Abstract
Blast-related traumatic brain injury (TBI) has been a common injury among returning troops due to the widespread use of improvised explosive devices in the Iraq and Afghanistan Wars. As most of the TBIs sustained are in the mild range, brain changes may not be detected by standard clinical imaging techniques such as CT. Furthermore, the functional significance of these types of injuries is currently being debated. However, accumulating evidence suggests that diffusion tensor imaging (DTI) is sensitive to subtle white matter abnormalities and may be especially useful in detecting mild TBI (mTBI). The primary aim of this study was to use DTI to characterize the nature of white matter abnormalities following blast-related mTBI, and in particular, examine the extent to which mTBI-related white matter abnormalities are region-specific or spatially heterogeneous. In addition, we examined whether mTBI with loss of consciousness (LOC) was associated with more extensive white matter abnormality than mTBI without LOC, as well as the potential moderating effect of number of blast exposures. A second aim was to examine the relationship between white matter integrity and neurocognitive function. Finally, a third aim was to examine the contribution of PTSD symptom severity to observed white matter alterations. One hundred fourteen OEF/OIF veterans underwent DTI and neuropsychological examination and were divided into three groups including a control group, blast-related mTBI without LOC (mTBI - LOC) group, and blast-related mTBI with LOC (mTBI + LOC) group. Hierarchical regression models were used to examine the extent to which mTBI and PTSD predicted white matter abnormalities using two approaches: 1) a region-specific analysis and 2) a measure of spatial heterogeneity. Neurocognitive composite scores were calculated for executive functions, attention, memory, and psychomotor speed. Results showed that blast-related mTBI + LOC was associated with greater odds of having spatially heterogeneous white matter abnormalities. Region-specific reduction in fractional anisotropy (FA) in the left retrolenticular part of the internal capsule was observed in the mTBI + LOC group as the number of blast exposures increased. A mediation analysis revealed that mTBI + LOC indirectly influenced verbal memory performance through its effect on white matter integrity. PTSD was not associated with spatially heterogeneous white matter abnormalities. However, there was a suggestion that at higher levels of PTSD symptom severity, LOC was associated with reduced FA in the left retrolenticular part of the internal capsule. These results support postmortem reports of diffuse axonal injury following mTBI and suggest that injuries with LOC involvement may be particularly detrimental to white matter integrity. Furthermore, these results suggest that LOC-associated white matter abnormalities in turn influence neurocognitive function.
Keywords: Fractional anisotropy, PTSD, Diffusion tensor imaging, OEF/OIF, Loss of consciousness
Highlights
-
•
Diffusion tensor imaging was used to examine blast mild traumatic brain injury.
-
•
114 Iraq and Afghanistan War veterans were studied.
-
•
mTBI + loss of consciousness (LOC) was associated with white matter abnormalities.
-
•
mTBI + LOC influenced verbal memory through white matter integrity.
1. Introduction
Traumatic brain injury (TBI) due to explosive ordnance has been one of the most frequent injuries sustained by Operation Enduring Freedom and Operation Iraqi Freedom (OEF/OIF) military personnel, accounting for over half of all head injuries requiring hospitalization (Wojcik et al., 2010). Due to troop drawdown from Iraq and Afghanistan, thousands of OEF/OIF soldiers have returned home and are living with mild TBI (mTBI). The long-term health consequences of blast-induced mTBI are not yet well known. However, there has been growing concern over chronic cognitive and psychological symptoms reported by many of these individuals, including memory problems, headaches, anxiety and personality changes, as well as negative long-term outcomes such as neurodegenerative disease and suicide (Bruce, 2010; Goldstein et al., 2012; Ursano et al., 2010). The negative outcomes observed may be related to changes in neuronal microstructure that occur as a result of traumatic axonal injury (TAI), the primary neuropathology of TBI. However, until recently, there was little direct evidence of chronic neuronal injury following blast-related mTBI in humans, and the impact of this type of injury on public health is still being debated (Wilk et al., 2012).
A significant challenge in detecting brain changes following blast-related mTBI is that axonal injury in mTBI is less extensive than in moderate and severe injuries (Blumbergs et al., 1995) and therefore unlikely to be detected by standard imaging procedures such as CT. Until recently, few tools were available to evaluate mTBI in vivo. Importantly, research has demonstrated that advanced neuroimaging techniques such as diffusion tensor imaging (DTI) are sensitive to changes in white matter microstructure following TBIs of varying severity and chronicity (Kraus et al., 2007). Fractional anisotropy (FA), one well-established measure of water anisotropy, may reflect the disruption to axonal integrity observed in TAI.
Recent DTI studies that have examined the impact of blast-induced mTBI on white matter integrity have reported mixed findings (Bazarian et al., 2013; Davenport et al., 2012; Jorge et al., 2012; Levin et al., 2010; Mac Donald et al., 2011). Whereas Mac Donald et al. (2011) reported that specific regions such as the cerebellum are impacted by blast, others have provided evidence for diffuse abnormalities in white matter not constrained to particular regions (Davenport et al., 2012; Jorge et al., 2012). Still other studies have not found a link between white matter abnormalities and blast-related mTBI using region-of-interest (ROI; Levin et al., 2010) or voxel-based (Bazarian et al., 2013) analyses. Further complicating interpretation of the mTBI literature, several of these studies made group comparisons using non-independent control samples (for discussion, see Watts et al. 2014), potentially biasing their data analyses and inflating the differences between the control and mTBI groups. Watts and colleagues demonstrated that group differences between a control and TBI group disappeared after adjusting for bias in the analysis. Thus, an accurate and consistent picture of the distribution of white matter changes following blast-related mTBI has yet to emerge.
Another factor that may contribute to the mixed findings is the difficulty in accurately diagnosing mTBI following explosive blast, particularly when alterations in consciousness, including feelings of confusion and memory loss, may be attributable to emotional trauma rather than concussion. By contrast, loss of consciousness (LOC) may be a reliable indicator of the presence and severity of concussion as it is more likely to be noticed and reported by witnesses than other alterations in consciousness. LOC has been shown to be an important predictor of brain volume loss over time (MacKenzie et al., 2002), postconcussive symptoms (Wilk et al., 2012) and psychosocial limitations, even after adjusting for psychological symptoms (Verfaellie et al., 2013). Two recent studies also suggest that LOC may be associated with greater white matter abnormalities, but these studies did not measure spatial heterogeneity (Matthews et al., 2012; Sorg et al., 2014). In light of these findings, as well as the proposal that mTBI with LOC is associated with more widespread structural damage (Ommaya and Gennarelli, 1974), we examined the extent to which mTBI with LOC was associated with greater white matter abnormalities than mTBI without LOC, using both measures of region-specific and spatially heterogeneous abnormality.
DTI findings of blast-induced mTBI are also likely to be complicated by the high PTSD comorbidity rate in this population, making it important to disentangle the contribution of these conditions to white matter abnormalities. Few studies have examined the impact of PTSD on white matter integrity following mTBI, despite some evidence that chronic PTSD may be associated with reductions in FA in specific white matter tracts (Schuff et al., 2011). Additional research is necessary to examine the unique contribution of PTSD to white matter integrity in this population.
In the present study, we used a non-biased approach in a large cohort of OEF/OIF veterans (N = 114) to address three primary issues that remain unresolved in the blast-related mTBI literature. These are: 1) the extent to which white matter abnormalities due to blast-related mTBI are region-specific or spatially heterogeneous, and the effect of LOC and repetitive blast exposure on white matter anisotropy; 2) the relationship between white matter abnormalities and neurocognitive function; and 3) the contribution of PTSD symptom severity to the observed white matter abnormalities.
