Abstract
Nasopharyngeal carcinoma (NPC) is endemic to Southeast Asia and over 40% of NPC tissues harbor PIK3CA amplifications. This study aims to study the preclinical activity of a novel PI3K inhibitor, BYL719, in 6 NPC cell lines: C666-1, CNE-2, HK1, HK1-EBV, HONE-1 and HONE-1-LMP1. Over 70% of growth inhibition was attained when NPC cell lines were exposed to increasing concentrations of BYL719, with IC50 values at the low micro-molar range. Two BYL719-sensitive cell lines that harbor PIK3CA mutations, CNE-2 and HONE-1, were selected for further analysis on the effect of BYL719 on cell cycle progression, apoptosis and PI3K signaling. BYL719 significantly reduced the phosphorylation of Akt, and the Akt-mTOR axis downstream effector S6 in these 2 cell lines, but a feedback activation of MAPK was observed at 72 hours post-treatment. BYL719 induced G0/G1 cell cycle arrest and apoptosis in both cell lines. In 3D cell culture models, the growth of NPC spheroids was significantly inhibited in a dose-depending manner. When BYL719 was combined with a MEK inhibitor (AZD6244) in a 3D cell culture system, strong synergism on NPC cell growth was observed with attenuation of MAPK activation. A synergistic inhibitory effect on growth was observed when BYL719 was combined with higher dose levels of cisplatin. These data suggest that BYL719 has preclinical activity in NPC cell lines especially in those which harbor PIK3CA mutation. Combination with a MEK inhibitor maybe a useful strategy that warrants further investigation.
Keywords: Nasopharyngeal carcinoma, PI3K inhibitor, BYL-719, MEK1/2 inhibitor, AZD6244, cisplatin
Introduction
Nasopharyngeal carcinoma (NPC) is an epithelial malignancy featured by its unique epidemiology and geographical distribution. Over 84,400 incident cases were diagnosed in 2008 and the incidence rate was the highest in Southeast Asia [1]. Concomitant chemoradiotherapy is the standard of care for locoregionally advanced NPC [2], but in spite of the overall improvement in survival for NPC in the last two decades, more than 50,000 people still died from NPC in 2008 worldwide, hence there is a need for better systemic treatment.
The phosphoinositide 3-kinase/protein kinase b (PI3K/Akt) pathway is often deregulated in NPC. The PI3K/Akt pathway has long been thought to play a critical role in carcinogenesis as activation of its downstream effectors such as mTOR have been associated with increased cellular proliferation and genomic instability, decreased apoptosis and alterations in the cytoskeleton [3]. Epstein-Barr virus (EBV) has been associated with many different types of human malignancies such as NPC [4]. The EBV latent membrane proteins (LMP)1 and LMP2A are frequently found in NPC and they are able to activate the PI3K/Akt and the mitogen-activated protein kinase (MAPK) signaling pathways [5]. The PI3K/Akt pathway is also essential in promoting cancer metastasis and drug resistance [6-8]. Genome-wide studies using array-based comparative genomic hybridization have shown that PIK3CA amplifications are present in 40-70% of NPC tissues [9,10]. Hot spot PIK3CA mutations have also been reported in low frequencies in NPC cell lines and tissues [10]. Hence, inhibition of this pathway could be an effective strategy against NPC.
BYL719 is a selective PI3Kα inhibitor that is equipotent against the somatic PI3Kα mutations and wild-type PI3Kα. Significant anti-tumor activity has been observed when BYL719 was administered orally to mice bearing PIK3CA-dependent tumor xenograft models. Compared to pan-PI3K inhibitors, BYL719 has a better safety profile in terms of glucose metabolism in animal model [11]. In vitro, BYL719 exhibits the highest potency against PI3Kα activation (IC50 = 4.6 nmol/L) than other isoforms such as PI3Kδ (IC50 = 290 nmol/L) and PI3Kβ (IC50 = 1,156 nmol/L) [12]. Moreover, the plasma level of insulin rose in proportion to the plasma concentration of BYL719 without any significant changes in the plasma glucose level in animal test. Interestingly, cells harboring PIK3CA mutations are more responsive to BYL719 [11]. BYL719 has been used in a phase I study regarding patients with PIK3CA-mutant ER-positive metastatic breast cancer (MBC). It was investigated with a favorable safety and PK profile, the MTD was established at 400 mg once daily and promising preliminary clinical activity was observed in MBC patients [13,14]. Hence, BYL719 warrants further investigation in other cancers
The objective of this study is to elucidate the effect of BYL719 on NPC growth, cellular apoptosis, cell cycle progression, and PI3K-Akt-mTOR signaling as well as the potential synergistic effect on growth when combined with cisplatin or a MEK inhibitor.
Materials and methods
Cell lines
Three EBV-associated NPC cell lines (HK-1-EBV, HONE-1-LMP1 and C666-1) and three non-EBV cell lines (HK-1, HONE-1 and CNE-2) were selected in this study. These cell lines were opted for this study as C666-1 and HK-1 cell lines carry PIK3CA amplifications while CNE-2 and HONE-1 harbor a PIK3CA mutation in exon 20 (missense mutation, H1047R) [10,15]. These cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (Hyclone, Thermo Fisher Scientific, Logan, UT), 1% of 10 mM sodium pyruvate and 1% mixture of penicillin (50 IU/ml) and streptomycin (50µg/ml) (P/S) (Gibco, Life Technologies, Carlsbad, CA).
