Abstract
miR-30c has been reported to act as a tumor suppressor and negatively regulate cancer metastasis by directly targeting metastasis associated genes; however, miR-30c has also been shown to promote the invasion of metastatic breast cancer cells, suggesting that miR-30c might be involved in cancer cell metastasis in different ways via targeting different genes. In this study, we demonstrated that over-expression and knockdown of immediate early response protein 2 (IER2) modulated the general capacity of the migration and invasion in hepatocellular carcinoma cell line SMMC-7721 and HepG2, whereas overexpression and knockdown of miR-30c decreased and promoted cell motility, respectively. Further studies revealed that miR-30c overexpression down-regulated the expression of IER2 protein but not its mRNA level, and miR-30c can directly target the 3’ untranslated region (3’UTR) of IER2, and subsequently reducing its expression. Moreover, we also showed that suppression of cell motility by miR-30c was partially rescued by IER2 re-expression. Our results indicated that miR-30c may function as a negative regulator in cell motility, with IER2 as a direct and functional target in SMMC-7721 and HepG2 cells.
Keywords: miR-30c, IER2, migration, invasion
Introduction
Human IER2 (also known as ETR101 or Chx1), initially identified and cloned from the HL-60 cells [1], is a member of the immediate early responsible family and can be rapidly or transiently up-regulated by extracellular stimuli, such as growth factors, cytokines, 12-O-tetradecanoylphorbol-13-acetate or Okadaic acid and some pathogens infection [1-7]. Currently, human IER2 has been characterized as a transcription regulator which acts as a fibroblast growth factor intracellular binding protein 1-interacting partner [8], and as a transcription factor or transcriptional co-activator for the human myo-inositol 1-phosphate synthase gene involved in the regulation of cellular responses [5,8]. Furthermore, data from another study indicated that IER2 is involved in the regulation of tumor progression and metastasis [9], although the precise role and signaling mechanism involved remains elusive.
MicroRNAs (miRNAs) are a class of small non-coding RNAs that regulate gene expression via binding to the 3’UTRs of the target genes, resulting in either translational repression or transcript degradation [10-14]. Although the role of miRNAs as oncogenes or/and tumor suppressors has been extensively studied [15-17], the role of miRNAs in tumorigenesis and their effective targets remain largely undetermined. Accumulating evidences suggested that miR-30 family members have been implicated in tumor suppression [18-25]. For example, miR-30c may act as a tumor suppressor and negatively regulate cancer metastasis by directly targeting metastasis associated genes [23-25,27-29]. However, a recent study demonstrated that miR-30c promoted the invasive phenotype of metastatic breast cancer cells [30], suggesting that miR-30c might be involved in cancer cell metastasis in different ways by targeting different genes and regulate key steps in the metastatic process of cancers.
Here, we found that miR-30c targeted IER2 for translational repression via a target site in its 3’UTR. Overexpression of miR-30c decreased cell migration and invasion, whereas knockdown of miR-30c promoted cell motility in SMMC-7721 and HepG2 cells. Furthermore, suppression of cell motility by miR-30c was partially abrogated by IER2 re-expression. Our results indicated that miR-30c may function as a negative regulator in cell motility via directly and functionally targeting IER2 in SMMC-7721 and HepG2 cells.
Materials and methods
Cell lines and culture conditions
The human hepatocellular carcinoma cell line HepG2, SMMC-7721, and the human embryonic kidney (HEK) 293T cells, obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China), were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% (vol/vol) heat-inactivated fetal bovine serum (FBS), and 1% penicillin-streptomycin. All of the cells were grown in a humidified incubator at 37°C with 5% CO2.
RNA extraction and real-time reverse transcription quantitative PCR (RT-qPCR)
Total RNA was extracted using the RNA Isolator Total RNA Extraction Reagent (VAZYME, Nanjing, China) according to the supplier’s instruction. The levels of miR-30c were determined by RT-qPCR using the SYBR®PrimeScriptTM miRNA RT-PCR Kit (Takara) following the manufacturer’s protocol and performed in an ABI 7500 real-time PCR system (Applied Biosystem, USA), and the levels of IER2 mRNA were also examined by RT-qPCR using the AceQ®qPCR SYBR® Green Master Mix kit (VAZYME) after the RNAs were reverse transcribed by using the HiScript 1st Strand cDNA Synthesis Kit (VAZYME). The relative expression of miR-30c and IER2 mRNA was calculated using the 2-ΔΔCT method, and normalized to the expression of internal miRNA control U6 snRNA and a housekeeping control gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively. Primers used for running qPCR were as follows: IER2 primers I, forward, 5’-TGGTGAAACTGGGCCAATCT-3’, and reverse, 5’-AAGAATCCACCGCACGAAAG-3’ (which spanned the 3’UTR of the IER2 gene and only recognized the endogenous IER2 mRNA), IER2 primers II, forward, 5’-CCAAAGTCAGCCGCAAACGA-3’, and reverse, 5’-TTTCTTCCAGACGGGCTTTCTTGC-3’ (which spanned the coding region and recognized both endogenous and exogenous IER2 mRNA), and GAPDH primers, forward, 5’-GCACCGTCAAGGCTGAGAAC-3’, and reverse, 5’-TGGTGAAGACGCCAGTGGA-3’, miR-30c forward primer, 5’-CAGTGTAAACATCCTACA CTC-3’, U6 primers, forward, 5’-CTCGCTTCGGCAGCACA-3’, and reverse, 5’-AA CGCTTCACGAATT TGCGT-3’.
