Abstract
F1FoATP synthase (ATP synthase) is a ubiquitous enzyme complex in eukaryotes. In general it is localized to the mitochondrial inner membrane and serves as the last step in the mitochondrial oxidative phosphorylation of ADP to ATP, utilizing a proton gradient across the inner mitochondrial membrane built by the complexes of the electron transfer chain. However some cell types, including tumors, carry ATP synthase on the cell surface. It was suggested that cell surface ATP synthase helps tumor cells thriving on glycolysis to survive their high acid generation. Angiostatin, aurovertin, resveratrol, and antibodies against the α and β subunits of ATP synthase were shown to bind and selectively inhibit cell surface ATP synthase, promoting tumor cell death. Here we show that ATP synthase β (ATP5B) is present on the cell surface of mouse pheochromocytoma cells as well as tumor cells of human SDHB-derived paragangliomas (PGLs), while being virtually absent on chromaffin primary cells from bovine adrenal medulla by confocal microscopy. The cell surface location of ATP5B was verified in the tissue of an SDHB-derived PGL by immunoelectron microscopy. Treatment of mouse pheochromocytoma cells with resveratrol as well as ATP5B antibody led to statistically significant proliferation inhibition. Our data suggest that PGLs carry ATP synthase on their surface that promotes cell survival or proliferation. Thus, cell surface ATP synthase may present a novel therapeutic target in treating metastatic or inoperable PGLs.
Keywords: Cell surface ATP synthase, paraganglioma, pheochromocytoma, resveratrol, mouse pheochromocytoma cells
Introduction
F1Fo-ATP synthase (ATP synthase) is a ubiquitous enzyme complex in eukaryotes. In general it is localized to the mitochondrial inner membrane. There it utilizes the proton gradient across the inner mitochondrial membrane, which is built by the complexes of the electron transfer chain, to catalyze the final step in the mitochondrial oxidative phosphorylation of ADP to ATP. Within the past decade, ATP synthase has been shown to play a role in cancer [1-3] and was suggested as a therapeutic target, particularly when expressed on the cell surface [4-6].
Initially cell surface ATP synthase was discovered on endothelial cells as a target of angiostatin [7]. Angiostatin was shown to inhibit tumor angiogenesis and thus was evaluated as a promising therapeutic agent. However, initial clinical trials were not as successful as hoped, because angiostatin proved to be rapidly degraded in the bloodstream and because it was used to target tumors nonspecifically. Nevertheless the combination of angiostatin with chemotherapy led to promising results (39.1% of patients showed a partial response and stable disease was observed in another 39.1% of patients) [8].
However, recently, cell surface ATP synthase has been also discovered on certain other cell types, including tumor cells, neurons, adipocytes, liver [9], heart [10], and immune cells [11,12]. Inhibition of cell surface ATP synthase with angiostatin, aurovertin, resveratrol, or antibodies against the α and β subunits of ATP synthase effectively and specifically inhibited proliferation of various tumor cells, including colon carcinoma [13], lung cancer [14-16], breast cancer [17], hepatoma [18], osteosarcoma [19], and glioma cells [20], especially under low pH conditions [6]. Cell types on which surface ATP synthase has been found, drugs that have been effectively used to target it, and its possible functions have recently been reviewed [9].
Thus, although the role of cell surface ATP synthase is still unclear, it has been suggested that it benefits tumor cells thriving on aerobic glycolysis by helping them survive their high acid generation by shuttling protons out of the cell to create both a physiological intracellular pH and an acidic extra-cellular environment [21]. An acidic micro-environment may trigger local destabilization of the extracellular matrix, facilitating tumor growth and tissue invasion [21]. Due to the acidic micro-environment surrounding many tumors, cell surface ATP-synthase inhibition holds the potential to specifically kill tumor cells, either directly or by killing endothelial cells of the microvessels that nourish the tumor.
As in most cancers, current treatment options for metastatic pheochromocytomas (PHEOs) and paragangliomas (PGLs), i.e. catecholamine producing tumors of the sympathetic nervous system, are limited and rarely curative. Thus, specific new therapeutic targets for the treatment of inoperable and metastatic PHEOs/PGLs need to be detected.
