Abstract
Recent evidence suggests that anti-diabetic drug metformin prevents cancer progression, but the mechanism by which metformin inhibits tumor growth remains elusive. In this study, we investigated the anticancer role of metformin in gastric cancer and explored the underlying mechanism. The expression of hypoxia inducible factor 1α (HIF1α) and pyruvate kinase M2 (PKM2) in different stages of gastric cancer tissues was detected by immunohistochemistry. Gastric cancer cell viability was evaluated by CCK-8 assay; apoptosis and cell cycle were analyzed by flow cytometry. The expression of PI3K, Akt, HIF1α, PARP, PKM2 and COX in gastric cancer cells was detected by immunofluorescence and Western blot analysis. We found that HIF1α and PKM2 protein expression levels were higher in advanced gastric cancer tissues than in gastritis tissues. Metformin reduced gastric cancer cell viability, invasion and migration. Metformin induced apoptosis and cell cycle arrest in part through inhibiting PARP expression. Metformin downregulated PI3K, Akt, HIF1α, PARP, PKM2 and COX expression. Moreover, overexpression of HIF1α increased gastric cancer cell viability, invasion and migration. In summary, metformin has profound antitumor effect for gastric cancer by inducing intrinsic apoptosis via the inhibition of HIF1α/PKM2 signaling pathway.
Keywords: Metformin, gastric cancer, hypoxia inducible factor 1α, pyruvate kinase M2
Introduction
Gastric cancer (GC) is the second most common cause of cancer-related death worldwide [1,2]. Tumor metabolism has been shown to play important role in tumorigenesis and tumor development [3]. Effect of tumor microenvironment on tumor metabolism has received more attention recently [4-7]. Hypoxia inducible factor 1α (HIF1α) and glucose metabolism cause the change of tumor microenvironment [8,9]. Targeting HIF-1 and tumor glucose metabolism at several levels reduce the antioxidant capacity of tumors, affect the tumor microenvironment, and sensitize various solid tumors to irradiation [10].
HIF1α plays a critical role in the regulation of tumor angiogenesis in response to hypoxia [11,12]. Upon hypoxia, PI3k/Akt/HIF1α signaling pathway is activated to regulate tumor glucose metabolism [13]. Metformin, a well-known anti-diabetic drug, has been shown to reduce the incidence of malignancies in patient with diabetes [14]. The use of metformin in patients with type 2 diabetes may reduce the risk of thyroid cancer [15]. A systematic review showed that metformin significantly reduced the occurrence of GC, liver cancer, lung cancer, colon cancer, esophageal cancer and reduced cancer-related mortality [16]. However, the mechanism by which metformin inhibits tumor growth remains elusive.
The aim of this study was to investigate the anticancer role of metformin in gastric cancer and explore the underlying mechanism. We detected the expression of HIF1α and pyruvate kinase M2 (PKM2) in different stages of GC and examined the efficacy and possible mechanism of metformin against human GC cells.
Materials and methods
Reagents
Metformin and 4’,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell counting kit-8 (CCK-8) was purchased from Dojindo Laboratories (Kumamoto, Japan). Alexa Fluor 488 conjugated goat anti-rabbit secondary antibody and Trizol were purchased from Invitrogen (Carlsbad, CA , USA). Annexin V Apoptosis Detection kit FITC was purchased from eBioscience (San Diego, CA, USA). Antibodies against PARP (9532), Akt (9272), and β-actin (4967) were purchased from Cell Signal Technology (Boston, MA, USA). Antibody against PI3K (Y467) and HIF1α (K377) were purchased from Bioworld Technology (USA). Antibody against HIF1α (ab113642) and COX (ab33985) were purchased from Abcam (USA). Rabbit secondary antibody was from Cell Signal Technology. PrimeScript™ RT Master Mix and SYBR Premix Ex Taq reagents were purchased from Takara Biotechnology (Dalian, China).
Samples and immunohistochemistry
A total of 20 superficial gastritis, 40 early GC and 40 advanced GC tissues were obtained from patients admitted at The Affiliated Drum Tower Hospital of Nanjing University after the approval of the local ethics committee and informed consent were obtained. Immunohistochemical staining of deparaffinized tumoral and gastritis tissues were performed according to standard protocols using HIF1α and PKM2 antibody. The staining intensities were graded as 0, 1, 2, and 3 by two pathologists, respectively.
