Abstract
This study investigated the relations of tectal volume and superior parietal cortex, as well as alterations in tectocortical white matter connectivity, with the orienting and executive control attention networks in individuals with spina bifida myelomeningocele (SBM). Probabilistic diffusion tractography and quantification of tectal and superior parietal cortical volume were performed on 74 individuals aged 8–29 with SBM and a history of hydrocephalus. Behavioral assessments measured posterior (covert orienting) and anterior (conflict resolution, attentional control) attention network functions. Reduced tectal volume was associated with slower covert orienting; reduced superior parietal cortical volume was associated with slower conflict resolution; and increased axial diffusivity and radial diffusivity along both frontal and parietal tectocortical pathways were associated with reduced attentional control. Results suggest that components of both the orienting and executive control attention networks are impaired in SBM. Neuroanatomical disruption to the orienting network appears more robust and a direct consequence of characteristic midbrain dysmorphology; whereas, executive control difficulties may emerge from parietal cortical anomalies and reduced frontal and parietal cortical–subcortical white matter pathways susceptible to the pathophysiological effects of congenital hydrocephalus.
Keywords: Attention network, Neuroimaging, Tectum, Superior parietal cortex, Tectocortical pathways, Robust correlations
Highlights
-
•
We use robust correlations to model structure–function relations.
-
•
We use a large sample of individuals with spina bifida myelomeningocele.
-
•
Reduced tectal volume is associated with slower covert orienting.
-
•
Reduced superior parietal cortical volume is associated with slower conflict resolution.
-
•
Tectocortical pathways are associated with reduced attentional control.
1. Introduction
Two distinct frontoparietal attention networks underlie attentional relevance and salience (Petersen and Posner, 2012). The executive control network is predominantly responsible for cognitively driven attention functions including conflict resolution and attentional control. This network involves dorsolateral frontal and superior parietal cortices, and connections with the thalamus and anterior cingulate cortex (Posner, 2012). Abnormalities of the brain structures subserving the executive control network have been linked to response control deficits in developmental disorders such as attention deficit hyperactivity disorder (ADHD; Dennis et al., 2008).
The orienting network, responsible for engaging, disengaging, and shifting attention (Posner, 1980), is subserved by the frontal eye fields, superior parietal lobule, and intraparietal sulcus, and the superior colliculus of the midbrain tectum (Posner and Petersen, 1990). Covert orienting is associated with unobservable, internal shifts of attention without engaging eye, head, or body movements (Klein, 2004). Tectal and posterior cortical abnormalities have been linked to covert orienting deficits in a variety of adult neurological disorders (Rafal et al., 1988).
Covert orienting deficits are a major characteristic of spina bifida myelomeningocele (SBM). This neurodevelopmental disorder is of particular interest because in addition to covert orienting deficits, developmental dysmorphologies in SBM commonly include congenital abnormalities of the tectum and reduced volume and atypical cortical thickness of the parietal lobes due to hydrocephalus. However, the relation of these attentional difficulties and brain abnormalities has not been quantitatively examined. Because SBM has a range of both cognitive and neural variability, one cannot assume specific links, and so it is important to demonstrate such links directly in a hypothesis driven manner.
1.1. Spina bifida myelomeningocele
Spina bifida myelomeningocele, a neural tube defect, is associated with the Chiari II malformation, which involves a small posterior fossa and associated pathology of the cerebellum and brainstem, often contributing to obstructive hydrocephalus and compression of the midbrain (Juranek and Salman, 2010). In addition, hypoplasia or partial dysgenesis of the corpus callosum (Hannay et al., 2009) is common, along with, significant variations in cortical thickness: frontal regions are often enlarged and posterior regions thinned (Juranek et al., 2008). Fig. 1 demonstrates frequently observed neurostructural abnormalities in SBM.
Fig. 1.
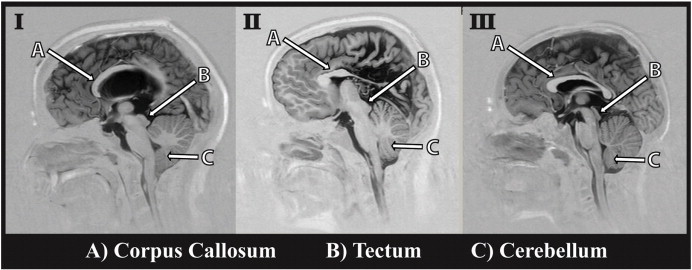
Depiction of variations in corpus callosum (A), tectum (B), and cerebellum (C) in SBM compared to typically developing individual. I) Individual with SBM showing partial dysgenesis of the corpus callosum, a normal appearing tectum, and downward herniation of the cerebellum; II) individual with SBM showing partial dysgenesis of the corpus callosum, beaking of the midbrain tectum and normal cerebellum; III) typically developing individual showing a fully formed corpus callosum, with normal appearing tectum and cerebellum.
