).
The additional tissue class image (binary lesion) for each subject was in an additional analysis to determine lesion volumes using the automated lesion identification algorithm (ALI toolbox) implemented in SPM8 (Seghier etal., 2008). These binary lesion images were also used to create a lesion overlap map (see Fig. 1) using the automated lesion overlap map toolbox in SPM. Lesion volumes between AOS and AOS-absent patients were tested with independent samples t-test, and not significantly different (p = .612).
Fig. 1.
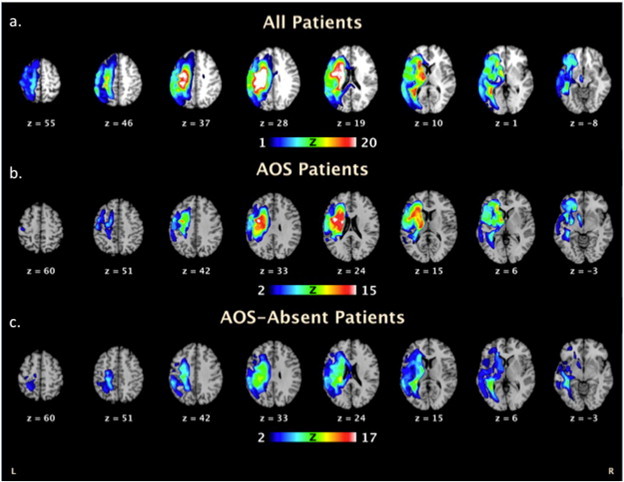
Stroke lesion overlap map for (a)the group of 32 stroke patients, (b)the 15 AOS diagnosed patients only and (c)the 17 AOS-absent patients displayed on axial slices of a template brain. Regions where voxels are included in their lesion are shown on a heat map scale where blue indicates very few patients had overlap in that region and red/white indicates multiple/all subjects had lesions in that region.
2.4.2. Functional image analysis
Prior to analysis, we ensured that all stroke patients did not have significantly more head motion than healthy controls. Independent sample t-tests of framewise displacement (FD) and root mean squared movement (RMS) were indeed not different between groups (Table 2). EPI images were first corrected for head movement by applying affine registration using a two-pass procedure in SPM8. A mean EPI image for each subject was created and then spatially normalized to the MNI single subject template using the “unified segmentation” approach (Ashburner and Friston, 2005). A deformation field was output from this process and then applied to the individual EPI volumes. Output images were then smoothed using a 5-mm FWHM Gaussian kernel. The following instances of variance were then removed from each voxel's time series in order to reduce spurious correlations by confounds such as physiological noise and motion (cf. Bandettini and Bullmore, 2008): i)the six motion parameters derived from the image realignment, ii) their first derivative, and iii) mean gray, white matter and CSF signal intensity (Jakobs etal., 2012; Reetz etal., 2012; Satterthwaite etal., 2012). Data were then band-pass filtered, preserving frequencies between 0.01 and 0.08 Hz (M.D. Fox and Raichle, 2007; zu Eulenburg etal., 2012).
Table 2.
Network location coordinates in MNI space.
| Region of interest | MNI coordinate (x, y, z) |
|---|---|
| Inferior frontal gyrus (BA44) (IFG) | −50 10 5 |
| 50 10 5 | |
| Anterior insula (aINS) | −32 15 2 |
| 32 15 2 | |
| Ventral premotor cortex (BA6) (PM) | −58 1 23 |
| 58 1 23 |
2.5. ROI selection
Functional connectivity was then investigated using a network of cortical regions proposed to have roles in AOS. Coordinates for each of these regions had also been identified from a meta-analysis of previous fMRI studies of overt speech and an effective connectivity analysis of this speech network [see Table 2;(Eickhoff etal., 2009)]. The regions selected were: i)IFG (BA 44), ii) aINS, and iii) PM (BA6). Coordinates of each region from Eickhoff etal. (2009) are displayed in Table 2. We examined both hemispheres in the same model to identify any altered connectivity between the affected and unaffected hemispheres and also any adaptive changes that may have occurred in the unaffected hemisphere. The time course for each of the identified seed regions was then extracted for each subject as the first eigenvariate of the resting-state signal time-series of all gray-matter voxels located within 5 mm of the respective peak coordinate. In particular, these regions were selected to test the different hypotheses associated with the role of each ROI in the pathogenesis of AOS.
2.6. Functional connectivity network analysis
For each subject we computed linear (Pearson) correlation coefficients between the extracted time series of each of the seed regions to examine connectivity within the specified network, after controlling for individual differences in head motion (see Table 3 for measures of head motion). These voxel-wise correlation coefficients were then transformed into Fisher's Z values where each score represents the functional connectivity strength for each connection in each subject. The connectivity was calculated for all seeds to identify regions showing significant coupling with other regions within the identified speech network. Functional network connectivity (cf. Langner etal., 2014; Roski etal., 2013) was assessed in both of the patient groups. Furthermore, we determined significant differences between patients and controls, and as a final comparison, significant differences in network connectivity between individuals diagnosed with AOS and those patients diagnosed as AOS-absent were identified using independent samples t-tests. Results for network connectivity within and between groups were thresholded at FDR-corrected p < 0.05.
Table 3.