Plasmid construction and oligonucleotides transfection
miR-30c expression vector was constructed by cloning the fragment, amplified from human genomic DNA by PCR with the primers 5’-AATCTCGAGCACCCACCCTTCCAACCCTA-3’ (forward) and 5’-CCGGAATTCATCAGACTGCAGCAACCCAC-3’ (reverse), into the XhoI and EcoRI sites of the pMSCV-PIG (Puro IRES GFP empty vector), a kind gift from Professor Yu (School of medicine, Yangzhou University, China). SMMC-7721 or HepG2 cells (1×105) were plated in the six-well culture plates, and then transfected with pMSCV-PIG as a negative control or pMSCV-PIG-miR-30c using lipofectamine 3000 (Invitrogen) following the manufacturer’s protocol. Transfected cells were selected by using puromycin (Santa Cruz) at the concentration of 0.5 μg/ml for SMMC-7721 cells and 1.0 μg/ml for HepG2 cells, and cell populations were selected for two weeks for following experiments, and the stably expressed cells were designated as miR-30c expressing cells or NC cells. Antisense oligonucleotides for miR-30c (referred to anti-miR-30c) or anti-miRNA negative control (ANC) purchased from Genepharma (Shanghai, China), were transfected into the cells using lipofectamine 3000 at a final concentration of 50 nM, and the cells were harvested 48 h after transfection. The vectors expressing IER2 (pEZ-Lv105-IER2) and null (pEZ-Lv105-null) were purchased from GeneCopoeia (USA). For IER2 rescue experiments, 2 µg pEZ-Lv105-null (null) or pEZ-Lv105-IER2 (IER2) was transfected into the miR-30c expressing cells using lipofectamine 3000, and the cells were harvested 72 h after transfection for transwell assays. The fragment of 3’ UTR of the IER2 with predicted miR-30c target binding sequence was amplified by PCR from human genomic DNA using primers 5’-CCGCTCGAGGTAGGTTCCCAGGTTCCAGC-3’ (forward) and 5’-ATAAGAATGCGGCCGCAAACGCCAGGTAGACGGAG-3’ (reverse), and subsequently cloned into the XhoI and NotI sites of the downstream of the Renilla luciferase reporter gene in psiCHECK-2 vector (Promega), and the construct was designated as psiCHECK-2-IER2-wt. The mutant construct of psiCHECK-2 -IER2-mut in the putative miR-30c binding site was also generated using psiCHECK-2-IER2-wt as the template with primers 5’-TCTGAGGGTCTGCTTATGTCCGCTTTCGTGCGGTGGAT-3’ (forward) and 5’-ATCCACCGCACGAAAGCGGACATAAGCAGACCCTCAGA-3’ (reverse) by using the QuikChange Site- Directed Mutagenesis Kit (Stratagene, USA) according to the manufacturer’s protocol. All of the constructs were confirmed by DNA sequencing.
Lentiviral production and infection
Packaging of the recombinant lentiviruses encoding IER2 (LV-IER2) or null (LV-CTL) were generated in HEK293T cells by co-transfecting the pEZ-Lv105-IER2 or pEZ-Lv105-null with the Lenti-Pac™ HIV Expression Packaging Kit obtained from GeneCopoeia according to the supplier’s instructions. Lentiviruses expressing shRNA specific targeting IER2 (LV-shR) and the non-silencing control shRNA (LV-shC) were obtained from GeneChem Corporation (Shanghai, China). For lentiviral infection, cells were plated at a concentration of 1×105 cells in the six-well culture plates, and then infected with indicated lentiviruses at a MOI of 30 for SMMC-7721 cells and 20 for HepG2 cells in the presence of 8 μg/ml polybrene, respectively. After infection for 72 h, puromycin was added to the media at the concentration of 0.5 μg/ml for SMMC-7721 cells and 1.0 μg/ml for HepG2 cells, and cell populations were selected for two weeks for following experiments.
Dual-luciferase reporter assay
For luciferase reporter detection, the luciferase reporter constructs (100 ng) were transiently transfected into the miR-30c expressing cells or NC cells in 24-well plates using lipofectamine 3000. After incubation for 48 h, luciferase activities were measured using the dual-luciferase reporter assay system (Promega). The Renilla luciferase activities were normalized to firefly luciferase activities.
Transwell migration and invasion assays
For transwell migration assay, 3×104 cells starved off serum overnight were prepared in serum-free DMEM and seeded into the 24-well transwell upper chambers (8.0 μm pore size; Costar, USA), and then were inserted into the lower wells containing DMEM with 10% FBS. After incubation for 24 h, the cells remaining on the upper surface of the chamber membrane were removed, and the cells that had migrated to the bottom of the membrane were fixed with methanol and stained with crystal violet. Cell invasion assay was also performed in the transwell chambers in the same manner as the migration assay with the minor modification that the upper chambers were pre-coated with 100 μg/ml Matrigel (BD Biosciences), and 5×104 cells suspended in serum-free DMEM were added to the upper chamber. The migrated and invaded cells in at least five randomly selected fields at 200× magnification were quantified, and images were captured using a phase contrast microscopy equipped with a digital image capturing system.
Western blot analysis
Cells were lysed with a total protein extraction kit including protease inhibitor mix (VAZYME). Protein concentrations were determined using Bradford Protein Quantification Kit (Thermo Fisher Scientific, USA). The samples were resolved by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene fluoride membranes (Millipore, USA), and blocked in 5% nonfat dried milk in PBS-Tween 20. Then, the membranes were incubated at 4°C overnight with mouse polyclonal anti-IER2 (1:1000, Abcam), or mouse monoclonal anti-GAPDH (1:1000, KangChen Bio-tech, Shanghai, China) antibodies, followed by incubation for 2 h at room temperature with horseradish peroxidase-conjugated goat anti-mouse IgG (1:2000, Cell Signaling Technology, USA), and visualized using the Pierce ECL Plus Western Blotting substrate (Thermo Fisher Scientific).
Statistical analysis
Data are expressed as means ± standard deviation (SD). Statistical significance was determined by the Student’s t-test, and was defined as p<0.05.