Like all cells in the body, PGL cells receive the majority of their energy from ATP. Recently, the energy metabolism in PHEOs/PGLs was much considered [22]. It has been shown that certain types of PHEOs/PGLs exhibit a glycolytic phenotype. In particular PHEOs/PGLs derived due to von Hippel-Lindau syndrome (VHL) or succinate dehydrogenase subunit B, C, and D (SDHB/C/D) mutations were shown to have an increased glycolytic activity [23,24], and thus, are susceptible to a low extracellular pH. Despite these similarities, the metastatic potentials of these types of PHEOs/PGLs are distinct: patients with VHL related PHEOs/PGLs almost never develop metastatic disease, while tumors due to SDHB mutations are at high risk to develop metastases. Recently, we have shown an increased expression of ATP5B in SDHB- compared to VHL-derived primary tumors [25]. Thus, we aimed to evaluate potential cell surface localization of ATP synthase particularly in aggressive PHEOs/PGLs and its potential as a therapeutic target.
Currently no human PHEO or PGL cell line exists. Thus, we evaluated the location of ATP synthase in mouse pheochromocytoma cells (MPC) as well as the more aggressive filial mouse tumor tissue (MTT) cells and cells from two human primary SDHB-derived retroperitoneal PGLs via immunocytochemistry. Bovine primary chromaffin cells were used as negative control tissue and HepG2 cells, which have been reported to carry ATP synthase on their surface, were included as positive controls [26-29]. The results were validated in an additional human SDHB-derived primary PGL by electron microscopy. Targeting of cell surface ATP synthase with resveratrol and an ATP5B antibody was attempted.
Cell surface ATP synthase may present a specific therapeutic target for the treatment of metastatic disease as well as in cases of inoperable PGLs.
Material and methods
Human PHEO/PGL tissue
PHEO/PGL tissue was collected with informed patients consent at the National Institutes of Health (NIH) and the Suburban Hospital in Bethesda, MD, under a protocol approved by the NICHD IRB. Genetic testing was performed, following informed patient consent, using established guidelines for testing [30] and consideration of biochemical phenotypes. Tumor tissue was routinely evaluated immunohistochemically for expression of markers for neuroendocrine tumors and characterized as PGL by the Pathology Department of the NIH.
Cell culture
Mouse pheochromocytoma cells (MPC 4/30PRR) were developed and provided by Dr. Tischler’s lab, Tufts Medical Center, Boston, MA [31]. Their more aggressive filial MTT (mouse tumor tissue-derived) cells were generated and maintained as described by Martiniova et al. [32]. Briefly, cells of liver tumors, established in nude mice after tail vein injection of MPC, were cultured and showed more aggressive behavior when re-injected than their parental MPCs. Catecholamine content was verified by HPLC for a range of passage numbers of both cell types, covering the ones used in this study.
MPC, MTT, and HepG2 cells were maintained at 5% CO2, 37°C in DMEM (Gibco, Grand Island, NY), supplemented with 10% heat-inactivated horse serum (Hyclone, Logan, UT), 5% fetal bovine serum (Gibco), HEPES (Gibco), and penicillin (10,000 units/ml)/streptomycin (100,000 µg/ml) (Gibco). Medium was changed every other day and cells were passaged when 80-90% confluence was reached.
Human primary PGL cells
Tissue was collected from two retroperitoneal PGLs - one from a 15-year-old male patient with SDHB germline mutation (PGL1), and one from a 41-year-old female without germline point mutations or deletions in the SDHB, -C, and -D genes (PGL2) - and placed in culture media. There was no evidence for one of the PGL syndromes in the latter patient. Central viable parts of tumor specimens were used for primary culture. Dissected tissues were washed three times in ice-cold PBS and minced into 1 mm3 cubes. Tissue was then dissociated in 0.025% trypsin and DNase I at 37°C for 20 min. The digested fluid was passed through a 40 μm cell strainer to obtain a single cell suspension. Cells were seeded on collagen coated chamber slides in a concentration of 5,000 cells/cm2. Cells were grown in DMEM supplemented with 10% FBS for at least one week before consequent experiments.
Bovine primary chromaffin cells
Bovine primary chromaffin cells were harvested and separated as previously described [33]. Cell identity was verified by immunocytochemical staining for tyrosine hydroxylase as described below.
Immunocytochemistry
Immunocytochemistry was performed in the same way for mouse MPC, MTT, HepG2, bovine primary chromaffin cells and human primary PGL cells.
