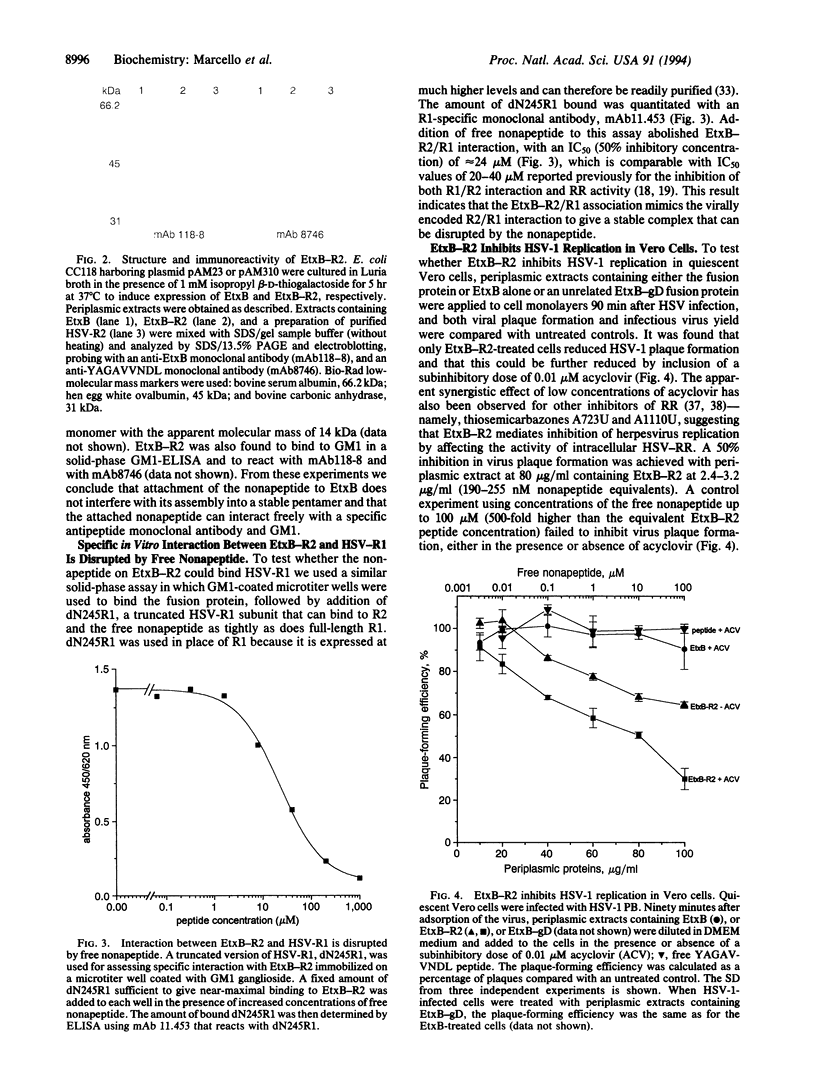
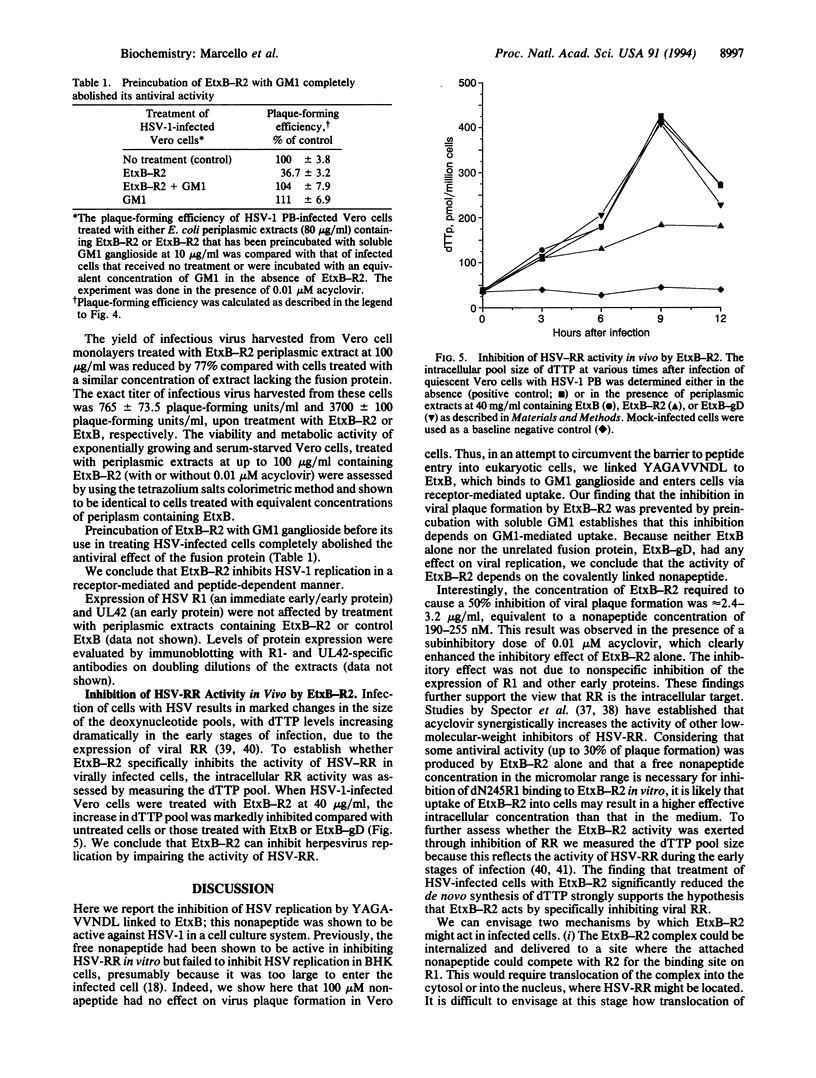
Abstract
Mimetic peptides capable of selectively disrupting protein-protein interactions represent potential therapeutic agents for inhibition of viral and cellular enzymes. This approach was first suggested by the observation that the peptide YAGAVVNDL, corresponding to the carboxyl-terminal 9 amino acids of the small subunit of ribonucleotide reductase of herpes simplex virus, specifically inhibited the viral enzyme in vitro. Evaluation and use of this peptide as a potential antiviral agent has, however, been thwarted by its failure to inhibit virus replication in vivo, presumably because the peptide is too large to enter eukaryotic cells unaided. Here, we show that the nontoxic B subunit of Escherichia coli heat-labile enterotoxin can be used as a recombinant carrier for the receptor-mediated delivery of YAGAVVNDL into virally infected cells. The resultant fusion protein specifically inhibited herpes simplex virus type 1 replication and ribonucleotide reductase activity in quiescent Vero cells. Preincubation of the fusion protein with soluble GM1 ganglioside abolished this antiviral effect, indicating that receptor-mediated binding to the target cell is necessary for its activity. This provides direct evidence of the usefulness of carrier-mediated delivery to evaluate the intracellular efficacy of a putative antiviral peptide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averett D. R., Lubbers C., Elion G. B., Spector T. Ribonucleotide reductase induced by herpes simplex type 1 virus. Characterization of a distinct enzyme. J Biol Chem. 1983 Aug 25;258(16):9831–9838. [PubMed] [Google Scholar]
- Bacchetti S., Evelegh M. J., Muirhead B. Identification and separation of the two subunits of the herpes simplex virus ribonucleotide reductase. J Virol. 1986 Mar;57(3):1177–1181. doi: 10.1128/jvi.57.3.1177-1181.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt C. R., Kintner R. L., Pumfery A. M., Visalli R. J., Grau D. R. The herpes simplex virus ribonucleotide reductase is required for ocular virulence. J Gen Virol. 1991 Sep;72(Pt 9):2043–2049. doi: 10.1099/0022-1317-72-9-2043. [DOI] [PubMed] [Google Scholar]
- Cameron J. M., McDougall I., Marsden H. S., Preston V. G., Ryan D. M., Subak-Sharpe J. H. Ribonucleotide reductase encoded by herpes simplex virus is a determinant of the pathogenicity of the virus in mice and a valid antiviral target. J Gen Virol. 1988 Oct;69(Pt 10):2607–2612. doi: 10.1099/0022-1317-69-10-2607. [DOI] [PubMed] [Google Scholar]
- Cohen E. A., Gaudreau P., Brazeau P., Langelier Y. Specific inhibition of herpesvirus ribonucleotide reductase by a nonapeptide derived from the carboxy terminus of subunit 2. Nature. 1986 May 22;321(6068):441–443. doi: 10.1038/321441a0. [DOI] [PubMed] [Google Scholar]