As part of the spectrum of abnormalities associated with Chiari II malformation, the midbrain is often mechanically distorted. The majority of individuals with SBM and Chiari II malformation have a beaked tectum that is stretched posteriorly and inferiorly (Behramn et al., 2003). Williams et al. (2013) used diffusion tensor imaging (DTI) to investigate the frontal and parietal tectocortical attention pathways in individuals with SBM relative to typically developing (TD) individuals. Compared to TD individuals, those with SBM had reduced tectal volume, decreased fractional anisotropy in parietal tectocortical pathways, and a greater discrepancy between frontal and parietal tectocortical diffusion metrics. Those with SBM and tectal beaking had increased axial diffusivity across frontal and parietal tectocortical pathways compared to individuals with SBM and no tectal beaking.
1.2. Attention in SBM
Impairment of the orienting network in SBM is well-established (Dennis and Barnes, 2010). Individuals with SBM and a beaked tectum have more difficulty disengaging attention from a current stimulus and redirecting it towards a new stimulus when compared to TD individuals and individuals with SBM and no tectal beaking (Dennis et al., 2005). Similar findings have been found in infants with SBM, who require more time to disengage and shift their attention towards new stimuli relative to TD infants (Taylor et al., 2010). Although these findings suggest a relation between orienting deficits and tectum, this relation has not been quantitatively evaluated.
Functioning of the executive control network in SBM is less clear. Individuals with SBM have demonstrated executive control difficulties on measures of top-down attention processes such as attention control and response inhibition (Ou et al., 2013). In contrast, on continuous performance tasks, several studies found that individuals with SBM make more commission errors relative to TD individuals (Swartwout et al., 2008), implicating response inhibition difficulties, although such findings have not been unequivocal (Colvin et al., 2003).
1.3. Objectives and hypotheses
The objective of the present study was to investigate the relations of tectal and superior parietal cortical volume, and tectocortical diffusivity metrics, with functioning of the orienting and executive control attention networks in SBM. Given the role of the superior colliculus in the orienting network, we hypothesized that lower tectal volume would be associated with poorer covert orienting, but not with executive control functions. Because the superior parietal cortex subserves both the orienting and executive control networks, we hypothesized that lower volume of the superior parietal cortex would be associated with poorer performance on both covert orienting and executive control tasks. Finally, we did not have any a priori expectations concerning the understudied relations of tectocortical pathways and attention outcomes. The present study is novel due to a large clinical sample, the simultaneous analysis of both the orienting and executive control attention networks, and the implementation of robust correlations and bootstrap procedures to increase the reliability of findings.
2. Materials and methods
2.1. Participants
Participants included 80 individuals with SBM (also reported in Williams et al., 2013) who had undergone structural MRI of the brain. These participants were recruited through clinics in Houston. Of these individuals, 74 had complete neuroimaging, orienting, and conflict resolution data (except for the superior parietal cortex, orienting, conflict resolution data where n = 73), and 59 had complete neuroimaging and attentional control data. Inclusion criteria consisted of a myelomeningocele at birth, evidence of hydrocephalus, and adequate upper limb control. Participants had no evidence of major psychiatric disorder. All participants had an IQ score of at least 70.
The sample was 13.70 years in age (SD = 4.81, age range: 7.90–29.11 years), 55% male, and 53% Hispanic. The sample was representative of other samples of SBM, with most showing the Chiari II malformation (88%), thinning (61%) or partial dysgenesis (35%) of the corpus callosum, lower spinal lesions (86%), ambulatory difficulties (68%), no seizure history (64%), and two to four shunt revisions (49%). Tectal beaking was present in 47 out of 74 participants with SBM. The study was approved by the human participants review boards at all institutions. Parents and participants gave written consent unless the participant was under 13, in which case the parent consented and the child assented.
2.2. MRI data acquisition and processing
High-resolution T1 scans were obtained on a Philips 3.0 T Intera system with the following acquisition parameters: slice thickness = 1.5 mm; TR/TE = 6.5 − 6.7/3.04–3.14 ms; flip angle = 8°; square FOV = 24 cm3; matrix = 256 × 256; in-plane pixel dimensions (x, y) = 0.94, 0.94; NEX = 2. DTI images were acquired using a single-shot spin-echo diffusion sensitized echo-planar imaging (EPI) sequence with the balanced Icosa21 encoding scheme with the following parameters: 44 slices total; square field of view (FOV) = 24 cm3; acquisition matrix = 256 × 256; slice thickness = 3 mm; TR/TE = 6100/84 ms; b-value = 1000 s/mm2. A single non-diffusion weighted image with a b-value = 0 s/mm2 was acquired concurrently as an anatomical reference volume.
All T1-weighted images were processed using FreeSurfer (https://surfer.nmr.mgh.harvard.edu). Resultant cortical labels were non-linearly co-registered using FMRIB's Linear Image Registration Tool (FLIRT) and transformed into native DTI space to serve as endpoint masks for tractography procedures. Cortical labels were dilated 2 mm into adjacent white matter to facilitate fiber-tracking procedures.