Drugs, chemicals and antibodies
BYL719 was provided by Novartis Pharma AG (Basal, Switzerland). AZD6244 and Cisplatin were purchased commercially from LC Laboratories (Woburn, MA) and Pharmachemie B.V. (Haarlem, The Netherlands) respectively. Amersham ECL Western blotting detection reagents were from GE Healthcare Biosciences (Pittsburgh, PA). The following antibodies were purchased from Cell Signaling Technology (Danvers, MA): antibodies recognizing cleaved PARP (#9541), p-p70S6K(Thr421/S424) (#9204), p-mTOR(Ser2448) (#2971), mTOR (#2972), p-kt(Ser473) (#9271), Akt(pan) (#4691), p-S6 (#2215), S6 (#2217), p-p44/42 MAPK(Thr202/Tyr204) (#9101), p44/42 MAPK (#9102) and GAPDH (#2118). Actin antibody (#CP01) was purchased from Calbiochem.
Assay of cytotoxicity of BYL719 alone or in combination
Cytotoxicity was assessed by a colorimetric assay using 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Cells were cultured in 48-well plates (5000-30000 cells per well) in respective culture medium. BYL719 alone or in combination with cisplatin/MEK inhibitor AZD6244 was added at 24 hours after cell plating and incubated at 37°C with 5% CO2 for 24, 48, and 72 hours in complete medium. Cell growth inhibition was expressed as the percentage of the absorbance of control cultures measured at 570 nm with a microplate reader (PerkinElmer 1420 Multilabel Counter VICTOR3, Waltham, Massachusetts, USA) and the 50% of the maximum growth inhibition (IC50) was calculated (GraphPad PRISM; Intuitive Software for Science, San Diego, CA). In each experiment, triplicate wells were performed for each drug concentration (n = 3), and assay was repeated in three independent experiments.
Western blot analysis
To study the effect of BYL719 on PI3K-Akt-mTOR signaling, two NPC cell lines that are most sensitive to BYL were treated with BYL719 at or near IC50 concentrations for 48 and 72 hours. Cells were lysed with the western lysis buffer for 10 min at 4°C and the lysate was then centrifuged at 4°C, 12,000 rpm for 10 min. Supernatant was collected for protein quantitation with the usage of Bicinchoninic Acid (BCA) Assay (Sigma Aldrich) and bovine serum albumin of known concentration as the standard. Twenty-five µg of total protein was resolved on SDS-PAGE gel and transferred onto the Trans-Blot nitrocellulose membrane using wet transfer machine (BioRad Laboratories, Hercules, CA). After protein transfer, the membrane was blocked with 5% non-fat dry milk, 0.2% Tween 20 in 1X PBS (TBST) for 2 hours at room temperature. The membrane was incubated with primary antibody at 4°C overnight and washed thrice with TBST for 15 min each. The membrane was then incubated with secondary antibody for 1 hour at room temperature and washed thrice for 15 min. The blot was developed with GE Amersham ECL chemiluminescent substrate by autoradiography.
Analysis of cell cycle and apoptosis
NPC cells were plated in 50 mm2 culture dishes at a number of 1.5 x 105 cells, and then treated either with control or 3 different concentrations of BYL719. Cells were collected at 16 and 24 hour by trypsinization, centrifugation, following fixation with 70% cold ethanol. DNA staining was performed with a solution containing RNase (0.2 mg/ml) and propidium iodide (0.05 μg/ml). Analysis was performed using a FACScan flow cytometer while data of cell cycle were processed with CELLQuest software (Becton Dickinson). Apoptotic effect of BYL719 was determined via the detection of cleaved poly (ADP-ribose) polymerase (PARP) by Western blotting.
3D culture
Cells were plated on matrigel (BD Biosciences) coated well and cultured in RPMI-1640 medium with 2% matrigel. DMSO control and three different doses at or near IC50 concentration of BYL719 were added to the spheroids after 48 hours of cell plating. The cells were then incubated for another 6 days and drug was replenished once within this period.
Synergistic effect in 3D culture
Synergistic effect with AZD6244 and cisplatin was performed on NPC cell lines that are most sensitive to BYL719 in 3D culture. 1 x 104 cells were plated in each matrigel-coated well in 48-well plates. Treatment started at 48 hours after cell plating (day 0) after cell plating and drug was replenished at day 3. Growth inhibition was determined by MTT assay at day 6. Briefly, 150 µl of 5 mg/ml MTT solution was added to each well, followed by 2 hours of incubation at 37°C. Cells were then incubated in 0.01 M HCl in 10% SDS at 37°C until all formazan dissolved. Cell growth inhibition was then determined as described above. Combination Index (CI) was calculated by Calcusyn software (Biosoft, Cambridge, UK). Based on the method described by Chou et al., the CI was calculated as D1/(Dx)1+D2/(Dx)2, in which ‘x’ represents the level of growth inhibition when the two drugs (D1 = dose of drug 1 and D2 = dose of drug 2) combined, while (Dx)1 represents the dose of drug 1 alone that was necessary to inhibit inhibition ‘x’ and (Dx)2 is the dose of drug 2 alone required to reach inhibition ‘x’. (Dx)1 and (Dx)2 were obtained from their individual dose-response curves. CI < 0.7 indicates that the drug combination is synergistic. Images of 3D culture spheroids were captured by Axio Observer Z1 using ZEN imaging software (Carl Zeiss, Germany).
Statistical analysis
Statistical analyses were performed using PRISM4 Software (GraphPad, La Jolla, CA). Unpaired T-test with Welch Correction was used unless specified. Findings were considered as statistically significant when P value < 0.05.
