Abstract
The axon guidance cues netrin-1 has been reported to be associated with cancer progression in various types of human cancers. However, the underlying molecular mechanism of netrin-1-mediated metastasis remains obscure. In this study, we found that overexpression of netrin-1 promoted HCC cell migration and invasion as determined by transwell assay and 3D cell culture assay. However, netrin-1 knockdown inhibited these processes. Further investigation indicated that netrin-1 decreased the expression of Blood Vessel Epicardial Substance (BVES), which was down-regulated in HCC. Interestingly, LY294002, a special inhibitor to PI3K/AKT signaling which was determined as a downstream pathway of netrin-1, restored the reduction in BVES caused by netrin-1. In addition, BVES exhibited an opposite effect on HCC cell metastasis to that of netrin-1. Importantly, up-regulating BVES expression significantly attenuated netrin-1-enhanced migration and invasion, whereas silencing BVES expression rescued the metastatic phenotype in netrin-1 knockdown cells. Moreover, netrin-1 expression was negatively correlated with BVES in HCC tissues and cell lines with different metastatic potential. Taken together, these results reveal that netrin-1 promotes HCC cell metastasis by regulating BVES expression via AKT activation.
Keywords: Netrin-1, hepatocellular carcinoma, metastasis, BVES, AKT
Introduction
Hepatocellular carcinoma (HCC) is a major global health problem, it has reached to the second leading cause of cancer death in China as reported by the National Central Cancer Registry (NCCR) in 2014 [1]. This high mortality is probably attributable to early metastasis. Loss of cell-cell adhesion and acquisition of increased migratory and invasive ability are two important steps for early tumor metastasis [2,3]. Therefore, identifying moleculars governing cell adhesion, migration and invasion will provide new opportunities for the diagnosis and prevention of HCC metastasis.
Netrin-1, adiffusible laminin-related protein, has been observed to play a major role in the control of neuronal navigation during the development of the nervous system [4,5]. However, more recently, netrin-1 along with other neuronal guidance proteins like semaphorins, has also been shown to widely expressed outside the nervous system, emerged as a completely different molecule that regulates cell adhesion, motility, proliferation, and differentiation in many non-neuronal tissues [6-9]. In cancer cells, a netrin-1 gain has recently been described in several human cancers, such as colorectal cancer [10], metastatic breast cancers [11], lung cancer [12], and neuroblastoma [13], and this netrin-1 overexpression has been shown as a selective advantage for tumor progression. Our previous study have observed an increased expression of netrin-1 in HCC [14], netrin-1 stably expressing cells acquired generally lost cell contact and increased migratory ability. However, the precise mechanisms of netrin-1 in the metastasis of HCC remain unknown.
Blood Vessel Epicardial Substance(BVES) belongs to the POPDC family, which shares the same Popeye structure within the intracellular C-terminus as an evolutionarily conserved transmembrane protein [15]. BVES was originally identified as a putative cell adhesion molecule and played an important role in maintaining epithelial integrity and regulating cell movement [16,17]. Injection of antisense morpholino targeting BVES mRNA into a 2-cell Xenopus embryo led to disorganized migration and disrupted organogenesis [18]. In recent years, BVES was reported to be frequent down-regulation in some epithelial tumors. BVES inhibition decreased cell-cell contacts, meanwhile increased RhoA activation to promote cell migration in human colorectal carcinoma and gastric cancer [19,20]; furthermore, abnormal expression of BVES was associated with progression and poor survival in gastric cancer [21]. BVES was also down-regulated in HCC as shown in our recent study, silencing BVES expression in HCC cells triggered epithelial-mesenchymal transition [22]. However, very little is known about the signaling events and transcriptional control regulating BVES expression.
The opposite role of netrin-1 and BVES on cancer cell metastasis lead us to speculate that the promotion role of netrin-1 on metastasis may depended on down-regulation of BVES in HCC cells. In this study, we desired to elucidate that netrin-1 is an upstream regulatory molecule of BVES via AKT activation in the metastatic process of HCC.
Materials and methods
Tumor specimens and cell culture
Thirty seven HCC samples and matched non-tumor tissues were collected at the time of surgical resection at Tongji Hospital. Tumor histopathology was confirmed by analysis of hematoxylin and eosin (H&E) stained tissue sections by a qualified neuropathologist. The present study was performed according to the guidelines of the Ethics Committee of the Tongji Hospital and approved in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. Human HCC cell lines Huh7, SMMC7721, HepG2, MHCC-LM3 and SK-Hep-1were cultured in DMEM medium supplemented with 10% FBS (HyClone, USA) and incubated in a 5% CO2 incubator at 37°C.
RNA extraction and quantitative real-time PCR
Total RNAs were isolated, cDNAs were synthesized and PCR amplifications were performed as described previously [22]. GAPDH was used as the internal control. Real-time PCR was performed using the SYBR Green PCR kit (Fermentas, Canada) according to the manufacturer’s recommended protocol. The primers were as follows: GAPDH: 5’-TCATTGACCTCAACTACATGGTTT-3’ (sense) and 5’-GAAGATGGTG ATGGGATTTC-3’ (antisense); BVES: 5’-CCTCTGCACACAGATCTCCA-3’ (sense) and 5’-CAAGGCAGCTGATGGACTTT-3’ (antisense); netrin-1: 5’-GGGTGCCC TTCCAGTTCTAC-3’ (sense) and 5’-GCGAGTTGTCGAAGTCGTG-3’ (antisense). The experiments were repeated in triplicates, and the relative expression levels were determined by the 2-ΔΔCt method.
Western blot analysis
A total of 30μg protein was separated in SDS-polyacrylamide gel and then transferred to PVDF membranes. Membranes were immunoblotted with antibodies against BVES and netrin-1 (Santa Cruz, USA), GAPDH (Epitomics, USA), p-AKT and AKT (Cell signaling Technology, USA), E-cadherin and vimentin (Abcam, UK). The membranes were washed and incubated with horseradish per-oxidase-conjugated secondary antibody for 1 h at room temperature. The immunoreactive bands were visualized by enhanced chemiluminescence using ECL detection reagent.
Confocal microscopy
Cells were fixed in 4% paraformaldehyde for 15 min and permeabilized with 0.3% Triton X-100 for 10 min on ice. For paraffin-embedded tissue sections, routine dewaxing, rehydration and antigen retrieval were performed before permeabilization. Cells were blocked with 1% BSA for 60 min, and incubated with primary antibody diluted in 1% BSA for 4°C overnight followed by 3 washes with PBS and finally incubated with secondary Alexa Fluor 594-conjugated anti-rabbit antibody or Alexa Fluor 488-conjugated anti-mouse antibody for 1 h at room temperature. After 3 washes with PBS, cells were stained with DAPI for detecting nuclei. Cells were mounted and examined under Olympus FluoView FV1000 confocal microscope.