- Cohen G. H. Ribonucleotide reductase activity of synchronized KB cells infected with herpes simplex virus. J Virol. 1972 Mar;9(3):408–418. doi: 10.1128/jvi.9.3.408-418.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner J., Furlong J., Murray J., Meighan M., Cross A., Marsden H., Clements J. B. Herpes simplex virus type 1 ribonucleotide reductase large subunit: regions of the protein essential for subunit interaction and dimerization. Biochemistry. 1993 Dec 14;32(49):13673–13680. doi: 10.1021/bi00212a036. [DOI] [PubMed] [Google Scholar]
- Conner J., Macfarlane J., Lankinen H., Marsden H. The unique N terminus of the herpes simplex virus type 1 large subunit is not required for ribonucleotide reductase activity. J Gen Virol. 1992 Jan;73(Pt 1):103–112. doi: 10.1099/0022-1317-73-1-103. [DOI] [PubMed] [Google Scholar]
- Cosentino G., Lavallée P., Rakhit S., Plante R., Gaudette Y., Lawetz C., Whitehead P. W., Duceppe J. S., Lépine-Frenette C., Dansereau N. Specific inhibition of ribonucleotide reductases by peptides corresponding to the C-terminal of their second subunit. Biochem Cell Biol. 1991 Jan;69(1):79–83. doi: 10.1139/o91-011. [DOI] [PubMed] [Google Scholar]
- Daikoku T., Yamamoto N., Maeno K., Nishiyama Y. Role of viral ribonucleotide reductase in the increase of dTTP pool size in herpes simplex virus-infected Vero cells. J Gen Virol. 1991 Jun;72(Pt 6):1441–1444. doi: 10.1099/0022-1317-72-6-1441. [DOI] [PubMed] [Google Scholar]
- Dutia B. M., Frame M. C., Subak-Sharpe J. H., Clark W. N., Marsden H. S. Specific inhibition of herpesvirus ribonucleotide reductase by synthetic peptides. Nature. 1986 May 22;321(6068):439–441. doi: 10.1038/321439a0. [DOI] [PubMed] [Google Scholar]
- Dutia B. M. Ribonucleotide reductase induced by herpes simplex virus has a virus-specified constituent. J Gen Virol. 1983 Mar;64(Pt 3):513–521. doi: 10.1099/0022-1317-64-3-513. [DOI] [PubMed] [Google Scholar]
- Filatov D., Ingemarson R., Gräslund A., Thelander L. The role of herpes simplex virus ribonucleotide reductase small subunit carboxyl terminus in subunit interaction and formation of iron-tyrosyl center structure. J Biol Chem. 1992 Aug 5;267(22):15816–15822. [PubMed] [Google Scholar]
- Frame M. C., Marsden H. S., Dutia B. M. The ribonucleotide reductase induced by herpes simplex virus type 1 involves minimally a complex of two polypeptides (136K and 38K). J Gen Virol. 1985 Jul;66(Pt 7):1581–1587. doi: 10.1099/0022-1317-66-7-1581. [DOI] [PubMed] [Google Scholar]
- Gaudreau P., Michaud J., Cohen E. A., Langelier Y., Brazeau P. Structure-activity studies on synthetic peptides inhibiting herpes simplex virus ribonucleotide reductase. J Biol Chem. 1987 Sep 15;262(26):12413–12416. [PubMed] [Google Scholar]
- Idowu A. D., Fraser-Smith E. B., Poffenberger K. L., Herman R. C. Deletion of the herpes simplex virus type 1 ribonucleotide reductase gene alters virulence and latency in vivo. Antiviral Res. 1992 Feb;17(2):145–156. doi: 10.1016/0166-3542(92)90048-a. [DOI] [PubMed] [Google Scholar]