Results
Effect of BYL719 on cell growth in NPC cell lines
Exposure to BYL719 for up to 72 hours resulted in over 80% of growth inhibition in HONE-1, HONE-1-LMP1, HK-1 and HK-1-EBV cell lines. Around 70% of growth inhibition was observed in C666-1 and CNE-2 (Figure 1A) cells. The respective IC50 values were in micro-molar range (in a descending order of sensitivity): HONE-1 = 0.56±0.04 µM; CNE-2 = 0.90±0.06 µM; HONE-1-LMP1 = 0.95±0.06 µM; HK-1-EBV = 1.49±0.25 µM; HK-1 = 1.53±0.12 µM; and C666-1 = 1.85±0.17 µM (Figure 1B). HONE-1 and CNE-2 cells were found to be most sensitive to the growth inhibitory effect of BYL719, and both cell lines harbor a PIK3CA mutation. The result suggests that cell lines carrying PIK3CA mutations could be more sensitive to BYL719 compared to those with wild-type PIK3CA.
Figure 1.
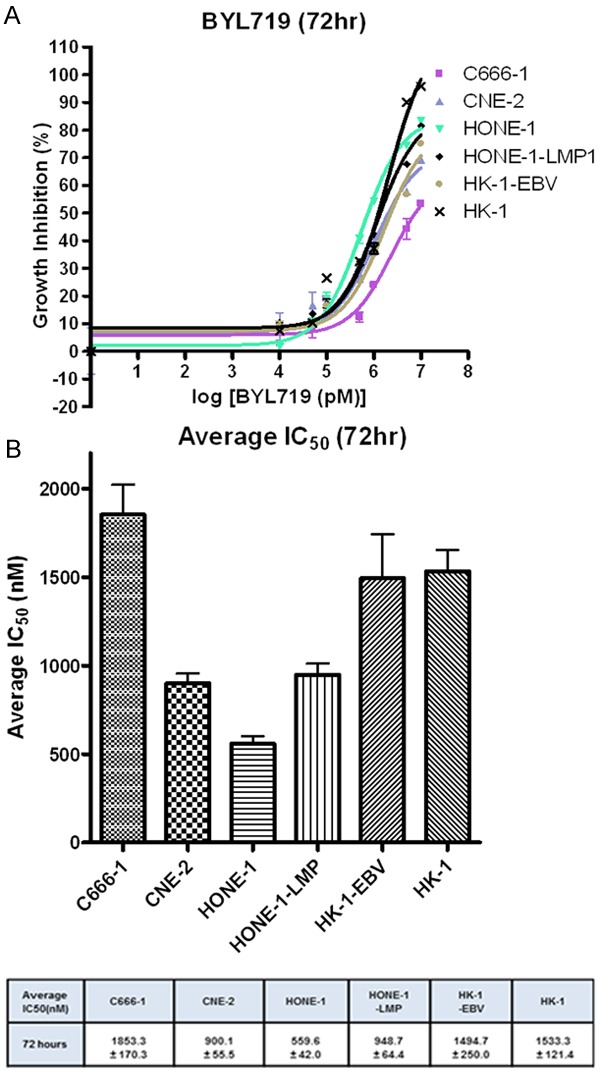
A. Representative dose response curve on C666-1, CNE-2, HONE-1, HONE-1-LMP1, HK-1-EBV and HK1, showing at least 50% of growth inhibition at 72 hours of incubation with BYL719. B. Corresponding IC50 at 72 hours incubation of BYL719.
Basal activation of PI3K-Akt-mTOR signaling in NPC cell lines
All NPC cell lines showed basal activation of Akt and its downstream effectors mTOR and p70S6K. C666-1, HONE-1-LMP1, HK-1-EBV and HK-1 overexpressed a higher basal level of p-Akt than HONE-1 and CNE-2 cells. Downstream to Akt, p-mTOR was strongly expressed in CNE-2 and HONE-1 compared to other cell lines. Most of the NPC cell lines expressed a high basal level of p-p70S6K in comparison with C666-1 cells (Figure 2). HONE-1 and CNE-2 cells were subsequently selected for further analysis on the effect of BYL719 on Akt signaling, cell cycle and apoptosis, as well as on cell growth when combined with cisplatin or a MEK inhibitor.
Figure 2.
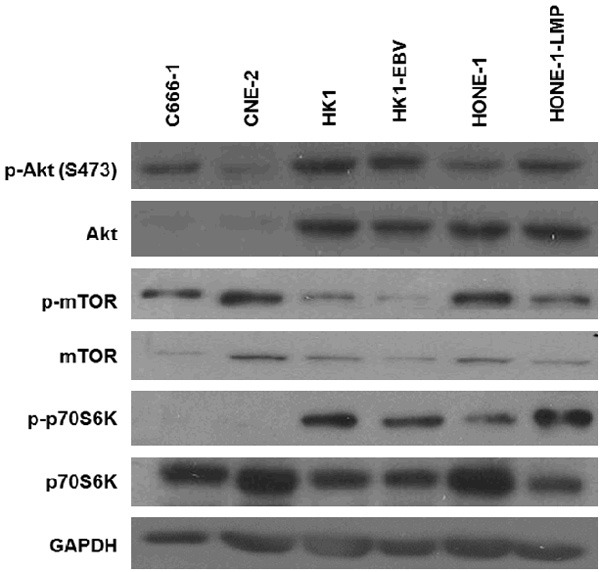
Akt, mTOR and p70S6K phosphorylation profile in NPC cell lines.