Plasmid and lentiviral transfection
Netrin-1 expression plasmid was gifted by Dr. P. Mehlen [8]. Netrin-1 shRNA plasmid was purchased from Santa Cruz. Cells stably overexpressing netrin-1 and netrin-1 shRNA were transfected with Lipofectin 2000 (Invitrogen) as previously described [14]. Lentiviral vectors expressing shRNA against BVES were obtained from Genechem Co., Ltd (Shanghai, China), and synthesized as follows: forward, 5’-CCUCCAGAUUUGUUCAGAAdTdT-3’, and reverse, 5’-dTdTGGAGGUCUAA ACAAGUCUU-3’. For overexpression of BVES, the open reading frame of BVES (NM_147147) was cloned into the lentiviral vector GV208. Transfections were performed using polybrene and enhanced infection solution (Genechem) according to the manufacturer’s recommended protocol.
Wound healing, cell invasion and migration assays
Indicated cells were plated to confluence in 6-well plates. Streaks were created in the monolayer with a pipette tip. Progression of migration was observed and photographed at 0 and 24 hours after wounding. Cell invasion and migration assays were analyzed using the transwell chambers assay (Costar; Corning Inc.), with or without coated Matrigel (BD Biosciences). A total of 5x104 cells in 0.2 ml media were plated in the upper chambers, the lower chamber of the transwell device was filled with 600 μl DMEM supplemented with 12% FBS. After 24 hours of incubation, cells invading into the bottom side of the inserts were fixed, stained, photographed, and quantified by counting them in 5 random fields.
RhoA-GTPase activation assay
The RhoA Activation Assay Kit (NewEast Biosciences) was used to determine RhoGTP levels. Briefly, Cells were lysed, and GTP-bound RhoA was immunoprecipitated using a conformation-specific antibody against active RhoA following the instructions of the manufacturer. Active and total RhoA were analyzed by western blotting of the immunoprecipitates and total cell lysates, respectively, using an antibody against total RhoA (NewEast Biosciences).
3D cell culture assay
3D cell culture assay was performed using hydrogel solution (BeaverNano™) according to the manufacturer’s instructions. Briefly, 1×105 cells were resuspended by 120 μl 10% sucrose solutions, and quickly mixed with equal volume of 0.5% hydrogel solution, cells were then immediately seeded in a Class Bottom Cell Culture Dish(NEST), the medium was refreshed every other day and cells were allowed to grow for six days and photographed by a microscopy.
Statistical analysis
Results are expressed as mean ± SD and representative of at least three independently performed experiments. For statistical analysis, the chi-square test (χ2 test) and Fisher’s exact test for non-parametric variables and Student t test for parametric variables were used. All tests were two-sided and p < 0.05 was considered statistically significant. Analysis was performed using SPSS software (version 18) or GraphPad Prism 5.0.
Results
Netrin-1 promotes migration and invasion of HCC cells
To investigate the possible role of netrin-1 in the metastasis of HCC cells, we created Huh7 cells with stably increased expression of netrin-1 by stable transfection and SK-Hep-1 cells with decreased expression of netrin-1 by shRNA interference. The efficiency of netrin-1 overexpression or interference were assessed by western blot (Figure 1A). To characterize the effects of netrin-1 on HCC cell migration and invasion, we used monolayer wound healing assay and transwell assay. As is shown in Figure 1B and 1C, overexpression of netrin-1 clearly enhanced cell migration and invasion in Huh7 cells, while silencing netrin-1 expression significantly decreased these capacities of SK-Hep-1 cells. Consistently, western blot data showed an increase in the expression of E-cadherin and a concomitant vimentin reduction in netrin-1 knockdown SK-Hep-1 cells, while opposite results were get in Huh7 cells overexpressing netrin-1 (Figure 1A).
Figure 1.
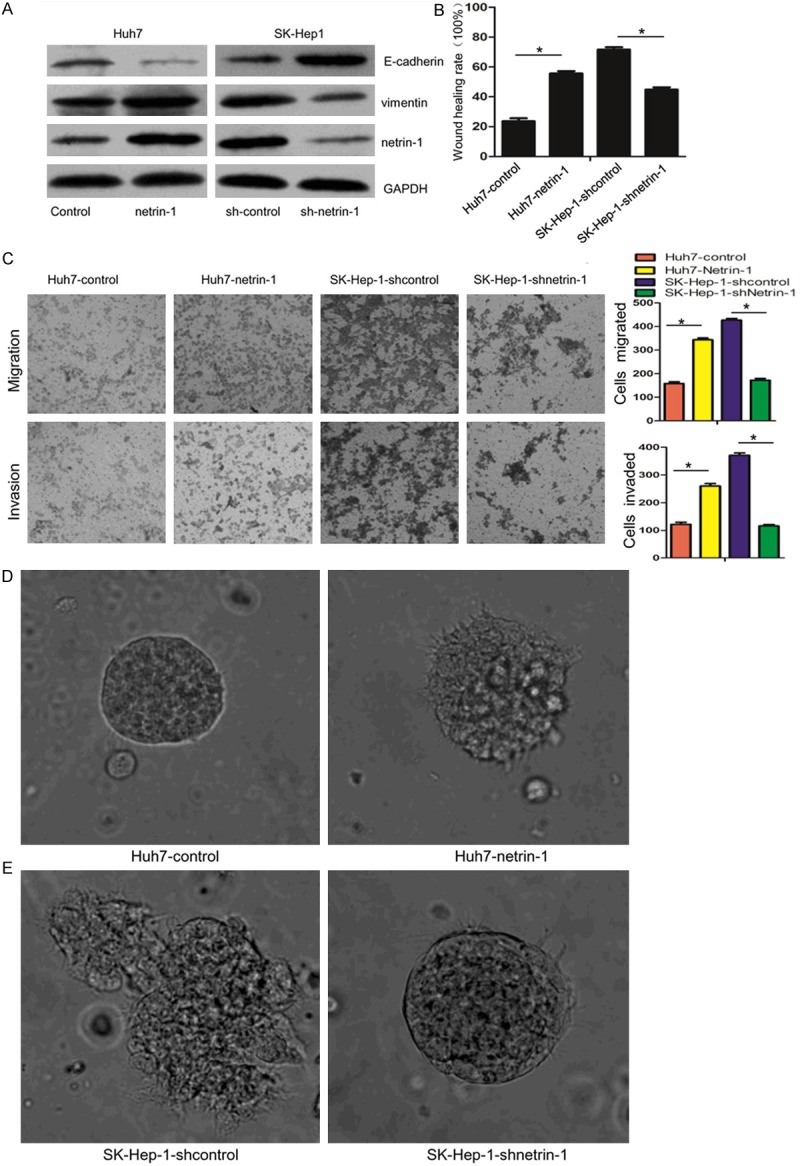
Netrin-1 promotes HCC cells migration and invasion. (A) Overexpression or interference efficiency of netrin-1 were examined by western blot, the EMT markers E-cadherin and vimentin are also detected after netrin-1 expression changed. (B) scratch wound healing assay was used to measure cell migration capability, dates were evaluated by wound healing rate at 24 h after wounding of the confluent cell layer. (C) Transwell assays were used to analyze cell migration and invasion. Overexpression of netrin-1 promoted Huh7 cells migration and invasion, while knockdown of netrin-1 in SK-Hep-1 cells suppressed them. In 3D culture assay, up-regulating netrin-1 in Huh7 cells increased the invasion phenotype (D), while down-regulation of netrin-1 inhibited the invasion capability in SK-Hep-1 cells (E).