Between-group matching for head motion.
| Group | FD mean (SD) | p (t-test) | RMS mean (SD) | p (t-test) |
|---|---|---|---|---|
| Controls | 0.33 (0.15) | 0.68 | 0.25 (0.13) | 0.97 |
| Patients | 0.35 (0.15) | 0.25 (0.11) |
To assess a possible relationship between functional connectivity and speech and language impairment, Spearman rank-correlation analyses were performed on the individual connectivity strengths between the regions of our network and expert diagnostic rating scale scores in the patient groups. Correlations between diagnostic scale scores and the neural network connectivity were thresholded at FDR-corrected p < 0.05.
3. Results
3.1. Characterization of communication impairment in patients with stroke
Across all 32 patients, the mean WAB Aphasia Quotient was moderate in severity (M = 65.66 ± 25.12). The patients were divided into two groups, 15 with AOS symptoms and 17 without AOS. Furthermore, of the AOS-absent group, three had mild word-finding aphasia, seven had anomia, one suffered from global aphasia, three from Broca's aphasia, one from transcortical sensory aphasia, one from Wernicke's aphasia, and one from conduction aphasia. Of the AOS group, two suffered from global aphasia, one from transcortical motor aphasia, four from conduction aphasia, four from Broca's aphasia, one from Wernicke's aphasia, and four from anomic aphasia. Two patients (one AOS, one AOS-absent) evidenced below normal nonverbal cognitive performance on Raven's Colored Progressive Matrices (M = 26 ± 6.86). Lastly, patients averaged 87.5% (M = 63 ± 17.17) on the Auditory Discrimination of Minimal Pairs subtest of the Psycholinguistic Assessments of Language Processing in Aphasia— 2. Finally, expert ratings of AOS and NVOA severity were moderately correlated (r = .718).
3.2. Lesion overlap data
Fig. 1 displays the results from the lesion overlap map. In the group of 15 patients diagnosed with AOS the regions of highest overlap were the left hemisphere caudate (head), insula and premotor regions (BA6). In the group of 17 patients without AOS symptoms, the highest areas of lesion overlap were the left hemisphere caudate (tail) and postcentral gyrus. Among the current regions of interest, 38% of patients had damage to the left IFG, 59% had damage to the left PM, and 56% had damage to the left aINS. Using post-hoctwo-tailedt-tests thresholded at Bonferroni-corrected p < .05, we found that patients categorized as non-verbal oral apraxia had significantly more damage to all ROIs (left IFG(p = .004), left PM (p < .001) and left aINS (p = .002)), compared to those without non-verbal oral apraxia. Using independent samples t-test thresholded at Bonferroni-corrected p < .05, percent damage to each 5 mm left hemisphere ROI was compared between AOS and AOS-absent patients. The groups did not significantly differ in percent damage to the left IFG (p = .064) or left aINS (p = .027). Percent damage to the left PM seed slightly differed between AOS and AOS-absent groups (p = .008). Multiple correlations determined that percent damage to any ROI was not related to connectivity strength in connections that included those damaged seeds.
3.3. Resting state connectivity in healthy controls and patients with and without AOS
Fig. 2 displays significant resting state connectivity (z-scores) within the identified network in healthy control subjects (a)and all patients (b). The network in healthy controls (Fig. 2a) showed strongest connectivity among bilateral seeds (left–right IFG: z = 5.77; left–right PM: z = 4.73; left–right aINS: z = 4.28). Inter- and intra-hemispheric connectivities between IFG and aINS were also consistent (right IFG–right aINS: z = 4.07; left IFG–left aINS: z = 3.83; left aINS–right IFG: z = 3.65; left IFG–right aINS: z = 3.34). The left IFG was also connected to the right PM (z = 2.94).
Fig. 2.
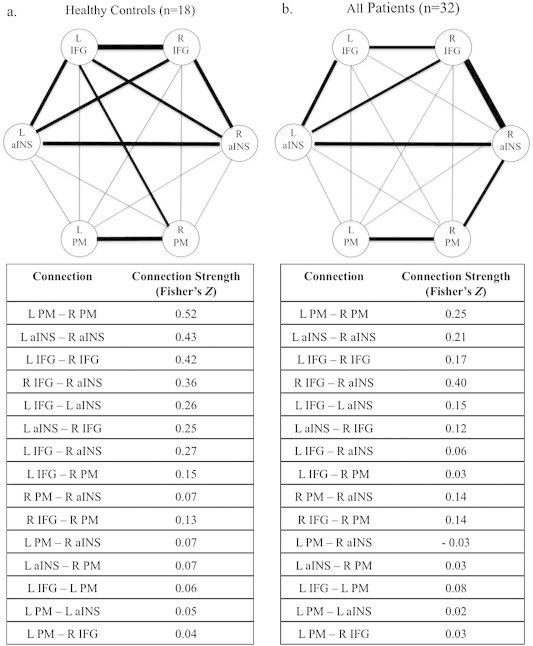
Resting state connectivity of the identified speech network in (a)healthy control subjects and (b)patients. Significant connections are identified with bold lines at a threshold of p < 0.05 FDR-corrected. IFG = inferior frontal gyrus; aINS = anterior insula; PM = premotor cortex.