Results
Expression of miR-30c and IER2 in SMMC-7721 and HepG2 cells
The expression of miR-30c and IER2 in SMMC-7721 and HepG2 cells were initially examined. The levels of miR-30c and IER2 mRNA from total RNA extracts were quantified using RT-qPCR, normalized to U6 and GAPDH, respectively, and the IER2 protein levels were determined by western blot analysis. As shown in Figure 1, markedly up-regulation of miR-30c was shown in miR-30c expressing SMMC-7721 and HepG2 cells as compared to that in indicated NC cells (Figure 1A), whereas anti-miR-30c transfection significantly down-regulated miR-30c as compared to ANC transfection (Figure 1B). Meanwhile, LV-IER2 transduction caused significant up-regulation of total IER2 mRNA (run qPCR by IER2 primers II), but no significant alteration to the endogenous IER2 mRNA (run qPCR by IER2 primers I) in SMMC-7721 cells (Figure 1C) as compared to that in empty vector-transduced (LV-CTL) and the un-transduced mock cells, indicating that LV-IER2 infection did not affect the expression of endogenous IER2, whereas knock-down of IER2 expression by LV-shR transduction was achieved at the endogenous IER2 mRNA levels (Figure 1D). Furthermore, western blot analysis demonstrated that over-expression and knock-down of IER2 protein were shown in LV-IER2- and LV-shR-transduced SMMC-7721 cells, respectively, as compared to that in LV-CTL- or LV-shC-transduced and the mock cells (Figure 1E-H). Similar results were also observed in HepG2 cells (data not shown). These results demonstrated that the indicated expressing vectors, oligonucleotides or lentiviruses were successfully transfected or transduced into SMMC-7721 and HepG2 cells.
Figure 1.
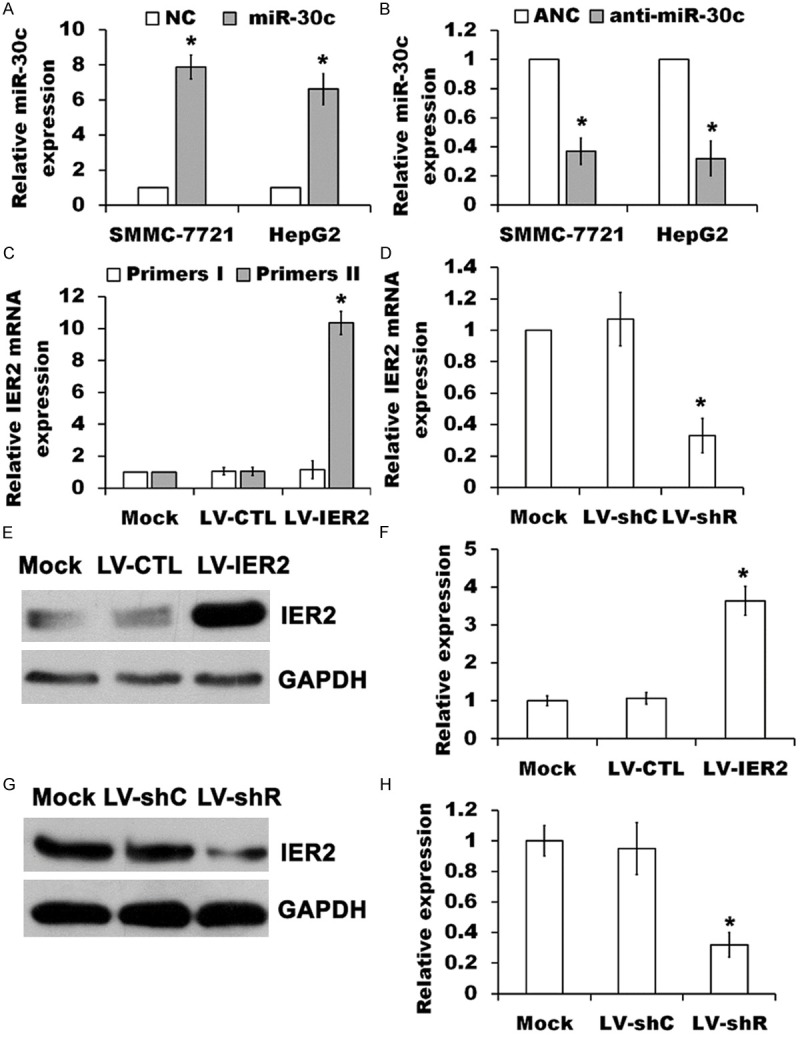
Expression of miR-30c and IER2 in SMMC-7721 and HepG2 cells. (A and B) miR-30c levels were measured by RT-qPCR in RNA extracts from the stably over-expressed miR-30c SMMC-7721 and HepG2 cells (miR-30c) relative to negative control (NC), or from the cells transfected with anti-miR-30c relative to transfection with anti-miRNA negative control (ANC), normalized to U6 RNA. The values represent the mean ± SD (n=5). *p<0.05, versus the NC (A) or ANC (B). (C) Levels of endogenous and total IER2 mRNA were quantified from the indicated lentivirus-transduced SMMC-7721 cells using primers I and primers II to run RT-qPCR, respectively, and normalized to GAPDH. The values represent the mean ± SD (n=5). *p<0.05, versus the mock. (D) Levels of endogenous IER2 mRNA were measured from the indicated lentivirus-transduced SMMC-7721 cells using RT-qPCR, normalized to GAPDH. The values represent the mean ± SD (n=5). *p<0.05, versus the mock. (E-H) Over-expression (E and F) and knock-down (G and H) of IER2 in SMMC-7721 cells were examined by SDS-PAGE immunoblotting with antibodies to IER2 and GAPDH, normalized to GAPDH. The values represented the mean ± SD (n=3). *p<0.05, versus the mock.