To closely mimic in vivo conditions, we next performed three-dimensional (3D) cultures to visualize tumor cell morphology and invasion. Results revealed that, compared with control Huh7 cells, Huh7 cells stably expressing netrin-1 grew into a more invasive phenotype with invasive projections emanating from cells and peripheral cells penetrating the surrounding gels (Figure 1D). While SK-Hep-1 cells, with high metastatic potential, formed nodule-like structures and well-defined borders after netrin-1 knockdown (Figure 1E). These results suggested that netrin-1 could promote migration and invasion of HCC cells in vivo-like culture environment.
Netrin-1 regulates BVES expression in HCC cells
BVES was reported to play an important role in maintaining epithelial integrity and regulating cell movement, our previous study also initially suggested that down-regulation of BVES triggered EMT in HCC cells. Therefore, we hypothesized that BVES inhibition might be involved in netrin-1-mediated migration and invasion of human HCC cells. Results showed that overexpressing of netrin-1 obviously decreased mRNA and protein expression of BVES in Huh7 cells, while knockdown of netrin-1 increased BVES level (Figure 2A and 2B). Netrin-1 is a secretory protein, it can be produced and released by hypoxic HCC cells. 100ng/ml concentration of recombinant human netrin-1 protein (rhnetrin-1) was enough to active signaling pathways downstream as suggested by previous studies [14,23]. Using this concentration, Huh7 cells treated with rhnetrin-1 were observed a time-dependently decrease in BVES level (Figure 2C and 2D). Immunofluorescence staining assay was performed to visually observe the decrease of BVES both in the cytomembrane and cytoplasm after rhnetrin-1 treatment (Figure 2E). Interestingly, the tight junction protein ZO-1, reported to be regulated by BVES [24], was also decreased (Figure 2E).
Figure 2.
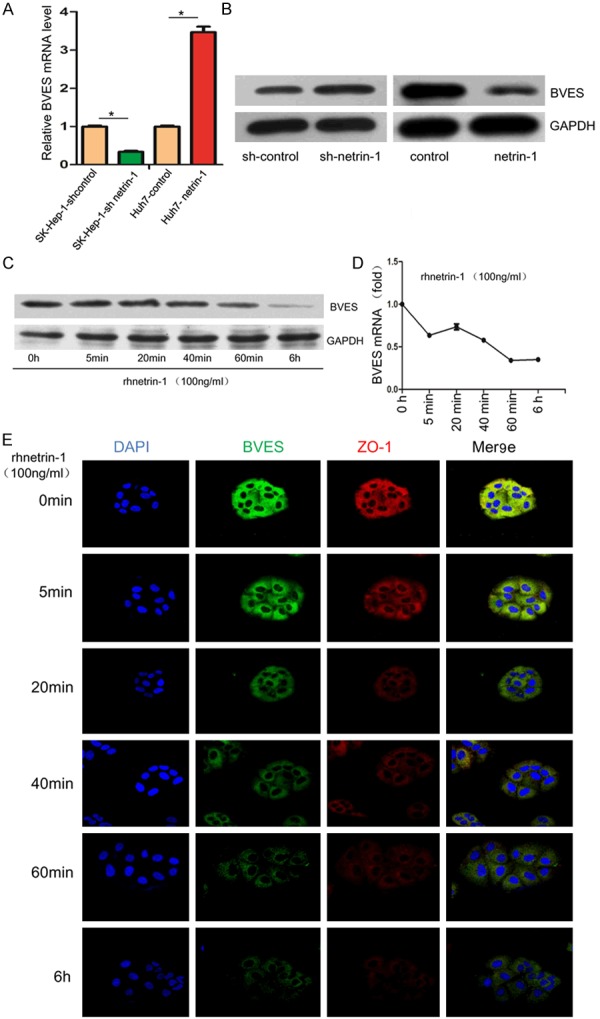
Netrin-1 regulates BVES expression. Both qRT-PCR (A) and western blot (B) demonstrated netrin-1 regulating BVES expression in Huh7 and SK-Hep-1 cells. BVES level was detected at different time points (0 h, 5 min, 20 min, 40 min, 60 min, 6 h) after rhnetrin-1 treatment by qRT-PCR(C) and western blot (D). (E) Immunofluorescence staining was performed to observe BVES (green) and ZO-1 (red) expression after rhnetrin-1 treatment, DAPI was used to show the location of the nucleus (blue).
Netrin-1 regulates BVES expressions via PI3K/AKT pathway
PI3K/AKT pathway plays an important role in tumor metastasis, we have proved PI3K/AKT signaling was activated in HCC, PI3K/AKT activation mediates EMT in hypoxic HCC cells [25], so we suppose it is also involved in netrin-1 induced BVES down-regulation in HCC. Increased p-AKT was found after rhnetrin-1 treatment in a time-dependent manner (Figure 3A). Next, Huh7 cells were pretreated with different concentration of PI3K/AKT inhibitor LY294002 before rhnetrin-1 treatment. As shown in Figure 3B, 50 µ mol/L LY294002 restored the decreased expression of BVES by rhnetrin-1 to a great extent at 6 hours. Immunofluorescence staining also observed increased expression of BVES and ZO-1 with 50 µ mol/L LY294002 pretreatment (Figure 3C). These results indicated that activation of PI3K/AKT was involved in netrin-1 mediated BVES down-regulation.
Figure 3.
