Abstract
S100A14 is an EF-hand calcium-binding protein that has been reported to exert its biological effects on different types of cells. However, the potential clinical significance and biological functions of S100A14 in cervical cancer has not yet been clarified. In this study, we firstly examined the correlation between S100A14 expression and clinical-pathological parameters in cervical cancers. Next, we observed the effect of S100A14 on cell cycle progression, cell proliferation, migration and invasion by employing lentiviral-mediated overexpression and knockdown of S100A14 in cervical cancer cells. Furthermore, we investigated the underlying mechanism of S100A14 affecting cell migration and invasion. Immunohistochemistry analysis demonstrated that S100A14 expression was associated with the International Federation of Gynecology and Obstetrics (FIGO) stage (P = 0.025) and lymph node (LN) metastasis (P = 0.001). Functional assays showed that S100A14 overexpression increased the proportion of G2/M phase, promoted cell proliferation, migration, and invasion, whereas S100A14 knockdown exhibited adverse effect on above properties. Mechanistic investigation demonstrated that S100A14 can act as a mediator of epithelial-mesenchymal transition (EMT). And overexpression of S100A14 increased expression of N-cadherin and Vimentin while decreased expression of E-cadherin. The opposite results were observed in S100A14-silenced cells. Taken together, our data indicate that S100A14 has a crucial role in cervical cancer progression. This study significantly increases our understanding of S100A14 functional roles in cervical cancer, which may lead to the development of a novel therapeutic target for cervical cancer.
Keywords: S100A14, cervical cancer, cell proliferation, migration, invasion, epithelial-mesenchymal transition (EMT)
Introduction
Cervical cancer is a common carcinoma of the female reproductive system that has the second highest incidence and the fifth highest mortality among all carcinomas [1]. Infection with high-risk human papillomavirus (HR-HPV), predominantly the type 16 and 18, can cause cervical cancer [2,3]. However, only a small number of HPV-infected patients develop cervical cancer, indicating that the occurrence of cervical cancer is a complex process that involves numerous factors [2]. The characteristic of highly invasive and diffusely metastatic is an indicator of poor prognosis and nearly always determines a fatal outcome in patients with solid cancers [4]. In the initial stage of invasion and metastasis, the morphogenetic changes due to conversion of polarized epithelial cells to motile mesenchymal cells, are referred as epithelial-mesenchymal transition (EMT) [5]. However, how cervical cancer cells acquire the ability to invade surrounding tissues and metastasize is not fully understood.
S100 proteins, a large subgroup of the EF-hand protein family, are small calcium-binding proteins that have a broad range of intracellular and extracellular functions. They exert their functions through the modulation of their subcellular localization and interaction with specific target proteins responsible for the regulation of inflammation, cell growth, differentiation, motility, survival and apoptosis. Altered expression of a large number of S100 proteins has been reported in various human cancers [6]. Several S100 proteins such as S100A2, S100A4, S100A6, S100A7, S100A8/A9 and S100P have been implicated in tumor invasion and metastasis [7-12]. The S100A14 gene was originally cloned and characterized in human lung cancer cell line, but was found to be differentially expressed in a variety of cell types. It was reported to be upregulated in several tumor types, including ovarian, lung, breast, and uterine cancer, but downregulated in others, such as kidney, colon, rectal, and esophageal cancer [13]. S100A14 can regulate oral squamous cell carcinoma cell invasion by modulating the expression of matrix metalloproteinase (MMP)-1 and MMP-9 [14]. Our previous studies showed that extracellular S100A14 can regulate cell proliferation and apoptosis by the interaction with RAGE [15], whereas intracellular S100A14 can induce G1/S arrest and act as a modulator of cell differentiation in esophageal cancer cells [16]. We also showed that S100A14 can directly interact with ErbB2 and functions as a modulator of ErbB2 signaling [17]. In addition, we have also demonstrated that S100A14 affects cell invasion by regulating MMP2 expression and function in a p53-dependent manner [18]. However, the role of S100A14 in cervical cancer development and the underlying molecular mechanisms remains unclear. In this study, we demonstrate that the overexpression of S100A14 is significantly associated with cervical cancer FIGO stage and LN metastasis. Furthermore, we demonstrate that S100A14 promotes cell cycle progression, cell growth, migration and invasion of cervical cancer cells. Mechanistic investigation shows that S100A14 is involved in the regulation of the main components of EMT including E-Cadherin, N-Cadherin and Vimentin.
Materials and methods
Tissue specimens
In this study, 98 cervical squamous cell carcinoma and 76 cervical intraepithelial neoplasia (CIN) cases were prospectively collected from patients who enrolled in the Affiliated Hospital of Qingdao University from March 2004 to March 2010, and received biopsy or primary surgery during that time. Also, 24 normal cervical tissue samples were collected as a control. All tumor tissues were histologically reviewed and only specimens with sufficient presence of tumor cells were included. Cervical cancer patients were clinically staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system and histologically classified and graded according to WHO grade. The treatment of cervical cancer consisted of radical hysterectomy with pelvic lymph node dissection and /or aortic lymph node dissection for FIGO stage I/II. Adjuvant radiotherapy or platinum-based concurrent chemo-radiation was performed in cases with increased risk of recurrent disease, such as positive resection margins, positive lymph nodes, or parametrial involvement. For FIGO stage III/IV cervical cancer primary chemo-radiation therapy was generally recommended, before this pelvic nodal status were diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI) and some patients underwent biopsy to confirm metastasis of the enlarged nodes (minimum diameter ≥ 1.0 cm). Clinicopathological factors include age, cancer stage, tumor differentiation, tumor size, lymphovascular space invasion (LVSI) and lymph node (LN) metastasis. Patients signed informed consents and research protocols for the use of human tissues were approved by and conducted in accordance with the policies of the Institutional Review Boards at Qingdao University.
