Abstract
The L-type amino acid transporter (LAT) family are Na+-independent transporters, which deliver neutral amino acids into cells. The four LATs, LAT1 (SLC7A5), LAT2 (SLC7A8), LAT3 (SLC43A1) and LAT4 (SLC43A2), are responsible for the majority of cellular leucine uptake. They show increased expression in many cancers, and are critical for control of protein translation and cell growth through the mTORC1 pathway. The increased transporter expression observed in cancers is regulated by transcriptional pathways such as hormone receptors, c-myc and nutrient starvation responses. We review the expression and function of the LAT family in cancer, as well as the recent development of specific inhibitors targeting LAT1 or LAT3. These LAT family inhibitors may be useful adjuvant therapeutics in multiple cancers.
Keywords: L-type amino acid, transport, cancer, mTORC1 pathway
Recent advances in therapeutics designed to target the PI3K/Akt/mTORC1 pathway have resulted in dozens of new anti-cancer compounds currently undergoing Phase I/II trials [1,2]. This critical cell growth pathway is also regulated by nutrients, in particular the essential amino acid leucine, which is required for activation of the mTORC1/Ragulator complex. Leucine is the most common of the 20 proteinogenic amino acids present in proteins. It is thought that mTORC1 can only begin translation when sufficient levels of leucine, arginine or glutamine are available. The L-type amino acid transporters (LATs) are the major transporters that mediate uptake of leucine into cells, thereby regulating mTORC1 signaling and protein synthesis. This critical requirement for intracellular leucine is reflected in the increased expression of LATs in the majority of cancers, and in the diverse transcription factors that regulate their expression. The classification, structure and function of LAT family have been well reviewed recently [3,4]. In our review, we provide an overview of recent studies focusing on the role and regulation of the four LAT family members (LAT1, LAT2, LAT3 and LAT4) in cancer. We have analyzed LAT family member expression levels, correlations with disease state and metastasis, and their role in cancer cell growth through the mTORC1 pathway. Furthermore, we discuss targeting of the LAT family as a novel anti-cancer approach and the current state of LAT inhibitors.
L-type amino acid transporter family
The L-type amino acid transporter (LAT) family consists of four Na+-independent neutral amino acid transporters. The members of this family are grouped in two sub-families, namely, SLC7 (LAT1 and LAT2) and SLC43 (LAT3 and LAT4). Each member of the LAT family is believed to contain 12 transmembrane domains, however there are no current structures solved for any of the human LAT proteins. LAT1 (SLC7A5) and LAT2 (SLC7A8) associate with the 4F2hc (4F2 antigen heavy chain; CD98 heavy chain) glycoprotein, forming a heterodimeric obligatory exchanger with a high affinity [5-9]. LAT3 (SLC43A1) and LAT4 (SLC43A2) are facilitated diffusers of neutral amino acids with a low affinity, and do not appear to require a binding partner [10,11]. LAT3 and LAT4 deliver a narrow range of neutral amino acids into cells, including leucine, isoleucine, valine, phenylalanine and methionine [10,11]. Similarly, LAT1 and LAT2 can transport these same neutral amino acids, including additional amino acids such as tyrosine, histidine and tryptophan [5,7-9] (Table 1).
Table 1.
LAT expression and function
| Protein | Gene | Substrates | Expression pattern |
|---|---|---|---|
| LAT1 | SLC7A5 | Leu, Ile, Phe, Met, Tyr, His, Trp, Val | Brain, spleen, placenta, ovary, testis, colon, blood-brain barrier, fetal liver, activated lymphocytes [5,6] |
| LAT2 | SLC7A8 | Gly, Ala, Ser, Thr, Asn, Gln, Met, Leu, Ile, Val, Phe, Tyr, Trp, His | Jejunum, ileum, kidney, placenta, brain, liver, skeletal muscle, prostate, ovaries, fetal liver, testis and heart [7-9] |
| LAT3 | SLC43A1 | Met, Leu, Ile, Val, Phe | Pancreas, skin, muscle, liver, kidney podocytes, prostate [10,20,23,25] |
| LAT4 | SLC43A2 | Met, Leu, Ile, Val, Phe | Placenta, kidney, peripheral blood leukocytes [11] |
In 1998, two independent groups cloned LAT1 from cancer cells [5,6]. LAT1 has 507 amino acids with a molecular weight of 55 kDa. LAT1 mRNA is strongly expressed in brain, spleen, placenta, testis and colon (Table 1) [5]. LAT2 was subsequently cloned due to its homology with LAT1 [7-9]. Human LAT2 and LAT1 shows an amino acid sequence identify of 50%. LAT2 has 535 amino acids with a molecular weight of 58 kDa [7]. LAT2 transcripts are strongly expressed in jejunum, ileum, kidney, placenta, brain, and also detected in liver, skeletal muscle, prostate, ovaries and heart [7-9,12]. Both LAT1 and LAT2 transport capacity is independent of sodium or chloride. However, binding of 4F2hc at cysteine 163 (LAT1) is required for the normal function and membrane localization of LAT1 and LAT2 [5,7,8]. Leucine transport by LAT1 is also dependent on L-glutamine, which is delivered by other amino acid transporters including ASCT2 (SLC1A5) [13-16].
LAT3 transcript was originally cloned from prostate cancer and named prostate cancer overexpressed gene 1 (POV1) [17,18]. Later, POV1 was identified as a transporter and named LAT3 by expression cloning from the hepatocarcinoma-derived cell line FLC [10]. Mouse LAT3 contains 564 amino acids with a molecular weight of 62.6 kDa [19], while human LAT3 has 559 amino acids. A long intracellular loop predicted to exist between transmembrane domains 6 and 7, contains putative protein kinase C-dependent phosphorylation sites and a tyrosine phosphorylation site [19]. Human LAT4 exhibits 57% identity to human LAT3 [11]. LAT3 and LAT4 have a broad expression pattern in human tissues. Northern blot analysis showed that LAT3 mRNA is expressed in pancreas, liver, skeletal muscle and fetal liver at a high levels [10,19] (Table 1). Human LAT4 mRNA is strongly expressed in placenta, kidney and peripheral blood leukocytes in human tissue. Mouse LAT4 is detected in intestine, kidney, brain, white adipose tissue, testis and heart, but not in liver [11] (Table 1). However, the physiologic functions of LAT3/LAT4 in these organs are still not fully understood. Although the expression of LAT3 in kidney is low, strong expression is detected at the apical plasma membrane of podocyte foot processes. LAT3 is important for the development and maintenance of podocyte function and structure [20,21]. A recent study also showed that red blood cell development requires LAT3 expression for hemoglobin production. In LAT4 knockout mice, newborn mice are smaller than wild type mice [22], suggesting that LAT4 is important for growth and development.
LAT family expression and function in cancer
While the LAT family clearly play important roles in development and function of normal tissues, they are frequently increased in cancer samples. To effectively review this area, and highlight the important role of LAT family members in cancer, we have summarized publications across multiple cancers (Table 2). In addition, we have performed new analyses of Oncomine microarray/sequencing datasets to further highlight which cancers show a significant increase in LAT expression compared to normal tissue (P < 0.05, Fold change > 2; Table 2). These data clearly show that LAT1 is most commonly upregulated in multiple cancers, and accordingly LAT1 has been the most studied of the LAT family members. While the Oncomine data suggest that LAT2 is upregulated in 9 different cancer types, there are few studies that have validated its role in cancer cell growth. LAT3 and LAT4 show a more restricted expression pattern in 5 or 4 different cancer types respectively, with multiple publications on the critical role of LAT3 in prostate cancer [17,18,23-25]. Table 2 shows the potential utility of targeting the LAT family in a variety of cancers, as well as highlighting a number of cancers that require further analysis of LAT family expression and function.