DTI acquisitions were processed using tools available through FreeSurfer and FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL) image analyses software (Behrens et al., 2007; Jenkinson et al., 2012). Diffusion volumes underwent eddy current and motion correction. Diffusion tensors were calculated for each voxel using a least-squares fit to the log of the diffusion signal. Maps for fractional anisotropy (FA), axial diffusivity [λ1] (AD) and radial diffusivity [(λ2 + λ3) / 2] (RD) were isolated for further FSL probabilistic tractography processing and analysis (Budde et al., 2007). Non-diffusion weighted images (T2) were skull stripped and used as a mask to remove non-brain tissue from calculated diffusion maps.
Fig. 2 provides a visualization of MRI-derived dependent variables. The midbrain tectum was manually defined for each participant by a single rater blinded to diagnosis in order to determine tectal volume and to function as a seed region in subsequent probabilistic tractography procedures (Williams et al., 2013). Hand drawn ROIs were traced in fslview in the axial plane of the T2-weighted lowb (e.g. b = 0) image of the DTI sequence such that the tectal label directly corresponded to the intended collicular structure in native DTI space (minimizing the need for spatial transformations reliant on co-registration procedures that were found to be less reliable for smaller mid-brain regions). Dice similarity coefficients (0.894) showed strong intra-rater reliability for the manual tracings (Zou et al., 2004). Volume of the superior parietal cortex within each hemisphere was acquired using the Freesurfer automatic cortical parcellation routine (Desikan et al., 2006). Probabilistic tractography was performed per hemisphere with seeding in the left or right collicular division. Tractography iterations were performed twice per hemisphere defining connectivity from the colliculi to separate parietal and frontal cortical endpoint regions, yielding four tracts for each participant: left hemisphere (LH) tectal-frontal, LH tectal-parietal, right hemisphere (RH) tectal-frontal, RH tectal-parietal. Tract normalization entailed waytotal normalization, thresholding to remove voxels with FA values less than 0.2 to minimize crossing fibers and partial voluming, with resultant output binarized to create tractography-derived masks for each pathway. Mean FA, RD, and AD was subsequently calculated for each pathway and served as dependent variables in subsequent statistical analysis.
Fig. 2.
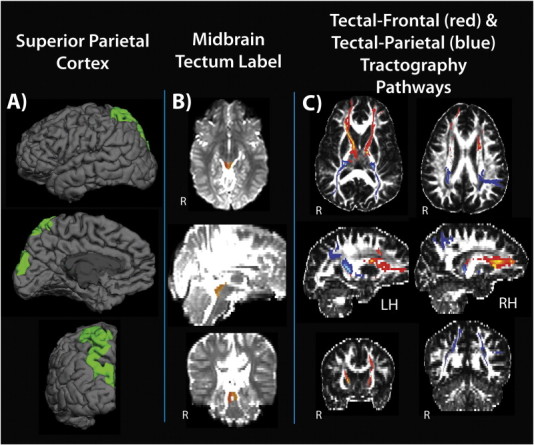
Visualization of MRI-derived dependent variables for individuals with SBM. (A) FreeSurfer semi-automated parcellation of the superior parietal cortex. (B) Manually defined regions of interest for left (yellow) and right (orange) tectum. (C) Results of probabilistic diffusion tractography (FSL) procedures defining left and right white matter pathways between the tectum and frontal (red) and parietal (blue) cortices.
2.3. Behavioral measures
2.4.1. The Child Attention Network Test
The Child Attention Network Test (Rueda et al., 2004) was designed to measure orienting and executive control attention networks in participants as young as 4 years of age (Rueda et al., 2004). Because the child version of the test is a computerized, timed test where ceiling effects are unlikely, it was possible to administer the same test to all participants regardless of their age. The test utilizes four types of cues along with three flanker conditions. The four cue types include: no cue (a fixation cross); a central cue (an asterisk in the place of the fixation cross); a double cue (asterisks above and below the fixation cross); and a spatial cue (an asterisk in the location of an upcoming target). The three flanker conditions include: congruent, incongruent, or neutral flankers. In the congruent flanker condition, the target and four distractors face the same direction. In the incongruent flanker condition, target and distracters face in opposite directions, creating a conflict. In the neutral flanker condition, the target appears without distracters (Rueda et al., 2004). The goal of the task is to determine in which direction the target is pointing using right or left button press. The primary measures for this study were composites associated with the orienting and executive control attentional networks. The covert orienting network was captured with the Orienting measure, equal to . The executive control network was captured with the Conflict Resolution measure, equal to . All mean RT were calculated on the correct trials only. Within sample reliability of orienting and conflict resolution measures was equal to r = .11 and r = .47, respectively.