Cell lines and cell culture
The human cervical cancer cell lines SiHa, HeLa, C33A, and CaSki were all purchased from the American Type Culture Collection (ATCC; Manassas, VA). SiHa, HeLa, and C33A cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma-Aldrich, St Louis, MO) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), 100 U/ml streptomycin, and 100 U/ml penicillin. CaSKi cells were cultured in McCoy’s 5A medium (Sigma-Aldrich) with 10% FBS, 100U/ml streptomycin, and 100 U/ml penicillin. All cell lines were cultured in complete medium in a 5% CO2 37°C incubator. The medium was changed at alternate days and cells were split before they reached confluency.
Lentiviral constructs, production of lentiviruses and lentiviral transduction
Full-length cDNA of human S100A14 was cloned between Xho I and Not I of pLVX-IRES-Neo to generate the constitutive lentiviral vector. 10 cm plate containing 6×106 293T cells was transfected using FuGENE HD (Roche) with 5 µg pLVX-IRES-Neo or pLVX-IRES-Neo-S100A14 lentiviral vector, 3.75 µg pCMV Δ8.91 and 1.25 µg VSVG. Supernatants were collected at 48 hours after transfection and frozen at -70°C. For lentiviral transduction, 1×105 cells/well were seeded in 6- well culture plates and infected the following day with lentiviruses. To generate stable cell lines, cells were selected for two weeks with 500 µg/ml Geneticin. An shRNA construct targeting GFP containing 19 bp sequence 5’-CGAGAAGCGCGATCACATG-3’. Two shRNA constructs targeting human S100A14 were cloned between BamHI and EcoRI of pSIH1-H1-Puro to generate the constitutive lentiviral vectors and had the following sequences 5’-AACGCAGAGGATGCTCAGG-3’ and 5’-ATGGGAAATGATTTGAATA-3’. Viruses were produced in 293T cells using 3 µg lentiviral vector, 3 µg pLP1, 3 µg pLP2 and 3 µg VSVG as described above. Cells were infected as described above and selected for 4-7 days in 2 µg/ml puromycin.
Quantitative real-time RT-PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) and cDNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time polymerase chain reaction was performed using Power SYBRs Green PCR Master Mix (Applied Biosystems) on the StepOne PlusTM Real-Time PCR System (Applied Biosystems) following the manufacturer’s instructions. The PCR primers were listed in Table 1.
Table 1.
Primers used in this study
| Genes | Primer sequences |
|---|---|
| S100A14-F | TCACCAAAGGACCAGACACA |
| S100A14-R | GCCCTCTCCACATCACTGAA |
| E-Cadherin-F | TGCCCAGAAAATGAAAAAGG |
| E-Cadherin-R | GTGTATGTGGCAATGCGTTC |
| N-Cadherin-F | ACAGTGGCCACCTACAAAGG |
| N-Cadherin-R | CCGAGATGGGGTTGATAATG |
| Vimentin-F | GAGAACTTTGCCGTTGAAGC |
| Vimentin-R | GCTTCCTGTAGGTGGCAATC |
| β-actin-F | GGCGGCACCACCATGTACCCT |
| β-actin-R | AGGGGCCGGACTCGTCATACT |
Western blotting
Protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking, the membranes were incubated with the appropriate primary antibodies at 4°C overnight. After washing, the membranes were incubated with secondary antibody at a dilution of 1:5,000 for 1 hour at room temperature. Proteins were detected with the enhanced chemiluminescence kit (Pierce, Rockford, IL, USA). Antibodies used were anti-S100A14 (a gift from Dr. Iver Petersen, University Hospital Charite’, Berlin, Germany), anti-E-Cadherin (Sc-7870), anti-Vimentin (Sc-6260) from Santa Cruz Biotechnology and anti-N-Cadherin (61920) from BD Biosciences. β-actin antibody (A5316, Sigma) was used to test for equal loading.
Immunohistochemistry staining
Tissue sections were dewaxed with xylene and rehydrated through gradient ethanol into water. After endogenous peroxidase activity was quenched with 3% H2O2 for 30 minutes, heated in citrate buffer (pH 6.0) at 95°C for 25 min, and cooled at room temperature. After PBS washes, nonspecific antibody binding was blocked by preincubating slides in 10% normal goat nonimmune serum at 37°C for 30 minutes. Sections were incubated with the polyclonal primary antibody against S100A14 at 1:200 dilution overnight at 4°C. IHC was performed using the PV-9000 Polymer Detection System for Immuno-Histological Staining kit (Beijing Golden Bridge Biotechnology Company). DAB was used to visualize the reaction, followed by counterstaining with Hematoxylin. All slides were scanned by Aperio scanning system (Aperio, San Diego, USA) and the Aperio Image Scope software was employed for quantitative analysis of S100A14 protein expression. About 4 to 6 different parts of the slide were randomly selected for analysis.
Fluorescence-activated cell sorting analysis
Cells were washed in PBS and fixed in methanol overnight. Subsequently, cells were washed and resuspended in PBS containing 50 μg/ml propidium iodide, 100 μg/ml RNase, and 0.1% Nonidet P-40 for 30 minutes at 37°C. The distribution of cells in different phases of the cell cycle was determined by measuring the nuclear DNA content using a FACS Calibur cell flow cytometer (Becton, Dickinson and Company).
Cell proliferation assay
MTS assay was applied to assess cell proliferation as instructed by the manufacturer (Promega). Cells were seeded at 3×103 cells/well in 100 µl/well using 96-well culture plates. The absorbance of the samples was measured at 490 nm on a scanning multi-well spectrophotometer. The experiment was repeated 3 times. Cell proliferation was compared at four time point (6 h, 24 h, 48 h, and 72 h) after the cells were seeded.