The network in stroke patients was similar among many of the seed regions, but differed qualitatively among certain nodes. The strongest connection in patients was that of the right IFG and right aINS (z = 6.98). Likewise, each bilateral seed connection was present (left–right aINS: z = 4.21; left–right PM: z = 4.02; left–right IFG: z = 3.26). Intrahemispheric connectivity was also represented among the left IFG and left aINS (z = 3.81), and the right PM and right aINS (z = 3.27). Finally, the left aINS was also connected to the right IFG (z = 3.20).
When comparing patients to healthy controls, each seed's bilateral connections were significantly reduced in patients (Fig. 3; left–right IFG: z = 3.33; left–right PM: z = 2.81; left–right aINS: z = 2.56).
Fig. 3.
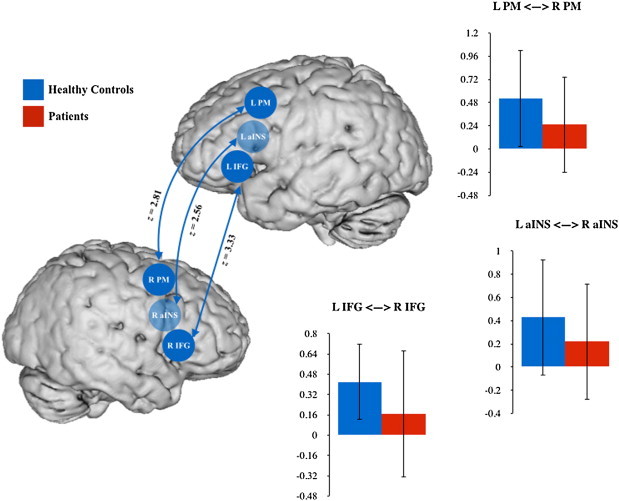
Resting state connectivity differences between healthy control subjects and stroke patients with and without AOS. Significant connections are identified with bold lines at a threshold of p < 0.05 FDR-corrected. IFG = inferior frontal gyrus; aINS = anterior insula; PM = premotor cortex.
In the AOS-absent group (Fig. 4a), there was coupling between the left aINS and right aINS (z = 3.64), right IFG (z = 3.02), and left IFG (z = 2.57). The right IFG was also connected to the left IFG (z = 2.37) and the right aINS (z = 5.01). Finally, the right PM was connected to the right aINS (z = 2.24) and the left PM (z = 3.91).
Fig. 4.
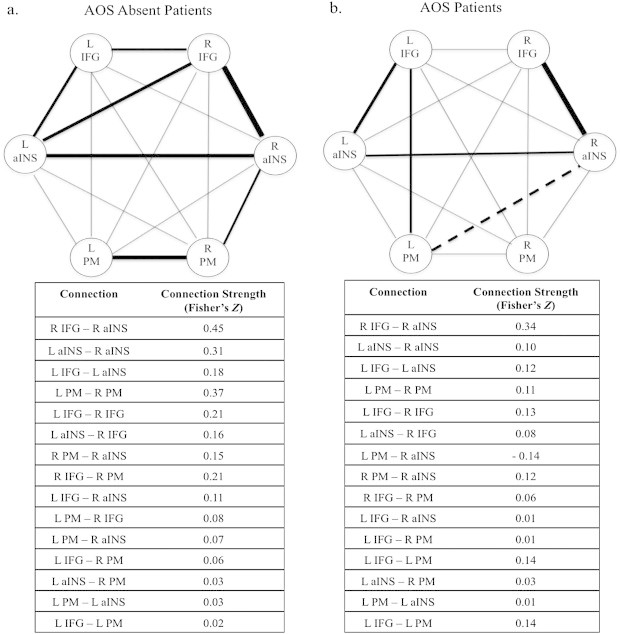
Resting state connectivity of the identified speech network in (a)AOS diagnosed patients, and (b)AOS-Absent patients. Significant connections are identified with bold lines at a threshold of p < 0.05 FDR-corrected. IFG = inferior frontal gyrus; aINS = anterior insula; PM = premotor cortex.
In the AOS group (Fig. 4b), the right aINS was positively connected to the right IFG (z = 4.79) and the left aINS (z = 2.21), but negatively connected to the left PM (z = 2.51). Finally, the left IFG was connected to the left aINS (z = 2.81), and the left PM (z = 2.07).
Group differences (AOS versus AOS-absent) reached significance between the left PM and right aINS (positive in AOS-absent and negative in AOS) and between the left and right PM (more positive for AOS-absent; see Fig. 5).
Fig. 5.
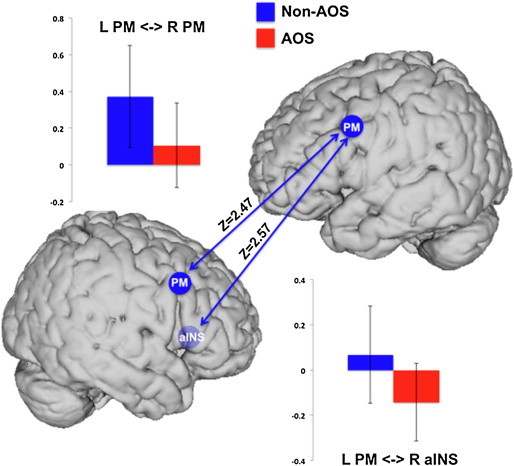
Significant decrease in functional connectivity of left PM and right aINS seeds in the AOS patients (red) when compared to the AOS-absent patients (blue). The blue arrows represent the connections where there was a significant decrease in the AOS group (p < 0.05, FDR corrected). aINS = anterior insula; PM = premotor cortex.