2.4.2. Test of Everyday Attention for Children, the Opposite Worlds subtest
Test of Everyday Attention for Children, the Opposite Worlds subtest (Manly et al., 1999), assessing Attentional Control, was used as an additional measure of the executive control attention network. The Opposite Worlds task involves two conditions, opposite world and same world. In the same world condition (the control condition), participants read aloud a list of “1” and “2” digits presented on a card. In the opposite world condition (the attentional control condition), participants are asked to say aloud the “opposite” of the digit appearing on the card (the correct verbal response for number “1” was two, and for number “2” was one; Baron, 2001). Participants receive two cards per condition. The order of presented cards is as follows: same world, opposite world, opposite world, same world. The time required to complete each condition is recorded. Incorrect responses result in a time penalty, as participants are not permitted to proceed with the task until they correct their response. Total time required to complete the two cards related to the opposite world condition was used as a measure of attentional control. Within sample reliability of the Opposite Worlds subtest was equal to r = .85.
2.4. Statistical analyses
Structure–function relations were estimated using the Pearson and percentage bend correlations, as well as the skipped correlation using the Donoho–Gasko median (DGM; Wilcox, 2003). The utility of the three correlations in examining structure–function relations is extensively discussed in Kulesz et al. (2015). Reaction time measures were log transformed. Statistical procedures were completed in R Version 3.0.2 (R Development Core Team, 2008) using the boot package Version 1.3-9, foreign package Version 0.8-55, MASS package Version 7.3-29, and custom written functions.
The bootstrap procedure was applied to improve accuracy of the inferences regarding investigated relations. Relying solely on findings from a single sample of data decreases reliability of the results as one cannot be sure that the same results will be replicated in the future. We sampled n observations with replacement 10,000 times for each relation (with n was equal to 74, 73 or 59 observations depending on the investigated relation and missing data points) to derive bootstrap samples. The three correlations were computed for a given relation on each of the 10,000 bootstrap samples. Empirical distributions of the three correlations were summarized using: mean and confidence intervals based on 2.5 and 97.5 percentiles.
The findings were evaluated in terms of their generalizability across different correlational estimates as well as single and bootstrap samples. Increased reliability of findings was assumed if: (a) significance tests for a single sample estimate yielded statistically significant results across all correlational estimates, and (b) confidence intervals based on the bootstrap distributions excluded 0 across all correlational estimates.
3. Results
Table 1 presents means and standard deviations for the tectum and superior parietal cortex volumes, FA, AD, and RD values for frontal and parietal tectocortical pathways, as well as measures of the orienting (i.e., covert orienting) and executive control (i.e., conflict resolution and attentional control) attention networks. Average diffusivity metrics were calculated for each participant based on the output of seed-based probabilistic tractography procedures modeling tectocortical connectivity for each hemisphere. Because there were no significant hemispheric differences with regard to indicators of WM tract integrity (Williams et al., 2013), left and right hemisphere measurements were averaged within participants to provide a single indicator depicting frontal and parietal tectocortical tract characteristics.
Table 1.
Descriptive statistics for behavioral and brain measures.
| Mean | SD | |
|---|---|---|
| Behavioral measures | ||
| Orienting | 0.05 | 0.07 |
| Conflict resolution | 0.17 | 0.12 |
| Attentional controla | 3.67 | 0.29 |
| Brain measures | ||
| Total tectal volume (mm3) | 1533.25 | 374.34 |
| Total SP volume (mm3)b | 31,429.86 | 6150.79 |
| FA | ||
| Frontal | 0.4403 | 0.0381 |
| Parietal | 0.4320 | 0.0324 |
| AD (× 10−3 mm2/s) | ||
| Frontal | 1.21 | 0.055 |
| Parietal | 1.26 | 0.051 |
| RD (× 10−3 mm2/s) | ||
| Frontal | 0.591 | 0.0445 |
| Parietal | 0.629 | 0.0502 |
SD, standard deviation; SP, superior parietal cortex; FA, fractional anisotropy; AD, axial diffusivity; RD, radial diffusivity.
Total N = 74.
N = 59.
N = 73.
Table 2, Table 3 present single sample estimates and mean values of estimates across 10,000 bootstrap samples (respectively) of the three correlations. As hypothesized, there was a significant negative relation of tectal volume with covert orienting, but not with either measure of the executive control network (i.e., conflict resolution and attentional control measures). Slower attentional shifts were associated with decreased tectal volume. Importantly, this result was statistically significant for both single and bootstrap samples across all correlational estimates, and therefore considered highly reliable.
Table 2.