Cell migration and invasion assay
In vitro cell migration and invasion assays were performed as described previously [18]. Cells growing in the log phase were trypsinized, re-suspended in serum-free medium, and seeded into Boyden chambers (8 μm pore size with polycarbonate membrane). The chambers were then inserted into transwell apparatus (Costar, Cambridge, MA, USA). The chambers were coated with Matrigel (BD Biosciences, San Jose, USA) when cell invasion assay was done. Medium with 10% FBS (600 μl) was added to the lower chamber. After incubation of 48 hours, cells on the top surface of the insert were removed by wiping with cotton swab. Cells that migrated to the bottom surface of the insert were stained in 0.3% crystal violet for 30 min, rinsed in PBS and then subjected to microscopic inspection. Images of four random fields (10×) were captured from each membrane, and the number of migratory or invasive cells was counted. The migration and invasion results were normalized by cell proliferation under the same treatment conditions. Triplicate assays were used or each experiment.
Statistical analysis
Statistical analyses were done using the SPSS 19.0 software version (SPSS, Inc., Chicago, IL). Mann-Whitney U nonparametric test was used for comparing two different groups such as tumor size, LN metastasis and Lymphovascular invasion, but if more than two groups were compared, Kruskal-Wallis nonparametric test was used. A value of p < 0.05 was considered statistically significant.
Results
S100A14 was highly expressed in human cervical cancer tissues
Clinical and experimental studies have suggested a link between S100A14 and multiple types of cancers [6]. However, the expression and potential clinical significance of S100A14 in cervical cancer are still unknown. Therefore, we firstly performed immunohistochemistry in a cohort of cervical cancer tissues from patients with CIN or invasive cervical cancer to detect S100A14 expression and analyze the correlation between S100A14 expression and clinical features of cervical cancer. Among 98 patients with cervical cancer, 62 patients of stage I, 26 of stage II and 10 of stage III, IV were included. The ages of the patients ranged from 29 to 74 years (median, 46.2 years). The tumor sizes ranged from 0.2 cm to 7.6 cm (mean, 2.2 cm). The following histological types were all squamous cell carcinomas. The length of patient follow-up time ranged from 5 months to 60 months, and median survival time at last follow-up was 40.5 months.
Our data indicated that there was almost no expression of S100A14 in normal cervical tissue slides, while S100A14 expression gradually increased according to the phase of cervical cancer progression, from normal tissues through low and high grade CINs to cervical cancer, and it was mainly located in the cytoplasm and cell membrane of cancer cells (Figure 1A). The finding was further supported by quantitative analysis of Aperio Image scope software that the score of S100A14 expression in squamous cell carcinomas is much higher than that in normal ovarian tissues. Next, the correlation between S100A14 expression with clinicopathological characteristics was evaluated. The results showed that S100A14 expression was associated with FIGO stage (P = 0.025) and LN metastasis (P = 0.001) (Table 2). S100A14 expression at stage III is higher than that at stage I & II (Figure 1B). And S100A14 expression in cases with LN metastasis was higher than that in cases without LN metastasis (Figure 1C). Furthermore, we examined the relationship of S100A14 expression with patient outcome. However, Kaplan-Meier plots demonstrated there was no significant difference between the two groups regarding the survival (data not shown). Altogether, these data suggested that S100A14 expression may be associated with aggressive behavior of cervical cancer and can serve as an indicator of LN metastasis in human cervical cancer.
Figure 1.
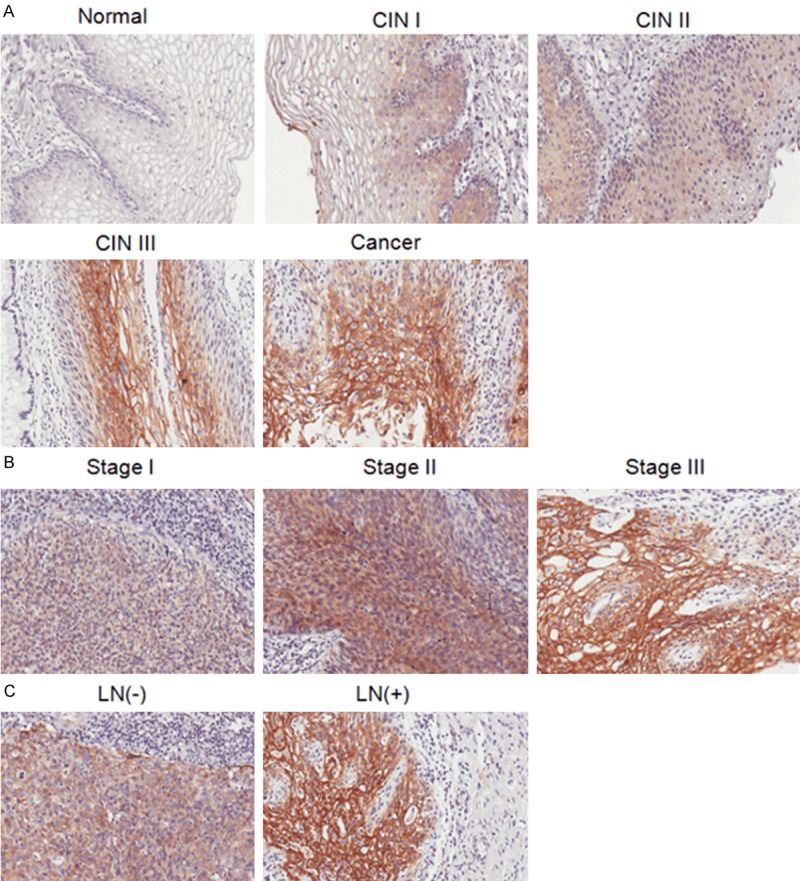
S100A14 expression in human cervical normal and neoplasia specimens. A. Representative immunohistochemistry staining images of S100A14 in normal cervix with almost no staining, weak staining intensity in low grade of cervical intra-epithelial neoplasia (CINI), gradually increased staining in CINII and CINIII, and strong intensity in squamous cell carcinoma. B. Representative immunohistochemistry staining images of S100A14 expression in different stages. C. Representative immunohistochemistry staining images of S100A14 expression in lymph node negative and lymph node positive tumor samples.