2. Materials and methods
2.1. Participants
Participants were 114 veterans who had been deployed in support of OEF/OIF (see Table 1 for participant demographics). Fifty-five veterans comprised a control group without TBI. Participants were excluded from the control group if they reported TBI from blast, blunt force, or any other mechanism of injury during their deployment. Thirty-seven (67%) of the individuals in this group had been exposed to deployment-related blast but reported no subsequent changes in mental status suggestive of TBI; the remaining 18 (33%) had not been exposed to blast. There were no differences in demographics and DTI measures between controls with and without blast exposure (see Inline Supplementary Table S1, Inline Supplementary Fig. S1). Fifty-nine veterans reported mTBI due to blast exposure within 100 m and were further divided into an mTBI without LOC (mTBI - LOC) group (n = 31), and an mTBI with LOC (mTBI + LOC) group (n = 28). To limit our TBI sample to individuals with mild severity, participants reporting posttraumatic amnesia >24 h or LOC >30 min were excluded. There were no participants with secondary blast injuries (e.g., fragments of shrapnel). A subset of participants in the mTBI groups reported having tertiary injuries (e.g., being thrown against an object; see Table 1). Thus, any reference to blast-related injuries in this study refers to blast mechanisms with additional tertiary injury in some cases. Because the goal of this study was to examine blast-related mTBI, participants with mTBI not associated with blast during their deployment were excluded. Participants were also excluded from the study if they reported a history of pre-deployment mTBI + LOC or, in the case of mTBI - LOC, if symptoms persisted more than 3 months after the injury. Participants were further excluded if they had structural brain abnormalities (e.g., hemorrhages, hematomas, skull fractures, tumors, excessive hyperintensities, hemispheric asymmetries) as determined by a board-certified neuroradiologist who reviewed FLAIR, susceptibility-weighted, and high resolution T1 scans. Participants who reported high levels of current alcohol use (>25 drinks per week) or showed questionable effort with raw scores below 45 on the retention trial of the Test of Memory Malingering (Tombaugh and Tombaugh, 1996) were also excluded. Participants were recruited through the VA Boston Polytrauma Network and through flyers and outreach events in the community. Study procedures took place outside the clinical context and were independent of diagnostic outcomes or treatment plans. Study procedures were approved by the VA Boston Institutional Review Board and all participants provided written informed consent consistent with the Declaration of Helsinki. Sixty-three participants (23 blast-exposed control, 21 mTBI - LOC, and 19 mTBI + LOC participants), whose neuropsychological findings were part of a separate report (Verfaellie et al., 2014), are also included in this study.
Table 1.
Demographic and clinical characteristics.
| Controls (n = 55) | mTBI − LOC (n = 31) | mTBI + LOC (n = 28) | Group comparison | |
|---|---|---|---|---|
| Age in years, M (SD) | 30.5 (6.7) | 29.6 (7.7) | 27.9 (4.2) | F(2,111) = 1.484, P = 0.231 |
| Males, no. (%) | 49 (89.1) | 30 (96.8) | 28 (100.0) | χ2(2) = 4.459, P = 0.108 |
| Education in years, M (SD) | 14.1 (2.2)a | 13.0 (1.6) | 12.9 (1.4) | F(2,111) = 4.465, P = 0.014 |
| WTAR z, M (SD) | 0.4 (0.8) | 0.5 (0.7) | 0.3 (0.8) | F(2,111) = 0.453, P = 0.637 |
| Blast exposures, M (SD) | 9.4 (11.7)b | 12.6 (12.2) | 7.2 (8.6) | F(2,93) = 1.852, P = 0.163 |
| Blast plus tertiaryc mechanism, no. (%) | 0b | 3 (13)d | 14 (78)e | χ2(1) = 18.192, P < 0.001 |
| Blast to MRI scan interval in months, M (SD) | 49.8 (36.3)b | 43.8 (27.2) | 56.3 (23.2) | F(2,93) = 1.836, P = 0.165 |
| CAPS total, M (SD) | 43.6 (29.7)a | 58.4 (24.6) | 63.2 (23.7) | F(2, 111) = 5.961, P = 0.003 |
| Current alcoholic drinks per week, M (SD) | 4.6 (6.2) | 3.4 (4.6) | 4.4 (7.2) | F(2,111) = 0.999, P = 0.372 |
Note: For ease of interpretation, mean (M) and standard deviation (SD) reflect non-transformed data for non-normal variables. TBI = traumatic brain injury. LOC = loss of consciousness. WTAR = Wechsler Test of Adult Reading. CAPS = Clinician-Administered PTSD Scale.
Group that was significantly different from other groups.
Includes only blast-exposed controls.
No subject had secondary blast injury.
For 7 subjects, it could not be determined if they experienced blast plus impact injury.
For 10 subjects, it could not be determined if they experienced blast plus impact injury.
Inline Supplementary Table S1.
Table S1.
Demographic and clinical characteristics comparing the no-blast controls to the blast-exposed controls.
| No-blast controls (n = 18) | Blast-exposed controls (n = 37) | Group comparison | |
|---|---|---|---|
| Age in years, M (SD) | 31.1 (7.7) | 30.2 (6.3) | ns, P > 0.05 |
| Males, no. (%) | 14 (78) | 35 (95) | ns, P > 0.05 |
| Education in years, M (SD) | 14.6 (2.4) | 13.8 (2.1) | ns, P > 0.05 |
| WTAR z, M (SD) | 0.4 (0.9) | 0.3 (0.8) | ns, P > 0.05 |
| CAPS total, M (SD) | 32.8 (31.4) | 48.8 (27.8) | ns, P > 0.05 |
| Current alcoholic drinks per week, M (SD) | 4.6 (5.7) | 4.6 (6.4) | ns, P > 0.05 |
Note: WTAR = Wechsler Test of Adult Reading; CAPS = Clinician-Administered PTSD Scale.
Inline Supplementary Figure S1.
Fig. S1.
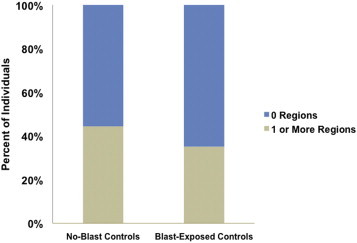
White matter abnormality distribution among control subgroups. There was no significant difference between no-blast controls and blast-exposed controls in percentage of individuals who had the presence of one or more abnormal ROI(s), (P > 0.05).
2.2. Clinical assessment procedure
A licensed clinical neuropsychologist with extensive experience in administering clinical interviews in outpatient, research, and rehabilitation settings conducted TBI, PTSD, and neuropsychological assessments.
Evaluation of TBI was based on an extensive clinical interview that queried participants about their blast exposure(s). The interview was structured in four parts: (1) determination of the ‘index event,’ which was the most severe blast exposure based on the length of LOC or posttraumatic amnesia; (2) in-depth description of the index event, including the participant's memory for the events preceding and subsequent to the blast, and presence of posttraumatic amnesia or LOC; (3) questions pertaining to the presence of neurological symptoms immediately after the blast that are consistent with TBI; and (4) inquiry regarding medical examination or reports by a witness. The TBI interview was modeled after a validated interview by Fortier et al. (2014), but was condensed in that it probed in detail only the most severe blast event. For individuals in the LOC group, in all but three cases information the participant obtained from a medic or peers who had witnessed the event was used to determine the presence and duration of LOC. Corroborative witness data were not available for individuals without LOC due to the fact that alterations in consciousness are often imperceptible. Participant interviews were transcribed and evaluated by two licensed clinical neuropsychologists who then sought consensus as to whether a minimal biomechanical threshold for concussion had plausibly been met, and any reported disorientation was the result of concussion rather than situational chaos and confusion.