Single sample estimates of population correlation relations.
| Pair of variables | Pearson correlation | Percentage bend correlation | Skipped correlation using DGM |
|---|---|---|---|
| Orienting–total tectal volume | −0.24 | −0.30 | −0.30 |
| Orienting–total superior parietal cortex volume | 0.19 | 0.20 | 0.17 |
| Orienting–frontal fractional anisotropy | 0.05 | 0.02 | −0.07 |
| Orienting–parietal fractional anisotropy | −0.03 | 0.01 | 0.04 |
| Orienting–frontal axial diffusivity | 0.17 | 0.15 | 0.14 |
| Orienting–parietal axial diffusivity | 0.03 | 0.05 | 0.10 |
| Orienting–frontal radial diffusivity | 0.03 | 0.09 | 0.10 |
| Orienting–parietal radial diffusivity | 0.04 | 0.04 | 0.04 |
| Conflict resolution–total tectal volume | 0.01 | 0.05 | 0.05 |
| Conflict resolution–total superior parietal cortex volume | −0.23 | −0.19 | −0.20 |
| Conflict resolution–frontal fractional anisotropy | −0.02 | −0.04 | 0.01 |
| Conflict resolution–parietal fractional anisotropy | −0.08 | −0.12 | 0.01 |
| Conflict resolution–frontal axial diffusivity | 0.05 | 0.05 | 0.07 |
| Conflict resolution–parietal axial diffusivity | −0.04 | −0.09 | 0.01 |
| Conflict resolution–frontal radial diffusivity | 0.02 | 0.08 | 0.06 |
| Conflict resolution–parietal radial diffusivity | 0.03 | 0.05 | 0.03 |
| Attentional control–total tectal volume | −0.02 | 0.00 | 0.09 |
| Attentional control–total superior parietal cortex volume | 0.11 | 0.10 | 0.10 |
| Attentional control–frontal fractional anisotropy | 0.03 | 0.09 | 0.01 |
| Attentional control–parietal fractional anisotropy | −0.19 | −0.14 | −0.20 |
| Attentional control–frontal axial diffusivity | 0.32 | 0.17 | 0.10 |
| Attentional control–parietal axial diffusivity | 0.32 | 0.04 | 0.06 |
| Attentional control–frontal radial diffusivity | 0.16 | 0.10 | 0.16 |
| Attentional control–parietal radial diffusivity | 0.37 | 0.20 | 0.21 |
N = 74 (relations with orienting and conflict resolution measures); N = 59 (relations with attentional control); N = 73 (relations with the superior parietal cortex); DGM, Donoho–Gasko median; boldface = statistically significant results based on p < 0.05.
Table 3.
Mean values of estimates across 10,000 bootstrap samples.
| Pair of variables | Pearson correlation | Percentage bend correlation | Skipped correlation using DGM |
|---|---|---|---|
| Orienting–total tectal volume | −0.24 | −0.30 | −0.28 |
| Orienting–total superior parietal cortex volume | 0.19 | 0.20 | 0.18 |
| Orienting–frontal fractional anisotropy | 0.05 | 0.02 | −0.02 |
| Orienting–parietal fractional anisotropy | −0.03 | −0.01 | 0.01 |
| Orienting–frontal axial diffusivity | 0.17 | 0.14 | 0.17 |
| Orienting–parietal axial diffusivity | 0.03 | 0.05 | 0.09 |
| Orienting–frontal radial diffusivity | 0.04 | 0.09 | 0.07 |
| Orienting–parietal radial diffusivity | 0.04 | 0.05 | 0.07 |
| Conflict resolution–total tectal volume | 0.01 | 0.05 | 0.04 |
| Conflict resolution–total superior parietal cortex volume | −0.23 | −0.18 | −0.19 |
| Conflict resolution–frontal fractional anisotropy | −0.02 | −0.04 | −0.02 |
| Conflict resolution–parietal fractional anisotropy | −0.08 | −0.11 | −0.03 |
| Conflict resolution–frontal axial diffusivity | 0.05 | 0.05 | 0.10 |
| Conflict resolution–parietal axial diffusivity | −0.04 | −0.09 | −0.01 |
| Conflict resolution–frontal radial diffusivity | 0.03 | 0.08 | 0.05 |
| Conflict resolution–parietal radial diffusivity | 0.03 | 0.05 | 0.06 |
| Attentional control–total tectal volume | −0.01 | 0.01 | 0.05 |
| Attentional control–total superior parietal cortex volume | 0.11 | 0.10 | 0.10 |
| Attentional control–frontal fractional anisotropy | 0.04 | 0.08 | 0.04 |
| Attentional control–parietal fractional anisotropy | −0.19 | −0.14 | −0.19 |
| Attentional control–frontal axial diffusivity | 0.31 | 0.17 | 0.12 |
| Attentional control–parietal axial diffusivity | 0.27 | 0.04 | 0.05 |
| Attentional control–frontal radial diffusivity | 0.16 | 0.11 | 0.15 |
| Attentional control–parietal radial diffusivity | 0.35 | 0.20 | 0.18 |
N = 74 (relations with orienting and conflict resolution measures); N = 59 (relations with attentional control); N = 73 (relations with the superior parietal cortex); DGM, Donoho–Gasko median; boldface = statistically significant results based on 2.5 and 97.5 percentile values taken from 10,000 bootstrap samples (i.e., confidence interval excluded 0).