Table 2.
The correlation between S100A14 expression and clinicopathologic characteristics in IHC analysis
| Clinical Variable | Relative expressiona of S100A14 and p-value of different categoriesb | ||
|---|---|---|---|
|
| |||
| N | Median | p-value | |
| Diagnostic category | < 0.001 | ||
| Normal | 24 | 0.37 | |
| Low grade CIN | 13 | 0.80 | |
| High grade CIN | 63 | 1.13 | |
| Cancer | 98 | 1.34 | |
| FIGO stage | 0.025 | ||
| I | 62 | 1.16 | |
| II | 26 | 1.43 | |
| III-IV | 10 | 2.13 | |
| Tumor grade | 0.698 | ||
| Well | 7 | 1.34 | |
| Moderate | 40 | 1.35 | |
| Poor | 33 | 1.48 | |
| Tumor size | 0.503 | ||
| < 4 cm | 74 | 1.235 | |
| ≥ 4 cm | 24 | 1.455 | |
| LN metastasis | 0.001 | ||
| Negative | 77 | 1.16 | |
| Positive | 21 | 1.81 | |
| Lymphovascular invasion | 0.615 | ||
| Negative | 85 | 1.34 | |
| Positive | 13 | 1.16 | |
Median of relative expression.
Mann-Whitney U and Kruskal-Wallis nonparametric test were used for comparing different groups.
S100A14 promotes G2/M phase progression and cell growth in cervical cancer cells
The above histological studies showed that S100A14 may play an important role in the tumorigenesis and progression of cervical cancer. So we set out to investigate the potential role of S100A14 in the development of a malignant phenotype in cervical cancer cells by altering intracellular S100A14 expression. Firstly, we detected S100A14 protein expression in four cervical cancer cell lines including SiHa, HeLa, C33A, and CaSki. We found a high level of S100A14 expression in CaSki cells but a low level in SiHa, C33A and HeLa cells (Figure 2A). To characterize the role of S100A14 in the development of malignant phenotype, we overexpressed S100A14 using pLVX-S100A14 lentivirus in SiHa and C33A cells and silenced S100A14 gene expression with lentiviral particles containing two independent shRNA sequences in CaSki cells. Overexpression of S100A14 in SiHa and C33A cells and attenuation of S100A14 expression in CaSki cells were confirmed at the protein and mRNA levels by Western Blot analysis (Figure 2B-D). Previous studies have documented a role for S100A14 in regulation of cell cycle progression and cell growth [16,19]. To determine the effect of S100A14 on cell proliferation, MTS assay was performed on SiHa, C33A and CaSki cells. The results showed that cell growth was highly enhanced in S100A14-overexpressing cells versus the corresponding control cells (Figure 3A, 3C). In contrast, cell growth was significantly decreased in S100A14-silenced cells compared to the control cells (Figure 3E). To verify whether the action of S100A14 on the proliferation of cervical cancer cells is associated with a change in cell cycle distribution, cell cycle analysis was performed using flow cytometry. The results showed that G2/M phase cells increased with a parallel decrease in G1-phase cells in S100A14-overexpressed SiHa and C33A cells (Figure 3B, 3D). In contrast, the proportion of cells in the G2/M phase decreased with a parallel increase in G1-phase in S100A14-silenced CaSki cells (Figure 3F). These results fully demonstrated that S100A14 is involved in the regulation of cell cycle progression and cell growth.
Figure 2.
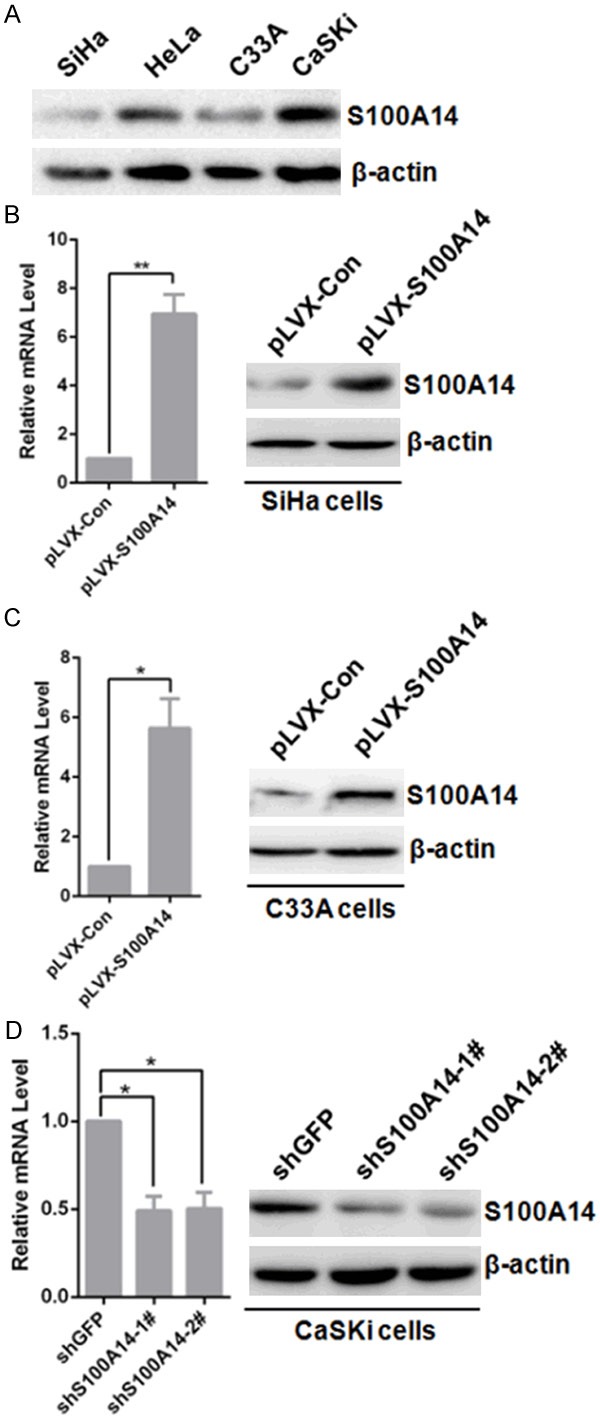
Establishment of stable cell lines of ectopic expression and knockdown of S100A14. A. The protein levels of S100A14 were detected in SiHa, HeLa, C33A and CaSKi cells. β-actin was used as a loading control. B, C. SiHa and C33A cells were infected with pLVX-Con and pLVX-S100A14 lentivirus, stable cells were established by Geneticin (G418) selection for about 2 weeks. Cells were harvested. S100A14 expression was detected by qRT-PCR (Left panel: [mean (n = 2) ± SD; 2-sided t test; *P < 0.05; **P < 0.01, normalized to β-actin]) and Western Blot (Right panel). And cells infected by pLVX-S100A14 lentivirus were named as SiHa-pLVX-S100A14, C33A-pLVX-S100A14 and the corresponding control cells were named as SiHa-pLVX-Control and C33A-pLVX-Control respectively. D. CaSKi cells were infected with shGFP, sh-S100A14-1#, and sh-S100A14-2# lentivirus, stable cells were established by puromycin selection for about 4 days. Cells were harvested. S100A14 expression was detected by qRT-PCR (Left panel: [mean (n = 2) ± SD; 2-sided t test; *P < 0.05, normalized to β-actin]) and Western Blot (Right panel). And cells infected by lentivirus containing two shRNA sequences targeting human S100A14 were named as CaSKi-shS100A14-1# and CaSKi-shS100A14-2# respectively, and the corresponding control cells were named as CaSKi-shGFP.