Table 2.
LAT expression in tumors
| LAT1(SLC7A5) | LAT2(SLC7A8) | LAT3(SLC43A1) | LAT4(SLC43A2) | 4F2hc(SLC3A2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Cancer | REF | ONC | REF | ONC | REF | ONC | REF | ONC | REF | ONC |
| Bladder | [92-94] | 2/12 | ||||||||
| Brain/CNS | [30,42] | 3/35 | ||||||||
| Breast | [34,77,95-97] | 15/52 | 10/50 | 1/45 | [34] | 1/53 | ||||
| Cervical | [98] | |||||||||
| Cholangiocarcinoma | [66] | |||||||||
| Colorectal | [34] | 23/34 | 4/34 | 2/34 | [99] | [34] | 6/37 | |||
| Esophageal | [100] | 3/9 | 1/6 | 1/9 | ||||||
| Gastric | [36] | 1/23 | ||||||||
| Head and Neck | [34,42,99,101] | 7/32 | 2/32 | [99] | [34] | 3/32 | ||||
| Kidney | 1/24 | 1/20 | 1/10 | 1/24 | ||||||
| Leukemia | [87,102] | 4/28 | 4/27 | [103] | 2/31 | |||||
| Liver | [102,104-107] | [108] | [33] | |||||||
| Lung | [26,35,37,67] | 14/31 | [37,67] | |||||||
| Lymphoma | 8/30 | 1/29 | 2/21 | |||||||
| Melanoma | [15,109] | 3/7 | 1/7 | 1/7 | ||||||
| Myeloma | [110-112] | 1/8 | [110] | |||||||
| Ovarian | [113,114] | 3/14 | ||||||||
| Pancreatic | [32,115,116] | |||||||||
| Pleural mesothelioma | [28] | |||||||||
| Prostate | [24,25,39] | 1/20 | [17,18,24,25] | 4/20 | 1/21 | |||||
| Sarcoma | [34,42] | 1/20 | [34] | |||||||
| Tongue cancer | [31] | [31] | ||||||||
| Thymic carcinomas | [29] | |||||||||
| Urinary tract | [92,117] | |||||||||
| Uterine leiomyoma | [118] | [79] | [79] | |||||||
| Other (seminoma) | 3/3 | |||||||||
| Other (skin) | 4/4 | 4/4 | 1/4 | |||||||
| Other (parathyroid) | 3/3 | |||||||||
Expression of LAT family members in a variety of cancers was assessed using Pubmed to find published references (REF) and Oncomine (ONC) to detect significant upregulation (P < 0.05, fold change > 2) for each transporter. Oncomine numbers represent Datasets with Significant Upregulation/Total Number of Datasets.
Immunohistochemical analysis in patient cohorts have shown that LAT1 is overexpressed in cancer and its expression correlates with cell proliferation and cancer progression. LAT1 is highly expressed in 52% of the large cell neuroendocrine carcinomas of the lung [26,27], 50% of pleural mesothelioma [28], 75% of thymic carcinomas [29], 25% of high-grade gliomas [30], 61% of tongue cancer [31], 53% of pancreatic cancer [32] and 61% in hepatocellular carcinoma [33]. In these studies, a significant correlation was found between LAT1 expression and proliferation marker Ki-67, suggesting that LAT1 is important for proliferation in cancer cells [27,29,31-37].
LAT1 has also been used as biomarker for malignant cancer. Using Kaplan-Meier analysis of patients, low LAT1 expression patients showed a significant longer overall survival compared to high LAT1 expression patients, indicating that LAT1 could be a prognostic marker for predicting poor outcome after surgery [32,33,38]. In prostate cancer, LAT1 expression is correlated with prognosis in poor survival patients [39]. In breast cancer, LAT1 (SLC7A5) is also part of the 5 gene MammostratTM immunohistochemistry panel, where high expression is used to predict recurrence in ER+ breast cancer during endocrine therapy [40,41].
Studies determining the function of LAT1 in cancer have utilized a well characterized LAT family inhibitor, BCH (2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid). BCH can inhibit all members of LAT family at a concentration above 10 mM. BCH treatment decreases leucine transport and suppresses mTORC1 signaling [13,24]. Expression of cell cycle regulators is altered, such as up-regulation of p21 in glioma cells [30] and p27 in prostate cancer cells [24], down-regulation of CDK1, CDC20 and E2F1 [25]. Therefore, cell proliferation and DNA synthesis are suppressed.
When LAT1 is blocked by BCH, several studies also showed that apoptosis was enhanced in glioma cells [30], oral epidermoid carcinoma cells, osteogenic sarcoma cells [42]. Cleaved caspase 3 and cleaved PARP levels are increased after BCH treatment [30]. However, no apoptosis was observed using either BCH or knockdown LAT1 or LAT3 in prostate cancer cell lines [24]. This suggests that LAT-related cell apoptosis may be dependent on the cell type.
LATs and mTORC1 signaling
Perhaps the most important role of LATs is to transport neutral amino acids for protein synthesis. One of the major LAT substrates, leucine, is not only an essential amino acid, but also a regulator of mTORC1 (mammalian target of rapamycin complex 1) signaling. mTOR is a member of the phosphoinositide-3-kinase related kinase (PIKK) family that possess catalytic activity as a protein serine-threonine kinase. mTOR is present within the cell bound in two major complexes, mTORC1 and mTORC2. mTORC1 is formed by mTOR complexed with mLST8, RAPTOR, PRAS40 and DEPTOR, activating S6 kinase while repressing eIF-4E-binding protein (4E-BP1), thereby regulating protein translation [43,44]. mTORC2 is formed by mTOR complexed with mLST8, RICTOR and mSin1, which can phosphorylate and activate Akt at Ser473 [45-47].
Both PI3K/Akt signaling and amino acids (especially leucine, arginine and glutamine) are required to activate mTORC1 signaling [13,44,48]. While the PI3K/Akt signaling pathway is well understood, the exact mechanism by which amino acids are sensed and activate mTORC1 remains unclear. Activated Akt phosphorylates TSC2 leading to the suppression of TSC2 activity. The inactivated TSC2 dissociates from the surface of lysosomes, releasing Rheb, a small GTPase, to activate mTORC1 on the lysosomal surface [49-53]. Recent studies have suggested that the intracellular level of leucine can be detected by a leucyl-tRNA synthetase (LRS), which can then catalyze the ATP-dependent ligation of L-leucine to leucyl-tRNA during protein synthesis [54,55]. In the leucine rich environment, LRS with leucine may then interact and activate the Rag GTPase complex. Rag proteins are Ras-related small GTP-binding proteins that include four mammalian members, RagA, RagB, RagC and RagD. They form heterodimers consisting of RagA or RagB with RagC or RagD [56,57]. Recent work showed that Rag GTPases are only essential for leucine- or arginine-activated mTORC1 signaling [58]. Glutamine-activated mTORC1 activation depends on adenosine diphosphate ribosylation factor-1 GTPase (Arf1) [58]. LRS may also bind to RAPTOR to activate mTORC1 signaling on the surface of lysosome [54,55]. While the mechanism of leucine sensing remains unclear, low levels of intracellular amino acids lead to Rag heterodimer binding and recruitment of the TSC complex to the lysosome, thereby inhibiting Rheb and mTORC1 signaling [52,53].
Another study has suggested that glutaminolysis and α-ketoglutarate are involved in glutamine and leucine sensing to activate mTORC1 signaling [48]. The enzyme glutaminase releases the amide group of glutamine to form glutamate. Leucine can directly bind and activate glutamate dehydrogenase, which subsequently converts glutamate to α-ketoglutarate (α-KG) [59]. α-KG is sufficient to stimulate recruitment of mTORC1 to the lysosome by activating RagB. The activated LRS or α-KG stimulates the transition of RagA/RagB GDP-RagC/RagD GTP to RagA/RagB GTP-RagC/RagD GDP [48].