3.4. Correlation of connectivity with expert clinical diagnosis scores
In order to assess whether connectivity within the specified network is associated with expert clinical diagnosis, correlations were performed between expert diagnosis ratings in all patients and connectivity strength of all connections. Results from the correlation analyses (Fig. 6) identified a significant negative correlation between connectivity between the left and right hemisphere PM regions, present in all stroke patients, and expert diagnosis rating of AOS (r = −0.59) in which patients rated with more severe AOS showed reduced connectivity between the PM regions. A significant negative correlation was also seen between severity ratings for non-verbal oral apraxia and connectivity between the left hemisphere PM and right hemisphere aINS regions (r = −0.56). In this case, patients who were rated as more impaired for non-verbal apraxia showed enhanced negative connectivity between the left PM and right aINS. We did not find any significant correlations between connectivity and clinical diagnosis ratings for dysarthria.
Fig. 6.
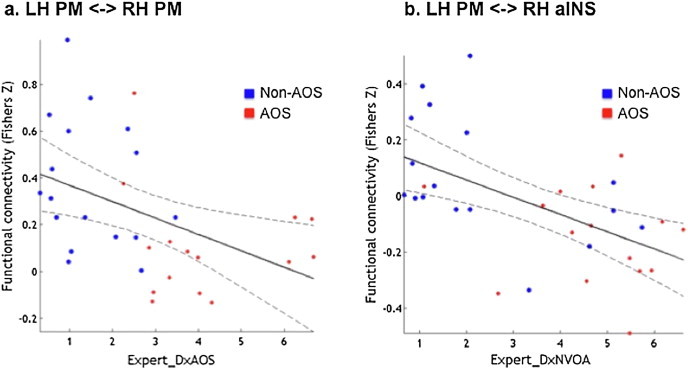
Significant correlation of (a)ExDx AOS with left and right PM coupling and (b)ExDx NVOA with left PM and right aINS coupling. AOS diagnosed patients (n = 15) are shown in red circles while AOS-absent patients (n = 17) are shown in blue.
4. Discussion
We investigated resting state functional connectivity between the PM, IFG and aINS regions in both hemispheres to provide insight into the neurobiological mechanisms of acquired AOS in three groups of subjects. These data add to the sparse neuroimaging information in AOS, provide insight into network connectivity hitherto unavailable for AOS, and provide insights from the brain at rest that are unbiased by task and that likely represent relatively stable brain states. Patients with speech and language problems were grouped based on the presence or absence of AOS. When comparing patient groups with healthy controls we found reductions in connectivity between each bilateral seed region in the patient groups. We found that stroke patients with AOS had reduced functional connectivity between the left and right premotor regions and the left premotor and right aINS regions in comparison to stroke patients without AOS. Importantly, the strength of bilateral PM connectivity was negatively correlated with severity of AOS in this patient group, which is expected and is supported by literature that provides evidence of changes in bilateral BOLD activation in chronic stroke in the motor cortex (James etal., 2009; Rehme and Grefkes, 2013). Furthermore, we did not find any instances of increased positive connectivity within the network in the AOS patients compared to the AOS-absent patients, though we did find that the left PM–right aINS connectivity was negative in the AOS patient network model.
Our findings of altered PM connectivity in AOS relative to AOS-absent patients may be understood in the context of DIVA. The model (Guenther etal., 2006; Tourville etal., 2008) has been recently updated to include a lateralized feedback control map in the right ventral premotor cortex. This region is thought to be involved in transforming error signals into corrective motor commands when incoming sensory feedback does not fall within the range of expected sensory consequences of the speech action. The reduced connectivity between the left and right hemisphere PM regions may be related to disordered preprogramming of speech units. Remapping of functions from the damaged left hemisphere to the right hemisphere PM region could be used in compensation for the interrupted left hemisphere networks. However, an alternative consideration regarding reduced positive left–right PM connectivity is that patients with AOS may be unable to fully compensate using the right PM region for the INT stage. In this case, it is possible that the right aINS may be recruited for compensation. Thisis supported by the negative connectivity seen in AOS patients between the left PM and right aINS. Imaging studies of perturbed speech production in healthy adults have also identified the ventral premotor cortex as having increased activation during the perturbed feedback condition when compared to the unperturbed condition (Golfinopoulos etal., 2011; Tourville etal., 2008; Toyomura etal., 2007), suggesting involvement of this region in speech error correction. Below, we discuss altered connectivity in stroke patients, followed by altered speech network connectivity specific to AOS.