PTSD was assessed using the Clinician-Administered PTSD Scale (CAPS) for DSM-IV (Blake et al., 1995). Continuous CAPS scores were used as a measure of PTSD symptom severity. CAPS scores were unavailable for three participants and were estimated based on their score on the PTSD Checklist-Military version (PCL-M, Weathers et al., 1991). The PCL is a self-report questionnaire that has good convergent validity with the CAPS (Wilkins et al., 2011). A linear regression model was generated using PCL scores of the remaining dataset as predictors of their CAPS scores. Using this model, the three individuals' PCL scores were entered into the equation to generate their predicted CAPS scores.
2.2.1. Neuropsychological assessment
Participants were administered the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) as an estimate of premorbid IQ as well as a battery of neuropsychological tests selected for their sensitivity to mTBI. For data reduction purposes, test z-scores were averaged to create five composite measures representing performance in the domains of attention, executive functions, verbal memory, visuospatial memory, and psychomotor speed. The attentional composite was based on number + letter sequencing time from the D-KEFS Trail Making Test, Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Span score, and WAIS-III Digit Symbol Coding score. The executive functioning composite was based on the D-KEFS Verbal Fluency Test score, the number-letter switching score from the D-KEFS trail Making Test, the inhibition score from the D-KEFS Color Word Interference Test, and the Auditory Consonant Trigrams Test score averaged over 9, 18 and 36 s delays. The verbal memory composite included measures of encoding and retrieval derived from the California Verbal Learning Test (CVLT), with trials 1–5 total, long delay free recall, and recognition discriminability as dependent measures. The visuospatial memory composite included total recall, delayed recall, and the recognition discrimination index of the Brief Visuospatial Memory Test (BVMT). The psychomotor speed composite was based on scores from the Finger Tapping Test, the Purdue Pegboard Test, and the D-KEFS Trail Making motor speed score. Test z-scores were calculated using published standardized normative data from civilians. For all tests for which scaled scores are available, z-scores were calculated from these normalized data. Three individuals had missing digit span data and two of those three also had missing digit symbol coding scores; their attention composite scores were based on the remaining measures included in this composite score.
2.3. Image acquisition and processing
Structural imaging data were acquired on a 3-Tesla Siemens Trio whole-body MRI scanner located at the VA Boston Healthcare System, Jamaica Plain Campus. Two T1-weighted anatomical MRI scans were collected for each participant. T1 scan parameters for 37 participants were the following: FOV = 256, Matrix = 240 × 256, 160 slices, 1 × 1 × 1.2 mm voxels, TR = 2300 ms, TE = 2.98 ms, flip angle = 9°. For the remaining participants, a slightly modified T1 sequence was used: FOV = 256, Matrix = 256 × 256, 176 slices, 1 × 1 × 1 mm voxels, TR = 2530 ms, TE = 3.32 ms, flip angle = 7°. DTI scan parameters for 37 participants were the following: two acquisitions of 30 directions averaged for a total of 60 diffusion weighted images, FOV = 256, Matrix = 128 × 128, TR = 8000 ms, TE = 83 ms, 2 × 2 × 2 mm voxels, b value = 700 s/mm2. For the remaining participants, a slightly modified DTI sequence was used: one acquisition of 60 directions, FOV = 256, Matrix = 128 × 128, TR = 10,000 ms, TE = 103 ms, 2 × 2 × 2 mm voxels, b value = 700 s/mm2. The sequences were modified in order to align them with the pulse sequence of a separate study protocol for data sharing. Several studies support the reproducibility of FA across sequences (Cercignani et al., 2003; Landman et al., 2007). Furthermore, all analyses were adjusted for DTI sequence as outlined in the Statistical Approach below. Roughly equivalent percentages of individuals in each group were scanned with sequence 1 (controls = 27%; mTBI − LOC = 39%; mTBI + LOC = 36%) and sequence 2 (controls = 73%; mTBI − LOC = 61%; mTBI + LOC = 64%; χ2 (2, N = 114) = 1.36, P > 0.51).
The data were analyzed using a combination of the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu) and The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) FSL software package (http://www.fmrib.ox.ac.uk/fsl). Images were corrected for motion and eddy currents using Freesurfer. FA images were created by fitting a tensor model using linear least squares to the raw diffusion data using the Freesurfer command dt_recon. Images were brain-extracted using BET (Smith, 2002) to remove non-brain voxels from the analysis.
To perform ROI analyses based on semi-automated methods, the John Hopkins White Matter Parcellation Atlas (JHU WMPA) and the Freesurfer-derived white matter parcellations were merged. These atlases provide complementary data, with the JHU WMPA atlas providing detailed labeling of deeper white matter structures, regions, and tracts and the Freesurfer parcellations providing a measure of white matter that extends to the cortical surface. The combined atlas was then multiplied by the mean FA skeleton mask derived from the FMRIB58_FA template in FSL's Tract Based Spatial Statistics program (TBSS; Smith et al., 2006) to limit segmentation to the white matter skeleton. Using the inverse-warp of each subject's nonlinear registration to standard space, the combined segmented atlas was then transformed back into each individual's respective native DTI space using FSL's tbss_deproject program. Once the segmented skeleton was in registration with each participant's native DTI image, the mean FA values were extracted for 38 ROIs hypothesized to be affected by mTBI, including: the genu, body, and splenium of the corpus callosum, the middle cerebellar peduncle, and the left and right hemispheres of the following ROIs: corticospinal tract, anterior limb of the internal capsule, posterior limb of the internal capsule, retrolenticular part of the internal capsule, anterior corona radiata, posterior corona radiata, sagittal stratum, external capsule, cingulum cingulate, cingulum hippocampus, fornix stria, superior longitudinal fasciculus, uncinate fasciculus, cerebellum white matter, inferior cerebellar peduncle, superior cerebellar peduncle, and posterior thalamic radiation.
2.4. Statistical approach
2.4.1. Demographic analysis
Statistical analyses were performed using SPSS, version 21 (IBM Corp., Armonk, NY). mTBI group differences in demographic characteristics were examined using one-way ANOVA for continuous data and chi-square for categorical data. Non-normal variables including age, education, WTAR, alcoholic drinks per week, blast load, and the time between injury and scan were first transformed before they were entered in group analyses. Education was added as a covariate to regression models but did not change the pattern of results in any of the analyses and is therefore not included in the analyses.
2.4.2. Region specific vs. spatially heterogeneous white matter abnormalities
To examine whether TBI and PTSD were associated with reduced FA in specific ROI tracts, separate hierarchical linear regression models were used for each ROI, correcting for multiple comparisons using the Benjamini–Hochberg false discovery rate method (FDR), q = 0.05. In recognition of the possibility that the FDR correction is overly conservative, particularly when ROIs are correlated, we also took the approach of Levin et al. (2010) and examined models that were significant at a more lenient uncorrected threshold of P < 0.01. In this analysis, mean FA in each of the 38 tracts was the outcome variable. Age, WTAR, and DTI sequence were included in the first step of the model as nuisance regressors, mTBI group status (controls, mTBI - LOC, and mTBI + LOC coded as dummy variables) was added in the second step, followed by PTSD symptom severity (i.e., CAPS total score) and the PTSD symptom severity by the mTBI group status interaction in the third step.