Effect of IER2 on the migration and invasion of SMMC-7721 and HepG2 cells
Since IER2 has been reported to regulate the tumor progression and metastasis [9], we initially performed the transwell assays to evaluate if IER2 affects the migration and invasion of SMMC-7721 and HepG2 cells. As shown in Figure 2, LV-IER2 transduction significantly promoted the migration and invasion of both SMMC-7721 and HepG2 cells, whereas knock-down of IER2 by LV-shR transduction obviously reduced the number of the motile cells in comparison with empty vectors-transduced cells and the mock. No significant differences in the cell migration and invasion were shown among the mock and the empty vectors-transduced cells, suggesting that depletion and over-expression of IER2 modulated the general capacity of the cell migration and invasion in SMMC-7721 and HepG2 cells, and the endogenous IER2 expression might be required for the cell motility.
Figure 2.
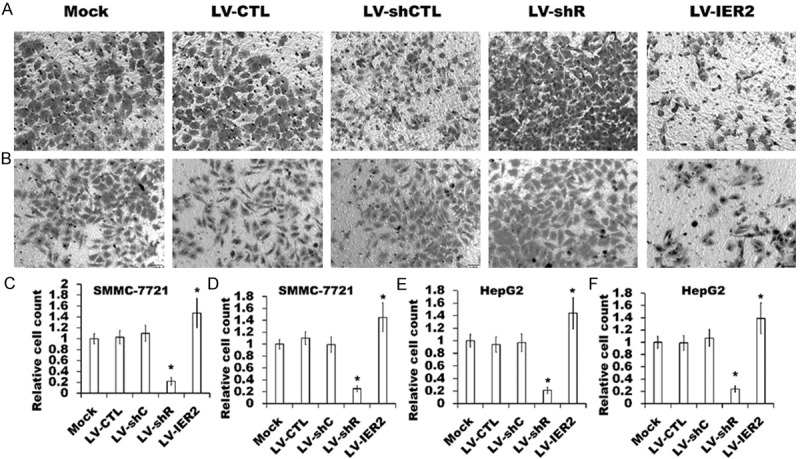
Effect of IER2 on cell migration and invasion. (A and B) Representative transwell migration assay (A) and invasion assay (B) in the indicated lentivirus-transduced SMMC-7721 cells, and similar results were obtained in three independent experiments. (C-F) Quantification of the migration and invasion of the lentivirus-transduced SMMC-7721 cells (C and D) and HepG2 cells (E and F) were shown. Assays were performed in triplicate for each experiment, and repeated three times. The values represented the mean ± SD. *p<0.05, versus the mock.
Effect of miR-30c on the migration and invasion of SMMC-7721 and HepG2 cells
Next, we also performed the transwell assays to investigate whether miR-30c influences the migration and invasion of SMMC-7721 and HepG2 cells. As shown in Figure 3, over-expression of miR-30c obviously reduced the migration and the invasive capacity of the cells of SMMC-7721 and HepG2 cells in transwell migration and invasion assays (Figure 3A, 3B and 3D) as compared to those in NC cells, which was in agreement with that LV-shR infection inhibited cell migration and invasion as mentioned above (Figure 2). Furthermore, knock-down of miR-30c by transfection with anti-miR-30c increased the cell migration and invasion as compared to ANC transfection (Figure 3A, 3C and 3E). These results demonstrated that miR-30c may suppress the migration and invasion of SMMC-7721 and HepG2 cells.
Figure 3.
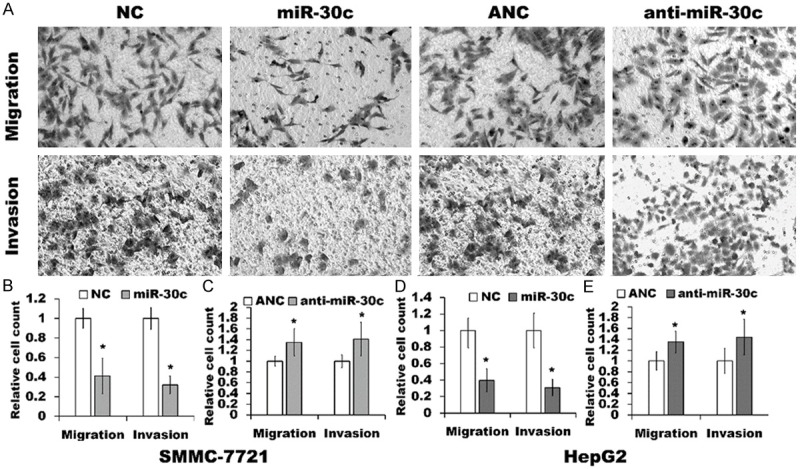
Effect of miR-30c on cell migration and invasion. (A) Representative transwell migration assay and invasion assay in the miR-30c expressing SMMC-7721 cells, NC cells, anti-miR-30c transfected cells and ANC cells, and the similar results were obtained in three independent experiments. (B-E) Quantification of the migration and invasion of SMMC-7721 cells (B and C) and HepG2 cells (D and E) were shown. Assays were performed in triplicate for each experiment, and repeated three times. The values represented the mean ± SD. *p<0.05, relative to NC (B and D) or ANC transfected cells (C and E).
miR-30c regulated IER2 expression
To determine whether IER2 is a potential target of miR-30c, six major miRNA-target prediction algorithms, including TargetScan (http://www.targetscan.org/), miRanda (www.microrna.org), PicTar (http://pictar.mdc-berlin.de/), EIMMO (http://www.mirz. unibas.ch/ElMMo2/), DIANA-microT (http://diana.cslab.ece.ntua.gr/microT/), and MicroCosm 5 (http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/), were used for the identification of putative miRNA target sites on the IER2 3’UTR. We found that IER2 was predicted to be the target of miR-30c by all of the six programs and thus need further experimental validation.