Cell culture
Human GC lines SGC7901 (moderately differentiated) and BGC-823 (poorly differentiated) were purchased from Shanghai Institute of Biochemistry, and cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 ng/L penicillin, and 100 ng/L streptomycin at 37°C in 5% CO2. HIF1α overexpression plasmid or control plasmid was transfected into BGC823 cells using Lipofectamine 2000 according to the manufacturer’s protocol.
Cell viability assay
Cell viability was detected by cell counting kit-8 (CCK-8) assay. Cells were seeded into 96-well plates at 1×104 cells/well and cultured overnight at 37°C. After treatment with metformin at indicated concentrations for 24, 48, 72 h, 10 µL CCK-8 was added to each well and incubated for 1 h at 37°C. The absorbance was measured at 450 nm. The data were presented as mean ± SD of triplicate samples from at least three independent experiments. The cell viability was calculated using the following formula: cell viability (%)=(As-Ab)/(Ac-Ab)×100%, where As represents the A value of the experimental well, Ac represents the A value in the control well, and Ab represents the A value of the blank well.
Annexin V-FIT C apoptosis assay
Cells were seeded in six-well plates at 4×105 cells/well and then treated with different concentrations of metformin for 24 h. Apoptotic cells were detected by flow cytometry using Annexin V-FITC kit according to the instructions.
Cell cycle analysis
Cell cycle distribution was analyzed by flow cytometry. After indicated treatments, cells were trypsinized, rinsed with PBS, fixed with 70% ethanol at 4°C overnight, and treated with RNaseA (0.02 mg/ml) in the dark at room temperature for 30 min. Cells were resuspended in 0.05 mg/ml propidium iodide and analyzed with flow cytometry. For each sample, at least 1×104 cells were recorded.
Cell invasion assay
Invasion assay was performed using 24-well Transwell units with 8μm pore size polycarbonate inserts. The polycarbonate membranes were cultured at 37°C for 1 h. Cells (1×104) suspended in 200 μl of RPMI1640 medium containing 1% fetal bovine serum were seeded in the upper compartment of the Transwell unit. 800 μl of RPMI1640 medium containing 10% fetal bovine serum was added into the lower compartment as a chemoattractant. After 24 h incubation, cells on the upper side of the membrane were removed, and the cells that migrated through the membrane to the underside were fixed and stained with 0.1% crystal violet. Cell numbers were counted in five separate fields using light microscopy at 400× magnification. The data were expressed as the mean value of cells in five fields based on three independent experiments.
Cell migration assay
Migration assay was performed using 24-well Transwell units with 8 μm pore size polycarbonate inserts. The polycarbonate membranes were coated with Matrigel (Becton Dickinson) and cultured at 37°C for 1 h. The next steps were the same as cell invasion assay described above.
Quantitative Real-time PCR
Total RNA was extracted using the Trizol Reagent and subsequently reverse transcribed using the PrimeScript RT Master Mix according to the manufacturer’s instructions. Quantitative Real-time PCR was performed with the 7500 Real-time PCR System (Applied Biosystems) using SYBR Premix Ex Taq reagents. PCR cycling conditions were: 40 cycles of 5 s at 95°C, 32-34 s at 60°C. Fold-induction was calculated using the formula 2-(ΔΔCt). The specific primers were as follows: HIF1α: sense: 5’-GTAGTGCTGACCCTGCACTCAA-3’ antisense: 3’-CCATCGGAAGGACTAGGTGTCT-5’; β-actin: sense: 5’-ACCGAGCGCGGCTACA-3’, antisense: 3’-CAGCCGTGGCCATCTCTT-5’.
Western blot analysis
Cells were lysed in RIPA buffer (50 mM Tris-HCl with pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP- 40, 1 mM EDTA). The proteins in cell lysates were resolved by 8-12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes. The membranes were blocked by 5% non-fat dry milk in Tris buffered saline containing 0.1% Tween-20 for 2 h at room temperature. Then the membranes were incubated with primary antibodies (1:1000 dilutions) overnight, followed by incubation with appropriate HRP-conjugated secondary antibodies (1:5000 dilutions). The blots were detected using Millipore Immobilon Western Chemiluminescent HRP Substrate according to the manufacturer’s instructions.