To examine whether blast-related mTBI was characterized by spatially heterogeneous reductions in FA rather than reductions in FA in specific white matter tracts, we followed previously published procedures (Kraus et al., 2007; Mac Donald et al., 2011) of calculating the mean and standard deviation (SD) of the control group for each ROI and deriving z-scores for each individual based on those values. Unlike previous methods that introduced bias by using the same reference group that was used to define abnormal white matter regions to compare group differences, we conducted a leave-one-out cross-validation analysis in which each control subject's z-score was derived from the mean and SD of the remaining control subjects' FA values for a particular ROI (see Watts et al., 2014). A dichotomous variable was created, reflecting the absence or presence of at least one abnormal ROI (i.e., ROIs with FA z-scores ≤ −2). To adjust for DTI sequence, z-scores for individuals scanned with the original DTI sequence were based on control subjects scanned with the original sequence and z-scores for individuals scanned with the modified sequence were based on control subjects scanned with the modified sequence.1 The dichotomized abnormal ROI variable is sensitive to spatial heterogeneity, as individuals with TBI could have any white matter tract counted as abnormal, rather than constraining all participants to have reductions in FA in the same tract as is the case for the mean FA ROI analysis described above. Hierarchical binary logistic regression analysis to predict the presence of abnormal ROIs was conducted with age and WTAR included in the first step of the model, TBI group status added in the second step, and PTSD symptom severity and the PTSD symptom severity by mTBI group status interaction added in the third step.
2.4.3. Loss of consciousness and extent of white matter abnormalities
Given prior work suggesting that LOC is associated with greater widespread structural damage than TBI without LOC, we next examined whether LOC was associated with a higher number of ROIs with z-scores ≤ −2. To use this metric as an outcome variable in later regression analyses, the total number of abnormal ROIs for each participant was square-root transformed to account for the positive skew of the data. Because the goal of this analysis was to examine the effect of LOC on extent of white matter abnormality, individuals with LOC were compared against all individuals without LOC grouped together. Although this involved combining individuals from the control group with the mTBI - LOC group, examination of the total number of ROIs showed that the two groups did not differ (see Inline Supplementary Fig. 2). A two-sample t-test was used to examine group differences.
Inline Supplementary Figure S2.
Fig. S2.
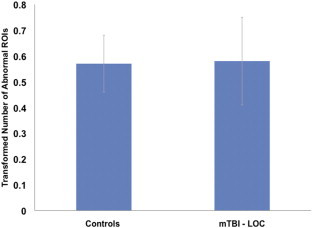
Number of abnormal ROIs among control and mTBI − LOC groups. There was no significant difference in number of abnormal ROIs (P > 0.05). mTBI − LOC = mild traumatic brain injury without loss of consciousness.
2.4.4. Blast injury characteristics and white matter abnormalities
To examine the effect of number of blast exposures (blast load), we first ran hierarchical linear regression analyses with mean FA in each ROI as the outcome variable. Age, WTAR, and DTI sequence were entered as nuisance variables in the first model, blast load was entered in next, and mTBI group status and the blast load by mTBI group status interaction were entered in the final model. The FDR method was used to correct for multiple comparisons. Similar to the region-specific analysis described in Section 2.4.2 above, we also examined models that were significant at a more lenient threshold of P < 0.01. The same factors entered in the linear regression were entered into a logistic regression model with age and WTAR as nuisance variables to examine whether blast load was a significant predictor of the presence of abnormal ROIs.
To examine the effect of mechanism of injury (i.e., primary blast vs primary blast plus tertiary injury), we first ran a hierarchical linear regression model with mean FA in each ROI as the outcome variable. Age, WTAR, and DTI sequence were entered as nuisance variables in the first model, and injury mechanism was entered as a predictor of mean FA in the second model. These models were first corrected for multiple comparisons using FDR and were also examined at a more lenient threshold of P < 0.01. Next, these same factors were entered into a logistic regression model with age and WTAR as nuisance variables to examine whether injury mechanism was a significant predictor of the presence of abnormal ROI(s). For 17 participants with blast-related TBI, it could not be determined whether they had additionally experienced an impact injury. These participants were excluded from the analyses pertaining to injury mechanism.
2.4.5. Mediation of neurocognitive function
Finally, a mediation analysis was performed to examine whether the relationship between mTBI and neurocognitive function was influenced by the number of abnormal ROIs. In simple mediation models, the independent variable (presence of LOC) can exert an indirect effect on the dependent variable (neurocognitive composite scores) through an intermediary variable (total number of abnormal ROIs). The model was tested with and without CAPS as a covariate. Direct and indirect effects were examined using the Mediate macro for SPSS (Hayes and Preacher, 2014). Bootstrapping was used to estimate the sampling distribution (n = 5000) and 95% confidence intervals for the indirect effect. Helmert coding was used in linear models to test the direct effect between mTBI group status (using LOC as the comparison group) and cognitive outcome, as well as the effect of mTBI group status on total number of abnormal ROIs. The only neurocognitive domain that was significantly associated with total number of abnormal ROIs was verbal memory. Thus, we tested the model that the presence of LOC had an indirect effect on verbal memory through its effect on the total number of abnormal ROIs.
3. Results
3.1. Demographic characteristics
The groups did not significantly differ in age, gender, WTAR, number of alcoholic drinks per week, number of blast exposures, or interval between time of index blast event and MRI scan. The two mTBI groups also did not significantly differ in PTSD symptom severity although both of them had greater PTSD symptoms than the control group. The control group had higher education than the other groups. The mTBI + LOC group had a higher number of individuals with tertiary blast injury than the other groups.
3.2. Region specific vs. spatially heterogeneous white matter abnormalities
Hierarchical linear regression failed to show an effect of group on mean FA for any specific ROI after controlling for multiple comparisons, consistent with previous reports that did not find mTBI-related differences using an ROI approach (Davenport et al., 2012; Levin et al., 2010). However, when examining the uncorrected results at P < 0.01, two ROIs showed reduced FA in the mTBI + LOC group, including the splenium of the corpus callosum and right sagittal stratum. These results are shown in Inline Supplementary Table 2. PTSD symptom severity or the PTSD by mTBI group status interaction was not significantly associated with mean FA in any ROI after multiple comparisons correction. However, when reducing the threshold to P < 0.01, a PTSD symptom severity by mTBI group status interaction was observed in the left retrolenticular part of the internal capsule such that the mTBI + LOC group had lower FA values in this region with increasing PTSD symptom severity (see Inline Supplementary Table 3).
Inline Supplementary Table S2.
Table S2.
ROIs that survive P < 0.01 for region specific analysis.
| ROI | mTBI + LOC v Controls |
mTBI + LOC v mTBI − LOC |
R2 | ΔR2 | ΔF (P) |
|---|---|---|---|---|---|
| β (P) | β (P) | ||||
| Splenium of corpus callosum | 0.30 (0.003) | 0.30 (0.003) | 0.33 | 0.07 | 5.78 (0.004) |
| Right sagittal stratum | 0.35 (0.002) | 0.25 (0.02) | 0.22 | 0.08 | 5.40 (0.006) |
Note: Coefficients are standardized values. Values represent significant effect of adding mTBI group status in hierarchical linear regression. mTBI = mild traumatic brain injury. LOC = loss of consciousness. P = P value. ROI = region of interest.
Inline Supplementary Table S3.
Table S3.
ROI that survive P < 0.01 for PTSD symptom severity by mTBI group status interaction.
| ROI | PTSD ∗ Controlsa |
PTSD ∗ mTBI − LOCb |
R2 | ΔR2 | ΔF (P) |
|---|---|---|---|---|---|
| β (P) | β (P) | ||||
| Left retrolenticular part of the internal capsule | 0.85 (0.001) | 0.60 (0.045) | 0.23 | 0.09 | 4.09 (0.009) |
Note: Values represent significant effect of adding CAPS score in hierarchical linear regression. Coefficients are standardized values. a mTBI+LOC is the reference group for comparison with Controls. b mTBI + LOC is the reference group for comparison with mTBI − LOC group. PTSD = posttraumatic stress disorder. CAPS = Clinician Administered PTSD Scale. P = P value. ROI = region of interest.