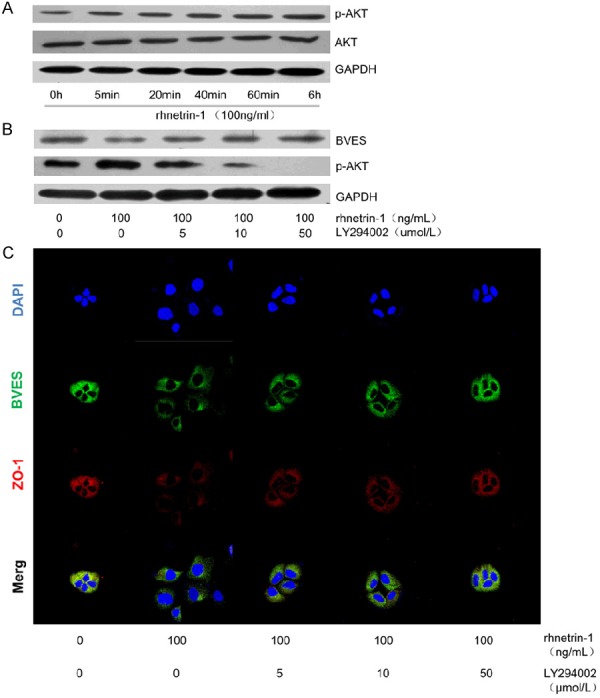
Netrin-1 regulates BVES expressions by PI3K/AKT pathway. (A) 100ng/ml rhnetrin-1 increased p-AKT level in a time-dependent manner, while total AKT level was not obviously increased. 50 µ mol/L LY294002 pretreatment decreased p-AKT level to a large extent (B), and restored the decreased expression of BVES by rhnetrin-1 to a great extent, as detected by western blot (B) and immunofluorescence staining (C).
BVES inhibits migration and invasion of HCC cells
To further address the effect of BVES on migration and invasion of HCC cells, we silenced or over-expressed BVES in Huh7 and SK-Hep-1 cells by lentiviral transfection, respectively. Monolayer wound healing as-say and transwell assay were employed to detect cell migration and invasion. As is shown in Figure 4A and 4B, BVES knockdown significantly increased the migration and invasion of Huh7 cells, while up-regulating BVES expression in SK-Hep-1 cells obviously enh-anced these capacities. Western blot data showed an decrease of E-cadherin and a concomitant increase of vimentin in Huh7 cells after BVES inhibition, while opposite results were get in BVES expressing SK-Hep-1 cells (Figure 4C). We also observed a regulatory role of BVES on the expression of ZO-1 and the activity of RhoA, which are important for cancer cell metastasis. As shown in Figure 4D, knockdown of BVES decreased the expression of ZO-1, increased RhoA activity in Huh7 cells, while overexpression of BVES increased ZO-1 expression, decreased the activity of RhoA. In 3D cultures, BVES silenced Huh7 cells grew into a more aggressive phenotype with invasive projections emanating from cells and peripheral cells penetrating the surrounding gels (Figure 4E). While SK-Hep-1 cells formed more nodule-like structures and well-defined borders after overexpression of BVES (Figure 4F). These results suggested that BVES had an opposite role on the migration and invasion of HCC cells as that of netrin-1.
Figure 4.
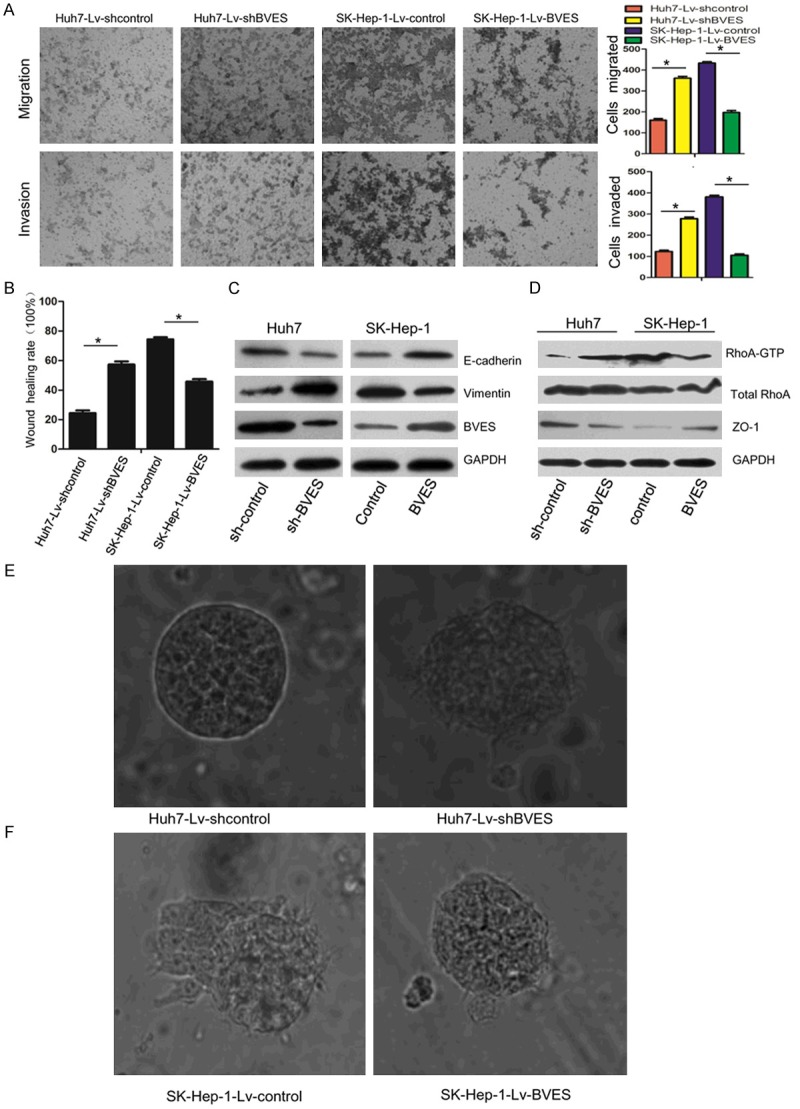
BVES inhibits migration and invasion of HCC cells. Scratch wound healing assay (A) and Transwell chambers assays (B) were used to analyze cell migration and invasion, BVES knockdown promoted Huh7 cells migration and invasion, while overexpression of BVES in SK-Hep-1 cells suppressed them. (C) EMT markers E-cadherin and vimentin were detected after BVES knockdown and overexpression. (D) BVES up-regulated ZO-1 expression, and decreased RhoA activity as detected by RhoA-GTPase activation assay. In 3D culture assay, down-regulation of BVES in Huh7 cells increased the invasion capability (E), while overexprssion of BVES inhibited the invasion capability in SK-Hep-1 cells (F).