4.1. Altered speech network connectivity in patients with stroke
The comparison of healthy control subjects to stroke subjects is complicated by the fact that the control subjects do not have a lesion in the left hemisphere. Although inter-hemispheric connectivity was significantly reduced across both patient groups following contrast analysis, these lateral connections were also consistently present in both healthy control and patient group models, individually. In addition, the ipsilateral IFG–aINS connection was present in both hemispheres in both patient groups and healthy controls. When looking at individual group models, the healthy controls' connectivity strength is similar in both the left and right hemispheres, but in the patient group, the right hemisphere IFG–aINS connection is similar in strength to healthy controls, yet the left hemisphere IFG–aINS connection appears to be weaker. Functional imaging of unimpaired speech production consistently reports activation of the aINS and IFG regions (e.g. Eickhoff etal., 2009). The left IFG (BA44) is associated with the speech sound map component of DIVA (Guenther etal., 2006) and it has been suggested that the left aINS region might represent involvement in speech motor sequence planning (Bohland and Guenther, 2006). The seemingly weaker connection strength between the left aINS and left IFG is most likely due to over half of the patient sample having extensive damage to the left aINS, but fewer having damage to the left IFG. This may alter the amount and nature of the BOLD signal as recorded during fMRI.
Group differences, which were found in bilateral seed connections, represent a reduction in interhemispheric synchronization of the BOLD signal following stroke. If reorganization of function from left to right hemisphere occurred, this would certainly alter the correlation of BOLD signal in time between the two hemispheres, causing the difference in connectivity. In the current study, we are unable to infer causation and directionality and can therefore only hypothesize about the cause of connectivity influences. One resting state study (James etal., 2009) employed an effective connectivity analysis, structural equation modeling (SEM), to examine changes following an upper limb rehabilitation therapy post-stroke. They found increases in connectivity from the affected hemisphere PM region to the unaffected hemisphere PM region. We can hypothesize that the reduced connectivity we see in stroke compared to healthy controls might be in the direction of the affected to the unaffected hemisphere for the IFG, PM, and aINS regions. Previous inter-hemispheric synchronization is no longer present following stroke, nor have the current patients received rehabilitation; thus, the speech and language functions of these regions degrade, and possibly reorganize to different remote regions in chronic stages. Though interhemispheric motor region resting state connectivity changes are common following stroke, interpretation may still be debatably over-simplified (Rehme and Grefkes, 2013).
4.2. Altered connectivity specific to AOS
Within the speech network hypothesized to be different in stroke patients with AOS, we found different trends of connectivity between two sets of nodes, both including the left PM. Similar to all stroke patients compared to healthy controls, bilateral PM connectivity was reduced in AOS compared to AOS-absent patients. This is further discussed below in the context of its relation to clinical ratings of AOS severity.
Furthermore, patients with AOS differed from those without AOS because they showed a negative relationship between the left PM and right aINS, e.g. lower activity in the left PM was associated with higher activity in the right aINS. This is in keeping with the possibility that the right aINS may play a role in compensating for left PM damage. These results point to the importance of the left PM region in its role in disordered planning and programming of speech following stroke. The nature of neural activity in these regions is unique to AOS. Furthermore, the effect of the two patients who scored below average on Raven's Colored Progressive Matrices is not presumed to be significant, as one was in the AOS group, and the other in the AOS-absent group.
In addition, the lesion overlap analysis shows more overlap on the left PM seed in the AOS group. Damage to the left PM cortex, as supported by intrinsic connectivity that also differentiates AOS and AOS-absent stroke patients, may be a unique factor in developing AOS. However, the current data are not capable of providing enough support to posit this argument. The relationship between neuronal death and BOLD signal is only beginning to be studied, particularly in cerebrovascular diseases (Hall etal., 2014).
4.3. Clinical apraxia severity correlates in the speech network
Previous studies examining resting state connectivity following stroke have found correlations in limb motor behavior or motor impairment with resting state connectivity measures (Chen and Schlaug, 2013; Yin etal., 2012; Yin etal., 2013). Here we found significant correlations with expert clinical ratings of AOS severity in the two connections that showed reduced connectivity in the AOS patient group. Specifically, we found the connectivity between the left and right PM regions to be negatively correlated with expert diagnosis ratings of AOS severity. The “inverse” connectivity between the left PM and right aINS regions was greater in the AOS patient group compared to the AOS-absent patient group and also negatively correlated with expert ratings of non-verbal orofacial apraxia severity. Insula activation is present in a wide range of cognitive and sensorimotor tasks, but the specific role of the right aINS region in speech is less documented. Ballard etal. (2014) previously found that gray matter intensity in the right insula correlated with measures of non-fluency during reading in individuals with progressive aphasia with and without progressive AOS, further emphasizing its involvement in sensory motor loops and cortical integration. The region's documented role in peripheral arousal relating to task difficulty (Kotani etal., 2009) could also suggest involvement in anticipation of sensory feedback during speech. It is likely that the insula is critical to many aspects of speech and integration of information and, therefore, increased negative connectivity of this region with the left PM in AOS, particularly those with severe non-verbal orofacial apraxia, suggests a compensating role of the right aINS, though perhaps unspecific to motor-speech pathology, following left hemisphere damage.