- Ingemarson R., Lankinen H. The herpes simplex virus type 1 ribonucleotide reductase is a tight complex of the type alpha 2 beta 2 composed of 40K and 140K proteins, of which the latter shows multiple forms due to proteolysis. Virology. 1987 Feb;156(2):417–422. doi: 10.1016/0042-6822(87)90422-3. [DOI] [PubMed] [Google Scholar]
- Jacobson J. G., Leib D. A., Goldstein D. J., Bogard C. L., Schaffer P. A., Weller S. K., Coen D. M. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology. 1989 Nov;173(1):276–283. doi: 10.1016/0042-6822(89)90244-4. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Bjursell G. Deoxyribonucleoside triphosphate pools in herpes simplex type 1 infected cells. J Gen Virol. 1976 Apr;31(1):101–113. doi: 10.1099/0022-1317-31-1-101. [DOI] [PubMed] [Google Scholar]
- Janicot M., Fouque F., Desbuquois B. Activation of rat liver adenylate cyclase by cholera toxin requires toxin internalization and processing in endosomes. J Biol Chem. 1991 Jul 15;266(20):12858–12865. [PubMed] [Google Scholar]
- Karlsson A., Harmenberg J. Effects of ribonucleotide reductase inhibition on pyrimidine deoxynucleotide metabolism in acyclovir-treated cells infected with herpes simplex virus type 1. Antimicrob Agents Chemother. 1988 Jul;32(7):1100–1102. doi: 10.1128/aac.32.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankinen H., McLauchlan J., Weir M., Furlong J., Conner J., McGarrity A., Mistry A., Clements J. B., Marsden H. S. Purification and characterization of the herpes simplex virus type 1 ribonucleotide reductase small subunit following expression in Escherichia coli. J Gen Virol. 1991 Jun;72(Pt 6):1383–1392. doi: 10.1099/0022-1317-72-6-1383. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., Delp C., Neutra M. R., Madara J. L. Mechanism of cholera toxin action on a polarized human intestinal epithelial cell line: role of vesicular traffic. J Cell Biol. 1992 Jun;117(6):1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- McClements W., Yamanaka G., Garsky V., Perry H., Bacchetti S., Colonno R., Stein R. B. Oligopeptides inhibit the ribonucleotide reductase of herpes simplex virus by causing subunit separation. Virology. 1988 Jan;162(1):270–273. doi: 10.1016/0042-6822(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Nashar T. O., Amin T., Marcello A., Hirst T. R. Current progress in the development of the B subunits of cholera toxin and Escherichia coli heat-labile enterotoxin as carriers for the oral delivery of heterologous antigens and epitopes. Vaccine. 1993;11(2):235–240. doi: 10.1016/0264-410x(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Palu' G., Biasolo M. A. Nucleotide sequence of the thymidine kinase gene of a new strain of herpes simplex virus type 1. Virus Genes. 1989 Mar;2(2):183–186. doi: 10.1007/BF00315261. [DOI] [PubMed] [Google Scholar]
- Paradis H., Gaudreau P., Brazeau P., Langelier Y. Mechanism of inhibition of herpes simplex virus (HSV) ribonucleotide reductase by a nonapeptide corresponding to the carboxyl terminus of its subunit 2. Specific binding of a photoaffinity analog, [4'- azido-Phe6] HSV H2-6(6-15), to subunit 1. J Biol Chem. 1988 Nov 5;263(31):16045–16050. [PubMed] [Google Scholar]