Hierarchical logistic regression was performed to examine whether mTBI group status and PTSD were associated with the presence of one or more abnormal ROIs, thus reflecting spatial heterogeneity of injury. Results showed that mTBI group status was the only significant predictor with a significant overall model [χ2 (4, N = 114) = 10.44, P < 0.04, Negelkerke R2 = 0.12] and significant change in the chi square equation [Δχ2 (2, N = 114) = 6.79, P < 0.04]. The mTBI + LOC group was 3.26 times more likely to have one or more abnormal ROIs than the control group (P < 0.02) and 3.36 times more likely than the mTBI - LOC group (P < 0.04). The control and mTBI - LOC groups did not differ from each other (P > 0.9). Percent of individuals in each group with one or more abnormal ROIs is shown in Fig. 1.
Fig. 1.
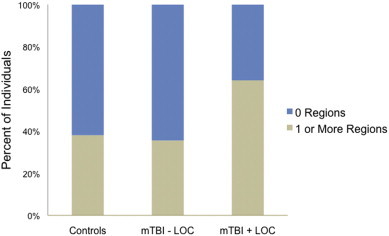
White matter abnormality distribution among groups. A greater percentage of individuals in the mTBI + LOC group than in the control group had at least one brain region of reduced FA. mTBI = mild traumatic brain injury; LOC = loss of consciousness.
For illustration purposes only, Fig. 2 demonstrates that the percentage of individuals who show abnormal FA in any particular ROI is low, suggesting that the abnormalities are spread throughout the brain. Inline Supplementary Fig. 3 shows the outlines of each white matter ROI displayed in Fig. 2.
Fig. 2.
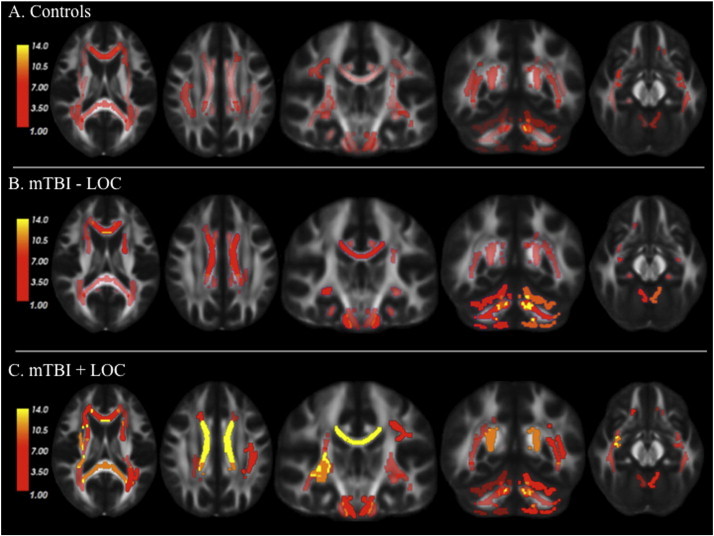
Spatially distributed reductions in FA among groups. This figure represents the percentage of individuals in each group who showed abnormal FA in the various ROIs in comparison to control values. Values vary from 1% to 14% (with low values indicated in dark red, intermediate values in light red, and high values in yellow) suggesting that there is limited spatial overlap in white matter abnormalities across individuals. mTBI-LOC = mild traumatic brain injury without loss of consciousness. mTBI + LOC = mild traumatic brain injury with loss of consciousness.
Inline Supplementary Figure S3.
Fig. S3.
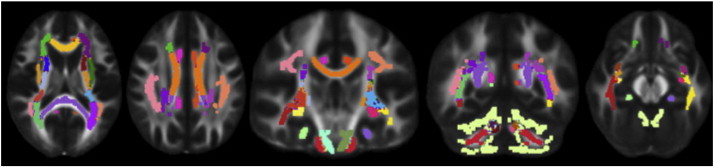
Visualization of white matter ROI tracts examined in the study. 38 ROIs were examined, including the genu, body, and splenium of the corpus callosum, the middle cerebellar peduncle, and the left and right hemispheres of the following ROIs: corticospinal tract, anterior limb of the internal capsule, posterior limb of the internal capsule, retrolenticular part of the internal capsule, anterior corona radiata, posterior corona radiata, sagittal stratum, external capsule, cingulum cingulate, cingulum hippocampus, fornix stria, superior longitudinal fasciculus, uncinate fasciculus, cerebellum white matter, inferior cerebellar peduncle, superior cerebellar peduncle, and posterior thalamic radiation.
By contrast, PTSD symptom severity was not significantly associated with one or more abnormal ROIs.
3.3. Loss of consciousness and extent of white matter abnormalities
We next examined whether individuals with LOC had a greater number of ROIs with low FA as an indicator of extent of white matter abnormalities. Consistent with the hypothesis that LOC is associated with greater extent of white matter abnormality, individuals with LOC had a significantly higher number of abnormal ROIs (square-root transformed M = 1.02, SD = 0.9) than individuals without LOC (square-root transformed M = 0.57, SD = 0.83), two-sample t(112) = 2.41, P < 0.02. The number of abnormal ROIs observed in the mTBI + LOC group in comparison to the number of ROIs expected based on the other two groups is shown in Fig. 3, illustrating that the mTBI + LOC group had greater-than-expected number of ROIs below control values.
Fig. 3.
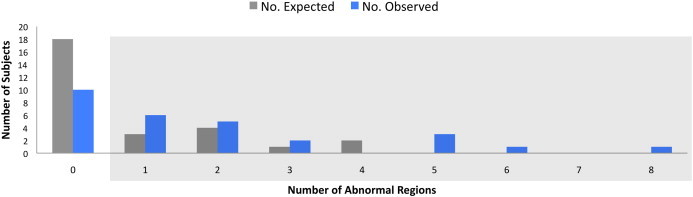
Number of abnormal ROIs expected versus observed in the mTBI + LOC group. The mTBI + LOC group had greater than expected abnormal ROIs (i.e., FA z-scores ≤ −2). The box indicates subjects with one or more abnormal ROIs. For illustration purposes, the total number of ROIs is shown in lieu of square-root transformed scores. mTBI + LOC = mild traumatic brain injury with loss of consciousness.
3.4. Blast injury characteristics and white matter abnormalities
Linear regression revealed a significant F change in the left retrolenticular part of the internal capsule in the final model [ΔF(4,105) = 5.41, P < 0.001, R2 = 0.22]. The blast load by mTBI group status interaction term was significant for both the control group versus mTBI + LOC group (P < 0.004) and the mTBI - LOC group versus the mTBI + LOC group (P < 0.03) such that for individuals with LOC, higher blast load was associated with lower FA in this region (see Fig. 4A). At an uncorrected threshold, this pattern was also observed in the right sagittal stratum [ΔF(4,105) = 4.13, P < 0.004, R2 = 0.28]. The blast load by mTBI group status interaction term was marginally significant for the control group versus mTBI + LOC group (P < 0.07) and significant for the mTBI - LOC group versus the mTBI + LOC group (P < 0.02) (see Fig. 4B). Results of the logistic regression indicated that blast load or the interaction term were not associated with the presence of abnormal ROIs.
Fig. 4.
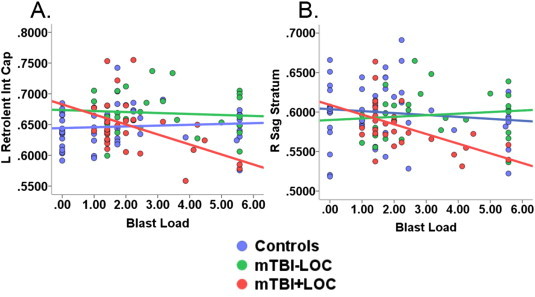
Blast load by mTBI group status interaction is significant in two specific ROIs. (A) The mTBI + LOC group had lower FA values in the left retrolenticular part of the internal capsule with increasing blast load after correcting for multiple comparisons. This pattern was not observed in the control or mTBI - LOC groups (B). The mTBI + LOC group had lower FA values in the right sagittal stratum with increasing blast load, with no effect of blast load in the control and mTBI-LOC groups. This effect was present only at an uncorrected threshold of P < 0.01. mTBI - LOC = mild traumatic brain injury without loss of consciousness. mTBI + LOC = mild traumatic brain injury with loss of consciousness. L Retrolent Int cap = left retrolenticular part of the internal capsule. R Sag Stratum = right sagittal stratum. Blast load was square-root transformed.