We also found a strong correlation between expert ratings of AOS and NVOA severity, consistent with our previous work (Ballard etal., 2000). Various functional imaging meta-analyses of speech (Brown etal., 2005; Turkeltaub etal., 2002) and non-speech orofacial motor systems (Takai etal., 2010) have shown similarity in regions activated in both speech and non-speech motor tasks. These findings suggest that the brain networks subserving speech and non-verbal orofacial motor systems overlap one another. Though there was a high overlap between AOS and non-verbal oral apraxia ratings in most of the AOS patients, other work in pure AOS following stroke supports the role of the left PM in AOS (Graff-Radford etal., 2014), though further experimental controls are needed to verify these findings. Furthermore, we did not find any significant correlations with connectivity and clinical diagnosis ratings for dysarthria, supporting AOS as a separate clinical entity and the utility of resting state network analysis as a potential clinical tool.
4.4. Limitations
The most notable limitation to the current study is that of the imbalance in gender, particularly between patients and controls. As healthy controls were recruited for this study, they were not explicitly matched to patients based on gender. In order to quantify the effect of gender in the current study, we ran a post-hoc t-test comparison of connectivity across genders, within the control group and the patient group. Though we found no difference in connectivity between males and females in healthy controls (p = .422), or in patients (p = .203), future work should enact gender matching.
Another caveat of the current methodology is that the relationship between structural and functional connectivity in stroke is unclear. A correlation in activity between two regions does not necessarily reflect either direct or indirect anatomical connections between the correlated regions. It could reflect two complementary systems working in synchrony (positive correlation) or opposition (negative correlation). More specifically, the relationship between neuronal death, particularly in populations such as chronic stroke, and the BOLD signal (nonetheless functional connectivity) is unknown. Future studies are needed to understand this relationship. Even if the differences in connectivity seen here are simply a result of impaired or absent anatomic connections between these regions, the results still inform us of the key connections involved in AOS, the impact of lesion on the function and integrity of the speech network and the key differences in patients with aphasia when AOS is judged as present or absent.
Finally, we would also need to examine groups based on lesion site or disorder (i.e.,non-AOS), while controlling for lesion volume, as the current sample did not provide enough patients with these matching characteristics. It might also be important to look at alternative regions, such as the postcentral gyrus, as it was one of the most common sites of lesion overlap, and in some studies it has been a common site of lesion overlap in persons with “pure” AOS, without concomitant dysarthria or aphasia (McNeil etal., 1990). The caudate, the other most common site of lesion overlap in our AOS sample, should also be included in future network connectivity studies.
5. Conclusions
These results suggest that bilateral PM is critically associated with AOS. Interestingly, we did not see connectivity differences between patient groups related to the IFG, given the postulated involvement in AOS. We show that reduced bilateral PM functional connectivity is related to AOS severity, while left PM–right aINS connectivity is related to non-verbal oral apraxia severity. Apraxia of speech severity was also more strongly related to the percent damage of the PM seed than was non-verbal oral apraxia severity. The decrease in connectivity between these regions in patients with AOS compared to AOS-absent patients may result from differences in the severity of aphasia that exist in both groups. However, the connectivity differences clearly relate to neural mechanisms of reorganization and/or symptom. These connectivity differences related to speech production impairment help inform us of the neural mechanisms driving differences in speech motor planning and programming impairment following stroke.
Funding
This work was supported by the National Health and Medical Research Council project grant 632763 (Principal Investigator: Kirrie J. Ballard, PhD).
References
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. 15955494 [DOI] [PubMed] [Google Scholar]
- Ballard K.J., Granier J.P., Robin D.A. Understanding the nature of apraxia of speech: theory, analysis, and treatment. Aphasiology. 2000;14(10):969–995. [Google Scholar]
- Ballard K.J., Savage S., Leyton C.E., Vogel A.P., Hornberger M., Hodges J.R. Logopenic and nonfluent variants of primary progressive aphasia are differentiated by acoustic measures of speech production. PLOS One. 2014;9(2):e89864. doi: 10.1371/journal.pone.0089864. 24587083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandettini P.A., Bullmore E. Endogenous oscillations and networks in functional magnetic resonance imaging. Hum. Brain Mapp. 2008;29(7):737–739. doi: 10.1002/hbm.20607. 18465798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland J.W., Guenther F.H. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. 16730195 [DOI] [PubMed] [Google Scholar]