Superior parietal cortex volume was significantly associated with conflict resolution, but not with attentional control or covert orienting, partially supporting our hypothesis. A slower pace of resolving conflicts between competing stimuli was associated with lower volume of the superior parietal cortex. This relation was significant for single and bootstrap samples for the Pearson correlations, but not for the percentage bend correlation or the skipped correlation using the DGM, suggesting that these findings are less reliable and may reflect general properties of a Pearson correlation which is less robust to violations of distributional assumptions and outliers.
Significant positive associations were found between attentional control and frontal AD, parietal AD, and parietal RD. These results suggest that poorer attentional control was associated with reduced indicators of white matter integrity along both frontal and parietal tectocortical attention pathways. Relations between DTI metrics and attentional control were significant for Pearson correlations, but not for the percentage bend correlation or the skipped correlation using the DGM, which as above may reflect the more robust properties of the latter two estimators. The relation with RD was significant only for the single sample estimate, suggesting that these findings may be sample-specific.
4. Discussion and conclusions
Despite the generalized nature of the neural abnormalities characteristic of SBM, covert orienting difficulties were specifically related to the midbrain anomalies that emerge as part of the Chiari II malformation. In contrast, problems with the executive control network were associated with reduced superior parietal volume, as well as reduced DTI indicators of WM integrity along tectocortical pathways, both potentially attributed to the pathological effects of hydrocephalus. These results indicate that the neural disruption associated with SBM occurs in a principled manner and can be detected with a sufficiently large sample, a quantitative, multi-modality approach to neuroimaging, and more sophisticated methods for estimating correlations.
Neuropsychological impairments in SBM have historically been assumed to result predominantly from the damaging effects of hydrocephalus on the brain. Recent investigations into the neuropsychological correlates of tectal beaking suggest that there are specific neuropsychological impairments that cannot be attributed solely to the effects of hydrocephalus; rather, they result from the characteristic brain malformation involving the midbrain and posterior fossa (Treble-Barna et al., 2014). As hypothesized, lower tectal volume was associated with poorer covert orienting in SBM, and not with functions of the executive control attention network. This finding is highly reliable given that it was statistically significant for both single and bootstrap samples across all correlational estimates. The specificity and reliability of this finding confirm previously speculated importance of the superior colliculus in covert orienting (Dennis et al., 2005).
Because superior parietal cortex subserves both the orienting and executive control networks, we hypothesized that lower volume of the superior parietal cortex would be associated with poorer performance on both covert orienting and executive control functions. In partial support of our hypothesis, lower volume of the superior parietal cortex was associated with poorer conflict resolution in SBM; however, we did not find expected associations with covert orienting or attentional control. Cortical volume is a less than optimal measure of brain morphology in SBM, possibly accounting for our only partially supported hypothesis. Cortical volume can be more finely characterized by contributory components including surface area, thickness, and gyrification. In SBM, although the parietal lobe is lower in cortical volume relative to TD individuals (Juranek et al., 2008), the superior parietal cortex is thicker but less gyrified relative to TD individuals (Treble et al., 2013). Thus, volume may be inadequately sensitive to associations between brain morphology and attention functions in SBM.
When examining WM microstructural morphology, poorer attentional control was associated with increased AD along frontal and parietal tectocortical pathways, and increased RD along parietal pathways. These findings suggest reduced white WM integrity and organization. This is interesting because in Williams et al. (2013), DTI indicators of WM integrity in those with SBM appeared more compromised along parietal tectocortical pathways compared to controls. However, present findings demonstrated positive associations between reaction time and AD along frontal and parietal pathways, whereas parietal pathways also revealed a significant association between RD and attentional control. The specific DTI metrics associated with reduced attention performance in each pathway suggest specific mechanisms of injury, with more significant damage in posterior regions.
Although the interpretation of DTI metrics is still a topic of active investigation (see Jones and Cercignani, 2010), early animal studies provide some ground for interpretation. AD is defined as the degree of diffusivity along the primary diffusion axis, and has been related to variations of axonal integrity in animal models. Conversely, RD is calculated as the average of the two secondary diffusion axes, and is thought to approximate the degree of flow restriction provided by membranes and myelin integrity (Song et al., 2003). Increasing values of both axial and radial diffusivity are considered indicators of decreased tissue integrity, and typically demonstrate an inverse relation to FA values. Increased AD along both frontal and parietal tectocortical pathways in association with reduced attentional control suggests widespread disruption to axonal integrity and organization along both anterior and posterior white matter pathways in SBM. Our finding that RD is also higher in the parietal tectocortical attention pathway suggests that posterior white matter regions sustain a double dose of injury, resulting in damage to axonal organization as well as myelin integrity. Although the hypothesized mechanisms contributing to reductions in DTI indicators of white matter integrity vary between frontal and parietal pathways, the results suggest that individuals with SBM may demonstrate executive control difficulties resulting from the effects of hydrocephalus on both the anterior and posterior neural substrates underlying the executive control attention network.