Immunofluorescence
Cells were cultured on 24-well plates, fixed with 4% paraformaldehyde, and blocked for 1 h with 5% normal goat serum, followed by incubation with monoclonal antibodies against HIF1α (1:200) and COX (1:100) overnight at 4°C. Cells were then rinsed with PBS and incubated with Alexa Fluor 488-conjugated goat anti-rabbit or goat anti-mouse secondary antibody. Cells were counter-stained with DAPI (2 μg/ml) and examined by fluorescence microscopy.
Statistical analysis
All data were presented as mean ± SD of three independent experiments at least. Statistical analysis was performed using SPSS22.0 and Prism 5 (GraphPad Software Inc., San Diego, USA). Single factor analysis of variance test was used for comparisons among multiple groups, and t test was used for comparisons between two groups. P<0.05 was considered statistically significant.
Results
High expression levels of HIF1α and PKM2 in GC tissue
We detected the expression of HIF1α and PKM2 in superficial gastritis, early GC and advanced GC tissues by immunohistochemistry. The expression level of HIF1α appeared to increase in early GC, but there was no significant difference compared with superficial gastritis. However, the expression level increased significantly in advanced GC compared with superficial gastritis and early GC tissues (Figure 1A). The expression level of PKM2 increased significantly in early and advanced GC compared with superficial gastritis tissue (Figure 1B).
Figure 1.
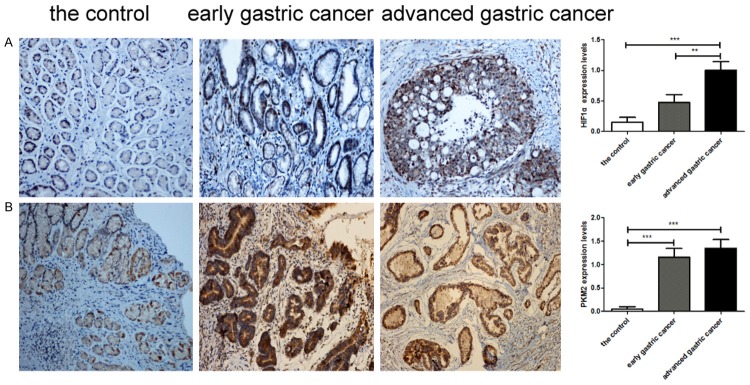
Immunohistochemical analysis of HIF1α and PKM2 expression in superficial gastritis, early gastric cancer, and advanced gastric cancer tissues. A. Representative images of HIF1α staining. B. Representative images of PKM2 staining. Original magnification 200×. **P<0.01, ***P<0.001.
Metformin decreases GC cell viability
We evaluated the effect of metformin on the viability of two GC cell lines: SGC7901 and BGC823. CCK-8 assay showed significant dose- and time-dependent decrease in the viability of SGC7901 and BGC823 cells after metformin treatment (Figure 2).
Figure 2.
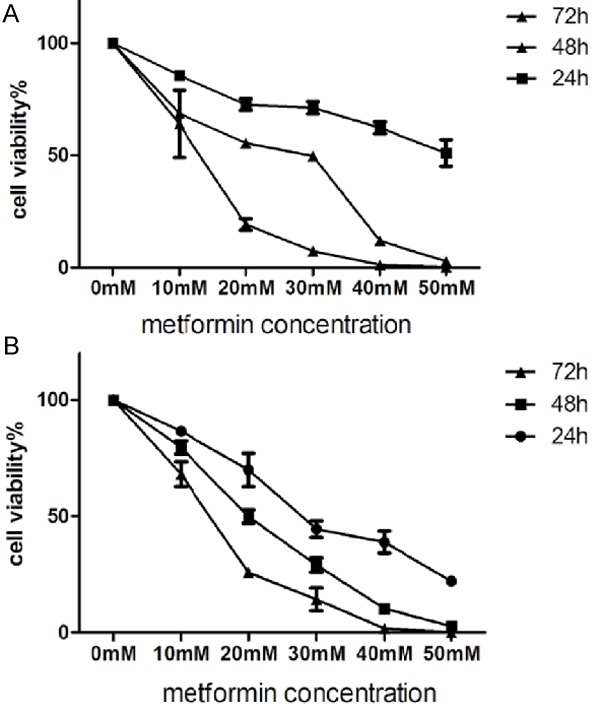
Metformin inhibits the viability of gastric cancer cells. SGC7901 cells (A) and BGC823cells (B) were treated with metformin (0-50 mM) for 24, 48, 72 h. Cell viability was evaluated by CCK-8 assy.