BYL719 induced G0/G1 cycle arrest in NPC cell lines
A dose-dependent increase in the percentage of cells undergoing G0/G1 cycle arrest was observed in HONE-1 and CNE-2. A dose-dependent increase in the proportion of cells entering the G0/G1 phase could be observed when HONE-1 cells were exposed to BYL719 at increasing concentrations at 16 and 24 hours. For instance at 24 hours after exposure to BYL719, the percentage of cells entering G0/G1 phase rose from a baseline of 46.82% to 53.79% at a concentration of 0.3 µM, to 53.14% at 0.6 µM, and to 60.61% at 0.9 µM. On the other hand, G0/G1 accumulation was observed in CNE-2 cells only at 24 hours following exposure to BYL719, with the proportion of cells entering G0/G1 phase increasing from a baseline of 39.42% to 43.17% at a concentration of BYL719 at 0.5 µM, 48.86% at 1.0 µM, and 52.17% at 1.5 µM) (Figure 3A).
Figure 3.
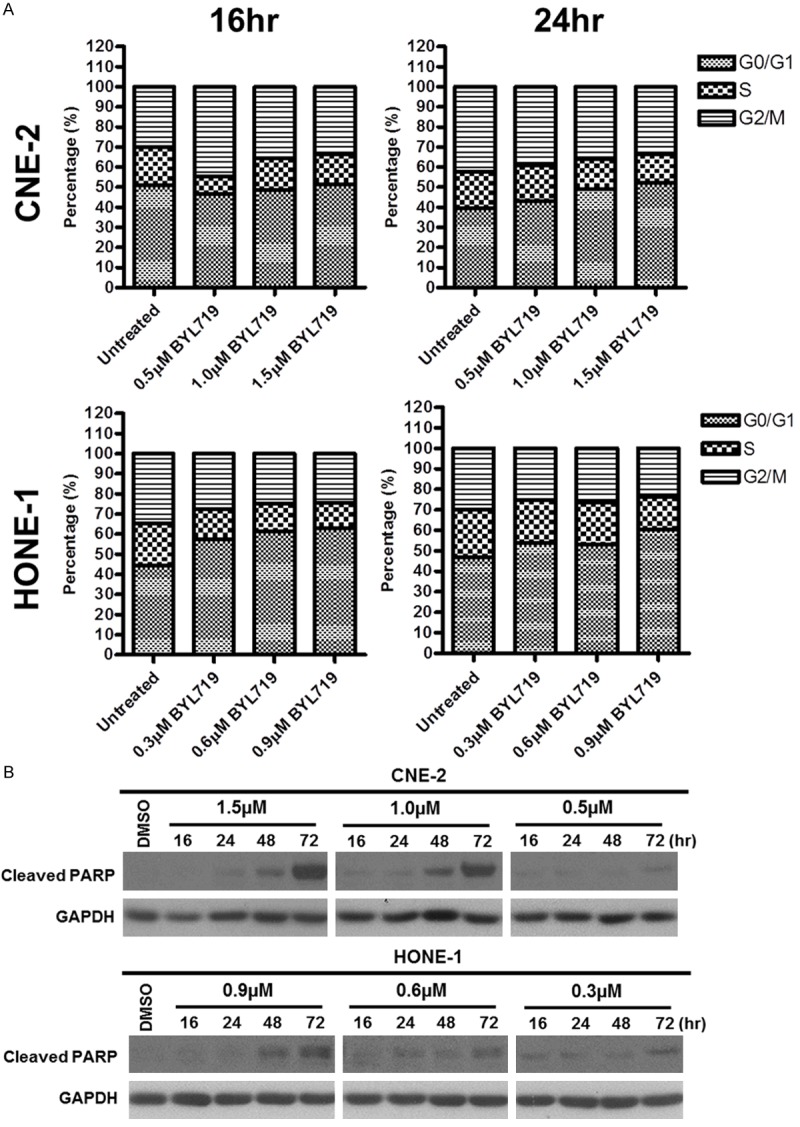
A. Effect of either 16 or 24 hours incubation of BYL719 on cell cycle distribution in CNE-2 and HONE-1 cell lines. Minor G1 phase accumulations were observed. B. Effect of BYL719 on apoptotic induction of CNE-2 and HONE-1. Cleaved PARP was detected at 48 or 72 hours of incubation with BYL719, in a time- and dose-dependent manner.
Using cleaved PARP as an indicator for apoptosis, it was found that apoptosis was not detected in both cell lines at 16 and 24 hours following exposure to BYL719 at concentrations at or near the IC50 values of the respective cell lines. Apoptosis was only detected following longer exposure at 48 to 72-hour in both HONE-1 and CNE-2 cells (Figure 3B).
BYL719’s effects on Akt and its downstream pathway signaling
The effect of BYL719 on PI3K-mediated signaling was assessed by analyzing the expression level of activated and total forms of its downstream targets. Upon treatment with BYL719 at concentrations at or near their respective IC50 values for 72 hours, the levels of phosphorylated and total forms of Akt and its downstream effectors were significantly reduced in HONE-1 and CNE-2. The effect on phosphorylated Akt and S6 was detected shortly after 16 hours treatment of BYL719 and became increasingly noticeable after 72 hours exposure (Figure 4).
Figure 4.
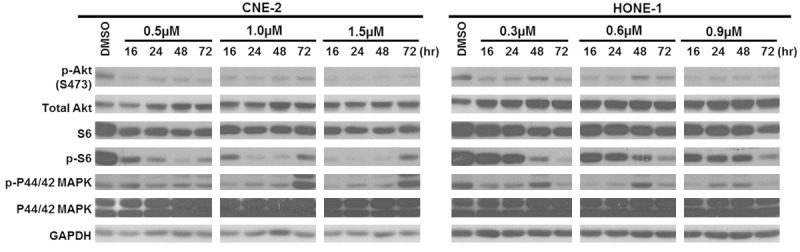
Protein expression was detected by immunoblotting after 16, 24, 48 and 72 hours treatment. Inhibition of Akt and S6 were observed up to 48 hours of treatment. At 72 hours, the inhibition of S6 was diminished and feedback activation of MAPK was observed.