Primary blast injury versus combination of primary and tertiary injury was not significantly associated with mean FA in any ROI, either with FDR correction or at the uncorrected threshold. This variable was also not associated with the presence of abnormal ROIs.
3.5. Mediation of neurocognitive function
A mediation analysis using ordinary least squares path analysis revealed that mTBI + LOC indirectly influenced verbal memory performance through its effect on the extent of white matter abnormalities (as measured by total number of abnormal ROIs). Individuals with LOC had a greater number of abnormal ROIs (a = −0.44, P < 0.03) and, in turn, the number of abnormal ROIs was negatively associated with verbal memory performance (b = −0.21, P < 0.05). The direct effect of mTBI on verbal memory was not significant (P > 0.3). A bias-corrected bootstrap confidence interval for the indirect effect of verbal memory (ab = 0.09) based on 5000 bootstrap samples did not encompass zero (95% CI [0.005, 0.29]). The results remained consistent after adjusting for PTSD symptom severity: individuals with LOC had a greater number of abnormal ROIs (a = −0.46, P < 0.02) and, in turn, total number of abnormal ROIs was negatively associated with verbal memory performance (b = −0.22, P < 0.04). A bias-corrected bootstrap confidence interval for the indirect effect of verbal memory (ab = 0.10) based on 5000 bootstrap samples did not encompass zero (95% CI [0.01, 0.32]). These results suggest that as white matter abnormalities accumulate, individuals with LOC show decreased verbal memory performance. PTSD symptom severity was associated with attention (Pearson's r = −0.24, P < 0.02), verbal memory (Pearson's r = −0.21, P < 0.03), and visuospatial memory (Pearson's r = −0.19, P < 0.05). However, the effect of CAPS on number of abnormal ROIs was not significant (P > 0.05), and therefore a mediation analysis was not further pursued.
4. Discussion
In a large cohort of OEF/OIF veterans, we examined the relationship between blast-related mTBI, PTSD, and white matter abnormalities using DTI. There were three main findings from the study. First, mTBI + LOC, but not mTBI - LOC was associated with white matter abnormalities. Importantly, these abnormalities were spatially heterogeneous, consistent with previous post-mortem and imaging work showing that white matter injury in mTBI is diffuse. Reductions in FA in specific white matter tracts were observed in the context of higher blast load for individuals with mTBI + LOC. Second, a mediation analysis revealed that mTBI + LOC was indirectly associated with lower verbal memory performance through its effect on white matter abnormalities. Third, PTSD symptom severity was not associated with presence of spatially heterogeneous white matter abnormalities, although at a reduced statistical threshold, symptom severity moderated the effect of mTBI + LOC on white matter in the left retrolenticular part of the internal capsule.
These results are notable given our unbiased methodology. Recently, it was observed that several studies reporting changes in white matter integrity in OEF/OIF veterans used methods that potentially biased their results in favor of finding control and TBI group differences (Watts et al., 2014). In their study, Watts and colleagues examined their data two ways; in the first, the authors computed z-scores of abnormal white matter voxels based on a reference group, and also included the reference group in group analyses. This method, considered standard in the literature, revealed FA differences in clusters of voxels across groups, suggesting white matter abnormalities in individuals with mTBI. However, when they used a leave-one-out cross-validation method to control for the potentially inflated reference group values, they no longer observed group differences, raising the possibility that these findings were an artifact of flawed methodology. Here, we used the leave-one-out cross-validation method in the largest DTI study to date in OEF/OIF veterans with blast-related mTBI. We found that mTBI + LOC secondary to blast events was associated with spatially heterogeneous white matter abnormalities. These white matter abnormalities were observed in the chronic stages of mTBI, years after the injury occurred.
Although the precise mechanisms triggering LOC are not entirely known, one influential model of TBI has posited that injury to the brain occurs in a centripetal fashion (Ommaya and Gennarelli, 1974). According to this view, injuries associated with LOC involve widespread damage of cortical and subcortical areas that result in disconnection of deep structures involved in consciousness such as the brainstem, whereas damage to more restricted cortical regions tends to be associated with alterations in consciousness in the absence of LOC, such as posttraumatic amnesia and confusion. Consistent with this view, the present results demonstrated that individuals with LOC had greater extent of injury. Notably, the centripetal hypothesis was formulated in the context of blunt impact head injury. The present results provide some initial evidence supporting its generalization to blast-related TBI.
In addition, LOC may have been a more reliable indicator of mTBI than posttraumatic amnesia or other alterations of consciousness, as the latter two may also occur following a psychologically traumatic event without head injury. LOC can also be more readily observed by a witness whereas other alterations in consciousness would be less apparent to an observer. In our sample, all but three individuals with LOC had learned of their injury from a witness.
Recent studies have also found a positive association between blast-related LOC and white matter changes. In an exploratory analysis, Sorg et al. (2014) showed that mTBI + LOC was associated with lower FA values in ventral-prefrontal white matter. In another recent study, Jorge and colleagues (2013) showed that individuals with LOC had a greater number of “potholes” or clusters of white matter abnormalities than individuals without LOC. The results reported here suggest that the association between LOC and white matter abnormality is a robust finding, as it survived using an independent comparison group. Studies with negative DTI findings did not consider individuals with LOC as a subgroup (i.e., Bazarian et al., 2013; Levin et al., 2010), which may in part explain the null results. Taken together, the results add to the growing literature showing that LOC is an important diagnostic marker of mTBI-related white matter abnormalities.
Our findings are likely to present a conservative estimate of chronic injury. A recent report has suggested that the leave-one-out approach may underestimate the actual level of difference between the control and mTBI groups (Mayer et al., 2014). Further, ROI-based approaches are less sensitive to small areas of injury than voxel-based approaches because in the ROI approach, voxels of abnormal signal may be averaged together with voxels of normal signal to obtain summary statistics across the entire ROI. Finally, the need to control for pulse sequence reduced the effective sample size of the control group used to determine the presence of ROIs with abnormal FA, thus likely reducing the power of this analysis. When the threshold was lowered to uncorrected P value < 0.01, mTBI + LOC was associated with decreased FA in the splenium of the corpus callosum and right sagittal stratum.
It is possible that changes in specific ROIs are only evident in the context of multiple blast exposures. Consistent with this notion, we found that multiple blast exposures in individuals with LOC were associated with reduced FA in the left retrolenticular part of the internal capsule and, at an uncorrected threshold, in the right sagittal stratum. However, higher blast load was not associated with more extensive spatially heterogeneous white matter abnormalities. The latter result is consistent with Davenport et al. (2012), who found no significant differences in white matter abnormalities between individuals with single and multiple blast exposures. Thus, the small literature to date paints an inconsistent picture with regard to the impact of multiple blasts on white matter in humans. This stands in contrast to the animal literature, which has provided relatively robust evidence for increased severity of brain injury with repeated blast exposure (Calabrese et al., 2014; Donovan et al., 2014; Mouzon et al., 2012; Wang et al., 2011). The reason for the inconsistency in results may stem from tighter control over experimental manipulations in animal studies, such as the timing of subsequent blasts. For example, research has provided evidence for a critical time window following the first TBI within which the second insult must occur to observe dose-dependent increases in brain injury. Thus, the animal literature may be more consistent due to precise timing of repeated injury. Further, animal studies have also measured outcomes much closer to the time of injury whereas outcomes of repetitive blast exposure were measured on the order of years in the current study.