Metformin induces apoptosis and cell cycle arrest in GC cells
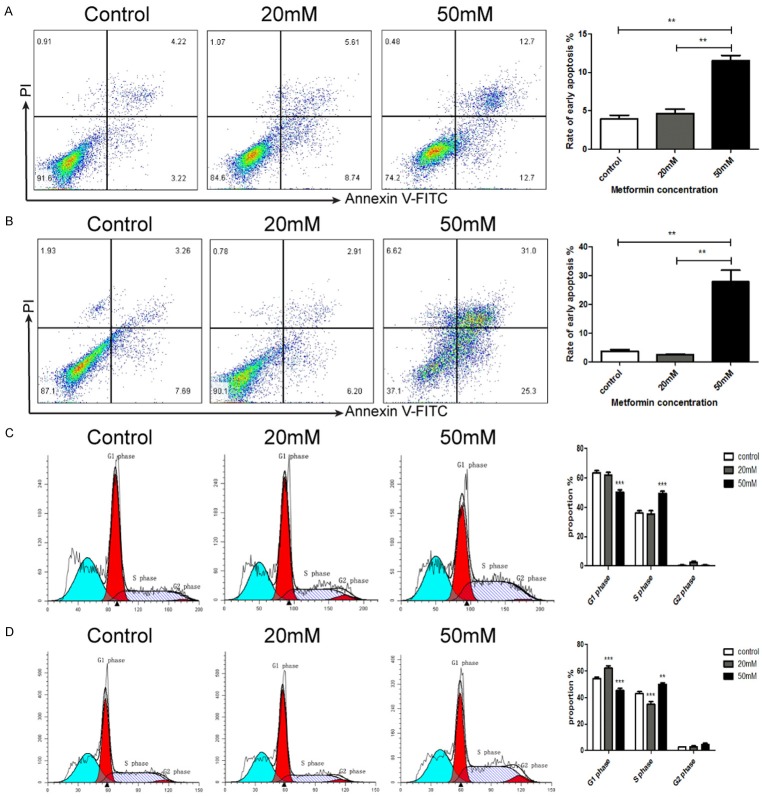
To elucidate the mechanism by which metformin decreases the viability of GC cells, we wondered whether metformin could induce apoptosis and cell cycle arrest in GC cells. By Annexin V-FITC and PI staining, we observed that metformin increased the proportion of apoptotic cells in SGC7901 and BGC823 cells in a dose dependent manner (Figure 3A, 3B). In addition, by flow cytometry analysis, we found that metformin induced cell cycle arrest in SGC7901 and BGC823 cells in a dose dependent manner (Figure 3C, 3D).
Figure 3.
Metformin induces apoptosis and cell cycle arrest in gastric cancer cells. SGC7901 cells (A, C) and BGC823 cells (B, D) were treated with metformin (0, 20, 50 mM) for 24 h. The proportions of early apoptosis cells (A, B) and cells in different phases of cell cycle (C, D) were assessed by flow cytometry. **P<0.01, ***P<0.001.
Metformin reduces HIF1α, PARP, COX and PKM2 expression in GC cells
Next we evaluated the effects of metformin on PI3k, Akt, HIF1α, PARP, COX, and PKM2 protein expression in GC cells. Immunofluorescence staining of HIF1α and COX in SGC7901 cells showed significant decrease in HIF1α and COX expression after metformin treatment (Figure 4A, 4B). Western blot analysis showed that metformin inhibited the expression level of PI3K, Akt, HIF1α, PARP, COX and PKM2 in SGC7901 and BGC823 cells (Figure 4C).
Figure 4.