Netrin-1 promotes HCC cells migration and invasion partially by regulating BVES expression
The above results showed that netrin-1 and BVES have an opposite effect on HCC cells migration and invasion, and netrin-1 could inhibit BVES expression by p-AKT. Thus, we wonder whether the effect of netrin-1 on HCC cells migration and invasion is mediated by BVES down-regulation. Therefore, we set up different cross-over experiments in SMMC7721 cell to test our hypothesis, as this cell line has balanced expression of netrin-1 and BVES (Figure 5A). As shown in Figure 5B and 5C, overexpression of netrin-1 alone inhibited cells migration and invasion, which was consistent with our above findings. Interestingly, when BVES was co-transfected with netrin-1, the migration and invasion significantly decreased, suggesting that overexpression of BVES partially abolished HCC cell migration and invasion induced by netrin-1 (Figure 5B and 5C). In line with above findings, the 3D culture also showed similar results (Figure 5E). In addition, when Lv-shBVES was co-transfected with netrin-1-shRNA, BVES inhibition obviously rescued the decreased metastasis by netrin-1 down-regulation (Figure 5B and 5D), the 3D culture also showed similar results (Figure 5F). Taken together, these results suggest that netrin-1 promotes HCC cell migration and invasion partially by regulating BVES expression.
Figure 5.
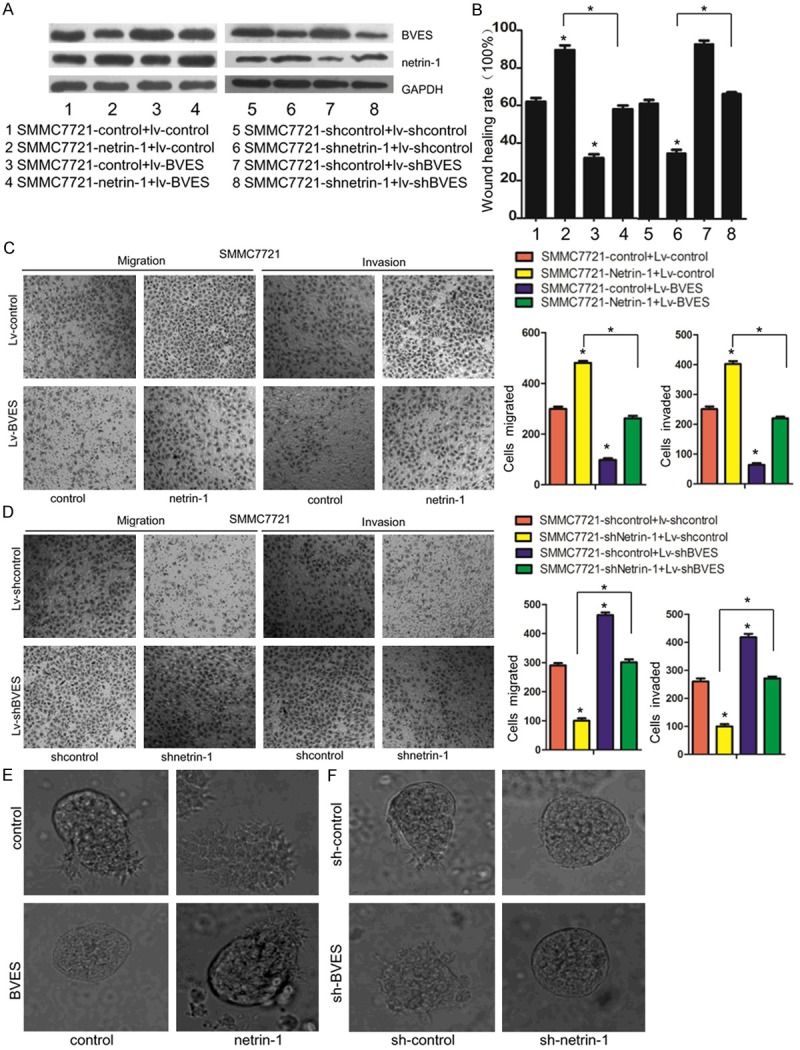
Netrin-1 promotes HCC cells migration and invasion partially by regulating BVES expression. (A) Representative blots showed the co-transfection of netrin-1 and Lv-BVES (1-4), and co-transfection of shnetrin-1 and Lv-shBVES (5-8). (B) Scratch wound healing assay showed that over-expression of netrin-1 increased cell migration was partially attenuated by over-expression of BVES, while BVES knockdown rescued the decreased cell migration inhibited by up-regulation of netrin-1. Transwell chambers assays showed that over-expression (C)/down-regulation (D) of netrin-1 increased/decreased cell migration was significantly attenuated/rescued by co-transfecting with Lv-BVES (C) or Lv-shBVES (D). 3D culture assay showed the more aggressive phenotype induced by overexpression of netrin-1 was attenuated by co-transfection with Lv-BVES (E), the less aggressive phenotype inhibited by down-regulation of netrin-1 was rescued by co-transfection with Lv-shBVES (F).
Inverse expression of netrin-1 and BVES in HCC tissues and HCC cell lines
Finally, we studied the clinical relevance of netrin-1 and BVES and their relationship in clinical HCC samples. As is shown in Figure 6A, the mRNA level of netrin-1 in HCC samples was approximately 2~3 fold higher than that of adjacent non-tumor tissues(p=0.0025), while the mRNA level of BVES was 2~3 fold decrease in HCC samples than that of adjacent non-tumor tissues (p < 0.0001; Figure 6B). Interestingly, there is a negative correlation between the netrin-1 and BVES levels (r = -0.484, p < 0.0001; Figure 6C). Similar result was obtained by immunofluorescence staining method to examine the protein expression and distribution of netrin-1 and BVES in HCC samples (Figure 6D), netrin-1 was mainly localized in the cytoplasm and less in the nucleus, while BVES was mainly localized in the cytoplasm and cytomembrane. The inverse expression of netrin-1 and BVES was also observed in HCC cell lines (Figure 6E).
Figure 6.