Injury mechanism did not affect white matter abnormality in the present study. To our knowledge, few, if any studies have directly compared white matter abnormalities associated with primary blast alone versus blast plus secondary/tertiary mechanisms. In a behavioral study, Kontos et al. (2013) did not find any neurocognitive differences between individuals with blast and blast + blunt combination injuries, although they did find increased risk of TBI and PTSD symptoms in the blast+blunt combination group versus the blast alone group. Given these mixed outcomes, the effects of primary blast versus combination injuries is an important topic for future investigation.
For the first time, we demonstrated that mTBI + LOC was associated with decreased performance in verbal memory through its influence on extent of white matter abnormality. These findings provide direct evidence that widespread white matter abnormalities negatively impact cognitive function following blast-related mTBI. The results support and extend those of Levin et al. (2010) who found that blast-related mTBI was associated with reductions in verbal memory. Here, we showed that extent of white matter abnormality is an important intervening variable in explaining the relationship between mTBI and verbal memory.
PTSD was not significantly associated with white matter abnormalities after multiple comparisons correction, a finding consistent with the results of several other studies in veterans with mTBI (Jorge et al., 2012; Morey et al., 2013; Taber et al., 2015). However, we found that at a reduced statistical threshold, PTSD moderated the relationship between mTBI + LOC and FA in the left retrolenticular part of the internal capsule. These results provide some evidence that mTBI + LOC is associated with greater white matter abnormality as PTSD symptom severity increases, although these results must be interpreted with caution because they did not survive multiple comparisons correction. PTSD symptom severity was also associated with lower attention, verbal memory, and visuospatial memory, consistent with a large body of work linking PTSD to impairments in these cognitive domains (Brandes et al., 2002; Kanagaratnam and Asbjørnsen, 2007; Leskin and White, 2007; Vasterling et al., 1998, 2002; Verfaellie et al., 2014).
Limitations of the current study should be noted. Similar to most previous studies of post-deployment health, mTBI diagnosis relied on retrospective self-reports with limited availability of corroborating medical record data. However, mTBI assessment was conducted using a structured and guided interview based on a validated interview (Fortier et al., 2014) to characterize the nature of each injury, currently the gold standard for diagnosis (Corrigan and Bogner, 2007). Furthermore, all but three individuals with LOC reported information obtained from a witness to the event, reducing reliance on self-report to infer LOC. In the current study, the control group consisted of individuals who were never exposed to blast-wind mechanisms as well as individuals who were exposed to blast without TBI. There is some evidence that individuals with blast exposure, even without mTBI, show white matter abnormalities (as an example, see Taber et al., 2015). Although we did not find any differences related to blast exposure itself in the current study, it is possible that our methods were not optimized to detect white matter abnormalities that may be associated with blast exposure. Taber et al. (2015) identified cluster areas of abnormal voxels not restricted to any particular white matter tract. This approach avoids the potential problem of null results due to averaging together healthy and damaged tissue in an ROI approach. Thus, future studies of subconcussive blast exposure may benefit from this approach. Finally, we note that because we did not measure neurocognitive functioning premorbidly, we cannot exclude the possibility that some neurocognitive differences were pre-existing. However, there were no group differences in a brief measure of premorbid IQ (i.e., the WTAR), providing some evidence against this possibility.
4.1. Conclusions
In summary, we report evidence for white matter abnormalities associated with blast-related mTBI, particularly in individuals who experienced LOC. These abnormalities were spatially heterogeneous in nature, not confined to any one particular white matter tract except in the presence of multiple blast exposure. One consequence of these white matter abnormalities may be reduced neurocognitive performance, particularly in verbal memory. PTSD was not associated with spatially heterogeneous white matter abnormalities, although there was a suggestion that at higher levels of PTSD symptom severity, LOC may be associated with a region-specific abnormality. Taken together, the results from this study underscore the consequences of blast-related mTBI on brain structure and provide evidence of the importance of using sensitive techniques, such as DTI, to detect brain injury following mTBI.
Funding
This work was supported by VA Merit Review 822-MR-18176 (awarded to M.V.), NIH grant K23MH084013 (awarded to J.P.H.), the National Center for PTSD, and the VA Clinical Science Research and Development Service.
Footnotes
For 9 control subjects who were scanned on sequence 1, sequence 2 data were also available. These data were included in the reference group to derive z-scores for individuals scanned with sequence 2.
References
- Bazarian J.J., Donnelly K., Peterson D.R., Warner G.C., Zhu T., Zhong J. The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J. Head Trauma Rehabil. 2013;28(1):1–12. doi: 10.1097/HTR.0b013e318256d3d3. 22647965 [DOI] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a Clinician-Administered PTSD Scale. J. Trauma. Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. 7712061 [DOI] [PubMed] [Google Scholar]
- Blumbergs P.C., Scott G., Manavis J., Wainwright H., Simpson D.A., McLean A.J. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J. Neurotrauma. 1995;12(4):565–572. doi: 10.1089/neu.1995.12.565. 8683607 [DOI] [PubMed] [Google Scholar]
- Brandes D., Ben-Schachar G., Gilboa A., Bonne O., Freedman S., Shalev A.Y. PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Res. 2002;110(3):231–238. doi: 10.1016/s0165-1781(02)00125-7. 12127473 [DOI] [PubMed] [Google Scholar]
- Bruce M.L. Suicide risk and prevention in veteran populations. Ann. N. Y. Acad. Sci. 2010;1208(1):98–103. doi: 10.1111/j.1749-6632.2010.05697.x. 20955331 [DOI] [PubMed] [Google Scholar]
- Calabrese E., Du F., Garman R.H., Johnson G.A., Riccio C., Tong L.C., Long J.B. Diffusion tensor imaging reveals white matter injury in a rat model of repetitive blast-induced traumatic brain injury. J. Neurotrauma. 2014;31(10):938–950. doi: 10.1089/neu.2013.3144. 24392843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cercignani M., Bammer R., Sormani M.P., Fazekas F., Filippi M. Inter-sequence and inter-imaging unit variability of diffusion tensor MR imaging histogram-derived metrics of the brain in healthy volunteers. A.J.N.R. Am. J. Neuroradiol. 2003;24(4):638–643. 12695195 [PMC free article] [PubMed] [Google Scholar]
- Corrigan J.D., Bogner J. Screening and identification of TBI. J. Head Trauma Rehabil. 2007;22(6):315–317. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- Davenport N.D., Lim K.O., Armstrong M.T., Sponheim S.R. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. Neuroimage. 2012;59(3):2017–2024. doi: 10.1016/j.neuroimage.2011.10.050. 22040736 [DOI] [PubMed] [Google Scholar]