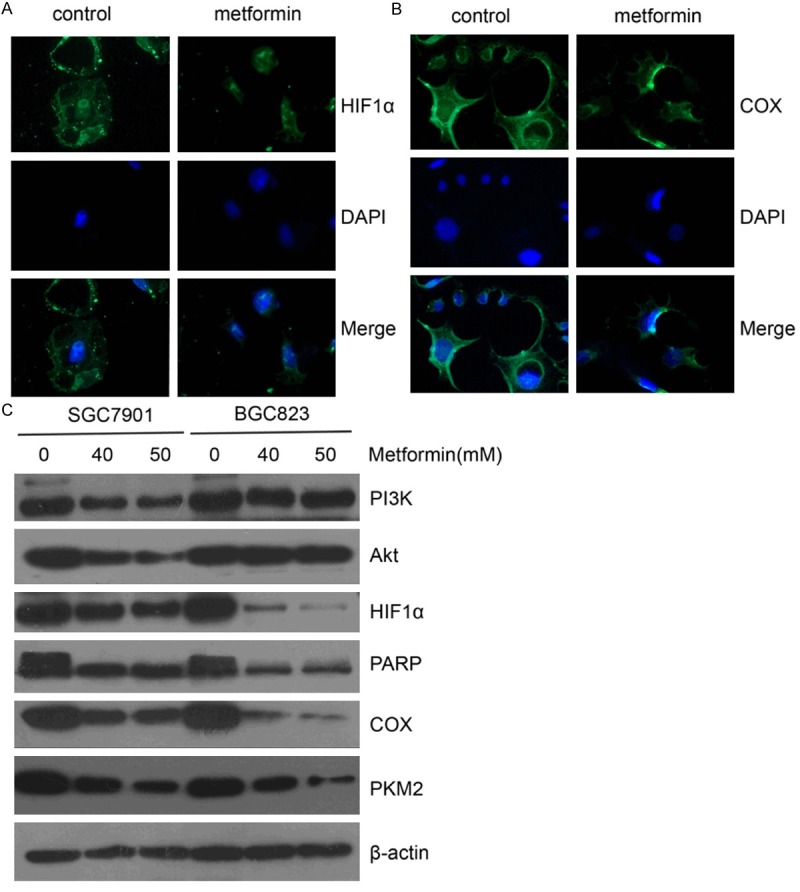
Metformin reduces HIF1α, PARP and COX protein expression in gastric cancer cells. (A, B) SGC7901 cells were treated with metformin (0, 40 mM) for 24 h and then analyzed for the expression of HIF1α (A) and COX (B) by immunofluorescence. Original magnification 400×. (C) SGC7901 and BGC823 cells were treated with metformin (0, 40, 50 mM) for 24 h and then protein expression of PI3K, Akt, HIF1α, PARP, COX, PKM2 was detected by Western blot analysis. β-actin was loading control.
Metformin inhibits the invasion and migration of GC cells
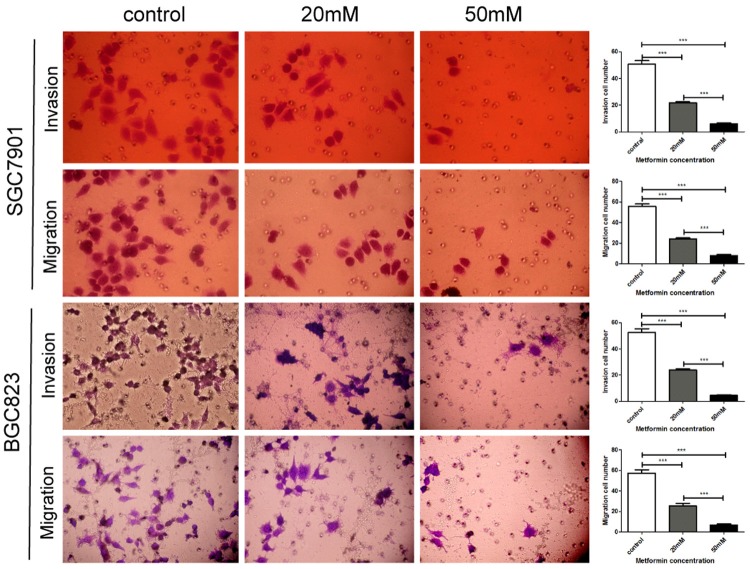
To investigate the activity of metformin against tumor metastasis, we examined the effects of metformin on the invasion and migration of GC cells. Transwell assay showed that metformin inhibited the invasion and migration of SGC7901 and BGC823 cells in a dose dependent manner (Figure 5).
Figure 5.
Metformin inhibits the invasion and migration of gastric cancer cells. SGC7901 and BGC823 cells were seeded on transwell for invasion and migration analysis. The numbers of invaded and migrated cells were counted in five separate fields using light microscopy. Original magnification 400×. The data were expressed as the mean value of cells in five fields based on three independent experiments. ***P<0.001.