- Preston V. G., Darling A. J., McDougall I. M. The herpes simplex virus type 1 temperature-sensitive mutant ts1222 has a single base pair deletion in the small subunit of ribonucleotide reductase. Virology. 1988 Dec;167(2):458–467. [PubMed] [Google Scholar]
- Preston V. G., Palfreyman J. W., Dutia B. M. Identification of a herpes simplex virus type 1 polypeptide which is a component of the virus-induced ribonucleotide reductase. J Gen Virol. 1984 Sep;65(Pt 9):1457–1466. doi: 10.1099/0022-1317-65-9-1457. [DOI] [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Sandkvist M., Hirst T. R., Bagdasarian M. Alterations at the carboxyl terminus change assembly and secretion properties of the B subunit of Escherichia coli heat-labile enterotoxin. J Bacteriol. 1987 Oct;169(10):4570–4576. doi: 10.1128/jb.169.10.4570-4576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., Wartna E. S., van Zanten B. A., Witholt B., Hol W. G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991 May 30;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Spangler B. D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992 Dec;56(4):622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Averett D. R., Nelson D. J., Lambe C. U., Morrison R. W., Jr, St Clair M. H., Furman P. A. Potentiation of antiherpetic activity of acyclovir by ribonucleotide reductase inhibition. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4254–4257. doi: 10.1073/pnas.82.12.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Harrington J. A., Morrison R. W., Jr, Lambe C. U., Nelson D. J., Averett D. R., Biron K., Furman P. A. 2-Acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (A1110U), a potent inactivator of ribonucleotide reductases of herpes simplex and varicella-zoster viruses and a potentiator of acyclovir. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1051–1055. doi: 10.1073/pnas.86.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J. Multicopy expression vectors carrying the lac repressor gene for regulated high-level expression of genes in Escherichia coli. Gene. 1987;51(2-3):255–267. doi: 10.1016/0378-1119(87)90314-3. [DOI] [PubMed] [Google Scholar]
- Stubbe J. Ribonucleotide reductases: amazing and confusing. J Biol Chem. 1990 Apr 5;265(10):5329–5332. [PubMed] [Google Scholar]
- Yang F. D., Spanevello R. A., Celiker I., Hirschmann R., Rubin H., Cooperman B. S. The carboxyl terminus heptapeptide of the R2 subunit of mammalian ribonucleotide reductase inhibits enzyme activity and can be used to purify the R1 subunit. FEBS Lett. 1990 Oct 15;272(1-2):61–64. doi: 10.1016/0014-5793(90)80449-s. [DOI] [PubMed] [Google Scholar]
- de Wind N., Berns A., Gielkens A., Kimman T. Ribonucleotide reductase-deficient mutants of pseudorabies virus are avirulent for pigs and induce partial protective immunity. J Gen Virol. 1993 Mar;74(Pt 3):351–359. doi: 10.1099/0022-1317-74-3-351. [DOI] [PubMed] [Google Scholar]