It is generally accepted that miRNAs regulate gene expression by targeting the 3’UTRs of the genes for either translational repression or transcript degradation [10-12]. To test whether IER2 could be regulated by miR-30c, we performed RT-qPCR and western blot analysis. The results from RT-qPCR demonstrated that the levels of IER2 mRNA did not change either in miR-30c expressing SMMC-7721 and HepG2 cells or anti-miR-30c-transfected cells as compared to that in indicated NC and ANC transfected cells (Figure 4A and 4B). Importantly, western blot analysis revealed that miR-30c over-expression markedly down-regulated the IER2 in SMMC-7721 cells as compared to that in NC cells, whereas IER2 protein level increased in anti-miR-30c-transfected cells as compared to that in ANC transfected cells (Figure 4C-E), and similar results were also observed in HepG2 cells (data not shown). These data suggested that IER2 gene expression is translationally suppressed by miR-30c in SMMC-7721 and HepG2 cells.
Figure 4.
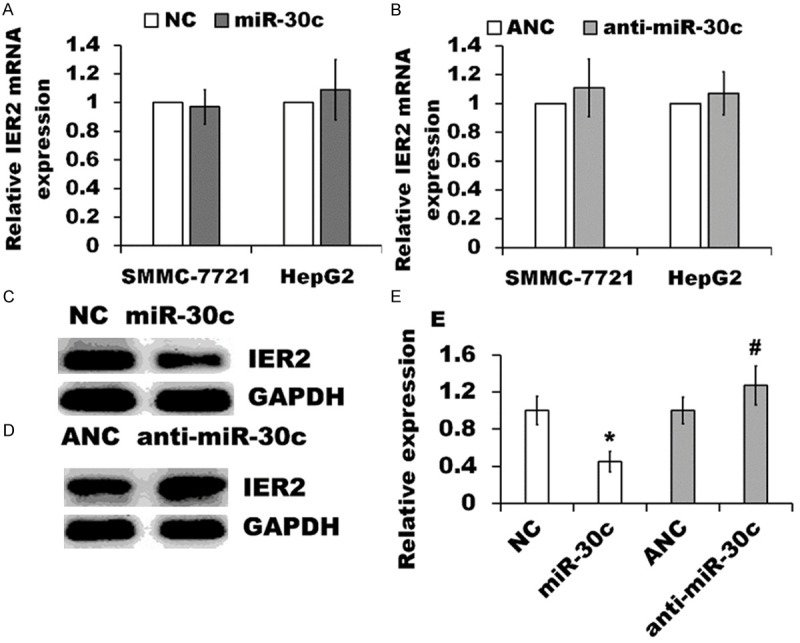
miR-30c regulated IER2 expression. (A and B) Levels of IER2 mRNA were quantified by RT-qPCR from the miR-30c expressing SMMC-7721 and HepG2 cells relative to indicated NC cells (A), or from the cells transfected with anti-miR-30c relative to transfection with ANC (B), normalized to GAPDH. The values represented the mean ± SD (n=5). (C and D) Western blot analysis of IER2 protein in miR-30c expressing SMMC-7721 cells and NC cells (C), or in anti-miR-30c transfected cells and ANC (D). Similar results were obtained from three independent experiments. (E) Quantification of the band intensity from the western blot analysis was performed with ImageJ software, and expressed as folds of the NC or ANC after being normalized with the GAPDH. The values represented the mean ± SD of three independent experiments. *p<0.05, versus the NC, and #p<0.05, versus the ANC.
miR-30c directly targeted the IER2 3’UTR
To confirm whether the observed decrease of IER2 expression is the consequence of the interaction of miR-30c with the predicted binding site on the IER2 3’UTR (Figure 5A), we performed the luciferase reporter gene assay. The luciferase reporter vectors psiCHECK-2-IER2-wt or psiCHECK-2-IER2-mut (Figure 5B) were transfected into the miR-30c expressing SMMC-7721 and HepG2 cells or indicated NC cells. We found that significant reduction of the relative Renilla luciferase activities were shown in psiCHECK-2-IER2-wt transfected miR-30c expressing cells (IER2 3’UTR-WT), but not in the psiCHECK-2-IER2-mut transfected cells (IER2 3’UTR-mut) in comparison with those in transfected NC cells (Figure 5C and 5D). Considering miR-30c overexpression down-regulated the levels of IER2 protein as mentioned above (Figure 4), our results strongly indicated that IER2 might be a direct target of miR-30c in SMMC-7721 and HepG2 cells, and the inhibition of miR-30c on IER2 expression requires miR-30c directly binding to the predicted binding sites on the IER2 3’UTR.
Figure 5.