Lysosomal membrane proteins, vacuolar adenosine triphosphatase (v-ATPase) and SLC-36A1, have been shown to interact with Rag GTPases, and may be necessary for mTORC1 activation by amino acids [60]. Lysosomal membrane amino acid transporter SLC38A9 was recently shown to interact with Ragulator/LAMTOR complex, four RAG GTPases and VA0D1 of the v-ATPase [61,62]. Purified SLC38A9 directly interacts with arginine making it a potential amino acid sensor for mTORC1 signaling [62]. When arginine binds to SLC38A9 substrate-binding site, SLC38A9 may undergo a conformational change which affects its interactors, such as v-ATPase, RAG GTPases and Ragulator.
Amino acid-activated signaling is also tightly regulated by proteins which interact with Rag GTPase or Ragulator. For example, folliculin and its interacting partner FNIP1/2 form a complex to activate RagC/D as a GTPase activating protein (GAP) [63]. Sestrins also bind to the heterodimeric Rag complexes and negatively regulate the activity of Rag complexes [64]. GATOR complexes interact with the Rag GTPase complex to negatively regulate leucine signaling. GATOR is composed of two subcomplexes named GATOR1 (DEPDC5, Nprl2 and Nprl3) and GATOR2 (Mios, WDR24, WDR59, Seh1L and Sec13) [65]. GATOR1 has GTPase-activating protein activity for RagA and RagB. GATOR1 components, such as NPRL2 and DEPDC5, contain deletion or mutation in multiple cancer cell lines, leading to hyperactivation of mTORC1 signaling and insensitivity to amino acid deprivation [65].
The role of LAT in metastasis
Several studies have suggested that LAT1 expression also correlates with metastasis. These data are across a range of cancers, including colon, breast, prostate, head and neck, lung, genital as well as soft-tissue sarcomas, all showing that LAT1 expression is significantly higher in the metastatic sites than in the primary sites [24,27,34]. LAT1 transport function may be critical in providing nutrients for metastatic cancer cells, as BCH treatment or knockdown of LAT1 expression by shRNA has been shown to decrease cell migration and invasion in cholangiocarcinoma cells in vitro [66]. This was also seen in prostate cancer, where LAT1 or LAT3 shRNA significantly inhibited metastasis in vivo, however this was confounded by a significant reduction in tumor size [25]. These effects were likely due to the transport function of the LATs, as microarray analysis showed significant downregulation of genes involved in cell cycle regulation, including CDK1, CDC20 and transcription factors E2F1 and E2F2 [25]. These same genes are highly expressed in metastatic prostate cancer, suggesting inhibition of LAT transporters may suppress metastatic prostate cancer proliferation [25].
LAT1 expression closely correlates with 4F2hc expression in human cancers, and has been shown to have a critical role in the metastatic process of diverse human neoplasms [31,33,34,37,67]. Apart from the transport activity of LAT1, regulation of metastasis may be mediated through integrin signaling, since 4F2hc has been shown to interact with β1-integrin and regulate β1-integrin affinity [68] and expression [69]. It was shown that the amino acid transporter function of LAT1 is not required for this effect of 4F2hc on integrin function. Further studies showed that 4F2hc interacts with the cytoplasmic domain of β1A integrin to reverse the suppression of integrin activation [70,71]. The 4F2hc transmembrane domain also binds to integrin αvβ3 [72], suggesting that perhaps LAT1/4F2hc may be important in interactions with the metastatic niche. It is also possible that 4F2hc binding to integrins allows the cell to use LAT1 to “probe” the environment for nutrients.
Induction of LAT expression
Several factors, such as hormone stimulation, Myc/Rb oncogenic transcription, nutrient starvation and environmental stress have been shown to induce LAT expression, thereby providing the neutral amino acids required for cancer cell growth, survival and progression. The diverse nature of these stimuli highlight the critical requirement for nutrient supply to the cancer cell. For example, in prostate cancer LAT3 expression is driven by androgen receptor (AR) signaling, leading to high expression in primary prostate cancer [24,25]. This is driven by direct AR transcription, confirmed by chromatin immunoprecipitation (ChIP) and promoter luciferase assays [24]. However, during anti-androgen therapies, LAT3 levels decrease, causing nutrient starvation. The reduction of amino acid levels activate the ATF4 nutrient stress signaling pathway through uncharged tRNAs in the cytoplasm. The general control non-derepressible 2 (GCN2) kinase binds to uncharged tRNAs, leading to phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α) on Serine 51 [73]. Activated eIF2α initiates the rapid translation of ATF4, which translocates to the nucleus, driving an adaptive response that includes transcription of amino acid transporters. The ATF4 knockout mice showed decreased expression of a number of amino acid transporters, with recent ChIP and promoter luciferase assays used to confirm ATF4 binding to amino acid response elements (AAREs) in LAT1, 4F2hc, ASCT2, ASCT1 and xCT [24,25,74-76]. Therefore, the ATF4 adaptive response to anti-androgen therapies restores intracellular amino acid levels through transporters including LAT1, allowing further protein translation and cell growth.
Other LAT family members also appear to be regulated by hormone receptors. In breast cancer cells, LAT1 expression is increased in response to estrodial which activates estrogen receptor (ER) [77]. LAT2 has been shown to increase expression in the presence of dihydrotestosterone (DHT), which activates AR [78]. Progesterone also activates ER to induce LAT2 mRNA level increase in primary human uterine leiomyoma smooth muscle (LSM) cells and tissues from premenopausal women [79]. These hormone driven responses are likely important drivers of proliferation during development, and their reactivation during oncogenic transformation is critical for subsequent cancer cell growth.
In addition to nutrient deprivation, oxygen-tension may also contribute to LAT1 expression. HIF2α binds to the SLC7A5 proximal promoter, increasing expression of LAT1 and activating mTORC1 signaling in renal carcinoma cells, as well as in normal liver and lung tissues [80]. These studies indicate that LAT1 is a key environmental sensor to regulate mTORC1 signaling.
The development of a LAT1 knockout mouse has provided further clues to regulation of LAT1 expression. Knockout T cells do not respond to antigen stimulation, thereby preventing T cell clonal expansion or effector cell differentiation [81]. Wild type T cells, however, respond to antigen or PKC activation (phorbol ester) by upregulating expression of LAT1 [81,82]. Conditional knockdown of LAT1 in activated T cells suppressed c-Myc translation but not transcription. This is an mTORC1 independent process, as rapamycin did not prevent TCR-mediated elevation of c-Myc expression [81]. Since c-Myc has a short half-life (~15 min) [83], sustained expression is required for the maintenance of c-Myc protein. Therefore, LAT1 is critical in sustaining c-Myc levels. c-Myc is also important for metabolic processes including glycolytic switch and regulation of glutaminolysis, as well as for cell proliferation [84].
LAT family inhibitors
Amino acids such as leucine (Figure 1A) contain amine and carboxylic acid groups, as well as side chains, which are recognized by substrate binding sites of transporters. Generation of LAT inhibitors has therefore focused primarily on compounds that mimic LAT substrates, and can therefore compete for amino acid binding. However, this strategy has in general resulted in high effective concentrations of inhibitors, as is seen with the leucine analogue BCH (Figure 1B; ~10 mM). Furthermore, since the LAT family shares the majority of substrates, BCH targets all members of LAT family, which is undesirable for clinical development. Recently, several new inhibitors were developed to more potently target LAT1 and/or LAT3 (Figure 1).