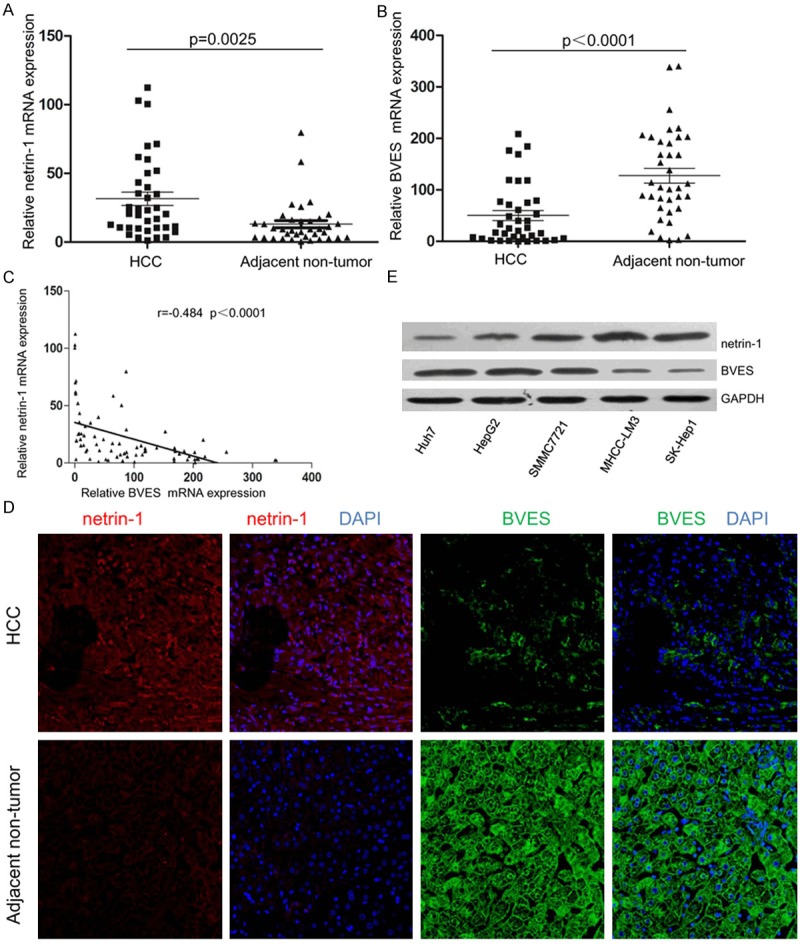
Comparison of netrin-1 and BVES expression in HCC tissues and HCC cell lines. Total RNA was extracted from liver biopsies to assess gene expression levels by qRT-PCR (A, B). As we previously reported, expression data confirmed netrin-1 up-regulation in HCC samples compared to their matched adjacent non-tumor tissues (A), n=37, p=0.0025. However, BVES was down-regulated in HCC samples compared to their matched non-tumor counterpart (B), n=37, p < 0.0001. (C) Correlation scatter plot (Spearman test) of netrin-1and BVES expression in HCC samples and adjacent non-tumor tissues, r=-0.484 p < 0.0001. (D) Representative images of netrin-1 (green) and BVES (red) from HCC samples and their matched adjacent non-tumor tissues determined by immunofluorescence staining. DAPI was used to show the location of the nucleus (blue). (E) Representative western blots showing inverse expression of netrin-1 and BVES in HCC cell lines with different metastatic potential.
Collectively, these results have suggested an inverse expression of netrin-1 and BVES in human HCC tissues and HCC cell lines, this further confirmed the regulatory role of netrin-1 on BVES expression in HCC migration and invasion.
Discussion
Hepatocellular carcinoma (HCC) is among the leading causes of cancer death globally, despite the advances in diagnosis and management of HCC, the biology of this tumor remains poorly understood [26]. The majority of cancer deaths are caused by metastasis, thus the elucidation of the molecular mechanisms underlying the early metastasis of HCC is critically important for the development of novel treatments for this disease.
We have previously reported that netrin-1 induces EMT activation and promotes metastasis in hypoxic HCC cells [14]. However, the molecular pathways downstream of netrin-1 were not well known. In this study, we show that netrin-1 promotes metastasis by the suppression of BVES expression in HCC cells. We also find that PI3K/AKT pathway involved in the inhibition of netrin-1 on BVES expression, the down-regulated BVES makes further effect on ZO-1 expression and RhoA activation, finally mediates cell migration and invasion phenotypes. Based on these results, we propose a new molecular pathway model for the function of netrin-1, and also for BVES, as the regulatory system of BVES expression was barely examined.
Netrin-1 has been considered as an oncogene in some cancers including colorectal cancer [8], breast cancer [11], lung cancer [12], pancreatic cancer [27] and neuroblastoma [13], gain of netrin-1 has been shown as a selective advantage for tumor progression. In this study, netrin-1 was also found to be increased in HCCs than their adjacent non-tumor tissues, however, netrin-1 did not have any significant association with patient’s clinical outcomes, such as gender, age, tumor stage, or tumor size (Table 1). Ganesan Ramesh et al. tested plasma netrin-1 levels in 300 cancer plasma samples from breast, renal, prostate, liver, meningioma, pituitary adenoma, glioblastoma, lung, pancreatic and colon cancer patients by ELISA, compared against 138 non-cancerous control plasma samples, plasma netrin-1 levels were significantly increased in the cancers [28]. They also did not find any significant association between netrin-1 level with patient’s gender, age, tumor stage, or tumor size, netrin-1 didn’t show stage-specific expression and was elevated in all stages of tumors [28]. These results suggest that gain of netrin-1 is a frequent event in early stage as well as advanced-stage tumors, and are consistent with the idea that critical changes in metastatic potential may be determined early during the development of cancer [29]. Therefore, netrin-1 is an independent predictor of the presence of a tumor, and it could be used as an early diagnostic biomarker of cancer.
Table 1.
Correlation between netrin-1 and BVES expression and clinicopathological characteristics of 37 HCCs
| Variable | Samples (n = 37) | netrin-1 | BVES | ||
|---|---|---|---|---|---|
|
| |||||
| high expression | P-value | low expression | P-value | ||
| Gender | |||||
| Male | 28 | 22 | 0.091 | 20 | 0.229 |
| Female | 9 | 4 | 4 | ||
| Age | |||||
| ≥ 60 | 8 | 5 | 0.672 | 4 | 0.413 |
| < 60 | 29 | 21 | 20 | ||
| Tumour number | |||||
| Multiple | 11 | 7 | 0.699 | 5 | 0.143 |
| Solitary | 26 | 19 | 19 | ||
| Tumour size (cm) | |||||
| ≥ 5 | 20 | 16 | 0.279 | 14 | 0.512 |
| < 5 | 17 | 10 | 10 | ||
| Tumour differentiation | |||||
| Moderately and poorly | 22 | 17 | 0.295 | 16 | 0.300 |
| Well | 15 | 9 | 8 | ||
| Portal invasion | |||||
| Present | 12 | 8 | 1.000 | 9 | 0.476 |
| Absent | 25 | 18 | 15 | ||
| Lymph node metastases | |||||
| Present | 10 | 7 | 1.000 | 7 | 1.000 |
| Absent | 27 | 19 | 17 | ||
| HBsAg | |||||
| Present | 30 | 22 | 0.403 | 19 | 1.000 |
| Absent | 7 | 4 | 5 | ||
Abbreviation: HBsAg, hepatitis B surface antige.
The promoting role of ne-trin-1 on cancer progress was mostly focused on regulating inflammation, angiogenesis, and apoptosis. In this study, we found netrin-1 could also promote HCC cells migration and invasion by monolayer wound healing assay and transwell assay, while down-regulation of netrin-1 inhibited cell migration and invasion. To generate tissue-like models in vitro, 3D spheroid culture systems were performed, cells overexpressing netrin-1 grew in 3D culture obtained a more aggressive phenotype with invasive projections emanating from cells and peripheral cells penetrating the surrounding gels. While netrin-1 knockdown made cells more nodule-like structures and well-defined borders. These results further suggested that netrin-1 could promote metastasis of HCC cells in vitro.