- Donovan V., Kim C., Anugerah A.K., Coats J.S., Oyoyo U., Pardo A.C., Obenaus A. Repeated mild traumatic brain injury results in long-term white-matter disruption. J. Cereb. Blood Flow Metab. 2014;34(4):715–723. doi: 10.1038/jcbfm.2014.6. 24473478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier C.B., Amick M.M., Grande L., McGlynn S., Kenna A., Morra L., Clark A., Milberg W.P., McGlinchey R.E. The Boston Assessment of Traumatic Brain Injury-Lifetime (BAT-L) semistructured interview: Evidence of research utility and validity. J. Head Trauma Rehabil. 2014;29(1):89–98. doi: 10.1097/HTR.0b013e3182865859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J.A. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 2012;4(134):134–160. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F., Preacher K.J. Statistical mediation analysis with a multicategorical independent variable. British Journal of Mathematical and Statistical Psychology. 2014 doi: 10.1111/bmsp.12028. 24188158 Retrieved from http://www.afhayes.com/public/hpcatx.pdf. [DOI] [PubMed] [Google Scholar]
- Jorge R.E., Acion L., White T., Tordesillas-Gutierrez D., Pierson R., Crespo-Facorro B., Magnotta V.A. White matter abnormalities in veterans with mild traumatic brain injury. Am. J. Psychiatry. 2012;169(12):1284–1291. doi: 10.1176/appi.ajp.2012.12050600. 23212059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagaratnam P., Asbjørnsen A.E. Executive deficits in chronic PTSD related to political violence. J. Anxiety Disord. 2007;21(4):510–525. doi: 10.1016/j.janxdis.2006.06.008. 16938424 [DOI] [PubMed] [Google Scholar]
- Kontos A.P., Kotwal R.S., Elbin R., Lutz R.H., Forsten R.D., Benson P.J., Guskiewicz K.M. Residual effects of combat-related mild traumatic brain injury. J. Neurother. 2013;30(8):680–686. doi: 10.1089/neu.2012.2506. [DOI] [PubMed] [Google Scholar]
- Kraus M.F., Susmaras T., Caughlin B.P., Walker C.J., Sweeney J.A., Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(10):2508–2519. doi: 10.1093/brain/awm216. 17872928 [DOI] [PubMed] [Google Scholar]
- Landman B.A., Farrell J.A., Jones C.K., Smith S.A., Prince J.L., Mori S. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage. 2007;36(4):1123–1138. doi: 10.1016/j.neuroimage.2007.02.056. 17532649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskin L.P., White P.M. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychol. 2007;21(3):275–284. doi: 10.1037/0894-4105.21.3.275. 17484590 [DOI] [PubMed] [Google Scholar]
- Levin H.S., Wilde E., Troyanskaya M., Petersen N.J., Scheibel R., Newsome M. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma. 2010;27(4):683–694. doi: 10.1089/neu.2009.1073. 20088647 [DOI] [PubMed] [Google Scholar]
- Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. 21631321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie J.D., Siddiqi F., Babb J.S., Bagley L.J., Mannon L.J., Sinson G.P., Grossman R.I. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. A.J.N.R. Am. J. Neuroradiol. 2002;23(9):1509–1515. 12372740 [PMC free article] [PubMed] [Google Scholar]
- Matthews S.C., Spadoni A.D., Lohr J.B., Strigo I.A., Simmons A.N. Diffusion tensor imaging evidence of white matter disruption associated with loss versus alteration of consciousness in warfighters exposed to combat in operations enduring and Iraqi freedom. Psychiatry Res. 2012;204(2–3):149–154. doi: 10.1016/j.pscychresns.2012.04.018. 23149025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R., Bedrick E.J., Ling J.M., Toulouse T., Dodd A. Methods for identifying subject-specific abnormalities in neuroimaging data. Hum. Brain Mapp. 2014;35(11):5457–5470. doi: 10.1002/hbm.22563. 24931496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Haswell C.C., Selgrade E.S., Massoglia D., Liu C., Weiner J. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Human Brain Mapping. 2013;34(11):2986–2999. doi: 10.1002/hbm.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouzon B., Chaytow H., Crynen G., Bachmeier C., Stewart J., Mullan M. Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. Journal of Neurotrauma. 2012;29(18):2761–2773. doi: 10.1089/neu.2012.2498. [DOI] [PubMed] [Google Scholar]
- Ommaya A.K., Gennarelli T.A. Cerebral concussion and traumatic unconsciousness. Correlation of experimental and clinical observations of blunt head injuries. Brain. 1974;97(4):633–654. doi: 10.1093/brain/97.1.633. 4215541 [DOI] [PubMed] [Google Scholar]
- Schuff N., Zhang Y., Zhan W., Lenoci M., Ching C., Boreta L. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. Neuroimage. 2011;54(Suppl. 1):S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. 20483375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. 12391568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. 16624579 [DOI] [PubMed] [Google Scholar]
- Sorg S.F., Delano-Wood L., Luc N., Schiehser D.M., Hanson K.L., Nation D.A. White matter integrity in veterans with mild traumatic brain injury: associations with executive function and loss of consciousness. J. Head Trauma Rehabil. 2014;29(1):21–32. doi: 10.1097/HTR.0b013e31828a1aa4. 23640539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber K.H., Hurley R.A., Haswell C.C., Rowland J.A., Hurt S.D., Lamar C.D., Morey R.A. White matter compromise in veterans exposed to primary blast forces. J. Head Trauma Rehabil. 2015 doi: 10.1097/HTR.0000000000000030. 24590156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T.N., Tombaugh P.W. Test of Memory Malingering: TOMM. Multi-Health Systems; North Tonawanda, NY: 1996. [Google Scholar]
- Ursano R.J., Goldenberg M., Zhang L., Carlton J., Fullerton C.S., Li H. Posttraumatic stress disorder and traumatic stress: from bench to bedside, from war to disaster. Annals of the New York Academy of Sciences. 2010;1208(1):72–81. doi: 10.1111/j.1749-6632.2010.05721.x. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Constans J.I., Sutker P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychol. 1998;12(1):125–133. doi: 10.1037//0894-4105.12.1.125. 9460740 [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Duke L.M., Brailey K., Constans J.I., Allain A.N., Jr., Sutker P.B. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16(1):5–14. doi: 10.1037//0894-4105.16.1.5. 11853357 [DOI] [PubMed] [Google Scholar]
- Verfaellie M., Lafleche G., Spiro A., Tun C., Bousquet K. Chronic postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. J. Int. Neuropsychol. Soc. 2013;19(1):1–10. doi: 10.1017/S1355617712000902. 23095177 [DOI] [PubMed] [Google Scholar]
- Verfaellie M., Lafleche G., Spiro A. III, Bousquet K. Neuropsychological outcomes in OEF/OIF veterans with self-report of blast exposure: associations with mental health, but not MTBI. Neuropsychol. 2014;28(3):337–346. doi: 10.1037/neu0000027. 24245929 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wei Y., Oguntayo S., Wilkins W., Arun P., Valiyaveettil M. Tightly coupled repetitive blast-induced traumatic brain injury: development and characterization in mice. Journal of Neurotrauma. 2011;28(10):2171–2183. doi: 10.1089/neu.2011.1990. [DOI] [PubMed] [Google Scholar]
- Watts R., Thomas A., Filippi C.G., Nickerson J.P., Freeman K. Potholes and molehills: bias in the diagnostic performance of diffusion-tensor imaging in concussion. Radiology. 2014;272(1):217–223. doi: 10.1148/radiol.14131856. 24635677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers F., Huska J., Keane T. The PTSD Checklist Military Version (PCL-M) National Center for PTSD; Boston, MA.: 1991. [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading: WTAR. Psychological Corporation; 2001. [Google Scholar]
- Wilk J.E., Herrell R.K., Wynn G.H., Riviere L.A., Hoge C.W. Mild traumatic brain injury (concussion), posttraumatic stress disorder, and depression in U.S. soldiers involved in combat deployments: association with postdeployment symptoms. Psychosom. Med. 2012;74(3):249–257. doi: 10.1097/PSY.0b013e318244c604. 22366583 [DOI] [PubMed] [Google Scholar]
- Wilkins K.C., Lang A.J., Norman S.B. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress. Anxiety. 2011;28(7):596–606. doi: 10.1002/da.20837. 21681864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik B.E., Stein C.R., Bagg K., Humphrey R.J., Orosco J. Traumatic brain injury hospitalizations of U.S. army soldiers deployed to Afghanistan and Iraq. Am. J. Prev. Med. 2010;38(1):S108–S116. doi: 10.1016/j.amepre.2009.10.006. 20117583 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