Figure 1.
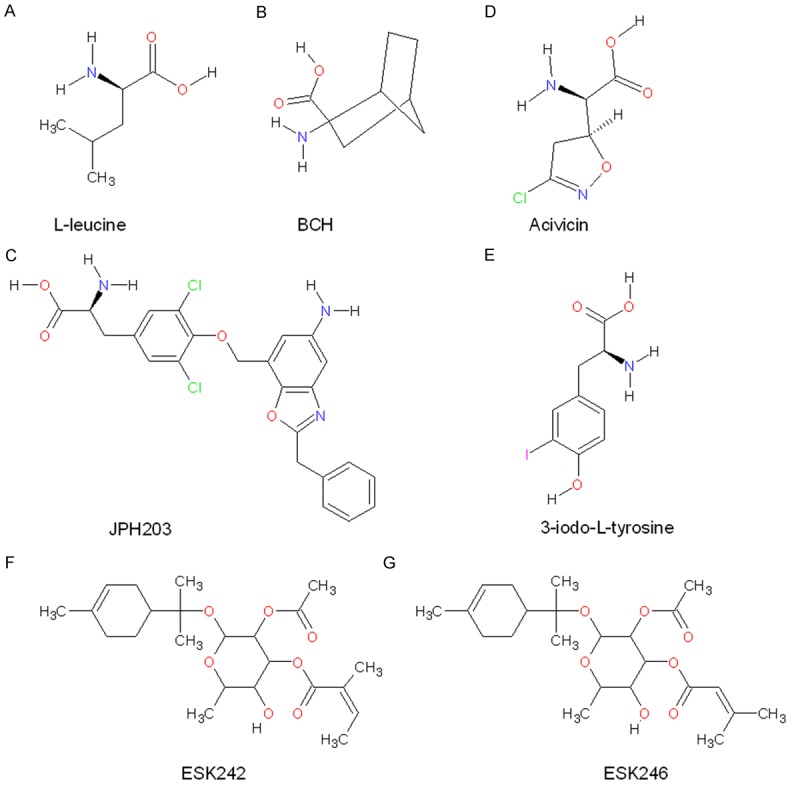
Structure of L-leucine and LAT family inhibitors. A. L-leucine; B. BCH; C. JPH203; D. Acivicin; E. 3-iodo-L-tyrosine; F. ESK242; G. ESK246.
JPH203 (also called KYT-0353; (S)-2-amino-3-(4-((5-amino-2-phenylbenzo[d]oxazol-7-yl)methoxy)-3,5-dichlorophenyl) propanoic acid; Figure 1C) is a novel tyrosine analog, which selectively inhibits LAT1 transport activity [85]. JPH203 showed a dramatic inhibition of leucine uptake (IC50=0.06 µM) and cell growth (IC50=4.1 µM) in human colon cancer cells (HT-29) [85], human oral cancer cells (YD-38) [86] and leukemic cells [87]. In nude mice, significant inhibitory effects on tumor growth were observed after 7 days treatment with 12.5 mg/kg of this compound [85]. JPH203 suppressed activation of mTORC1 and Akt, decreased expression of c-myc in T-ALL (T-cell acute lymphoblastic leukemia) and T-LL (T-cell lymphoblastic lymphoma) [87]. JPH203 induced ATF4 translation initiation and an unfolded protein response mediated by CHOP (the C/EBP homologous protein), followed by cell death [87]. Importantly, JPH203 had no toxic effect on normal murine thymocytes, lymphocytes, erythrocytes, platelets, bone marrow mature cells, stem cells and early progenitors. Preclinical data from four patients showed no apoptotic effects of JPH203 on normal peripheral blood lymphocytes cells or cord blood mononuclear cells ex vivo [87]. Therefore, therapies targeting LAT1 in T-ALL is an attractive strategy that appears to have little side effects in normal cells. However, JPH203 biotransformation via phase II metabolism produces N-acetyl-JPH203 (NAc-JPH203), which may accumulate in the liver and kidney, and will need to be considered for future pre-clinical testing [88,89]. Combined with chemotherapeutic drugs, such as rapamycin, dexamethasone, doxorubicin, Velcade and L-asparaginase, JPH203 showed synergistic effects, decreasing cell survival. The highest synergy was observed in combination with rapamycin [87]. Therefore, JPH203 could be an adjuvant therapeutic strategy to treat hematopoietic malignancies. However, the specificity of this compound was only examined for LAT1 and LAT2 in human colon cells HT-29 and mouse renal proximal tubule cells S2. It remains to be determined whether JPH203 can also inhibit LAT3 or LAT4 [85].
Recent structural analysis of membrane proteins have led to a number of publications modelling transporter structures [90]. The LAT1 structure was modelled based on the crystal structure of the arginine/agmatine transporter AdiC from E. coli in the outward–facing conformation. Virtual screening was then performed using DOCK3.5.54, to filter compounds from KEGG (Kyoto Encyclopedia of Genes and Genomes) DRUG and KEGG LIGAND COMPOUND database against the LAT1 model. The top-scoring compounds were validated in vitro, discovering two novel LAT1 ligands, acivicin (Figure 1D) and 3-iodo-L-tyrosine (Figure 1E) [90]. The IC50 of 3,5-diiodo-L-tyrosine (similar to 3-iodo-L-tyrosine) and acivicin is 7.9 µM and 340 µM, respectively. Both 3-iodo-L-tyrosine and acivicin were shown to suppress GBM cancer proliferation [90].
Other than these in silico screening approaches, conventional high throughput screening strategies have also led to the discovery of novel LAT inhibitors. Using a natural compounds library (Nature Bank), two new monoterpene glycosides ESK242 (Figure 1F) and ESK246 (Figure 1G) were isolated, which inhibit LATs with a low IC50 [91]. These compounds were screened from more than 4500 fractions of biota samples, and specificity was determined using Xenopus oocytes expressing LAT1/4F2hc, LAT2/4F2hc, LAT3 or LAT4. ESK242 was found to inhibit LAT1 and LAT3 mediated leucine uptake, while ESK246 preferentially inhibits LAT3. So far, ESK246 is the first reported LAT3 specific inhibitor, which may be used to study the physiological function of LAT3 in the future. Comparison of these new inhibitors with BCH (IC50=4060 µM in LNCaP prostate cancer cells), showed they are ~2 orders of magnitude more effective at inhibiting leucine uptake, with ESK246 and ESK242 having IC50 values of 8.1 µM and 29.6 µM respectively. ESK246 was also shown to significantly suppress LNCaP cell proliferation and cell cycle regulator expression at 50 µM [91]. While these compounds do not contain distinct amine and carboxylic acid groups, ESK242 has a side chain similar to isoleucine and ESK246 similar to leucine. Further studies are required to determine if these side chains mediate binding to LAT1/3. These data would assist in the development of more drug-like inhibitors in the absence of LAT family structural information.
Conclusion
Over recent years, there has been substantial progress made on both the understanding of LAT family regulation and function in cancer, as well as the development of new inhibitors for this family of transporters. However, despite these advances, analysis of Oncomine data clearly shows that there are many more cancers where LAT family proteins may play an important role. Furthermore, a number of questions remain to be answered: 1) Since LAT1 and ASCT2 cooperate to regulate leucine transport, is it possible to target both transporters to more effectively suppress tumor growth? 2) Are there any proteins (other than 4F2hc) that directly interact with LATs to regulate amino acid transport? 3) Are there post-translational modifications, such as phosphorylation, that can regulate the LAT family? The answer to these questions may provide additional avenues for therapeutic strategies modulating LAT functions. In conclusion, while increased expression of the L-type amino acid transporter family is important for cancer growth and progression, further development of current inhibitors are required in order to reach their full therapeutic potential.