Figure 3.
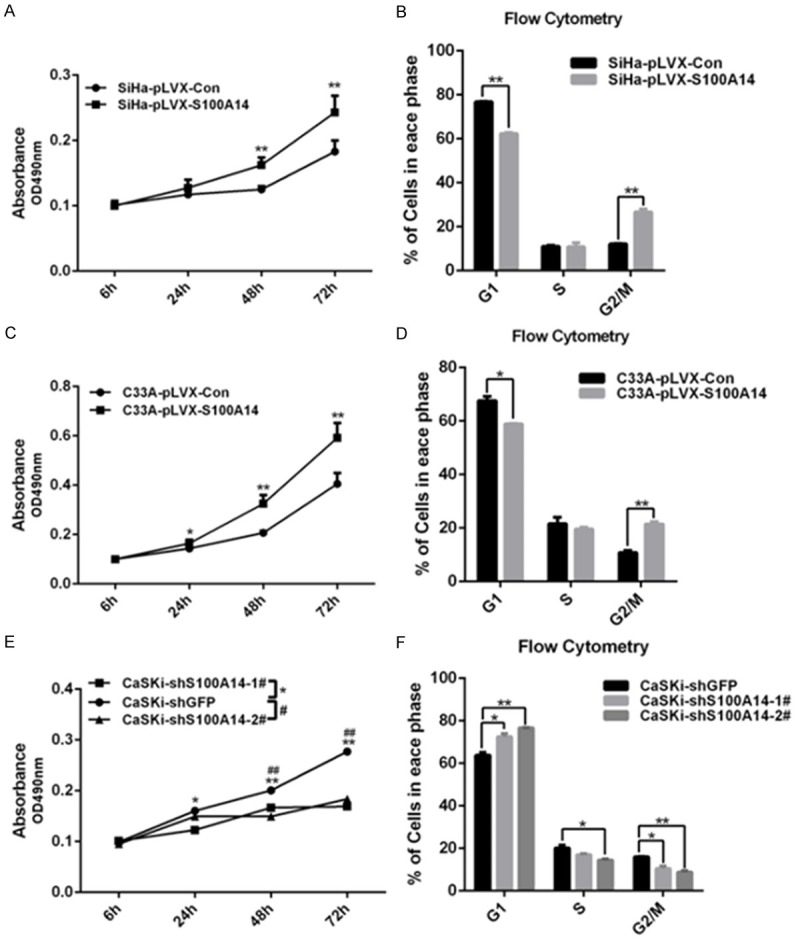
S100A14 promotes cervical cancer cell proliferation and cell cycle progression. Cell proliferation was determined by MTS assay. And overexpression of S100A14 markedly promoted cell proliferation of SiHa and C33A cells (A, C). In contrast, knockdown of S100A14 significantly inhibited cell proliferation of CaSKi cells (E). Cell-cycle distribution was analyzed by FACS, and a significant G2/M phase increase and G1 phase decrease were observed in S100A14-overexpressed cells compared with their corresponding control group [mean (n = 2) ± SD; 2-sided t test; *P < 0.05; ** P < 0.01] (B. SiHa cells; D. C33A cells). In contrast, G2/M phase cell decrease with a parallel increase in G1 phase cells after S100A14 silencing in CaSKi cells (F).
S100A14 enhances cell migration and invasion in cervical cancer cells
Since S100A14 expression significantly correlated with cervical cancer lymph node metastasis and therefore may affect cell migration and invasion. Moreover, functional assays have demonstrated that S100A14 is involved in the regulation of cell migration and invasion. And our previous studies showed that overexpression of S100A14 increases migration and invasion capabilities of breast cancer cell lines [18,20]. We next performed cell migration and invasion transwell assays to examine the effect on cell migration and invasion capabilities of S100A14. The results showed that overexpression of S100A14 significantly enhanced migration and invasion of SiHa and C33A cells compared with their corresponding control cells (Figure 4A, 4B). On the contrary, silencing of S100A14 inhibited cell migration and invasion in CaSki cells (Figure 4C). Taken together, these results support the notion that S100A14 plays an important role in cervical cancer cell migration and invasion.
Figure 4.