BVES plays an important part in the early stage of cancer metastasis, Williams et al. analyzed a large colorectal cancer expression array data set to reveal decreased BVES at all stages of CRC [19], which further implicated BVES in tumorigenesis, loss of BVES in HCE cells is associated with reduced E-cadherin level and the redistribution of β-catenin both in cytoplasm and nucleus, these results suggest that BVES modulates canonical WNT signaling and contributes to tumorigenesis [19]. BVES also influences TJ-associated signaling by regulating the sequestration of GEF-H1 (an activator of RhoA) at the cell membrane [17], this, in turn, modulates RhoA activation and then promotes cancer cell migration and invasion. In our study, BVES was also found to be decreased in HCC tissues and cell lines, down-regulating BVES expression in Huh7 cells increased cells migration and invasion, while overexpression of BVES in SK-Hep-1 cells inhibited them. These results were further confirmed by 3D culture model, in which BVES inhibited the invasive phenotype. In line with previous studies, we further revealed that BVES could regulate ZO-1 protein levels and RhoA activity, which are two important molecules regulating cell adhesion and migration [30,31]. These results provide an important molecular mechanism of BVES function.
Although the underline mechanisms of BVES on cell adhesion and movement have become increasingly clearer, very little is known about the signaling events and transcriptional control regulating BVES expression [15]. Lin et al. reported that BVES was downstream of Gurken (Grk)/EGFR signaling in Drosphila, and BVES expression was negatively regulated by Grk [32]. M. Kim et al further proved the regulation role of EGF on BVES expression in human gastric cancer cell, SNU-216 cells treated with 20 ng/ml EGF showed rapidly down-regulation of BVES mRNA [20]. In the present study, we found BVES expression was down-regulated after overexpression of netrin-1 in HCC cells, Huh7 cells treated with 100 ng/ml rhnetrin-1 protein exhibited decreased BVES expression in a time-dependent manner. In addition, PI3K/AKT pathway was activated after rhnetrin-1 treatment, which has been confirmed a downstream of netrin-1 in other studies [33,34]. Interestingly, PI3K/AKT pathway inhibitor LY94002 restored the decreased expression of BVES by rhnetrin-1 to a great extent. We also found an inverse expression between netrin-1 and BVES in human HCC tissues and HCC cell lines. These dates suggest that netrin-1 may be an upstream regulatory molecules of BVES via PI3K/AKT pathway. However, there may be other signaling events and molecules involved in netrin-1 regulated BVES expression, netrin-1 structurally has three cystein-rich EGF modules and has been confirm to up-regulating EGF expression in some conditions [23,35], and crosstalk always exist between the EGFR signaling and PI3K/AKT pathway [36,37], further studies are needed to elucidate the regulatory pathway of BVES, especially in tumorigenesis and tumor progression.
In summary, we showed that netrin-1 and BVES alone could promote or inhibit cancer cell metastasis in HCC, respectively. For the first time, we find the regulatory role of netrin-1 on BVES expression, and PI3K/AKT pathway is crucial in this process. Decreased expression of BVES contributes to reduced ZO-1 expression and increased RhoA activation, thus enabling HCC cell migration and invasion. We suggest that netrin-1/BVES pathway may be an important target for HCC therapies and are candidate biomarkers for HCC detection and prognosis.
Acknowledgements
This study is supported by the National Natural Science Foundation of China (NO:81472311, 81401992 and 81270507), the Fundamental Research Funds for the Central Universities (2014ZHYX020 and 2014TS077), the Project sponsored by SRF for ROCS, SEM (2014-1685) and Hubei Province health and family planning scientific research project (WJ2015Q006).
Disclosure of conflict of interest
We have no conflicts of interest to declare.
References
- 1.Chen WQ, Zheng RS, Zhang SW, Zeng HM, Zou XN. The incidences and mortalities of major cancers in China, 2010. Chin J Cancer. 2014;33:402–405. doi: 10.5732/cjc.014.10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radisky DC. Epithelial-mesenchymal transition. J Cell Sci. 2005;118:4325–4326. doi: 10.1242/jcs.02552. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 5.Forcet C, Stein E, Pays L, Corset V, Llambi F, Tessier-Lavigne M, Mehlen P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- 6.Paradisi A, Mehlen P. Netrin-1, a missing link between chronic inflammation and tumor progression. Cell Cycle. 2010;9:1253–1262. doi: 10.4161/cc.9.7.11072. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu A, Nakayama H, Wang P, Konig C, Akino T, Sandlund J, Coma S, Italiano JE Jr, Mammoto A, Bielenberg DR, Klagsbrun M. Netrin-1 promotes glioblastoma cell invasiveness and angiogenesis by multiple pathways including activation of RhoA, cathepsin B, and cAMP-response element-binding protein. J Biol Chem. 2013;288:2210–2222. doi: 10.1074/jbc.M112.397398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazelin L, Bernet A, Bonod-Bidaud C, Pays L, Arnaud S, Gespach C, Bredesen DE, Scoazec JY, Mehlen P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 9.Gu C, Giraudo E. The role of semaphorins and their receptors in vascular development and cancer. Exp Cell Res. 2013;319:1306–1316. doi: 10.1016/j.yexcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko SY, Blatch GL, Dass CR. Netrin-1 as a potential target for metastatic cancer: focus on colorectal cancer. Cancer Metastasis Rev. 2014;33:101–113. doi: 10.1007/s10555-013-9459-z. [DOI] [PubMed] [Google Scholar]
- 11.Fitamant J, Guenebeaud C, Coissieux MM, Guix C, Treilleux I, Scoazec JY, Bachelot T, Bernet A, Mehlen P. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci U S A. 2008;105:4850–4855. doi: 10.1073/pnas.0709810105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delloye-Bourgeois C, Brambilla E, Coissieux MM, Guenebeaud C, Pedeux R, Firlej V, Cabon F, Brambilla C, Mehlen P, Bernet A. Interference with netrin-1 and tumor cell death in non-small cell lung cancer. J Natl Cancer Inst. 2009;101:237–247. doi: 10.1093/jnci/djn491. [DOI] [PubMed] [Google Scholar]