Acknowledgements
This work was supported by grants from Movember through the Prostate Cancer Foundation of Australia (YI0813 to Q.W.; PG2910 to J.H.; YI0707 to J.H.); and the Australian Movember Revolutionary Team Award Targeting Advanced Prostate Cancer, J.H., Q.W.); National Breast Cancer Foundation (ECF-12-05 J.H.) and the National Health and Medical Research Council (1051820 to J.H.).
Disclosure of conflict of interest
No potential conflicts of interest were disclosed.
References
- 1.Kruczek K, Ratterman M, Tolzien K, Sulo S, Lestingi TM, Nabhan C. A phase II study evaluating the toxicity and efficacy of single-agent temsirolimus in chemotherapy-naive castration-resistant prostate cancer. Br J Cancer. 2013;109:1711–1716. doi: 10.1038/bjc.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Templeton AJ, Dutoit V, Cathomas R, Rothermundt C, Bartschi D, Droge C, Gautschi O, Borner M, Fechter E, Stenner F, Winterhalder R, Muller B, Schiess R, Wild PJ, Ruschoff JH, Thalmann G, Dietrich PY, Aebersold R, Klingbiel D, Gillessen S Swiss Group for Clinical Cancer Research (SAKK) Phase 2 trial of single-agent everolimus in chemotherapy-naive patients with castration-resistant prostate cancer (SAKK 08/08) Eur Urol. 2013;64:150–158. doi: 10.1016/j.eururo.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 3.Fotiadis D, Kanai Y, Palacin M. The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med. 2013;34:139–158. doi: 10.1016/j.mam.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Bodoy S, Fotiadis D, Stoeger C, Kanai Y, Palacin M. The small SLC43 family: facilitator system l amino acid transporters and the orphan EEG1. Mol Aspects Med. 2013;34:638–645. doi: 10.1016/j.mam.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 6.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 7.Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- 8.Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Kuhn LC. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]
- 9.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- 10.Babu E, Kanai Y, Chairoungdua A, Kim DK, Iribe Y, Tangtrongsup S, Jutabha P, Li Y, Ahmed N, Sakamoto S, Anzai N, Nagamori S, Endou H. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278:43838–43845. doi: 10.1074/jbc.M305221200. [DOI] [PubMed] [Google Scholar]
- 11.Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- 12.Park SY, Kim JK, Kim IJ, Choi BK, Jung KY, Lee S, Park KJ, Chairoungdua A, Kanai Y, Endou H, Kim DK. Reabsorption of neutral amino acids mediated by amino acid transporter LAT2 and TAT1 in the basolateral membrane of proximal tubule. Arch Pharm Res. 2005;28:421–432. doi: 10.1007/BF02977671. [DOI] [PubMed] [Google Scholar]
- 13.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol. 2005;15:254–266. doi: 10.1016/j.semcancer.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Beaumont KA, Otte NJ, Font J, Bailey CG, van Geldermalsen M, Sharp DM, Tiffen JC, Ryan RM, Jormakka M, Haass NK, Rasko JE, Holst J. Targeting glutamine transport to suppress melanoma cell growth. Int J Cancer. 2014;135:1060–1071. doi: 10.1002/ijc.28749. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R, Wong JJ, Metierre C, Pinello N, Otte NJ, Lehman ML, Gleave M, Nelson CC, Bailey CG, Ritchie W, Rasko JE, Holst J. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236:278–89. doi: 10.1002/path.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuaqui RF, Englert CR, Strup SE, Vocke CD, Zhuang Z, Duray PH, Bostwick DG, Linehan WM, Liotta LA, Emmert-Buck MR. Identification of a novel transcript up-regulated in a clinically aggressive prostate carcinoma. Urology. 1997;50:302–307. doi: 10.1016/s0090-4295(97)00194-5. [DOI] [PubMed] [Google Scholar]
- 18.Cole KA, Chuaqui RF, Katz K, Pack S, Zhuang Z, Cole CE, Lyne JC, Linehan WM, Liotta LA, Emmert-Buck MR. cDNA sequencing and analysis of POV1 (PB39): a novel gene up-regulated in prostate cancer. Genomics. 1998;51:282–287. doi: 10.1006/geno.1998.5359. [DOI] [PubMed] [Google Scholar]
- 19.Fukuhara D, Kanai Y, Chairoungdua A, Babu E, Bessho F, Kawano T, Akimoto Y, Endou H, Yan K. Protein characterization of NA+-independent system L amino acid transporter 3 in mice: a potential role in supply of branched-chain amino acids under nutrient starvation. Am J Pathol. 2007;170:888–898. doi: 10.2353/ajpath.2007.060428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekine Y, Nishibori Y, Akimoto Y, Kudo A, Ito N, Fukuhara D, Kurayama R, Higashihara E, Babu E, Kanai Y, Asanuma K, Nagata M, Majumdar A, Tryggvason K, Yan K. Amino acid transporter LAT3 is required for podocyte development and function. J Am Soc Nephrol. 2009;20:1586–1596. doi: 10.1681/ASN.2008070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung J, Bauer DE, Ghamari A, Nizzi CP, Deck KM, Kingsley PD, Yien YY, Huston NC, Chen C, Schultz IJ, Dalton AJ, Wittig JG, Palis J, Orkin SH, Lodish HF, Eisenstein RS, Cantor AB, Paw BH. The mTORC1/4E-BP pathway coordinates hemoglobin production with L-leucine availability. Sci Signal. 2015;8:ra34. doi: 10.1126/scisignal.aaa5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guetg A, Mariotta L, Bock L, Herzog B, Fingerhut R, Camargo SM, Verrey F. Essential amino acid transporter Lat4 (Slc43a2) is required for mouse development. J Physiol. 2015;593:1273–89. doi: 10.1113/jphysiol.2014.283960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard C, Mecham B, Dumpit R, Coleman I, Bhattacharjee M, Chen Q, Sikes RA, Nelson PS. Conserved gene expression programs integrate mammalian prostate development and tumorigenesis. Cancer Res. 2009;69:1739–1747. doi: 10.1158/0008-5472.CAN-07-6817. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Bailey CG, Ng C, Tiffen J, Thoeng A, Minhas V, Lehman ML, Hendy SC, Buchanan G, Nelson CC, Rasko JE, Holst J. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 2011;71:7525–7536. doi: 10.1158/0008-5472.CAN-11-1821. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Tiffen J, Bailey CG, Lehman ML, Ritchie W, Fazli L, Metierre C, Feng YJ, Li E, Gleave M, Buchanan G, Nelson CC, Rasko JE, Holst J. Targeting amino acid transport in metastatic castration-resistant prostate cancer: effects on cell cycle, cell growth, and tumor development. J Natl Cancer Inst. 2013;105:1463–1473. doi: 10.1093/jnci/djt241. [DOI] [PubMed] [Google Scholar]
- 26.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. Br J Cancer. 2008;98:742–748. doi: 10.1038/sj.bjc.6604235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Iijima H, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. Expression of L-type amino acid transporter 1 (LAT1) in neuroendocrine tumors of the lung. Pathol Res Pract. 2008;204:553–561. doi: 10.1016/j.prp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Kaira K, Oriuchi N, Takahashi T, Nakagawa K, Ohde Y, Okumura T, Murakami H, Shukuya T, Kenmotsu H, Naito T, Kanai Y, Endo M, Kondo H, Nakajima T, Yamamoto N. L-type amino acid transporter 1 (LAT1) expression in malignant pleural mesothelioma. Anticancer Res. 2011;31:4075–4082. [PubMed] [Google Scholar]