Previously, it was reported that the inhibition of mTOR would lead to the activation of MAPK pathway in other human cancers [16,17]. Since BYL719 exerts potent inhibitory effects on the Akt-mTOR signaling, we investigated its effect on the expression level of MAPK and its activation in the NPC cell lines. Treatment with BYL719 resulted in an increase in the level of phosphorylated and total p-p44/42 MAPK at 48 and 72 hours in HONE-1 and CNE-2 cells respectively, whereas MAPK activation in HONE-1 was diminished at 72 hours. In general, BYL719 had a more rapid onset of inhibitory effect on Akt and its downstream signaling pathways in CNE-2 cells, which is also more sustainable compared to HONE-1.
The effect of combining BYL719 and cisplatin or the MEK inhibitor AZD6244 on NPC cell growth
3D culture was used to further test the inhibitory effects of BYL719. Over 50% of growth inhibition was observed in HONE-1 spheroids for a 6-day treatment at concentrations at or near their IC50 values (Figure 5A). HONE-1 was then opted for the investigation of synergistic effect of BYL719 in combination with cisplatin/MEK inhibitor AZD6244.
Figure 5.
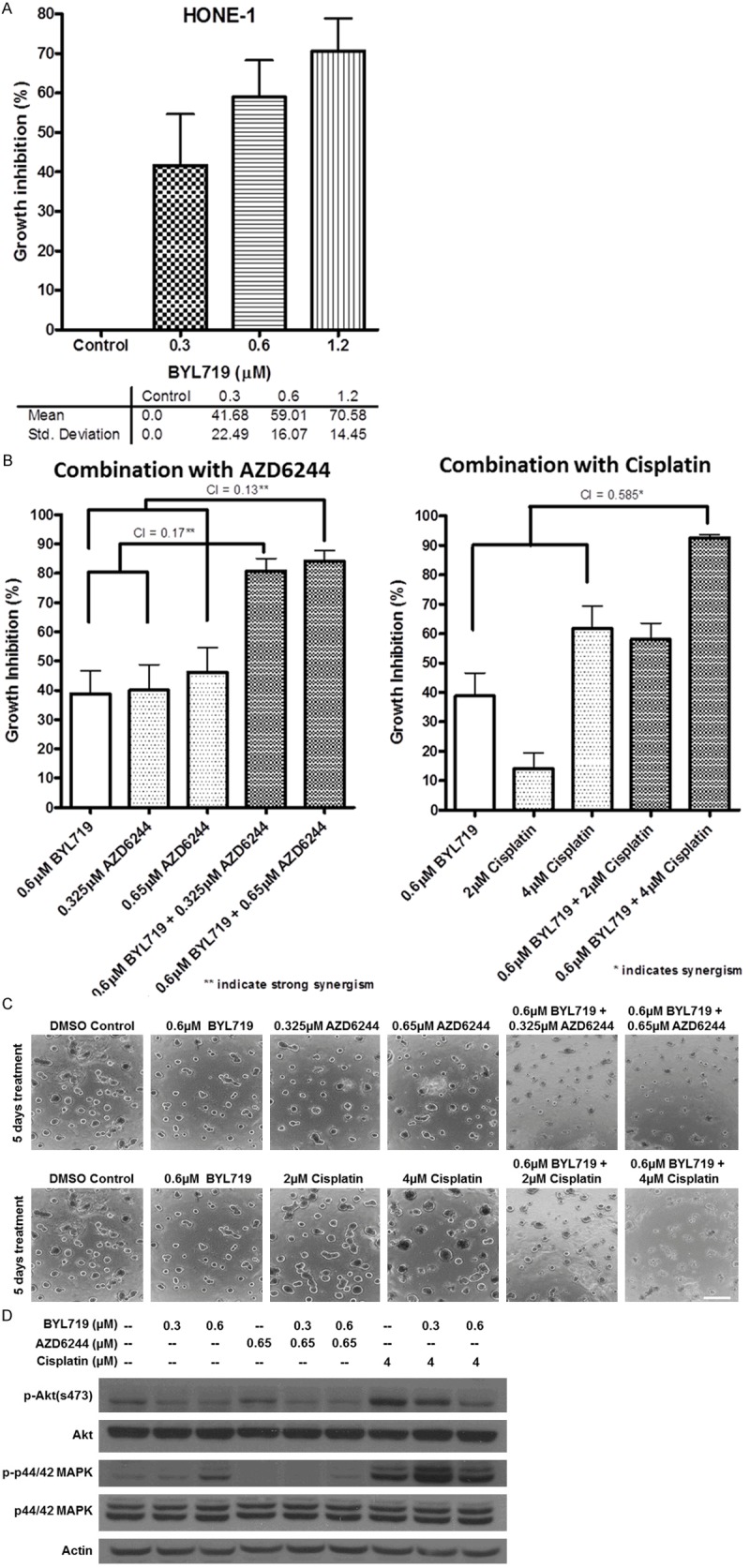
A. Efficacy of BYL719 was evaluated by HONE-1 3D cultures. A dose dependent growth inhibition was observed. B. Synergistic study of BYL719 and AZD6244 or cisplatin on HONE-1 3D cultures. Strong synergistic effects were observed from the combined treatment of BYL719 and AZD6244. Synergism was observed in BYL719 treatment with high dose of cisplatin only. C. Microscopic images showing 6 days treatment of BYL719, AZD6244, cisplatin or in combinations with matrigel 3D culture of HONE-1 cells. The combined treatments had shown significant inhibition to the growth of spheroids compared to untreated control and single drug treatment. Bar represents 200 μm. D. Protein was extracted for western blot. The induced feedback activation of MAPK at 72 hours was successfully inhibited by the co-treatment with AZD6244. The combined treatment with cisplatin did not significantly change the signaling profile of BYL719 treatment; the beneficial effects of the combined treatment might be due to the addition of DNA damaging effect.