Lab-TekTM II chamber slide (Nalge Nunc International, Rochester, NY, USA) surfaces were evenly covered with 0.1 mg/ml rat tail collagen type VII (Sigma-Aldrich) in cell culture grade H2O with 0.01% acetic acid. Chamber slides were allowed to air dry in a fume hood. When dry, 0.5 mill cells/ml (MPC and MTT) or 0.25 mill cells/ml (bovine primary chromaffin cells and primary SDHB-derived cells) were seeded in DMEM culture media supplemented as described above. Mitochondria were labeled during 15 min incubation with 500 nM MitoTracker® Red CM-H2-XRos at 37°C (Invitrogen, Molecular Probes, Eugene OR, USA). After washing the cells in PBS, they were fixed in 4% paraformaldehyde solution. Half of the cells were permeabilized with methanol, pre-cooled to -20°C for 3 minutes, while the other half was not permeabilized. Cells were blocked in 10% donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 h before incubation in a 1:1000 dilution of monoclonal anti ATP5B antibody (ab14730) from Abcam (Cambridge, MA, USA) for 4 hours (except “no primary” control wells). For HepG2, primary bovine chromaffin cells, and PGL2 cells, the previously non-permeabilized cells were then permeabilized and all wells, except the “no primary” control wells, were incubated with anti-tyrosine hydroxylase antibody for 4 hours (1:1000 ab93291, abcam). The secondary antibodies were Alexa Fluor® 647 donkey anti-rabbit and Alexa Fluor® 488 donkey anti-mouse (Invitrogen, Molecular Probes). The labeled cells were cover-slipped in a 0.12 M tris solution, consisting of 30% glycerol, 12% mowiol 4-88 (Calbiochem, EMD Chemicals, Inc., Gibbstown, NJ, USA), 2.5% 1,4-diazobicyclo-[2.2.2]-octane (DABCO) (Sigma-Aldrich), pH 6.8. Images were taken with a Zeiss 510 inverted microscope.
Immunoelectron microscopy
Tissue from an SDHB-derived cardiac PGL of a male 53-year-old patient with multiple tumors was selected for immunoelectron microscopy. A carotid body tumor invading the nasopharynx was removed 2 month previously, but there were no signs of metastatic disease. Tissue was cut from a paraffin block and labeled as previously described [34]. The same ATP5B antibody was used as for immunocytochemistry (ab14730, abcam) at 1:500. Identity of the examined tissue as PGL was confirmed by the presence of secretory granules.
In vitro drug response
MPC and MTT cells were cultured at 1 mill cells/ml in collagen I coated 96 well plates (Biocoat, Becton Dickinson Labware, Bedford, MD) in DMEM media, supplemented as described above. After 24 h, media was exchanged to the respective drug solutions in pH 6.5 and pH 7.0 supplemented DMEM. Drug solutions were monoclonal anti-ATP5B antibody; (Novus Biologicals, Littleton, CO, USA) and non-immune IgG (Novus Biologicals): 50, 25, 10 μg/ml; oligomycin (Cell Signaling Technology, Dauvers, MA, USA): 10 μg/ml; and resveratrol (Sigma-Aldrich Corp., St. Louis, MO, USA): 100, 50, 10, 5 μM.
Cell proliferation was measured with the CellTitre-Flour cell viability assay (Promega, Madison WI, USA) after 24 h for antibody, and over 4 consecutive days (24, 48, 72, 96 h) for resveratrol with the cell proliferation kit II (XTT) (Roche Diagnostics Corporation, Indianapolis, IN USA) following manufacturer recommendations. Drug solutions were exchanged every 48 h.
Statistical tests were done in Prism 4 (GraphPad Software Inc., LaJolla, CA, USA) with one-way ANOVAs for comparing, in different cell types, different antibody and IgG solutions (treatments), followed when appropriate by Dunnett’s multiple comparison post-hoc test to compare multiple treatments vs. a common vehicle. In addition a Bonferroni multiple comparison post-hoc test was employed to evaluate statistical differences between respective antibody and IgG concentrations. Differences between cell types were evaluated by one-way ANOVA and differences between pH were evaluated with unpaired T-test for each cell type. To estimate significance of the effect of the different resveratrol solutions on the subsequent days of measurement, two-way ANOVA with Bonferonni post-hoc test was used for each cell type and pH separately comparing the ratio of each treatment to vehicle and the ratio of media only to vehicle.