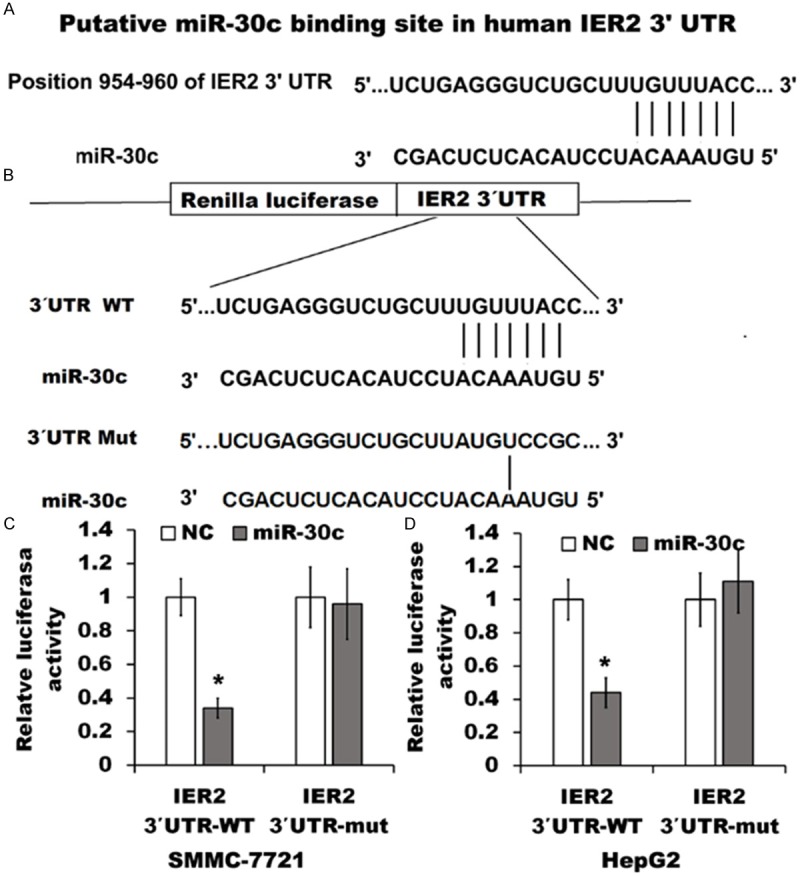
miR-30c directly targeted the 3’UTR of IER2. (A) Putative miR-30c- binding site in the 3’UTR of IER2. (B) Wild-type (WT) of IER2 3’UTR sequence that are targeted by miR-30c or mutant IER2 3’UTR (Mut) was cloned into a psiCHECK-2 vector. (C and D) miR-30c expressing SMMC-7721 cells (C) and HepG2 cells (D) or indicated NC cells were transfected with psiCHECK-2-IER2-wt or psiCHECK-2-IER2-mut vectors for 48 h, and the Renilla luciferase activity was measured and normalized to firefly luciferase activity. The data represented the mean ± SD from four independent experiments. *p<0.05, relative to NC cells.
miR-30c inhibited the cell migration and invasion via regulation of IER2
Our data have demonstrated that both IER2 and miR-30c can regulate the migration and invasion of SMMC-7721 and HepG2 cells (Figures 2 and 3). Subsequently, we further verified whether miR-30c regulated cell motility through IER2 using a transwell assay. As shown in Figure 6, transient transfection with pEZ-Lv105-IER2 (IER2), which expressed exogenous IER2 (without 3’-UTR), into the miR-30c expressing SMMC-7721 cells (A, miR-30c + IER2) and HepG2 cells (B, miR-30c + IER2) significantly increased the cell migration and invasion relative to transfection with pEZ-Lv105-null (miR-30c + null), and partially abolished miR-30c-induced suppression of cell migration and invasion, whereas transfection with pEZ-Lv105-IER2 into the NC cells (NC + IER2) obviously increased the cell motility as compared to transfection with pEZ-Lv105-null (NC + null). Taken together, these results revealed that IER2 altered the general capacity of cell motility, and miR-30c acted as a negative regulator in cell migration and invasion, and down-regulation of IER2 by miR-30c contributed, at least in part, to the reduction of the migration and invasion in SMMC-7721 and HepG2 cells.
Figure 6.
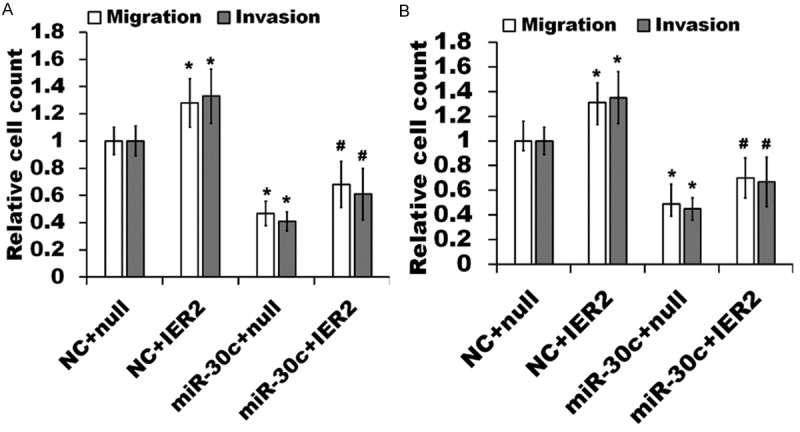
miR-30c inhibition of cell migration and invasion is partially rescued by IER2. miR-30c expressing SMMC-7721 cells (A) and HepG2 cells (B) or indicated NC cells were transfected with pEZ-Lv105-IER2 (miR-30c + IER2, NC + IER2) or pEZ-Lv105-null (miR-30c + null, NC + null) and the transwell migration and invasion assays were then performed. The number of migrated and invaded cells in each condition was assessed by cell counting and expressed as the fold of NC + null transfected cells. The data represented the mean ± SD from three independent experiments. *p<0.05, relative to NC + null, and #p<0.05, relative to miR-30c + null.
Discussion
The findings presented here revealed a role for miR-30c as a regulator of cell migration and invasion through IER2 in human hepatocellular carcinoma cell line SMMC-7721 and HepG2. We demonstrated that knockdown and over-expression of IER2 modulated the general capacity of cell migration and invasion, while miR-30c can also regulate the cell motility. We further demonstrated that miR-30c may function as a negative regulator in cell motility partially through directly binding to IER2 3’UTR and leading to translational suppression of IER2 in SMMC-7721 and HepG2 cells.
Although IER2 has been reported to play a role in tumor cell motility and metastasis [9], the mechanism involved is only partly understood, and the function of IER2 expression in human hepatocellular carcinoma cell migration and invasion has not been addressed. In our initial experiments, we used the SMMC-7721 and HepG2 cells to investigate the role of IER2 in cell migration and invasion. Our findings that IER2 over-expression obviously promoted the cell migration and invasion and that IER2 knock-down by lentiviral mediated shRNA specific targeting IER2 decreased the cell motility suggested a mechanism by which over-expression and knock-down of IER2 could regulate the general capacity of cell motility in SMMC-7721 and HepG2 cells and the endogenous IER2 expression might be required for the cell motility, which is in accordance with that of Neeb and coworkers [9], who reported that IER2 over-expression promoted tumor cell motility.