HONE-1 and CNE-2 cell lines were treated with BYL719 at IC50 concentration (0.6 µM) in combination with a MEK inhibitor AZD6244 (0.325 µM, 0.65 µM) or cisplatin (2 µM, 4 µM) for up to 72 hours. BYL719 had shown synergistic effect on growth inhibition when combined with AZD6244 or cisplatin. The combination indices (CIs) for BYL719 (0.6 µM) plus AZD6244 at 2 different concentrations of 0.325 µM and 0.65 µM were 0.17 and 0.13 respectively, indicating strong synergisms. The CIs for BYL719 (0.6 µM) plus cisplatin at 2 different concentrations (2 µM and 4 µM) were 1.109 and 0.585, indicating a mild synergism on growth inhibition (Figure 5B). Microscopic images had shown that the combined treatments could significantly inhibit the growth and formation of tumor spheroids compared with untreated control or single drug treatment (Figure 5C). The results were further verified by Western blotting. The addition of AZD6244 resulted in the suppression of the MAPK feedback activation induced by BYL719 treatment (Figure 5D). The abrogation of the MAPK feedback activation could have partly accounted for the synergistic effect between BYL719 and AZD6244. Treatment of NPC cells with cisplatin also resulted in a very strong Akt and MAPK activation. The inhibitory effect observed from BYL719 plus higher doses of cisplatin could be due to the suppression of Akt activation by BYL719 in vitro (Figure 5D). BYL719 had demonstrated a better inhibitory effect on NPC cell growth when combined with AZD6244 in both 2D and 3D NPC culture models.
Discussion
The PI3K pathway has always been seen as a critical component in cell growth, proliferation, survival and protein synthesis. Previous findings affirmed that Akt signaling and its downstream effectors are over-active in most NPC cell lines. We have previously evaluated the preclinical activity of an inhibitor of Akt (MK-2206), mTOR (RAD001) and dual inhibitors of PI3K-mTOR (PF-04691502 and BEZ235) [18-21]. A common phenomenon observed with TORC1 inhibitors is the induction of Akt activation in some NPC cell lines, which has been well described with rapamycin analogues owing to its effect on the Akt feedback loop [22].
In this study, the alpha-isoform specific PI3K inhibitor, BYL719, was effective in suppressing cell growth in 6 NPC cell lines (C666-1, CNE-2, HONE-1, HONE-1-LMP1, HK-1-EBV and HK-1) at micro-molar concentrations, while CNE-2 and HONE-1 cells were found to be most sensitive to BYL719. Around 80% of growth inhibition was attained at 72 hours of treatment. BYL719 could also induce apoptosis and G0/G1 cycle arrest in CNE-2 and HONE-1 cells in a dose dependent manner. In addition, this inhibitory effect was also observed in 3D matrigel cultured spheroids in HONE-1 cells. Mechanistically, BYL719 was effective in inhibiting the activation of Akt and its downstream pathways such as S6, which is their downstream effector of Akt-mTOR axis. This could have impaired protein synthesis and driven the cells to G1 cycle arrest. Furthermore, a MAPK activation feedback loop was induced by BYL719 treatment, which was possibly due to the inhibition of mTOR and its downstream effectors as reported in other types of cancers [16].
Previous reports have indicated a supra-additive effect on growth inhibition when an Akt inhibitor like MK-2206, or TORC1 inhibitor such as RAD001 was combined with cisplatin in NPC cell lines [18,19]. However, only a modest additive effect was resulted when the dual inhibitor PF-04691502 was combined with cisplatin [20]. For BYL719, a strong synergistic effect was observed in the 3D culture model when it was combined with MEK inhibitor AZD6244. The same drug combination was also reported to be synergistic against KRAS mutant NSCLC cells [23]. The feedback activation of MAPK induced by BYL719 could have diminished the growth inhibitory effect since MAPK signaling plays an important role in controlling cell growth, proliferation and protein synthesis [24]. Inhibition of this feedback activation of p44/42 MAPK via the MEK inhibitor, AZD6244, could have explained the strong synergism observed when BYL719 was combined with AZD6244. This supports a combinatorial approach in the clinical development of BYL719.
In this study, cisplatin treatment alone was found to induce very strong Akt and MAPK activation in NPC cell lines. BYL719 was able to suppress this Akt activation, but had minimal effect on MAPK activation. This probably contributed to the lesser degree of synergism on cell growth observed with the BYL719-cisplatin combination, compared with the BYL719-AZD6244 combination. Overall, the results of this study support the clinical evaluation of BYL719 in NPC, preferably in combination with MEK/MAPK inhibitor or cisplatin.