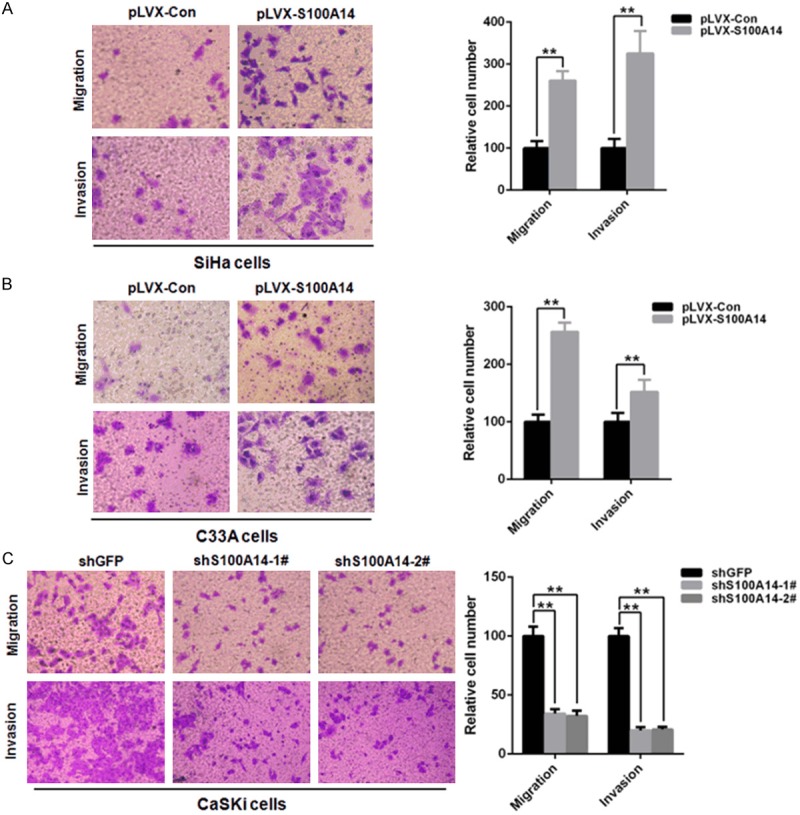
S100A14 promotes cervical cancer cell migration and invasion. (A, B) Transwell migration and Matrigel invasion assays in S100A14-overexpressed cells and their corresponding control cells (A. SiHa cells; B. C33A cells). Left panel: Representative image of cell migration and invasion. Right panel: Quantitative results of migration and invasion assays. The stained cells were manually counted from 4 randomly selected fields and normalized with cell proliferation [2-sided t test; *P < 0.05; **P < 0.01]. (C) Transwell migration and Matrigel invasion assays in S100A14-silenced CaSKi cells and control cells. Left panel: Representative image of cell migration and invasion. Right panel: Quantitative results of migration and invasion assays. The stained cells were manually counted from 4 randomly selected fields and normalized with cell proliferation [2-sided t test; *P < 0.05; **P < 0.01].
S100A14 mediates epithelial-to-mesenchymal transition (EMT)
Epithelial-to-mesenchymal transition (EMT) is one of the key mechanisms for cancer cells to acquire the ability of metastasis and invasion [21]. Emerging evidences support that EMT has a crucial role in the metastasis of primary tumors and provides molecular mechanisms for cervical cancer metastasis [22]. To evaluate whether S100A14 has influence on the EMT-related protein in cervical cancer cells, we carried out Western Blots to detect the expression of E-cadherin, N-cadherin, and Vimentin. The results showed that overexpression of S100A14 in SiHa and C33A cells significantly increased the mRNA expression of N-cadherin and Vimentin, while obviously decreased the expression of E-cadherin (Figure 5A, 5C). Furthermore, the changes of N-cadherin, Vimentin and E-cadherin at protein levels were confirmed by Western Blot (Figure 5B, 5D). On the contrary, the attenuation of S100A14 reduced the expression of N-cadherin and Vimentin, while the expression of E-cadherin increased obviously (Figure 5E, 5F). All the results illustrated that S100A14 was involved in EMT-related gene regulation and may contribute to the conversion of cervical tumors into invasive malignancies.
Figure 5.
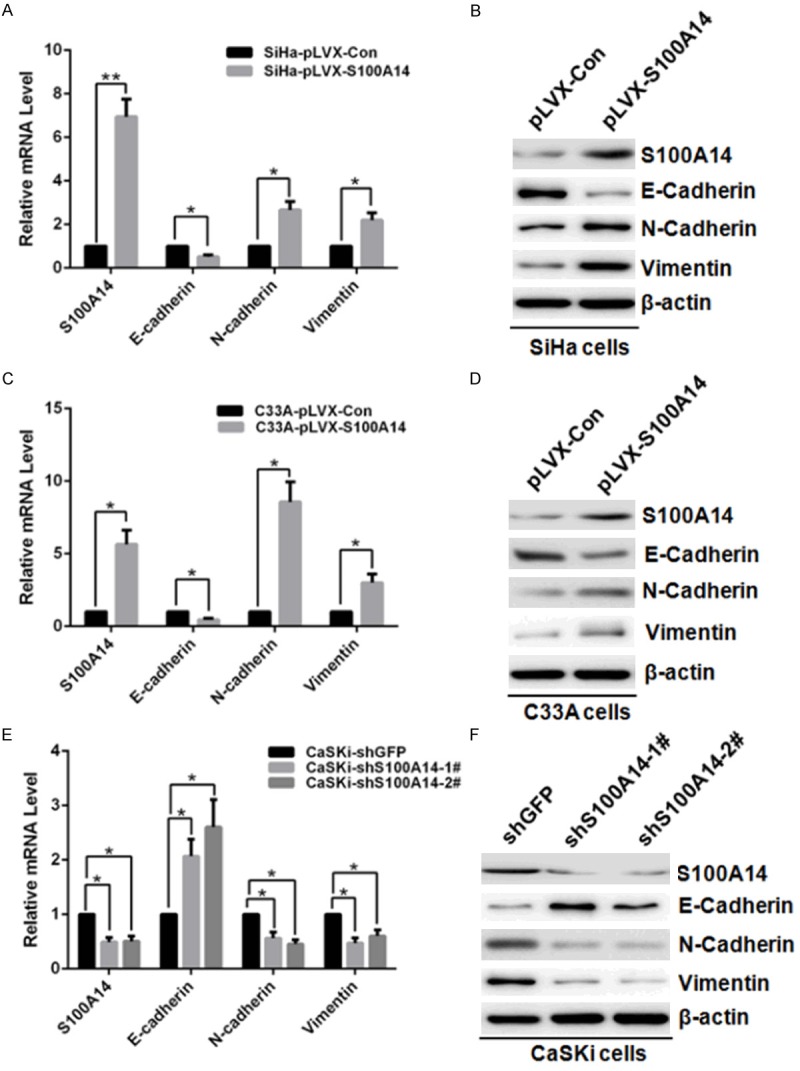
S100A14 modulates expression levels of EMT markers in cervical cancer cells. Relative mRNA expression of epithelial maker E-Cadherin and mesenchymal makers (N-Cadherin and Vimentin) in S100A14-overexpressed SiHa cells and control cells (A), and in S100A14-overexpressed C33A cells and control cells (C) or in CaSKi cells after S100A14 silencing (E) determined by qRT-PCR. [mean (n = 2) ± SD; 2-sided t test; *P < 0.05; **P < 0.01, normalized to β-actin]. Western Blot examined the protein level of epithelial marker E-cadherin and mesenchymal marker (N-cadherin and Vimentin) after overexpression of S100A14 in SiHa cells (B) and C33A cells (D), or knockdown of S100A14 in CaSKi cells (F). β-actin is used as a loading control.
Discussion
Increasing evidence suggests the important roles of S100 proteins in tumorigenesis and cancer metastasis. S100 proteins have emerged as key regulators of cell cycle progression, cell growth, cell invasion and migration etc. And their altered expression has been associated with numerous human cancers [6]. During the last few years, our interest has focused on the role of S100A14 in tumorigenesis and tumor progression. And our study showed that S100A14 acts as either a tumor suppressor or an oncogene depending on the cell and tissue types [15-17,20,23]. However, the role of S100A14 in cervical cancer remains unclear. Here we show for the first time an important role of