The results of the present study suggest neural correlates (disruption of superior parietal cortex and tectocortical white matter pathways) and pathophysiological mechanisms (reduced cortical volume and axonal and myelin damage resulting from hydrocephalus) that may contribute to difficulties in the executive control of attention in individuals with SBM. Further characterization of the functioning of the executive control network in individuals with SBM relative to their TD peers is needed in order to establish whether the identified differences in neural structure (Williams et al., 2013), and the association between that structure and function in the present study, result in significant impairment. Although individuals with SBM are able to adequately sustain their attention, they are impaired in the ability to successfully disengage and shift it in response to the environment and may also be impaired in their ability to exert executive control over it.
The present study was limited by a 3 mm DTI slice thickness and the use of 21 directions for DTI acquisition, which are below current standards. However, at the time the study began in 2005, these decisions were consistent with current scanner technology and the need to reduce acquisition time for young children. The output adequately reflected the intended pathways and the presence of significant structure–function associations further supports our confidence in the results. Although we found several significant structure–function associations, only those involving covert orienting were reliably significant for both single and bootstrap samples across all correlational estimates, suggesting that replication of the findings in other samples is warranted. It would be of interest for future studies to replicate tractography of tectocortical pathways in SBM, as well as to investigate association pathways, such as the superior longitudinal fasciculus, the integrity of which may be even more strongly related to functioning of the attention networks. Future studies should also further characterize the functioning of the attention networks in SBM in relation to TD individuals, especially that of the executive control network.
Overall, neuroanatomical disruption to the orienting attention network in SBM appears more robust and a direct consequence of characteristic midbrain dysmorphology. Executive control attention network difficulties in SBM may alternatively emerge from parietal cortical anomalies and reduced frontal and parietal cortical–subcortical connectivity related to the pathophysiological effects of congenital hydrocephalus.
Acknowledgments
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant (P01 HD35946-06, “Spina Bifida: Cognitive and Neurobiological Variability”). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. All the authors state that they have no conflict of interests related to this manuscript.
Maureen Dennis passed away on July 15th, 2014.
References
- Baron I.S. Test of everyday attention for children. Child Neuropsychol. 2001;7(3):190–195. doi: 10.1076/chin.7.3.190.8742. 12187475 [DOI] [PubMed] [Google Scholar]
- Behramn R.E., Kliegman R.M., Jenson B.H. Congenital anomalies of the central nervous system. In: Behramn R.E., Kliegman R.M., Jenson B.H., editors. Nelson Textbook of Pediatrics. Saunders; Philadelphia, PA: 2003. pp. 1983–1985. [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007;34(1):144–155. doi: 10.1016/j.neuroimage.2006.09.018. 17070705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde M.D., Kim J.H., Liang H.F., Schmidt R.E., Russell J.H., Cross A.H., Song S.K. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn. Reson. Med. 2007;57(4):688–695. doi: 10.1002/mrm.21200. 17390365 [DOI] [PubMed] [Google Scholar]
- Colvin A.N., Yeates K.O., Enrile B.G., Coury D.L. Motor adaptation in children with myelomeningocele: comparison to children with ADHD and healthy siblings. J Int Neuropsychol Soc. 2003;9(4):642–652. doi: 10.1017/S1355617703940045. 12755176 [DOI] [PubMed] [Google Scholar]
- Dennis M., Barnes M.A. The cognitive phenotype of spina bifida meningomyelocele. Dev. Disabil. Res. Rev. 2010;16(1):31–39. doi: 10.1002/ddrr.89. 20419769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M., Edelstein K., Copeland K., Frederick J., Francis D.J., Hetherington R., Blaser S.E., Kramer L.A., Drake J.M., Brandt M.E., Fletcher J.M. Covert orienting to exogenous and endogenous cues in children with spina bifida. Neuropsychologia. 2005;43(6):976–987. doi: 10.1016/j.neuropsychologia.2004.08.012. 15716168 [DOI] [PubMed] [Google Scholar]
- Dennis M., Sinopoli K.J., Fletcher J.M., Schachar R. Puppets, robots, critics, and actors within a taxonomy of attention for developmental disorders. J Int Neuropsychol Soc. 2008;14(5):673–690. doi: 10.1017/S1355617708080983. 18764966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. 16530430 [DOI] [PubMed] [Google Scholar]
- Hannay H.J., Dennis M., Kramer L., Blaser S., Fletcher J.M. Partial agenesis of the corpus callosum in spina bifida meningomyelocele and potential compensatory mechanisms. J. Clin. Exp. Neuropsychol. 2009;31(2):180–194. doi: 10.1080/13803390802209954. 19052950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. 21979382 [DOI] [PubMed] [Google Scholar]