Results
Fluorescent labeling for ATP5B led to a strong signal in the mitochondria of both MPC and MTT cells, as shown by overlapping red (mitotracker) and green (ATP5B antibody) which appears as a yellow signal. In addition, a positive signal for ATP5B that was spatially unrelated to the mitochondria was detected (Figure 1). In non-permeabilized cells there was no overlap in staining of ATP5B and the mitochondria (Figure 1). In the absence of primary antibody, no signal was detected in the green channel (Figure 1). When examining bovine primary chromaffin cells with the same methods, we detected only minute amounts of ATP5B signal that were not related to the mitochondria (Figure 2A). To assure chromaffin cell origin, cells were co-labeled with anti-tyrosine hydroxylase antibody (Figure 2A). For comparison of the ATP synthase staining pattern of PGL to that of cells that have been previously reported to carry ATP synthase on their surface, we also carried out immunocytochemical staining of HepG2 cells (Figure 2B). The labeling pattern of MPC and MTT cells closely resembles that of HepG2 cells.
Figure 1.
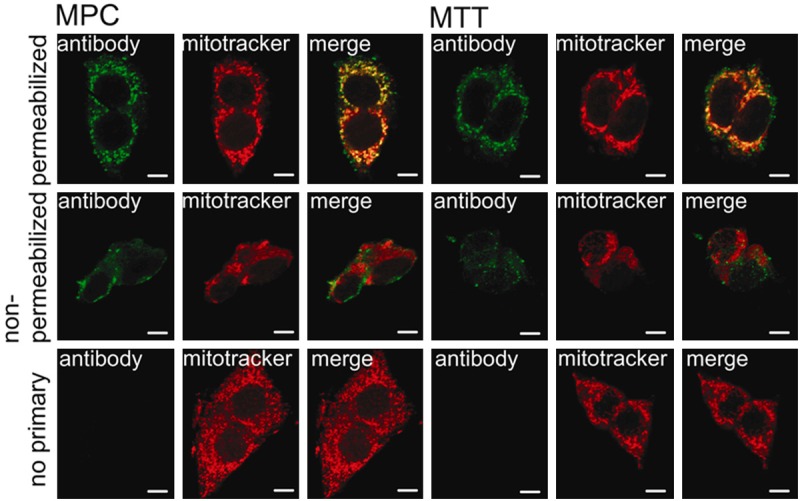
ATP synthase is present on the surface of mouse pheochromocytoma cells. Separate confocal microscopy channels of MPC (left) and MTT (right) cells labeled with ATP5B antibody (green signal) and mitotracker (red signal) and merged channels. The top row shows results for permeabilized cells, the center panel shows non-permeabilized cells, and the bottom panel shows no primary antibody negative controls. Both MPC and MTT showed strong positive staining for ATP5B that greatly, but not entirely, overlapped with the mitochondria (top row). Signal for ATP5B on non-permeabilized cells does not overlap with mitochondrial staining, indicating cell surface location of ATP5B (second row). Application of secondary antibody only did not lead to any detectable signal (bottom row). Scale bar indicates 10 μm.
Figure 2.
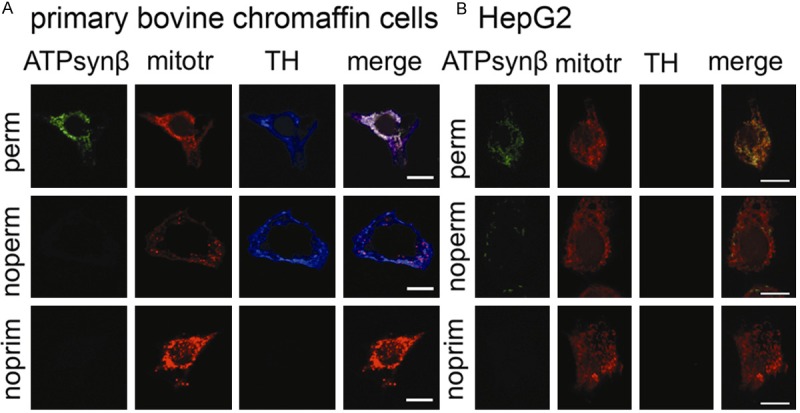
ATP synthase is absent from the surface of normal bovine chromaffin cells. Confocal images of primary bovine chromaffin cells (A) and HepG2 cells (B) labeled with mouse anti-ATP5B (ATPsynβ) or tyrosine hydroxylase (TH) antibody and mitotracker (mitotr) in permeabilized cells (perm, top row), non-permeabilized cells (noperm, second row), and no primary antibody negative controls (noprim, third row). Merged channels for ATP5B and mitotracker show complete overlap in permeabilized cells (top row), while no signal for ATP5B was evident in non-permeabilized cells (second row) or the negative control (third row) for primary bovine chromaffin cells (A). Positive staining for TH confirms chromaffin cell origin. Non-permeabilized HepG2 cells showed punctate ATP5B signal. As expected, no TH signal was detectable for HepG2 cells. In conclusion cell surface localization of ATP5B was non-detectable in primary chromaffin cells while present on HepG2 cells. Scale bar indicates 10 μm.