HIF1α over-expression increases the viability, invasion and migration of GC cells
Since metformin inhibited protein expression of HIF1α in GC cells, we wondered whether HIF1α might be the key factor to mediate the effects of metformin on GC cells. We transfected HIF1α over-expression plasmid into BGC823 cells, and confirmed the expression of HIF1α by RT-PCR and Western blot analysis (Figure 6A, 6C). We found that HIF1 overexpression increased the viability, invasion and migration of BGC823 cells (Figure 6B, 6D).
Figure 6.
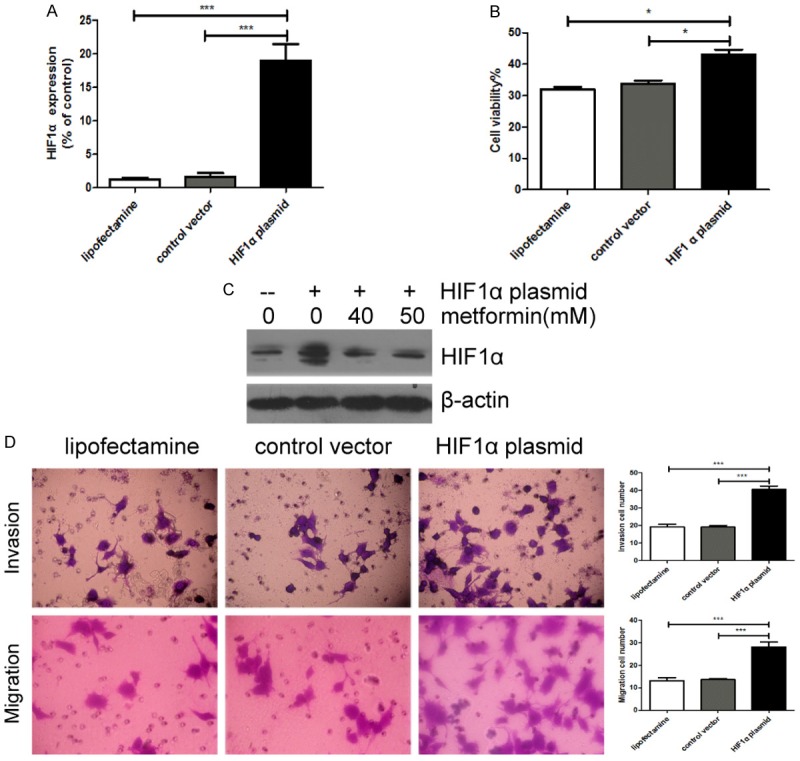
HIF1α over-expression increases the viability, invasion and migration of BGC823 cells. A, C. Lipofectamine, control vector or HIF1α overexpression plasmid were transfected into BGC823 cells, and HIF1α mRNA and protein expression were detected by RT-PCR and Western blot analysis. β-actin was loading control. B. Cell viability was detected by CCK-8 assay. D. The numbers of invaded and migrated cells were counted in five separate fields using light microscopy. Original magnification 400×. The data were expressed as the mean value of cells in five fields based on three independent experiments. *P<0.05, ***P<0.001.
Discussion
In this study, we revealed the high expression of HIF1α and PKM2 in GC tissues, and found that metformin significantly induced apoptosis, inhibited cell invasion and migration of GC cells. The mechanism by which metformin exhibits anti-tumor activities is through the induction of apoptosis and the inhibition of HIF1α.
Recent studies showed that overexpression of HIF1α are implicated in tumorigenesis, tumor chemotherapy resistance, tumor angiogenesis, and tumor glycolysis [17-20]. Increased HIF-1α level is associated with increased risk of mortality in many human cancers, including gastric cancer [11]. HIF1α inhibitor inhibited tumor growth and angiogenesis [12]. In this study we found that metformin inhibited the expression of HIF1α in GC cells, suggesting that metformin may inhibit tumor cell growth and metastasis via HIF1α inhibition. However, further studies are needed to confirm our conclusion.
Targeting of tumor metabolism is emerging as a novel therapeutic strategy against cancer [21]. According to the “Warburg effect”, tumor cells exhibit an increased dependence on glycolytic pathway for ATP generation both in normoxia or hypoxia conditions [22]. PKM2 is an important executor downstream of HIF1α signaling and acts as the key enzyme of glycolysis [23]. In this study we found high expression of PKM2 in GC tissues by immunohistochemistry, indicating the important role of glycolysis in the development of GC. Our study showed that metformin inhibited the expression of PKM2 protein, especially in poorly differentiated BGC823 cells. These data suggest that metformin reduces the energy supply of GC by inhibiting HIF1α/PKM2 pathway.