S100A14 in tumorigenesis and progression of cervical carcinoma. Our immunohistochemistry results show that S100A14 is overexpressed in human cervical cancer and the expression of S100A14 correlates with the tumor FIGO stage and LN metastasis. Next, we directly evaluated the role of S100A14 in cell cycle regulation, cell growth, invasion and migration by lentiviral-mediated overexpression and lentiviral shRNA-mediated knockdown of S100A14 using a series of functional assays. Overexpression of S100A14 enhances the percentage of cells in G2/M phase, promotes cell growth, invasion and migration. In contrast to S100A14-overexpression experiments, depletion of S100A14 decreased the proportion of cells in G2/M phase, inhibited cell growth, migration and invasion. Taken together, all these findings highlight the importance of aberrant expression and biological function of S100A14 in cervical cancer.
Previous studies showed that S100A14 has a dual effect on cell proliferation. And S100A14 inhibits proliferation of oral carcinoma and esophageal cancer cells by inducing G1 arrest [16,19]. On the contrary, S100A14 promotes cell proliferation of hepatocellular carcinoma cells [24]. The disparity of the regulation of cell proliferation by S100A14 may reflect the different characteristics of different tissues or cell types. Our results showed S100A14 promotes cell proliferation of cervical cancer cells by regulating G2/M progression. To further characterize the effect of S100A14 on the pathways regulating cell cycle progression and cell proliferation, gene expression profiling analysis needs to be performed in future study.
Metastasis is a main cause of cancer-related death in patients, and epithelial to mesenchymal transition (EMT) is essential for cancer metastasis, which includes local invasion, intravasation, extravasation, and proliferation at distant sites and is a complicated and currently uncontrolled process. A key feature of EMT is the switch from E-cadherin to N-cadherin, cells undergoing EMT downregulate the expression of E-cadherin accompanied by an increased expression of N-cadherin which promotes the interaction with endothelial and stromal components [21]. Notably, here we show that overexpression of S100A14 leads to a reduction of E-cadherin, and an increase of N-cadherin and Vimentin in cervical cancer cell lines at both mRNA and protein levels, which indicates that S100A14 induces EMT in the cervical cancer cells. In contrast, knockdown of S100A14 exhibits adverse effect on the regulation of the main components of EMT including E-Cadherin, N-Cadherin and Vimentin. Our data suggest that S100A14 induces EMT, and therefore is important in cervical cancer progression and metastasis. However, the detailed mechanism of regulation of EMT components by S100A14 needs to be further elucidated. A complete understanding of the role of S100A14 in controlling EMT in tumors is crucial for the use of S100A14 as a potential therapeutic target in cervical cancer.
Taken together, we have identified S100A14 associated with metastasis in human cervical cancer. High level expression of S100A14 might be valuable for prediction of metastasis. We further showed that S100A14 regulates cell cycle progression, cell proliferation, migration and invasion. Notably, we demonstrated that S100A14 can act as a regulator of EMT. For the first time, this study implicates a role for S100A14 in the metastatic process of human cervical cancer.
Acknowledgements
This work was supported by National Natural Science Foundation of China Grants 81472452. We also thank Dr. Iver Petersen for providing the S100A14 antibodies.