In addition, toxicity is a crucial issue that we could not neglect as the PI3K/Akt/mTOR and MEK/ERK pathways have an important role in human cell physiology and the simultaneous blockage of both pathways could have undesirable consequences. In clinical trials, the safety profile of PI3K inhibitors regarding feudal events is acceptable and the adverse events have been manageable. Some of the toxicities observed in early phase clinical trials have included hyperglycemia, maculopapular rash, gastrointestinal intolerance (anorexia, nausea, vomiting, dyspepsia, diarrhea), and stomatitis [23]. Since p110α is primarily the intracellular mediator of insulin response via the interactions with insulin receptor substrate (IRS), nearly 50% of patients treated with BYL719 in phase I trial encountered hyperglycemic event for at least once [25-27]. MEK inhibitors might bring about vascular leakage, mild and reversible visual disturbance [28]. For instance, patients in the phase I single agent study of MEK inhibitor AS703026 were reported developing blurred vision, abnormal perception of colors and field defects, the symptoms were resolved spontaneously with the on-going treatment [13]. It might also lead to rashes, however, distinctive to the ones caused by PI3K inhibitors. In a study of 225 patients with advanced cancer who were treated with PI3K inhibitors alone (54%), MAPK inhibitors alone (16%) or in combination (54%), a higher percentage of drug-related grade 3 or 4 adverse events (AEs) were observed in patients who received MAPK and PI3K inhibitors together (46%) compared to those who received either of these agents alone (17%). Five out of nine patients had demonstrated tumor regression after co-treatment, in which they were either having simultaneous PI3K pathway genetic alternations, K-Ras or B-Raf mutations, suggesting dual blockage strategy was effective in patients with these mutations. Hence, the results suggested that concurrent blockade of both pathways may be efficacious but at the expense of increased toxicities [13,29]. The result of an ongoing phase Ib study of MEK162 and BYL719 co-treatment in adult patients with selected advanced solid tumors is eagerly awaited (NCT01449058).
The profiling results of Cancer Cell Line Encyclopedia (CCLE) confirmed that PIK3CA mutation status affects BYL719’s sensitivity and it was found to be the most significant mutation feature for predicting the response of BYL719. The response rate for PIK3CA mutant cell lines was 64% while the response rate for PIK3CA wild-type group was only 22%. The PIK3CA mutation feature illustrated a 3-fold improvement in response rate against wild-type and a 2.5-fold over random. PIK3CA amplification and NRAS mutation have also been found to have a positive effect on BYL719’s response [11]. In NPC, the incidence of PIK3CA hotspot mutations has been reported to be between 0 to 9.6% 15, 25. However, these studies examined relatively small number of tumor samples and thus the actual incidence remains to be defined.
It was reported that persistent mTORC1 activity was found in BYL719 resistant cell lines. A phase 1 clinical trial of BYL719 in patients with PIK3CA mutant solid tumors showed that mTORC1 inhibition is crucial for the growth inhibitory effect of BYL719 in PIK3CA mutant cancers. Strong suppression of S6 phosphorylation was observed in breast cancer biopsies derived from patients who had responded to BYL719. mTORC1 inhibition also enhanced the efficacy of BYL719, and the combination of BYL719 and RAD001 was found to yield better anti-tumor activity than if either of these drugs were used alone [30]. Clinically, BYL719 is being evaluated in various cancers, including esophageal squamous cell carcinoma (ESCC), metastatic head and neck squamous cell carcinoma, pancreatic and ovarian cancers. In the on-going clinical trials, BYL719 is combined with MEK inhibitor, MEK162, in the treatment of advanced solid tumors (NCT01449058). It is also being combined with ERBB antibodies, cetuximab (NCT01602315) and LJM716 (NCT01922613). Combinational therapy is a promising approach for the development of BYL719.
Conclusion
The result of the current study supports the clinical evaluation of BYL719 in NPC in combination with inhibitors of MEK signaling or cisplatin-based chemotherapy. The association between sensitivity to BYL719 and PIK3CA mutations warrants further investigations in animal models.
Acknowledgements
This work is supported by an independent research grant from Novartis. The authors would like to thank The Charlie Lee Charitable Foundation for the generous support of this study. The study was presented at American Association for Cancer Research Annual Meeting 2014 and the 19th Annual Scientific Symposium of the Hong Kong Cancer Institute.
Disclosure of conflict of interest
Brigette Ma received honorarium for consultancy and research grants from Novartis. The rest of the authors declare that they have no conflict of interest in this study.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Caponigro F, Longo F, Ionna F, Perri F. Treatment approaches to nasopharyngeal carcinoma: a review. Anti-Cancer Drugs. 2010;21:471–477. doi: 10.1097/CAD.0b013e328337160e. [DOI] [PubMed] [Google Scholar]
- 3.Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10:610–616. doi: 10.1111/j.1467-789X.2009.00607.x. [DOI] [PubMed] [Google Scholar]
- 4.Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10:878–889. doi: 10.1038/nrc2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks L, Yao QY, Rickinson AB, Young LS. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falasca M. PI3K/Akt Signalling Pathway Specific Inhibitors: A Novel Strategy to Sensitize Cancer Cells to Anti-Cancer Drugs. Curr Pharm Des. 2010;16:1410–1416. doi: 10.2174/138161210791033950. [DOI] [PubMed] [Google Scholar]