- Brown S., Ingham R.J., Ingham J.C., Laird A.R., Fox P.T. Stuttered and fluent speech production: an ALEmeta-analysis of functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):105–117. doi: 10.1002/hbm.20140. 15846815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.R., Astafiev S.V., Lang C.E., Connor L.T., Rengachary J., Strube M.J. Resting state inter-hemispheric fMRI connectivity predicts performance after stroke. Ann. Neurol. 2009;67(3):365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.L., Schlaug G. Resting state interhemispheric motor connectivity and white matter integrity correlate with motor impairment in chronic stroke. Front. Neurol. 2013;4:178. doi: 10.3389/fneur.2013.00178. 24223571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabul B.L. Apraxia Battery for Adults. second edition. 2000. -Language-Hearing Association, p. ABA-2: ProEd. [Google Scholar]
- Dronkers N.F. A new brain region for coordinating speech articulation. Nature. 1996;384(6605):159–161. doi: 10.1038/384159a0. 8906789 [DOI] [PubMed] [Google Scholar]
- Duffy J.R. Motor Speech Disorders. Mosby; St. Louis: 2005. [Google Scholar]
- Duffy J.R. Motor Speech Disorders. Mosby; St. Louis: 2013. [Google Scholar]
- Eickhoff S.B., Grefkes C. Approaches for the integrated analysis of structure, function and connectivity of the human brain. Clin. E.E.G. Neurosci. 2011;42(2):107–121. doi: 10.1177/155005941104200211. 21675600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Heim S., Zilles K., Amunts K. A systems perspective on the effective connectivity of overt speech production. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 2009;367(1896):2399–2421. doi: 10.1098/rsta.2008.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. 17704812 [DOI] [PubMed] [Google Scholar]
- Golfinopoulos E., Tourville J.A., Bohland J.W., Ghosh S.S., Nieto-Castanon A., Guenther F.H. fMRI investigation of unexpected somatosensory feedback perturbation during speech. Neuroimage. 2011;55(3):1324–1338. doi: 10.1016/j.neuroimage.2010.12.065. 21195191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J., Jones D.T., Strand E.A., Rabinstein A.A., Duffy J.R., Josephs K.A. The neuroanatomy of pure apraxia of speech in stroke. Brain Lang. 2014;129:43–46. doi: 10.1016/j.bandl.2014.01.004. 24556336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther F.H., Ghosh S.S., Tourville J.A. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain Lang. 2006;96(3):280–301. doi: 10.1016/j.bandl.2005.06.001. 16040108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A., O'Farrell F.M. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55–60. doi: 10.1038/nature13165. 24670647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis A.E., Work M., Barker P.B., Jacobs M.A., Breese E.L., Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127(7):1479–1487. doi: 10.1093/brain/awh172. 15090478 [DOI] [PubMed] [Google Scholar]
- Jakobs O., Langner R., Caspers S., Roski C., Cieslik E.C., Zilles K. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus–context integration. Neuroimage. 2012;60(4):2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. 22387170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James G.A., Lu Z.L., VanMeter J.W., Sathian K., Hu X.P., Butler A.J. Changes in resting state effective connectivity in the motor network following rehabilitation of upper extremity poststroke paresis. Top. Stroke Rehabil. 2009;16(4):270–281. doi: 10.1310/tsr1604-270. 19740732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J., Lesser R., Coltheart M. Hove. Psychology Press; East Sussex: 1992. PALPA: psycholinguistic assessments of language processing in aphasia. [Google Scholar]
- Kertesz A. Western Aphasia Battery— Revised. Harcourt Assessment; San Antonio, TX.: 2006. [Google Scholar]
- Klapp S.T. Reaction time analysis of two types of motor preparation for speech articulation: action as a sequence of chunks. J. Mot. Behav. 2003;35(2):135–150. doi: 10.1080/00222890309602129. 12711585 [DOI] [PubMed] [Google Scholar]
- Kotani Y., Ohgami Y., Kuramoto Y., Tsukamoto T., Inoue Y., Aihara Y. The role of the right anterior insular cortex in the right hemisphere preponderance of stimulus-preceding negativity (SPN): an fMRI study. Neurosci. Lett. 2009;450(2):75–79. doi: 10.1016/j.neulet.2008.11.032. 19028549 [DOI] [PubMed] [Google Scholar]
- Langner R., Cieslik E.C., Behrwind S.D., Roski C., Caspers S., Amunts K., Eickhoff S.B. Aging and response conflict solution: behavioural and functional connectivity changes. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0758-0. 24718622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Zhou M., Yu B., Ma Z., Chen S., Gong Q. Altered default mode and affective network connectivity in stroke patients with and without dysphagia. J. Rehabil. Med. 2014;46(2):126–131. doi: 10.2340/16501977-1249. 24213671 [DOI] [PubMed] [Google Scholar]
- Maas E., Robin D.A., Austermann Hula S.N., Freedman S.E., Wulf G., Ballard K.J., Schmidt R.A. Principles of motor learning in treatment of motor speech disorders. Am. J. Speech Lang. Pathol. 2008;17(3):277–298. doi: 10.1044/1058-0360(2008/025). 18663111 [DOI] [PubMed] [Google Scholar]
- McNeil M.R. Clinical Management of Sensorimotor Speech Disorders. Thieme; New York: 2011. [Google Scholar]
- McNeil M.R., Robin D.A., Schmidt R.A. Clinical Management of Sensorimotor Speech Disorders. Thieme; New York: 1997. Apraxia of speech: definition, differentiation, and treatment; pp. 311–344. [Google Scholar]
- McNeil M.R., Weismer G., Adams S., Mulligan M. Oral structure nonspeech motor control in normal, dysarthric, aphasic and apraxic speakers: isometric force and static position control. J. Speech Hear. Res. 1990;33(2):255–268. doi: 10.1044/jshr.3302.255. 2359266 [DOI] [PubMed] [Google Scholar]