It has been generally accepted that miRNAs perform their functions by regulating the gene expression via binding to the 3’UTRs of the target genes [10-12]. miR-30c has been reported to negatively regulate cancer metastasis by directly targeting metastasis associated genes [27-29], however, a role of miR-30c in promotion of cellular invasion was recently reported in the metastatic breast cancer cells [30], suggesting that miR-30c might be involved in cancer cell metastasis in different ways by targeting different genes. Our findings that miR-30c over-expression obviously reduced the cell migration and invasion, and that miR-30c knock-down by antisense oligonucleotides specific for miR-30c increased the cell motility suggested that miR-30c may function as a negative regulator in cell motility of SMMC-7721 and HepG2 cells.
Since we have demonstrated that IER2 and miR-30c functioned as the regulators of the migration and invasion in SMMC-7721 and HepG2 cell as mentioned above, we speculated if IER2 is a potential target of miR-30c. Firstly, bioinformatics analysis from six major miRNA-target prediction algorithms revealed that IER2 3’UTR contains a complementary site for the seed region of miR-30c (Figure 5A) and IER2 might be a potential target of miR-30c. We next performed RT-qPCR and western blot analysis to verify whether IER2 could be regulated by miR-30c. Our findings that over-expression of miR-30c obviously down-regulated the IER2 protein and that knock-down of miR-30c up-regulated the IER2 protein, and neither over-expression nor knock-down of miR-30c affected the level of IER2 mRNA, which is different from the knock-down of IER2 by LV-shR infection resulting in transcriptional degradation, suggested that miR-30c resulted in translational suppression of IER2 expression in SMMC-7721 and HepG2 cells. To verify the predicted target site of miR-30c on the 3’UTR of IER2, we then performed the luciferase reporter gene assays. We demonstrated that over-expression of miR-30c efficiently repressed the relative Renilla luciferase activity of the reporter construct containing wild-type but not the mutant IER2 3’UTR, and as expected, no significant alteration to the relative Renilla luciferase activity of the reporter construct containing the mutation of the putative miR-30c target site was observed, suggesting that IER2 might be a direct target of miR-30c in SMMC-7721 and HepG2 cells and that the inhibition of miR-30c on IER2 expression requires miR-30c directly binding to the predicted binding site on the 3’UTR of IER2. Finally, to further confirm whether miR-30c regulated cell motility through IER2, we performed the IER2 rescue experiments. Results from the transwell assays demonstrated that transfection with pEZ-Lv105-IER2, which expressed exogenous IER2 (without 3’-UTR), into the miR-30c expressing cells partially rescued cell migration and migration, suggesting that miR-30c overexpression inhibited cell motility, at least in part, due to down-regulation of IER2.
In conclusion, we demonstrated that IER2 modulated the general capacity of cell migration and invasion of the SMMC-7721 and HepG2 cells, and identified that miR-30c may function as a negative regulator of cell motility by targeting the IER2 for translational suppression in SMMC-7721 and HepG2 cells. Study of the underlying mechanism for effect of IER2 expression on the regulation of cell motility is under way, however, our present findings maybe reinforce the role for IER2 and miR-30c as novel molecular targets for metastatic carcinoma.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (no. 81172278).
Disclosure of conflict of interest
None.
References
- 1.Shimizu N, Ohta M, Fujiwara C, Sagara J, Mochizuki N, Oda T, Utiyama H. Expression of a novel immediate early gene during 12-O-tetradecanoylphorbol-13-acetate-induced macrophagic differentiation of HL-60 cells. J Biol Chem. 1991;266:12157–12161. [PubMed] [Google Scholar]
- 2.Deng YJ, Huang ZX, Zhou CJ, Wang JW, You Y, Song ZQ, Xiang MM, Zhong BY, Hao F. Gene profiling involved in immature CD4+ T lymphocyte responsible for systemic lupus erythematosus. Mol Immunol. 2006;43:1497–1507. doi: 10.1016/j.molimm.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 3.Shen QY, Zheng SS. Identification of genes differentially expressed in monocyte-derived dendritic cells with 1á, 25-dihydroxyvitamin D3 using cDNA arrays. J Zhejiang Univ SCI. 2004;5:222–225. doi: 10.1007/BF02840927. [DOI] [PubMed] [Google Scholar]
- 4.Zeng F, Hon CC, Sit WH, Chow KY, Hui RK, Law IK, Ng VW, Yang XT, Leung FC, Wan JM. Molecular characterization of Coriolus versicolor PSP-induced apoptosis in human promyelotic leukemic HL-60 cells using cDNA microarray. Int J Oncol. 2005;27:513–523. [PubMed] [Google Scholar]
- 5.Takaya T, Kasatani K, Noguchi S, Nikawa J. Functional analyses of immediate early gene ETR101 expressed in yeast. Biosci Biotechnol Biochem. 2009;73:1653–1660. doi: 10.1271/bbb.90162. [DOI] [PubMed] [Google Scholar]
- 6.Chen L, Ma S, Li B, Fink T, Zachar V, Takahashi M, Cuttichia J, Tsui LC, Ebbesen P, Liu X. Transcriptional activation of immediate-early gene ETR101 by human T-cell leukaemia virus type I Tax. J Gen Virol. 2003;84:3203–3214. doi: 10.1099/vir.0.19283-0. [DOI] [PubMed] [Google Scholar]