- 13.Delloye-Bourgeois C, Fitamant J, Paradisi A, Cappellen D, Douc-Rasy S, Raquin MA, Stupack D, Nakagawara A, Rousseau R, Combaret V, Puisieux A, Valteau-Couanet D, Benard J, Bernet A, Mehlen P. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J Exp Med. 2009;206:833–847. doi: 10.1084/jem.20082299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan W, Han P, Zhou Z, Tu W, Liao J, Li P, Liu M, Tian D, Fu Y. Netrin-1 induces epithelial-mesenchymal transition and promotes hepatocellular carcinoma invasiveness. Dig Dis Sci. 2014;59:1213–1221. doi: 10.1007/s10620-013-3016-z. [DOI] [PubMed] [Google Scholar]
- 15.Hager HA, Bader DM. Bves: ten years after. Histol Histopathol. 2009;24:777–787. doi: 10.14670/hh-24.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osler ME, Chang MS, Bader DM. Bves modulates epithelial integrity through an interaction at the tight junction. J Cell Sci. 2005;118:4667–4678. doi: 10.1242/jcs.02588. [DOI] [PubMed] [Google Scholar]
- 17.Smith TK, Hager HA, Francis R, Kilkenny DM, Lo CW, Bader DM. Bves directly interacts with GEFT, and controls cell shape and movement through regulation of Rac1/Cdc42 activity. Proc Natl Acad Sci U S A. 2008;105:8298–8303. doi: 10.1073/pnas.0802345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ripley AN, Osler ME, Wright CV, Bader D. Xbves is a regulator of epithelial movement during early Xenopus laevis development. Proc Natl Acad Sci U S A. 2006;103:614–619. doi: 10.1073/pnas.0506095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams CS, Zhang B, Smith JJ, Jayagopal A, Barrett CW, Pino C, Russ P, Presley SH, Peng D, Rosenblatt DO, Haselton FR, Yang JL, Washington MK, Chen X, Eschrich S, Yeatman TJ, El-Rifai W, Beauchamp RD, Chang MS. BVES regulates EMT in human corneal and colon cancer cells and is silenced via promoter methylation in human colorectal carcinoma. J Clin Invest. 2011;121:4056–4069. doi: 10.1172/JCI44228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim M, Jang HR, Haam K, Kang TW, Kim JH, Kim SY, Noh SM, Song KS, Cho JS, Jeong HY, Kim JC, Yoo HS, Kim YS. Frequent silencing of popeye domain-containing genes, BVES and POPDC3, is associated with promoter hypermethylation in gastric cancer. Carcinogenesis. 2010;31:1685–1693. doi: 10.1093/carcin/bgq144. [DOI] [PubMed] [Google Scholar]
- 21.Luo D, Huang H, Lu ML, Zhao GF, Chang J, Zheng MY, Wang Y. Abnormal expression of adhesion protein Bves is associated with gastric cancer progression and poor survival. Pathol Oncol Res. 2012;18:491–497. doi: 10.1007/s12253-011-9472-x. [DOI] [PubMed] [Google Scholar]
- 22.Han P, Fu Y, Luo M, He J, Liu J, Liao J, Tian D, Yan W. BVES inhibition triggers epithelial-mesenchymal transition in human hepatocellular carcinoma. Dig Dis Sci. 2014;59:992–1000. doi: 10.1007/s10620-013-2992-3. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A. 2006;103:6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russ PK, Kupperman AI, Presley SH, Haselton FR, Chang MS. Inhibition of RhoA signaling with increased Bves in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;51:223–230. doi: 10.1167/iovs.09-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan W, Fu Y, Tian D, Liao J, Liu M, Wang B, Xia L, Zhu Q, Luo M. PI3 kinase/Akt signaling mediates epithelial-mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;382:631–636. doi: 10.1016/j.bbrc.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 26.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 27.Dumartin L, Quemener C, Laklai H, Herbert J, Bicknell R, Bousquet C, Pyronnet S, Castronovo V, Schilling MK, Bikfalvi A, Hagedorn M. Netrin-1 mediates early events in pancreatic adenocarcinoma progression, acting on tumor and endothelial cells. Gastroenterology. 2010;138:1595–606. 1606.e1–8. doi: 10.1053/j.gastro.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 28.Ramesh G, Berg A, Jayakumar C. Plasma netrin-1 is a diagnostic biomarker of human cancers. Biomarkers. 2011;16:172–180. doi: 10.3109/1354750X.2010.541564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15:87–96. doi: 10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Runkle EA, Mu D. Tight junction proteins: from barrier to tumorigenesis. Cancer Lett. 2013;337:41–48. doi: 10.1016/j.canlet.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gen Y, Yasui K, Zen K, Nakajima T, Tsuji K, Endo M, Mitsuyoshi H, Minami M, Itoh Y, Tanaka S, Taniwaki M, Arii S, Okanoue T, Yoshikawa T. A novel amplification target, ARHGAP5, promotes cell spreading and migration by negatively regulating RhoA in Huh-7 hepatocellular carcinoma cells. Cancer Lett. 2009;275:27–34. doi: 10.1016/j.canlet.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Lin S, Zhao D, Bownes M. Blood vessel/epicardial substance (bves) expression, essential for embryonic development, is down regulated by Grk/EFGR signalling. Int J Dev Biol. 2007;51:37–44. doi: 10.1387/ijdb.052108sl. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Reeves WB, Ramesh G. Netrin-1 increases proliferation and migration of renal proximal tubular epithelial cells via the UNC5B receptor. Am J Physiol Renal Physiol. 2009;296:F723–729. doi: 10.1152/ajprenal.90686.2008. [DOI] [PubMed] [Google Scholar]
- 34.Son TW, Yun SP, Yong MS, Seo BN, Ryu JM, Youn HY, Oh YM, Han HJ. Netrin-1 protects hypoxia-induced mitochondrial apoptosis through HSP27 expression via DCC- and integrin alpha6beta4-dependent Akt, GSK-3beta, and HSF-1 in mesenchymal stem cells. Cell Death Dis. 2013;4:e563. doi: 10.1038/cddis.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Y, Shao Y, Lin Z, Qu YL, Wang H, Zhou Y, Chen W, Chen Y, Chen WL, Hu FR, Li W, Liu Z. Netrin-1 simultaneously suppresses corneal inflammation and neovascularization. Invest Ophthalmol Vis Sci. 2012;53:1285–1295. doi: 10.1167/iovs.11-8722. [DOI] [PubMed] [Google Scholar]
- 36.Bhat FA, Sharmila G, Balakrishnan S, Arunkumar R, Elumalai P, Suganya S, Raja Singh P, Srinivasan N, Arunakaran J. Quercetin reverses EGF-induced epithelial to mesenchymal transition and invasiveness in prostate cancer (PC-3) cell line via EGFR/PI3K/Akt pathway. J Nutr Biochem. 2014;25:1132–1139. doi: 10.1016/j.jnutbio.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Yip WK, Seow HF. Activation of phosphatidylinositol 3-kinase/Akt signaling by EGF downregulates membranous E-cadherin and betacatenin and enhances invasion in nasopharyngeal carcinoma cells. Cancer Lett. 2012;318:162–172. doi: 10.1016/j.canlet.2011.12.018. [DOI] [PubMed] [Google Scholar]