- 7.Chen J. The Src/PI3K/Akt signal pathway may play a key role in decreased drug efficacy in obesity-associated cancer. J Cell Biochem. 2010;110:279–280. doi: 10.1002/jcb.22572. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Huang XF, Katsifis A. Activation of signal pathways and the resistance to anti-EGFR treatment in colorectal cancer. J Cell Biochem. 2010;111:1082–1086. doi: 10.1002/jcb.22905. [DOI] [PubMed] [Google Scholar]
- 9.Hui ABY, Lo KW, Teo PML, To KF, Huang DP. Genome wide detection of oncogene amplifications in nasopharyngeal carcinoma by array based comparative genomic hybridization. Int J Oncol. 2002;20:467–473. [PubMed] [Google Scholar]
- 10.Or YYY, Hui ABY, To KF, Lam CNY, Lo KW. PIK3CA mutations in nasopharyngeal carcinoma. Int J Cancer. 2006;118:1065–1067. doi: 10.1002/ijc.21444. [DOI] [PubMed] [Google Scholar]
- 11.Fritsch C, Huang A, Chatenay-Rivauday C, Schnell C, Reddy A, Liu M, Kauffmann A, Guthy D, Erdmann D, De Pover A, Furet P, Gao H, Ferretti S, Wang Y, Trappe J, Brachmann SM, Maira SM, Wilson C, Boehm M, Garcia-Echeverria C, Chene P, Wiesmann M, Cozens R, Lehar J, Schlegel R, Caravatti G, Hofmann F, Sellers WR. Characterization of the Novel and Specific PI3Kα Inhibitor NVP-BYL719 and Development of the Patient Stratification Strategy for Clinical Trials. Mol Cancer Ther. 2014;13:1117–1129. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 12.Furet P, Guagnano V, Fairhurst RA, Imbach-Weese P, Bruce I, Knapp M, Fritsch C, Blasco F, Blanz J, Aichholz R, Hamon J, Fabbro D, Caravatti G. Discovery of NVP-BYL719 a potent and selective phosphatidylinositol-3 kinase alpha inhibitor selected for clinical evaluation. Bioorg Med Chem Lett. 2013;23:3741–3748. doi: 10.1016/j.bmcl.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE, Piccart-Gebhart MJ. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev. 2013;39:935–946. doi: 10.1016/j.ctrv.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Juric D, Argiles G, Burris H, Gonzalez-Angulo A, Saura C, Quadt C, Douglas M, Demanse D, De Buck S, Baselga J. Phase I study of BYL719, an alpha-specific PI3K inhibitor, in patients with PIK3CA mutant advanced solid tumors: preliminary efficacy and safety in patients with PIK3CA mutant ER-positive (ER+) metastatic breast cancer (MBC) Cancer Res. 2012;72:P6–10. [Google Scholar]
- 15.Or YY, Hui ABY, Tam KY, Huang DP, Lo KW. Characterization of chromosome 3q and 12q amplicons in nasopharyngeal carcinoma cell lines. Int J Oncol. 2005;26:49–56. [PubMed] [Google Scholar]
- 16.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turke AB, Song Y, Costa C, Cook R, Arteaga CL, Asara JM, Engelman JA. MEK Inhibition Leads to PI3K/AKT Activation by Relieving a Negative Feedback on ERBB Receptors. Cancer Res. 2012;72:3228–3237. doi: 10.1158/0008-5472.CAN-11-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma BB, Lui VW, Hui CW, Lau CP, Wong CH, Hui EP, Ng MH, Tsao S, Li Y, Chan AT. Preclinical evaluation of the AKT inhibitor MK-2206 in nasopharyngeal carcinoma cell lines. Invest New Drugs. 2013;31:567–575. doi: 10.1007/s10637-012-9896-5. [DOI] [PubMed] [Google Scholar]
- 19.Chan SL, Wong CH, Lau CP, Zhou Q, Hui CW, Lui VW, Ma BB, Chan AT, Yeo W. Preclinical evaluation of combined TKI-258 and RAD001 in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2013;71:1417–1425. doi: 10.1007/s00280-013-2139-4. [DOI] [PubMed] [Google Scholar]
- 20.Wong CH, Loong HH, Hui CW, Lau CP, Hui EP, Ma BB, Chan AT. Preclinical evaluation of the PI3K-mTOR dual inhibitor PF-04691502 as a novel therapeutic drug in nasopharyngeal carcinoma. Invest New Drugs. 2013;31:1399–1408. doi: 10.1007/s10637-013-0007-z. [DOI] [PubMed] [Google Scholar]
- 21.Ma BB, Lui VW, Hui CW, Lau CP, Wong CH, Hui EP, Ng MH, Cheng SH, Tsao SW, Tsang CM. Preclinical evaluation of the mTOR-PI3K inhibitor BEZ235 in nasopharyngeal cancer models. Cancer Lett. 2014;343:24–32. doi: 10.1016/j.canlet.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 24.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372–83. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 25.Juric D, Rodon J, Gonzalez-Angulo A, Burris H, Bendell J, Berlin J, Middleton M, Bootle D, Boehm M, Schmitt A. BYL719, a next generation PI3K alpha specific inhibitor: Preliminary safety, PK, and efficacy results from the first-in-human study. Cancer Res. 2012:72. [Google Scholar]
- 26.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 27.Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the Point of Inhibition: A Comparative Review of PI3K/AKT/mTOR Pathway Inhibitors. Mol Cancer Ther. 2014;13:1021–1031. doi: 10.1158/1535-7163.MCT-13-0639. [DOI] [PubMed] [Google Scholar]
- 28.Clermont AC, Cahill M, Salti H, Rook SL, Rask-Madsen C, Goddard L, Wong JS, Bursell D, Bursell SE, Aiello LP. Hepatocyte growth factor induces retinal vascular permeability via MAP-kinase and PI-3 kinase without altering retinal hemodynamics. Invest Ophthalmol Vis Sci. 2006;47:2701–2708. doi: 10.1167/iovs.05-0071. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, Smith LS, Gunn S, Smetzer L, Mays TA, Kaiser B, Wick MJ, Alvarez C, Cavazos A, Mangold GL, Patnaik A. The Clinical Effect of the Dual-Targeting Strategy Involving PI3K/AKT/mTOR and RAS/MEK/ERK Pathways in Patients with Advanced Cancer. Clin Cancer Res. 2012;18:2316–2325. doi: 10.1158/1078-0432.CCR-11-2381. [DOI] [PubMed] [Google Scholar]
- 30.Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, Serra V, Rodrik-Outmezguine V, Hazra S, Singh S, Kim P, Quadt C, Liu M, Huang A, Rosen N, Engelman JA, Scaltriti M, Baselga J. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med. 2013;5:196ra99. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]