The most important function of mitochondrial respiratory chain is to generate ATP by oxidation phosphorylation (OXPHOS). After sequential electron transfer, two respiratory chains generate ATP through being catalyzed by the respiratory chain enzyme complexes IV- cytochrome C oxidase (COX). In energy-rich conditions, the mitochondria of tumor cells maintain “well-being” state and effectively shut off apoptotic machinery, resulting in the protection against cell death, even when challenged with toxic drugs. Conversely, when the mitochondria of tumor cells are in the condition of “stress”, they induce the apoptosis of tumor cells [24]. One study showed recently that metformin inhibited mitochondrial complex I of cancer cells to reduce tumorigenesis [25]. In this study we found that metformin inhibited the expression level of COX in SGC7901 and BGC823 cells.
Poly(ADP)-ribose polymerase (PARP) plays a crucial role in DNA repair and the maintenance of genome stability. The proteolytic degradation of PARP is caused by a variety of stimuli [26]. In the present study, the expression of PARP was decreased significantly in GC cells treated with metformin. At the same time, cell apoptosis ratio increased remarkably.
In order to confirm that HIF1α mediates the effects of metformin on GC cell proliferation, apoptosis, invasion, and migration, we transfected HIF1α overexpression plasmid into BGC823 cells; and found that cell viability, invasion and migration were obviously enhanced in the cells transfected with HIF1α plasmid. These data indicate that metformin inhibits GC cell proliferation, invasion and metastasis by inhibiting the expression of HIF1α.
To the best of our knowledge, this is the first report demonstrating HIF1α/PKM2 signal pathway as a target of metformin in GC cells. Metformin exhibit potent effects to inhibit malignant behaviors of GC cells through decreasing the expression of HIF1α and PKM2. However, how metformin inhibits HIF1α/PKM2 signal pathway is not clear and needs further exploration.
In conclusion, our study provides evidence that metformin inhibits GC growth and metastasis. The main mechanism responsible for the anti-tumor effects of metformin might be inducing intrinsic apoptosis and tumor glucose metabolism via the inhibition of HIF1α. These findings suggest that metformin is a promising therapeutic agent for GC.
Acknowledgements
This study was supported by The National Natural Science Foundation of China (No. 81101814, 81272742, 81472756), Jiangsu Provincial Commission of Health and Family Planning (No. Q201413), Medical Youth Talent Reserve of Xuzhou, Xuzhou Science and Technology Plan (No. KC14SH007).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Macintyre AN, Rathmell JC. Activated lymphocytes as a metabolic model for carcinogenesis. Cancer Metab. 2013;1:5. doi: 10.1186/2049-3002-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar A, Kant S, Singh SM. Antitumor and chemosensitizing action of dichloroacetate implicates modulation of tumor microenvironment: a role of reorganized glucose metabolism, cell survival regulation and macrophage differentiation. Toxicol Appl Pharmacol. 2013;273:196–208. doi: 10.1016/j.taap.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Tavares-Valente D, Baltazar F, Moreira R, Queirós O. Cancer cell bioenergetics and pH regulation influence breast cancer cell resistance to paclitaxel and doxorubicin. J Bioenerg Biomembr. 2013;45:467–475. doi: 10.1007/s10863-013-9519-7. [DOI] [PubMed] [Google Scholar]
- 6.Brauer HA, Makowski L, Hoadley KA, Casbas-Hernandez P, Lang LJ, Romàn-Pèrez E, D’Arcy M, Freemerman AJ, Perou CM, Troester MA. Impact of tumor microenvironment and epithelial phenotypes on metabolism in breast cancer. Clin Cancer Res. 2013;19:571–585. doi: 10.1158/1078-0432.CCR-12-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carito V, Bonuccelli G, Martinez-Outschoorn UE, Whitaker-Menezes D, Caroleo MC, Cione E, Howell A, Pestell RG, Lisanti MP, Sotgia F. Metabolic remodeling of the tumor microenvironment: migration stimulating factor (MSF) reprograms myofibroblasts toward lactate production, fueling anabolic tumor growth. Cell Cycle. 2012;11:3403–3414. doi: 10.4161/cc.21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar V, Gabrilovich DI. Hypoxia inducible factors in regulation of immune responses in tumor microenvironment. Immunology. 2014;143:512–519. doi: 10.1111/imm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohashi T, Akazawa T, Aoki M, Kuze B, Mizuta K, Ito Y, Inoue N. Dichloroacetate improves immune dysfunction caused by tumor-secreted lactic acid and increases antitumor immunoreactivity. Int J Cancer. 2013;133:1107–1118. doi: 10.1002/ijc.28114. [DOI] [PubMed] [Google Scholar]