In addition, primary cells of human retroperitoneal PGLs were evaluated for cell surface localization of ATP5B. The cell surface signal of ATP5B in the non-permeabilized human primary PGL1 was not as pronounced as in the MPC and MTT cells. However, primary cells from human PGL2 showed similar signal intensity for ATP synthase to that observed in MPC, MTT and HepG2 (Figure 3A, 3B). For PGL1 co-staining with the TH antibody has not been performed.
Figure 3.
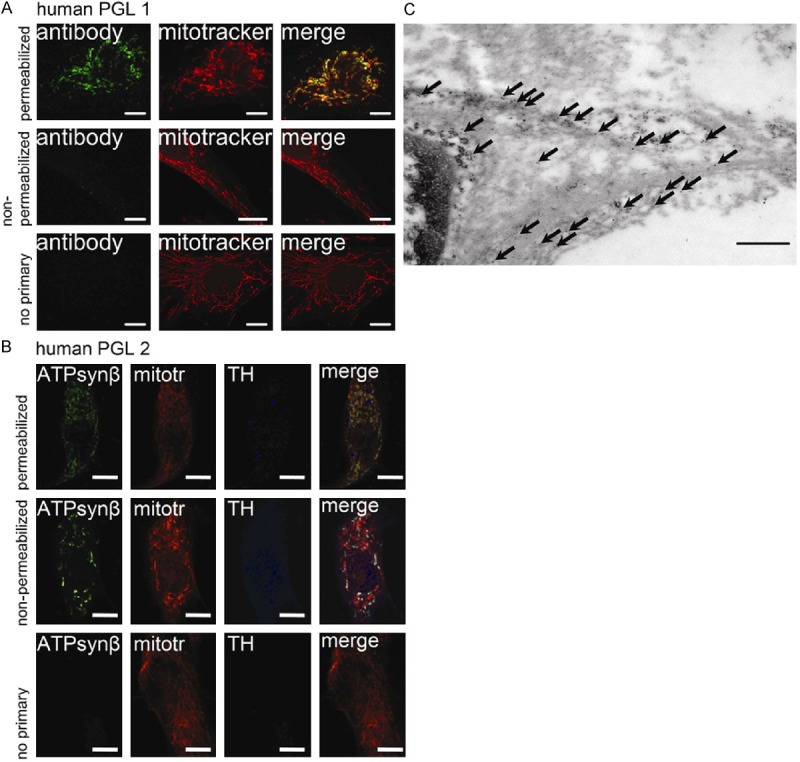
Cell surface ATP synthase in human paragangliomas. Confocal images of SDHB-derived primary paraganglioma cells (A: PGL1, B: PGL2) labeled with mouse anti-ATP5B antibody in permeabilized cells (top row), non-permeabilized cells (second row), and negative control (secondary antibody only, third row). PGL2 was additionally labeled with anti-tyrosine hydroxylase antibody. Merged channels for ATP5B and mitotracker show overlap in permeabilized cells. However, focal regions of ATP5B signal do not correspond to mitochondria (top row). Focal regions of positive ATP5B signal were also evident in non-permeabilized cells, while no overlap with the mitochondrial staining is evident (second row). Signals for the green and blue channels (were applicable) were absent on the negative control (third row). Scale bar indicates 10 μm. (C) Electron microscopy image of an SDHB-derived paraganglioma labeled for ATP5B with immuno-gold. Arrows indicate location of ATP5B labeled with gold particles within the cytosol as well as on the cell membrane. Scale bar indicates 500 nm. Abbreviations: ATPsynβ: ATP5B, mitotr: mitotracker, TH: tyrosine hydroxylase.
To support cell surface localization of ATP synthase in human PGL tissue, immuno-gold labeling of another SDHB-derived PGL was performed. As expected, transmission electron microscopy revealed the presence of gold particles in the cytosol in the vicinity of mitochondria. In addition, accumulation of gold particles at the plasmalemmal membrane was observed (Figure 3C).