- 29.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. L-Type Amino Acid Transporter 1 (LAT1) Is Frequently Expressed in Thymic Carcinomas but Is Absent in Thymomas. J Surg Oncol. 2009;99:433–438. doi: 10.1002/jso.21277. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K, Ohnishi A, Promsuk J, Shimizu S, Kanai Y, Shiokawa Y, Nagane M. Enhanced tumor growth elicited by L-type amino acid transporter 1 in human malignant glioma cells. Neurosurgery. 2008;62:493–503. doi: 10.1227/01.neu.0000316018.51292.19. discussion 503-494. [DOI] [PubMed] [Google Scholar]
- 31.Toyoda M, Kaira K, Ohshima Y, Ishioka NS, Shino M, Sakakura K, Takayasu Y, Takahashi K, Tominaga H, Oriuchi N, Nagamori S, Kanai Y, Oyama T, Chikamatsu K. Prognostic significance of amino-acid transporter expression (LAT1, ASCT2, and xCT) in surgically resected tongue cancer. Br J Cancer. 2014;110:2506–2513. doi: 10.1038/bjc.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaira K, Sunose Y, Arakawa K, Ogawa T, Sunaga N, Shimizu K, Tominaga H, Oriuchi N, Itoh H, Nagamori S, Kanai Y, Segawa A, Furuya M, Mori M, Oyama T, Takeyoshi I. Prognostic significance of L-type amino-acid transporter 1 expression in surgically resected pancreatic cancer. Br J Cancer. 2012;107:632–638. doi: 10.1038/bjc.2012.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namikawa M, Kakizaki S, Kaira K, Tojima H, Yamazaki Y, Horiguchi N, Sato K, Oriuchi N, Tominaga H, Sunose Y, Nagamori S, Kanai Y, Oyama T, Takeyoshi I, Yamada M. Expression of amino acid transporters (LAT1, ASCT2 and xCT) as clinical significance in hepatocellular carcinoma. Hepatol Res. 2014 doi: 10.1111/hepr.12431. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Tanaka S, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. l-type amino acid transporter 1 and CD98 expression in primary and metastatic sites of human neoplasms. Cancer Sci. 2008;99:2380–2386. doi: 10.1111/j.1349-7006.2008.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imai H, Kaira K, Oriuchi N, Yanagitani N, Sunaga N, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. L-type amino acid transporter 1 expression is a prognostic marker in patients with surgically resected stage I non-small cell lung cancer. Histopathology. 2009;54:804–813. doi: 10.1111/j.1365-2559.2009.03300.x. [DOI] [PubMed] [Google Scholar]
- 36.Ichinoe M, Mikami T, Yoshida T, Igawa I, Tsuruta T, Nakada N, Anzai N, Suzuki Y, Endou H, Okayasu I. High expression of L-type amino-acid transporter 1 (LAT1) in gastric carcinomas: comparison with non-cancerous lesions. Pathol Int. 2011;61:281–289. doi: 10.1111/j.1440-1827.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 37.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Ishizuka T, Kanai Y, Endou H, Nakajima T, Mori M. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in early stage squamous cell carcinoma of the lung. Cancer Sci. 2009;100:248–254. doi: 10.1111/j.1349-7006.2008.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaira K, Toyoda M, Shino M, Sakakura K, Takahashi K, Tominaga H, Oriuchi N, Kanai Y, Oyama T, Chikamatsu K. Clinicopathological significance of L-type amino acid transporter 1 (LAT1) expression in patients with adenoid cystic carcinoma. Pathol Oncol Res. 2013;19:649–656. doi: 10.1007/s12253-013-9624-2. [DOI] [PubMed] [Google Scholar]
- 39.Sakata T, Ferdous G, Tsuruta T, Satoh T, Baba S, Muto T, Ueno A, Kanai Y, Endou H, Okayasu I. L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathol Int. 2009;59:7–18. doi: 10.1111/j.1440-1827.2008.02319.x. [DOI] [PubMed] [Google Scholar]
- 40.Bartlett JMS, Thomas J, Ross DT, Seitz RS, Ring BZ, Beck RA, Pedersen HC, Munro A, Kunkler IH, Campbell FM, Jack W, Kerr GR, Johnstone L, Cameron DA, Chetty U. Mammostrat (R) as a tool to stratify breast cancer patients at risk of recurrence during endocrine therapy. Breast Cancer Res. 2010;12:R47. doi: 10.1186/bcr2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ring BZ, Seitz RS, Beck R, Shasteen WJ, Tarr SM, Cheang MC, Yoder BJ, Budd GT, Nielsen TO, Hicks DG, Estopinal NC, Ross DT. Novel prognostic immunohistochemical biomarker panel for estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006;24:3039–3047. doi: 10.1200/JCO.2006.05.6564. [DOI] [PubMed] [Google Scholar]
- 42.Kim CS, Cho SH, Chun HS, Lee SY, Endou H, Kanai Y, Kim do K. BCH, an inhibitor of system L amino acid transporters, induces apoptosis in cancer cells. Biol Pharm Bull. 2008;31:1096–1100. doi: 10.1248/bpb.31.1096. [DOI] [PubMed] [Google Scholar]
- 43.Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 44.Hara K, Yonezawa K, Kozlowski MT, Sugimoto T, Andrabi K, Weng QP, Kasuga M, Nishimoto I, Avruch J. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272:26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- 45.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 46.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 47.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 48.Duran RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell. 2012;47:349–358. doi: 10.1016/j.molcel.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 49.Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y, Jiang Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science. 2007;318:977–980. doi: 10.1126/science.1147379. [DOI] [PubMed] [Google Scholar]
- 50.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase-activating protein complex toward Rheb. Curr Biol. 2003;13:1259–1268. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–581. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 52.Demetriades C, Doumpas N, Teleman AA. Regulation of TORC1 in response to amino acid starvation via lysosomal recruitment of TSC2. Cell. 2014;156:786–799. doi: 10.1016/j.cell.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 55.Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46:105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sener A, Malaisse WJ. L-leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase. Nature. 1980;288:187–189. doi: 10.1038/288187a0. [DOI] [PubMed] [Google Scholar]