- Jones D.K., Cercignani M. Twenty-five pitfalls in the analysis of diffusion MRI data. N.M.R. Biomed. 2010;23(7):803–820. doi: 10.1002/nbm.1543. 20886566 [DOI] [PubMed] [Google Scholar]
- Juranek J., Fletcher J.M., Hasan K.M., Breier J.I., Cirino P.T., Pazo-Alvarez P., Diaz J.D., Ewing-Cobbs L., Dennis M., Papanicolaou A.C. Neocortical reorganization in spina bifida. Neuroimage. 2008;40(4):1516–1522. doi: 10.1016/j.neuroimage.2008.01.043. 18337124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J., Salman M.S. Anomalous development of brain structure and function in spina bifida myelomeningocele. Dev. Disabil. Res. Rev. 2010;16(1):23–30. doi: 10.1002/ddrr.88. 20419768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R.M. On the control of visual orienting. In: Posner M.I., editor. Cognitive Neuroscience of Attention. Guilford Books; New York, NY: 2004. pp. 29–44. [Google Scholar]
- Kulesz P.A., Tian S., Juranek J., Fletcher J.M., Francis D.J. Relations between volumetric measures of brain structure and attentional function in spina bifida: utilization of robust statistical approaches. Neuropsychology. 2015;29(2):212–225. doi: 10.1037/neu0000166. 25495830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly T., Robertson I.H., Anderson V., Nimmo-Smith I. The Test of Everyday Attention for Children: Manual. Thames Valley Test Company Limited; Bury St Edmunds, UK: 1999. [Google Scholar]
- Ou X., Snow J.H., Byerley A.K., Hall J.J., Glasier C.M. Decreased activation and increased lateralization in brain functioning for selective attention and response inhibition in adolescents with spina bifida. Child Neuropsychol. 2013;19(1):23–36. doi: 10.1080/09297049.2011.639754. 22145814 [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. 22524787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I. Orienting of attention. Q. J. Exp. Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. 7367577 [DOI] [PubMed] [Google Scholar]
- Posner M.I. Imaging attention networks. Neuroimage. 2012;61(2):450–456. doi: 10.1016/j.neuroimage.2011.12.040. 22227132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M.I., Petersen S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. 2183676 [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R:A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. [Google Scholar]
- Rafal R.D., Posner M.I., Friedman J.H., Inhoff A.W., Bernstein E. Orienting of visual attention in progressive supranuclear palsy. Brain. 1988;111(2):267–280. doi: 10.1093/brain/111.2.267. 3378136 [DOI] [PubMed] [Google Scholar]
- Rueda M.R., Fan J., McCandliss B.D., Halparin J.D., Gruber D.B., Lercari L.P., Posner M.I. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. 15093142 [DOI] [PubMed] [Google Scholar]
- Song S.K., Sun S.W., Ju W.K., Lin S.J., Cross A.H., Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. 14642481 [DOI] [PubMed] [Google Scholar]
- Swartwout M.D., Cirino P.T., Hampson A.W., Fletcher J.M., Brandt M.E., Dennis M. Sustained attention in children with two etiologies of early hydrocephalus. Neuropsychol. 2008;22(6):765–775. doi: 10.1037/a0013373. 18999350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H.B., Landry S.H., Barnes M., Swank P., Cohen L.B., Fletcher J. Early information processing among infants with and without spina bifida. Infant Behav. Dev. 2010;33(4):365–372. doi: 10.1016/j.infbeh.2010.03.005. 20488543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treble A., Juranek J., Stuebing K.K., Dennis M., Fletcher J.M. Functional significance of atypical cortical organization in spina bifida myelomeningocele: relations of cortical thickness and gyrification with IQ and fine motor dexterity. Cereb. Cortex. 2013;23(10):2357–2369. doi: 10.1093/cercor/bhs226. 22875857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treble-Barna A., Kulesz P.A., Dennis M., Fletcher J.M. Covert orienting in three etiologies of congenital hydrocephalus: the effect of midbrain and posterior fossa dysmorphology. J. Int. Neuropsychol. Soc. 2014;20(3):268–277. doi: 10.1017/S1355617713001501. 24528548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox R.R. Inferences based on multiple skipped correlations. Computational Statistics & Data Analysis. 2003;44(1–2):223–236. [Google Scholar]
- Williams V.J., Juranek J., Stuebing K., Cirino P.T., Dennis M., Fletcher J.M. Examination of frontal and parietal tectocortical attention pathways in spina bifida meningomyelocele using probabilistic diffusion tractography. Brain Connectivity. 2013;3(5):512–522. doi: 10.1089/brain.2013.0171. 23937233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K.H., Warfield S.K., Bharatha A., Tempany C.M., Kaus M.R., Haker S.J., Wells W.M., Jolesz F.A., Kikinis R. Statistical validation of image segmentation quality based on a spatial overlap index. Acad. Radiol. 2004;11(2):178–189. doi: 10.1016/S1076-6332(03)00671-8. 14974593 [DOI] [PMC free article] [PubMed] [Google Scholar]