- 7.Hess S, Rheinheimer C, Tidow F, Bartling G, Kaps C, Lauber J, Buer J, Klos A. The reprogrammed host: Chlamydia trachomatis-induced up-regulation of glycoprotein 130 cytokines, transcription factors, and antiapoptotic genes. Arthritis Rheum. 2001;44:2392–2401. doi: 10.1002/1529-0131(200110)44:10<2392::aid-art404>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 8.Hong SK, Dawid IB. FGF-dependent left-right asymmetry patterning in zebrafish is mediated by Ier2 and Fibp1. Proc Natl Acad Sci U S A. 2009;106:2230–2235. doi: 10.1073/pnas.0812880106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neeb A, Wallbaum S, Novac N, Dukovic-Schulze S, Scholl I, Schreiber C, Schlag P, Moll J, Stein U, Sleeman JP. The immediate early gene Ier2 promotes tumor cell motility and metastasis, and predicts poor survival of colorectal cancer patients. Oncogene. 2012;31:3796–3806. doi: 10.1038/onc.2011.535. [DOI] [PubMed] [Google Scholar]
- 10.Guo X, Wu Y, Hartley RS. MicroRNA-125a represses cell growth by targeting HuR in breast cancer. RNA Biol. 2009;6:575–583. doi: 10.4161/rna.6.5.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 12.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 13.Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Plasterk RH. Micro RNAs in animal development. Cell. 2006;124:877–881. doi: 10.1016/j.cell.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 16.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 17.White NM, Fatoohi E, Metias M, Jung K, Stephan C, Yousef GM. Metastamirs: a stepping stone towards improved cancer management. Nat Rev Clin Oncol. 2011;8:75–84. doi: 10.1038/nrclinonc.2010.173. [DOI] [PubMed] [Google Scholar]
- 18.Boominathan L. The guardians of the genome (p53, TA-p73, and TAp63) are regulators of tumor suppressor miRNAs network. Cancer Metastasis Rev. 2010;29:613–639. doi: 10.1007/s10555-010-9257-9. [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. Mir-30 reduction maintains self- renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29:4194–4204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 20.Quintavalle C, Donnarumma E, Iaboni M, Roscigno G, Garofalo M, Romano G, Fiore D, De Marinis P, Croce CM, Condorelli G. Effect of miR-21 and miR-30b /c on TRAIL-induced apoptosis in glioma cells. Oncogene. 2013;32:4001–4008. doi: 10.1038/onc.2012.410. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, Miao R, Li G, Wu Y, Robson SC, Yang X, Zhao Y, Zhao H, Zhong Y. Identification of recurrence related microRNAs in hepatocellular carcinoma after surgical resection. Int J Mol Sci. 2013;14:1105–1118. doi: 10.3390/ijms14011105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bockhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, Yee K, Chang YF, Huo D, Wen Y, Swanson KE, Qiu T, Lu J, Park SY, Dolan ME, Perou CM, Olopade OI, Clarke MF, Greene GL, Liu H. MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun. 2013;4:1393. doi: 10.1038/ncomms2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong K, Chen K, Han L, Li B. MicroRNA-30b/c inhibits non-small cell lung cancer cell proliferation by targeting Rab18. BMC Cancer. 2014;14:703. doi: 10.1186/1471-2407-14-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong Z, Xia Y, Wang P, Liu B, Chen Y. Low expression of microRNA-30c promotes invasion by inducing epithelial mesenchymal transition in non-small cell lung cancer. Mol Med Rep. 2014;10:2575–2579. doi: 10.3892/mmr.2014.2494. [DOI] [PubMed] [Google Scholar]
- 25.Kong X, Xu X, Yan Y, Guo F, Li J, Hu Y, Zhou H, Xun Q. Estrogen regulates the tumour suppressor MiRNA-30c and its target gene, MTA-1, in endometrial cancer. PLoS One. 2014;9:e90810. doi: 10.1371/journal.pone.0090810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oksuz Z, Serin MS, Kaplan E, Dogen A, Tezcan S, Aslan G, Emekdas G, Sezgin O, Altintas E, Tiftik EN. Serum microRNAs; miR-30c-5p, miR-223-3p, miR-302c-3p and miR-17-5p could be used as novel non-invasive biomarkers for HCV-positive cirrhosis and hepatocellular carcinoma. Mol Biol Rep. 2015;42:713–20. doi: 10.1007/s11033-014-3819-9. [DOI] [PubMed] [Google Scholar]
- 27.Suh SS, Yoo JY, Cui R, Kaur B, Huebner K, Lee TK, Aqeilan RI, Croce CM. FHIT suppresses epithelial-mesenchymal transition (EMT) and metastasis in lung cancer through modulation of microRNAs. PLoS Genet. 2014;10:e1004652. doi: 10.1371/journal.pgen.1004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinzelmann J, Unrein A, Wickmann U, Baumgart S, Stapf M, Szendroi A, Grimm MO, Gajda MR, Wunderlich H, Junker K. MicroRNAs with prognostic potential for metastasis in clear cell renal cell carcinoma: a comparison of primary tumors and distant metastases. Ann Surg Oncol. 2014;21:1046–1054. doi: 10.1245/s10434-013-3361-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhou H, Xu X, Xun Q, Yu D, Ling J, Guo F, Yan Y, Shi J, Hu Y. microRNA-30c negatively regulates endometrial cancer cells by targeting metastasis-associated gene-1. Oncol Rep. 2012;27:807–812. doi: 10.3892/or.2011.1574. [DOI] [PubMed] [Google Scholar]
- 30.Dobson JR, Taipaleenmäki H, Hu YJ, Hong D, van Wijnen AJ, Stein JL, Stein GS, Lian JB, Pratap J. hsa-mir-30c promotes the invasive phenotype of metastatic breast cancer cells by targeting NOV/CCN3. Cancer Cell Int. 2014;14:73. doi: 10.1186/s12935-014-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