- 60.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science. 2011;334:678–683. doi: 10.1126/science.1207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rebsamen M, Pochini L, Stasyk T, de Araujo ME, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, Scorzoni S, Filipek PA, Huber KV, Bigenzahn JW, Heinz LX, Kraft C, Bennett KL, Indiveri C, Huber LA, Superti-Furga G. SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature. 2015;519:477–81. doi: 10.1038/nature14107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang S, Tsun ZY, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, Wang T, Bar-Peled L, Zoncu R, Straub C, Kim C, Park J, Sabatini BL, Sabatini DM. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2015;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The Folliculin Tumor Suppressor Is a GAP for the RagC/D GTPases That Signal Amino Acid Levels to mTORC1. Molecular Cell. 2013;52:495–505. doi: 10.1016/j.molcel.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng M, Yin N, Li MO. Sestrins Function as Guanine Nucleotide Dissociation Inhibitors for Rag GTPases to Control mTORC1 Signaling. Cell. 2014;159:122–133. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janpipatkul K, Suksen K, Borwornpinyo S, Jearawiriyapaisarn N, Hongeng S, Piyachaturawat P, Chairoungdua A. Downregulation of LAT1 expression suppresses cholangiocarcinoma cell invasion and migration. Cell Signal. 2014;26:1668–1679. doi: 10.1016/j.cellsig.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, Hisada T, Kawashima O, Kamide Y, Ishizuka T, Kanai Y, Nakajima T, Mori M. Prognostic significance of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (CD98) expression in surgically resectable stage III non-small cell lung cancer. Exp Ther Med. 2010;1:799–808. doi: 10.3892/etm.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH. Complementation of dominant suppression implicates CD98 in integrin activation. Nature. 1997;390:81–85. doi: 10.1038/36349. [DOI] [PubMed] [Google Scholar]
- 69.Kim SM, Hahn JH. CD98 activation increases surface expression and clusteringof beta1 integrins in MCF-7 cells through FAK/Src- and cytoskeleton-independent mechanisms. Exp Mol Med. 2008;40:261–270. doi: 10.3858/emm.2008.40.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zent R, Fenczik CA, Calderwood DA, Liu S, Dellos M, Ginsberg MH. Class- and splice variant-specific association of CD98 with integrin beta cytoplasmic domains. J Biol Chem. 2000;275:5059–5064. doi: 10.1074/jbc.275.7.5059. [DOI] [PubMed] [Google Scholar]
- 71.Kolesnikova TV, Mannion BA, Berditchevski F, Hemler ME. Beta1 integrins show specific association with CD98 protein in low density membranes. BMC Biochem. 2001;2:10. doi: 10.1186/1471-2091-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prager GW, Feral CC, Kim C, Han J, Ginsberg MH. CD98hc (SLC3A2) interaction with the integrin beta subunit cytoplasmic domain mediates adhesive signaling. J Biol Chem. 2007;282:24477–24484. doi: 10.1074/jbc.M702877200. [DOI] [PubMed] [Google Scholar]
- 73.Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 74.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 75.Ren P, Yue M, Xiao D, Xiu R, Gan L, Liu H, Qing G. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. J Pathol. 2015;235:90–100. doi: 10.1002/path.4429. [DOI] [PubMed] [Google Scholar]
- 76.Sato H, Nomura S, Maebara K, Sato K, Tamba M, Bannai S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem Biophys Res Commun. 2004;325:109–116. doi: 10.1016/j.bbrc.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 77.Shennan DB, Thomson J, Gow IF, Travers MT, Barber MC. L-leucine transport in human breast cancer cells (MCF-7 and MDA-MB-231): kinetics, regulation by estrogen and molecular identity of the transporter. Biochim Biophys Acta. 2004;1664:206–16. doi: 10.1016/j.bbamem.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 78.Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechan- ism. J Physiol. 2011;589:3623–3640. doi: 10.1113/jphysiol.2011.207175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo X, Yin P, Reierstad S, Ishikawa H, Lin Z, Pavone ME, Zhao H, Marsh EE, Bulun SE. Progesterone and mifepristone regulate L-type amino acid transporter 2 and 4F2 heavy chain expression in uterine leiomyoma cells. J Clin Endocrinol Metab. 2009;94:4533–4539. doi: 10.1210/jc.2009-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elorza A, Soro-Arnáiz I, Meléndez-Rodríguez F, Rodríguez-Vaello V, Marsboom G, de Cárcer G, Acosta-Iborra B, Albacete-Albacete L, Ordóñez A, Serrano-Oviedo L, Giménez-Bachs J, Vara-Vega A, Salinas A, Sánchez-Prieto R, Martín Del Río R, Sánchez-Madrid F, Malumbres M, Landázuri M, Aragonés J. HIF2α Acts as an mTORC1 Activator through the Amino Acid Carrier SLC7A5. Mol Cell. 2012;48:681–91. doi: 10.1016/j.molcel.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 81.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nii T, Segawa H, Taketani Y, Tani Y, Ohkido M, Kishida S, Ito M, Endou H, Kanai Y, Takeda E, Miyamoto K. Molecular events involved in up-regulating human Na+-independent neutral amino acid transporter LAT1 during T-cell activation. Biochem J. 2001;358:693–704. doi: 10.1042/0264-6021:3580693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hann SR, Eisenman RN. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984;4:2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dang CV. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oda K, Hosoda N, Endo H, Saito K, Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y, Endou H. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci. 2010;101:173–179. doi: 10.1111/j.1349-7006.2009.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yun DW, Lee SA, Park MG, Kim JS, Yu SK, Park MR, Kim SG, Oh JS, Kim CS, Kim HJ, Kim JS, Chun HS, Kanai Y, Endou H, Wempe MF, Kim do K. JPH203, an L-type amino acid transporter 1-selective compound, induces apoptosis of YD-38 human oral cancer cells. J Pharmacol Sci. 2014;124:208–217. doi: 10.1254/jphs.13154fp. [DOI] [PubMed] [Google Scholar]
- 87.Rosilio C, Nebout M, Imbert V, Griessinger E, Neffati Z, Benadiba J, Hagenbeek T, Spits H, Reverso J, Ambrosetti D, Michiels JF, Bailly-Maitre B, Endou H, Wempe MF, Peyron JF. L-type amino acid transporter 1 (LAT1): a therapeutic target supporting growth and survival of t-cell lymphoblastic lymphoma/t-cell acute lymphoblastic leukemia. Leukemia. 2015;29:1253–66. doi: 10.1038/leu.2014.338. [DOI] [PubMed] [Google Scholar]
- 88.Wempe MF, Rice PJ, Lightner JW, Jutabha P, Hayashi M, Anzai N, Wakui S, Kusuhara H, Sugiyama Y, Endou H. Metabolism and pharmacokinetic studies of JPH203, an L-amino acid transporter 1 (LAT1) selective compound. Drug Metab Pharmacokinet. 2012;27:155–161. doi: 10.2133/dmpk.dmpk-11-rg-091. [DOI] [PubMed] [Google Scholar]
- 89.Toyoshima J, Kusuhara H, Wempe MF, Endou H, Sugiyama Y. Investigation of the role of transporters on the hepatic elimination of an LAT1 selective inhibitor JPH203. J Pharm Sci. 2013;102:3228–38. doi: 10.1002/jps.23601. [DOI] [PubMed] [Google Scholar]
- 90.Geier EG, Schlessinger A, Fan H, Gable JE, Irwin JJ, Sali A, Giacomini KM. Structure-based ligand discovery for the Large-neutral Amino Acid Transporter 1, LAT-1. Proc Natl Acad Sci U S A. 2013;110:5480–5485. doi: 10.1073/pnas.1218165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang Q, Grkovic T, Font J, Bonham S, Pouwer RH, Bailey CG, Moran AM, Ryan RM, Rasko JE, Jormakka M, Quinn RJ, Holst J. Monoterpene glycoside ESK246 from Pittosporum targets LAT3 amino acid transport and prostate cancer cell growth. ACS Chem Biol. 2014;9:1369–1376. doi: 10.1021/cb500120x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eltz S, Comperat E, Cussenot O, Roupret M. Molecular and histological markers in urothelial carcinomas of the upper urinary tract. BJU Int. 2008;102:532–535. doi: 10.1111/j.1464-410X.2008.07659.x. [DOI] [PubMed] [Google Scholar]