Disclosure of conflict of interest
None declared.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 4.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 5.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Xu C, Jin Q, Liu Z. S100 protein family in human cancer. Am J Cancer Res. 2014;4:89–115. [PMC free article] [PubMed] [Google Scholar]
- 7.Bulk E, Sargin B, Krug U, Hascher A, Jun Y, Knop M, Kerkhoff C, Gerke V, Liersch R, Mesters RM, Hotfilder M, Marra A, Koschmieder S, Dugas M, Berdel WE, Serve H, Muller-Tidow C. S100A2 induces metastasis in non-small cell lung cancer. Clin Cancer Res. 2009;15:22–29. doi: 10.1158/1078-0432.CCR-08-0953. [DOI] [PubMed] [Google Scholar]
- 8.Grum-Schwensen B, Klingelhofer J, Berg CH, El-Naaman C, Grigorian M, Lukanidin E, Ambartsumian N. Suppression of tumor development and metastasis formation in mice lacking the S100A4 (mts1) gene. Cancer Res. 2005;65:3772–3780. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- 9.Luu HH, Zhou L, Haydon RC, Deyrup AT, Montag AG, Huo D, Heck R, Heizmann CW, Peabody TD, Simon MA, He TC. Increased expression of S100A6 is associated with decreased metastasis and inhibition of cell migration and anchorage independent growth in human osteosarcoma. Cancer Lett. 2005;229:135–148. doi: 10.1016/j.canlet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Kataoka K, Ono T, Murata H, Morishita M, Yamamoto KI, Sakaguchi M, Huh NH. S100A7 promotes the migration and invasion of osteosarcoma cells via the receptor for advanced glycation end products. Oncol Lett. 2012;3:1149–1153. doi: 10.3892/ol.2012.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon CH, Moon HJ, Park HJ, Choi JH, Park do Y. S100A8 and S100A9 promotes invasion and migration through p38 mitogen-activated protein kinase-dependent NF-kappaB activation in gastric cancer cells. Mol Cells. 2013;35:226–234. doi: 10.1007/s10059-013-2269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteman HJ, Weeks ME, Dowen SE, Barry S, Timms JF, Lemoine NR, Crnogorac-Jurcevic T. The role of S100P in the invasion of pancreatic cancer cells is mediated through cytoskeletal changes and regulation of cathepsin D. Cancer Res. 2007;67:8633–8642. doi: 10.1158/0008-5472.CAN-07-0545. [DOI] [PubMed] [Google Scholar]
- 13.Pietas A, Schluns K, Marenholz I, Schafer BW, Heizmann CW, Petersen I. Molecular cloning and characterization of the human S100A14 gene encoding a novel member of the S100 family. Genomics. 2002;79:513–522. doi: 10.1006/geno.2002.6744. [DOI] [PubMed] [Google Scholar]
- 14.Sapkota D, Bruland O, Costea DE, Haugen H, Vasstrand EN, Ibrahim SO. S100A14 regulates the invasive potential of oral squamous cell carcinoma derived cell-lines in vitro by modulating expression of matrix metalloproteinases, MMP1 and MMP9. Eur J Cancer. 2011;47:600–610. doi: 10.1016/j.ejca.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Jin Q, Chen H, Luo A, Ding F, Liu Z. S100A14 stimulates cell proliferation and induces cell apoptosis at different concentrations via receptor for advanced glycation end products (RAGE) PLoS One. 2011;6:e19375. doi: 10.1371/journal.pone.0019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Ma J, Sunkel B, Luo A, Ding F, Li Y, He H, Zhang S, Xu C, Jin Q, Wang Q, Liu Z. S100A14: novel modulator of terminal differentiation in esophageal cancer. Mol Cancer Res. 2013;11:1542–1553. doi: 10.1158/1541-7786.MCR-13-0317. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Chen H, Wang X, Gao J, Che Y, Li Y, Ding F, Luo A, Zhang S, Liu Z. S100A14, a member of the EF-hand calcium-binding proteins, is overexpressed in breast cancer and acts as a modulator of HER2 signaling. J Biol Chem. 2014;289:827–837. doi: 10.1074/jbc.M113.469718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Yuan Y, Zhang C, Luo A, Ding F, Ma J, Yang S, Tian Y, Tong T, Zhan Q, Liu Z. Involvement of S100A14 protein in cell invasion by affecting expression and function of matrix metalloproteinase (MMP)-2 via p53-dependent transcriptional regulation. J Biol Chem. 2012;287:17109–17119. doi: 10.1074/jbc.M111.326975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sapkota D, Costea DE, Blo M, Bruland O, Lorens JB, Vasstrand EN, Ibrahim SO. S100A14 inhibits proliferation of oral carcinoma derived cells through G1-arrest. Oral oncology. 2012;48:219–225. doi: 10.1016/j.oraloncology.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 20.He H, Li S, Chen H, Li L, Xu C, Ding F, Zhan Y, Ma J, Zhang S, Shi Y, Qu C, Liu Z. 12-O-tetradecanoylphorbol-13-acetate promotes breast cancer cell motility by increasing S100A14 level in a Kruppel-like transcription factor 4 (KLF4)-dependent manner. J Biol Chem. 2014;289:9089–9099. doi: 10.1074/jbc.M113.534271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alderton GK. Metastasis: Epithelial to mesenchymal and back again. Nat Rev Cancer. 2013;13:3. doi: 10.1038/nrc3428. [DOI] [PubMed] [Google Scholar]
- 22.Talbot LJ, Bhattacharya SD, Kuo PC. Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. Int J Biochem Mol Biol. 2012;3:117–136. [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Yu D, Luo A, Tan W, Zhang C, Zhao D, Yang M, Liu J, Lin D, Liu Z. Functional role of S100A14 genetic variants and their association with esophageal squamous cell carcinoma. Cancer Res. 2009;69:3451–3457. doi: 10.1158/0008-5472.CAN-08-4231. [DOI] [PubMed] [Google Scholar]
- 24.Zhao FT, Jia ZS, Yang Q, Song L, Jiang XJ. S100A14 promotes the growth and metastasis of hepatocellular carcinoma. Asian Pac J Cancer Prev. 2013;14:3831–3836. doi: 10.7314/apjcp.2013.14.6.3831. [DOI] [PubMed] [Google Scholar]