- 10.Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012;18:5585–5594. doi: 10.1158/1078-0432.CCR-12-0858. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. HIF1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–3671. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu GT, Bu LL, Zhao YY, Liu B, Zhang WF, Zhao YF, Zhang L, Sun ZJ. Inhibition of mTOR reduce Stat3 and PAI related angiogenesis in salivary gland adenoid cystic carcinoma. Am J Cancer Res. 2014;4:764–775. [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Jia X, Duan Y, Xiao H, Sundqvist KG, Permert J, Wang F. Excess glucose induces hypoxia-inducible factor-1α in pancreatic cancer cells and stimulates glucose metabolism and cell migration. Cancer Biol Ther. 2013;14:428–435. doi: 10.4161/cbt.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McFarland MS, Cripps R. Diabetes mellitus and increased risk of cancer: focus on metformin and the insulin analogs. Pharmacotherapy. 2010;30:1159–1178. doi: 10.1592/phco.30.11.1159. [DOI] [PubMed] [Google Scholar]
- 15.Tseng CH. Metformin reduces thyroid cancer risk in taiwanese patients with type 2 diabetes. PLoS One. 2014;9:e109852. doi: 10.1371/journal.pone.0109852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, Nicolucci A. Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One. 2013;2:e71583. doi: 10.1371/journal.pone.0071583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goscinski MA, Nesland JM, Giercksky KE, Dhakal HP. Primary tumor vascularity in esophagus cancer - CD34 and HIF1-α expression correlate with tumor progression. Histol Histopathol. 2013;28:1361–1368. doi: 10.14670/HH-28.1361. [DOI] [PubMed] [Google Scholar]
- 18.Tong Y, Li QG, Xing TY, Zhang M, Zhang JJ, Xia Q. HIF1 regulates WSB-1 expression to promote hypoxia-induced chemoresistance in hepatocellular carcinoma cells. FEBS Lett. 2013;587:2530–2535. doi: 10.1016/j.febslet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Li Z, Bai S, Zhang H, Tang M, Lei Y, Chen L, Liang S, Zhao YL, Wei Y, Huang C. Mechanism of cancer cell adaptation to metabolic stress: proteomics identification of a novel thyroid hormone-mediated gastric carcinogenic signaling pathway. Mol Cell Proteomics. 2009;8:70–85. doi: 10.1074/mcp.M800195-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. HIF1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar A, Kant S, Singh SM. Antitumor and chemosensitizing action of dichloroacetate implicates modulation of tumor microenvironment: a role of reorganized glucose metabolism, cell survival regulation and macrophage differentiation. Toxicol Appl Pharmacol. 2013;273:196–208. doi: 10.1016/j.taap.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 23.Chaneton B, Gottlieb E. Rocki ng cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci. 2012;37:309–316. doi: 10.1016/j.tibs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Martinez-Outschoorn UE, Pestell RG, Howell A, Tykocinski ML, Nagajyothi F, Machado FS, Tanowitz HB, Sotgia F, Lisanti MP. Energy transfer in “parasitic” cancer metabolism: Mitochondria are the powerhouse and Achilles’ heel of tumor cells. Cell Cycle. 2011;10:4208–4216. doi: 10.4161/cc.10.24.18487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, Chandel NS. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim MY, Hashim NM, Mohan S, Abdulla MA, Kamalidehghan B, Ghaderian M, Dehghan F, Ali LZ, Arbab IA, Yahayu M, Lian GE, Ahmadipour F, Ali HM. α-Mangostin from Cratoxylum arborescens demonstrates apoptogenesis in MCF-7 with regulation of NF-κB and Hsp70 protein modulation in vitro, and tumor reduction in vivo. Drug Des Devel Ther. 2014;8:1629–1647. doi: 10.2147/DDDT.S66105. [DOI] [PMC free article] [PubMed] [Google Scholar]