- 93.Kim DK, Kanai Y, Choi HW, Tangtrongsup S, Chairoungdua A, Babu E, Tachampa K, Anzai N, Iribe Y, Endou H. Characterization of the system L amino acid transporter in T24 human bladder carcinoma cells. Biochim Biophys Acta. 2002;1565:112–121. doi: 10.1016/s0005-2736(02)00516-3. [DOI] [PubMed] [Google Scholar]
- 94.Baniasadi S, Chairoungdua A, Iribe Y, Kanai Y, Endou H, Aisaki K, Igarashi K, Kanno J. Gene expression profiles in T24 human bladder carcinoma cells by inhibiting an L-type amino acid transporter, LAT1. Arch Pharm Res. 2007;30:444–452. doi: 10.1007/BF02980218. [DOI] [PubMed] [Google Scholar]
- 95.Furuya M, Horiguchi J, Nakajima H, Kanai Y, Oyama T. Correlation of L-type amino acid transporter 1 and CD98 expression with triple negative breast cancer prognosis. Cancer Sci. 2012;103:382–389. doi: 10.1111/j.1349-7006.2011.02151.x. [DOI] [PubMed] [Google Scholar]
- 96.Shennan DB, Thomson J. Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol Rep. 2008;20:885–889. [PubMed] [Google Scholar]
- 97.Shennan DB, Thomson J, Barber MC, Travers MT. Functional and molecular characteristics of system L in human breast cancer cells. Biochim Biophys Acta. 2003;1611:81–90. doi: 10.1016/s0005-2736(03)00028-2. [DOI] [PubMed] [Google Scholar]
- 98.Uno K, Kuwabara H, Terado Y, Kojima K, Kawakami T, Kamma H, Sakurai H, Sakamoto A, Kurata A. Divergent expression of L-type amino acid transporter 1 during uterine cervical carcinogenesis. Hum Pathol. 2011;42:1660–1666. doi: 10.1016/j.humpath.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 99.Haase C, Bergmann R, Fuechtner F, Hoepping A, Pietzsch J. L-type amino acid transporters LAT1 and LAT4 in cancer: uptake of 3-O-methyl-6-18F-fluoro-L-dopa in human adenocarcinoma and squamous cell carcinoma in vitro and in vivo. J Nucl Med. 2007;48:2063–2071. doi: 10.2967/jnumed.107.043620. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi H, Ishii Y, Takayama T. Expression of L-type amino acid transporter 1 (LAT1) in esophageal carcinoma. J Surg Oncol. 2005;90:233–238. doi: 10.1002/jso.20257. [DOI] [PubMed] [Google Scholar]
- 101.Yamauchi K, Sakurai H, Kimura T, Wiriyasermkul P, Nagamori S, Kanai Y, Kohno N. System L amino acid transporter inhibitor enhances anti-tumor activity of cisplatin in a head and neck squamous cell carcinoma cell line. Cancer Lett. 2009;276:95–101. doi: 10.1016/j.canlet.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 102.Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto K, Takeda E, Goya T, Endou H. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 103.Xu SM, Tang K, Meng L, Tang Y. Suppression of amino acid transporter LAT3 expression on proliferation of K562 cells. J Huazhong Univ Sci Technolog Med Sci. 2013;33:632–635. doi: 10.1007/s11596-013-1171-2. [DOI] [PubMed] [Google Scholar]
- 104.Ohkame H, Masuda H, Ishii Y, Kanai Y. Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in liver tumor lesions of rat models. J Surg Oncol. 2001;78:265–271. doi: 10.1002/jso.1165. discussion 271-262. [DOI] [PubMed] [Google Scholar]
- 105.Tamai S, Masuda H, Ishii Y, Suzuki S, Kanai Y, Endou H. Expression of L-type amino acid transporter 1 in a rat model of liver metastasis: positive correlation with tumor size. Cancer Detect Prev. 2001;25:439–445. [PubMed] [Google Scholar]
- 106.Storey BT, Fugere C, Lesieur-Brooks A, Vaslet C, Thompson NL. Adenoviral modulation of the tumor-associated system L amino acid transporter, LAT1, alters amino acid transport, cell growth and 4F2/CD98 expressionwith cell-type specific effects in cultured hepatic cells. Int J Cancer. 2005;117:387–397. doi: 10.1002/ijc.21169. [DOI] [PubMed] [Google Scholar]
- 107.Kondoh N, Imazeki N, Arai M, Hada A, Hatsuse K, Matsuo H, Matsubara O, Ohkura S, Yamamoto M. Activation of a system A amino acid transporter, ATA1/SLC38A1, in human hepatocellular carcinoma and preneoplastic liver tissues. Int J Oncol. 2007;31:81–87. [PubMed] [Google Scholar]
- 108.Ritchie JW, Taylor PM. Tryptophan and iodothyronine transport interactions in HepG2 human hepatoma cells. Amino Acids. 2010;38:1361–1367. doi: 10.1007/s00726-009-0344-6. [DOI] [PubMed] [Google Scholar]
- 109.Ochiai H, Morishita T, Onda K, Sugiyama H, Maruo T. Canine Lat1: molecular structure, distribution and its expression in cancer samples. J Vet Med Sci. 2012;74:917–922. doi: 10.1292/jvms.11-0353. [DOI] [PubMed] [Google Scholar]
- 110.Kuhne A, Kaiser R, Schirmer M, Heider U, Muhlke S, Niere W, Overbeck T, Hohloch K, Trumper L, Sezer O, Brockmoller J. Genetic polymorphisms in the amino acid transporters LAT1 and LAT2 in relation to the pharmacokinetics and side effects of melphalan. Pharmacogenet Genomics. 2007;17:505–517. doi: 10.1097/FPC.0b013e3280ea77cd. [DOI] [PubMed] [Google Scholar]
- 111.Kuhne A, Tzvetkov MV, Hagos Y, Lage H, Burckhardt G, Brockmoller J. Influx and efflux transport as determinants of melphalan cytotoxicity: Resistance to melphalan in MDR1 overexpressing tumor cell lines. Biochem Pharmacol. 2009;78:45–53. doi: 10.1016/j.bcp.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 112.Isoda A, Kaira K, Iwashina M, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Oyama T, Asao T, Matsumoto M, Sawamura M. Expression of L-type amino acid transporter 1 (LAT1) as a prognostic and therapeutic indicator in multiple myeloma. Cancer Sci. 2014;105:1496–1502. doi: 10.1111/cas.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fan X, Ross DD, Arakawa H, Ganapathy V, Tamai I, Nakanishi T. Impact of system L amino acid transporter 1 (LAT1) on proliferation of human ovarian cancer cells: a possible target for combination therapy with anti-proliferative aminopeptidase inhibitors. Biochem Pharmacol. 2010;80:811–818. doi: 10.1016/j.bcp.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 114.Kaji M, Kabir-Salmani M, Anzai N, Jin CJ, Akimoto Y, Horita A, Sakamoto A, Kanai Y, Sakurai H, Iwashita M. Properties of L-type amino acid transporter 1 in epidermal ovarian cancer. Int J Gynecol Cancer. 2010;20:329–336. doi: 10.1111/IGC.0b013e3181d28e13. [DOI] [PubMed] [Google Scholar]
- 115.Yanagisawa N, Ichinoe M, Mikami T, Nakada N, Hana K, Koizumi W, Endou H, Okayasu I. High expression of L-type amino acid transporter 1 (LAT1) predicts poor prognosis in pancreatic ductal adenocarcinomas. J Clin Pathol. 2012;65:1019–1023. doi: 10.1136/jclinpath-2012-200826. [DOI] [PubMed] [Google Scholar]
- 116.Hayashi K, Jutabha P, Endou H, Anzai N. c-Myc is crucial for the expression of LAT1 in MIA Paca-2 human pancreatic cancer cells. Oncol Rep. 2012;28:862–866. doi: 10.3892/or.2012.1878. [DOI] [PubMed] [Google Scholar]
- 117.Nakanishi K, Ogata S, Matsuo H, Kanai Y, Endou H, Hiroi S, Tominaga S, Aida S, Kasamatsu H, Kawai T. Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Arch. 2007;451:681–690. doi: 10.1007/s00428-007-0457-9. [DOI] [PubMed] [Google Scholar]
- 118.Luo X, Coon VJS, Su E, Pearson EK, Yin P, Ishikawa H, Bulun SE. LAT1 Regulates Growth of Uterine Leiomyoma Smooth Muscle Cells. Reprod Sci. 2010;17:791–797. doi: 10.1177/1933719110372419. [DOI] [PubMed] [Google Scholar]