- Nielsen J.M. Agnosia, Apraia, Aphasia: Their Value in Cerebral Localization. Hafner Company, Inc; New York: 1936. [Google Scholar]
- Ogar J., Willock S., Baldo J., Wilkins D., Ludy C., Dronkers N. Clinical and anatomical correlates of apraxia of speech. Brain Lang. 2006;97(3):343–350. doi: 10.1016/j.bandl.2006.01.008. 16516956 [DOI] [PubMed] [Google Scholar]
- Park C.H., Chang W.H., Ohn S.H., Kim S.T., Bang O.Y., Pascual-Leone A., Kim Y.H. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42(5):1357–1362. doi: 10.1161/STROKEAHA.110.596155. 21441147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J.C. Colored Progressive Matrices Sets A, Ab, B. Oxford. Oxford Psychologists Press Ltd; 1947. [Google Scholar]
- Reetz K., Dogan I., Rolfs A., Binkofski F., Schulz J.B., Laird A.R. Investigating function and connectivity of morphometric findings— exemplified on cerebellar atrophy in spinocerebellar ataxia 17 (SCA17) Neuroimage. 2012;62(3):1354–1366. doi: 10.1016/j.neuroimage.2012.05.058. 22659444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehme A.K., Grefkes C. Cerebral network disorders after stroke: evidence fromimaging-based connectivity analyses of active and resting brain states in humans. J. Physiol. (Lond.) 2013;591(1):17–31. doi: 10.1113/jphysiol.2012.243469. 23090951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin D.A., Jack A., Ramage A.E. The neural substrates of apraxia of speech as uncovered by brain imaging: A critical review. In: Ingham R.J., editor. Neuroimaging in communication sciences and disorders. Plural Publishing; San Diego: 2008. pp. 129–154. [Google Scholar]
- Roski C., Caspers S., Langner R., Laird A.R., Fox P.T., Zilles K., Amunts K., Eickhoff S.B. Adult age-dependent differences in resting-state connectivity within and between visual-attention and sensorimotor networks. Front. Aging Neurosci. 2013;5(67):67. doi: 10.3389/fnagi.2013.00067. 24194718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Loughead J., Ruparel K., Elliott M.A., Hakonarson H. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. 22233733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Müller V.I., Hoffstaedter F., Clos M., Goya-Maldonado R., Gruber O., Eickhoff S.B. Meta-analytically informed network analysis of resting state FMRI reveals hyperconnectivity in an introspective socio-affective network in depression. PLOS One. 2014;9(4):e94973. doi: 10.1371/journal.pone.0094973. 24759619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Ramlackhansingh A., Crinion J., Leff A.P., Price C.J. Lesion identification using unified segmentation–normalisation models and fuzzy clustering. Neuroimage. 2008;41(4):1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. 18482850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai O., Brown S., Liotti M. Representation of the speech effectors in the human motor cortex: somatotopy or overlap? Brain Lang. 2010;113(1):39–44. doi: 10.1016/j.bandl.2010.01.008. 20171727 [DOI] [PubMed] [Google Scholar]
- Thompson C.K., den Ouden D.B., Bonakdarpour B., Garibaldi K., Parrish T.B. Neural plasticity and treatment-induced recovery of sentence processing in agrammatism. Neuropsychologia. 2010;48(11):3211–3227. doi: 10.1016/j.neuropsychologia.2010.06.036. 20603138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourville J.A., Reilly K.J., Guenther F.H. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39(3):1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. 18035557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomura A., Koyama S., Miyamaoto T., Terao A., Omori T., Murohashi H., Kuriki S. Neural correlates of auditory feedback control in human. Neuroscience. 2007;146(2):499–503. doi: 10.1016/j.neuroscience.2007.02.023. 17395381 [DOI] [PubMed] [Google Scholar]
- Trupe L.A., Varma D.D., Gomez Y., Race D., Leigh R., Hillis A.E., Gottesman R.F. Chronic apraxia of speech and Broca's area. Stroke. 2013;44(3):740–744. doi: 10.1161/STROKEAHA.112.678508. 23362082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar A.M., Snaphaan L., Shumskaya E., Rijpkema M., Fernandez G., Norris D.G., de Leeuw F.E. Default mode network connectivity in stroke patients. PLOS One. 2013;8(6):e66556. doi: 10.1371/journal.pone.0066556. 23824302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–780. doi: 10.1006/nimg.2002.1131. 12169260 [DOI] [PubMed] [Google Scholar]
- Van den Heuvel M.P., Hulshoff Pol H.E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010;20(8):519–534. doi: 10.1016/j.euroneuro.2010.03.008. 20471808 [DOI] [PubMed] [Google Scholar]
- Wertz R.T., LaPointe L.L., Rosenbek J.C. Apraxia of Speech in Adults: The Disorder and Its Management. Grune & Stratton; Orlando: 1984. [Google Scholar]
- Yin D., Luo Y., Song F., Xu D., Peterson B.S., Sun L. Functional reorganization associated with outcome in hand function after stroke revealed by regional homogeneity. Neuroradiology. 2013;55(6):761–770. doi: 10.1007/s00234-013-1146-9. 23417103 [DOI] [PubMed] [Google Scholar]
- Yin D., Song F., Xu D., Peterson B.S., Sun L., Men W. Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PLOS One. 2012;7(12):e52727. doi: 10.1371/journal.pone.0052727. 23285171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorkston, K., Beukelman, D., Traynor, C. (1984), Computerized Assessment of Intelligibility of Dysarthric Speech. CC Publications
- Zu Eulenburg P., Caspers S., Roski C., Eickhoff S.B. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage. 2012;60(1):162–169. doi: 10.1016/j.neuroimage.2011.12.032. 22209784 [DOI] [PubMed] [Google Scholar]