The effect of treating MPC and MTT cells with an antibody against ATP5B in pH 7.0 and pH 6.5 media for 24 hours was determined fluorometrically. Cell proliferation in MPC and MTT cells was significantly decreased when treated with 50 μg/ml antibody (MPC pH 6.5 and pH 7.0: p<0.05, MTT pH 6.5 and pH 7.0: p<0.01) compared to vehicle (Figure 4). Statistical evaluation of differences between antibody and the respective IgG concentrations showed a significant difference in proliferation between cells treated with 50 µg/ml ATP5B antibody and non-immune IgG for MPC at pH 6.5, and for MTT at both pH conditions tested (p<0.05). However, no significance was reached for MPC treated at pH 7.0. There was no difference in effect between the different pH media or cell types. Oligomycin was used as a positive control and showed a slightly higher decrease in proliferation than the highest dose of ATP synthase antibody.
Figure 4.
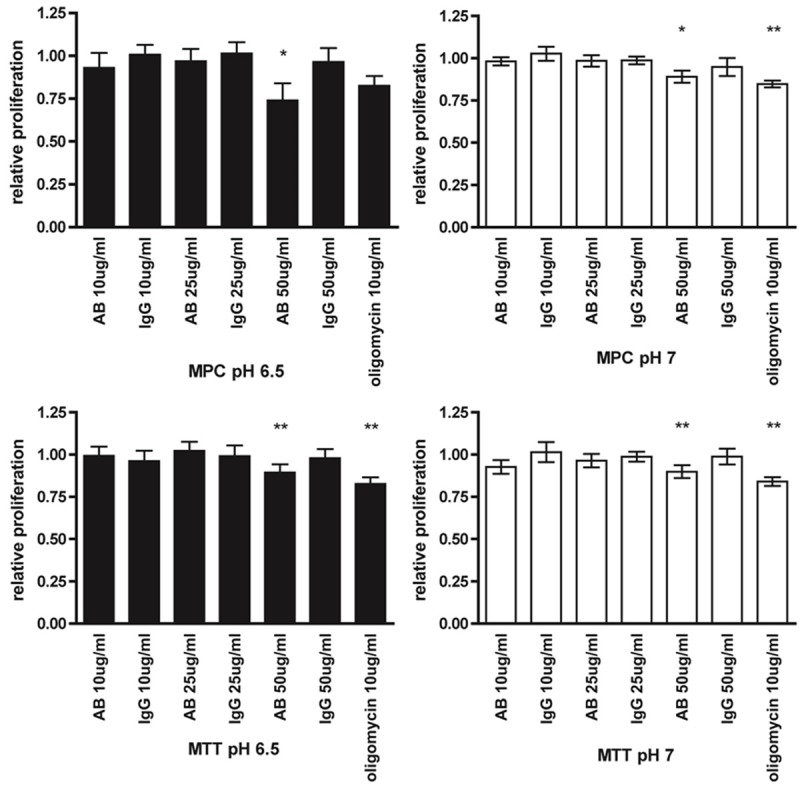
Targeted treatment with ATP synthase β antibody. Proliferation of MPC (top) and MTT (bottom) cells, treated for 24 h with the indicated concentrations of ATP5B antibody (AB), non-immune IgG, and oligomycin relative to vehicle in pH 7.0 (right) and pH 6.5 solution (left), detected with the Cell-titre fluor assay (Promega). Under all test conditions, 50 μg/ml AB inhibited proliferation with slightly less efficiency than oligomycin. *: p<0.05, **: p<0.01.
Next we evaluated the response of MPC and MTT cell proliferation to treatment with the ATP synthase inhibitor resveratrol (5 μM, 10 μM, 50 μM, 100 μM) in pH 7.0 and pH 6.5 media over 4 days. The relative proliferation compared to vehicle (0.1% ethanol in media) decreased with increasing dose and treatment duration (Figure 5). A concentration of 100 μM significantly decreased proliferation compared to media from day 2 on under all conditions (MPC: day 2, day 3, and day 4, p<0.001 for both pH conditions; MTT: day 2, day 3, and day 4, p<0.001 for both pH conditions). Treatment with 50 μM resveratrol led to significantly decreased cell proliferation for all tested conditions in MPC and MTT from day 3 on (p<0.001), and for MPC at pH 6.5 even on day 2 (p<0.01). A dose of 10 μM resveratrol led to a significant difference from media for MPC at pH 7.0 and MTT at pH 6.5 on day 3, as well as for MPC at pH 6.5 on day 4. Proliferation of cells treated with 5 μM resveratrol led to a slightly but significantly decreased value relative to media for MPC pH 7.0 and MTT at pH 6.5 on day 3 and MPC and MTT at pH 6.5 on day 4 (p<0.05). The efficacy of resveratrol was independent of different pH conditions and cell type treated.
Figure 5.
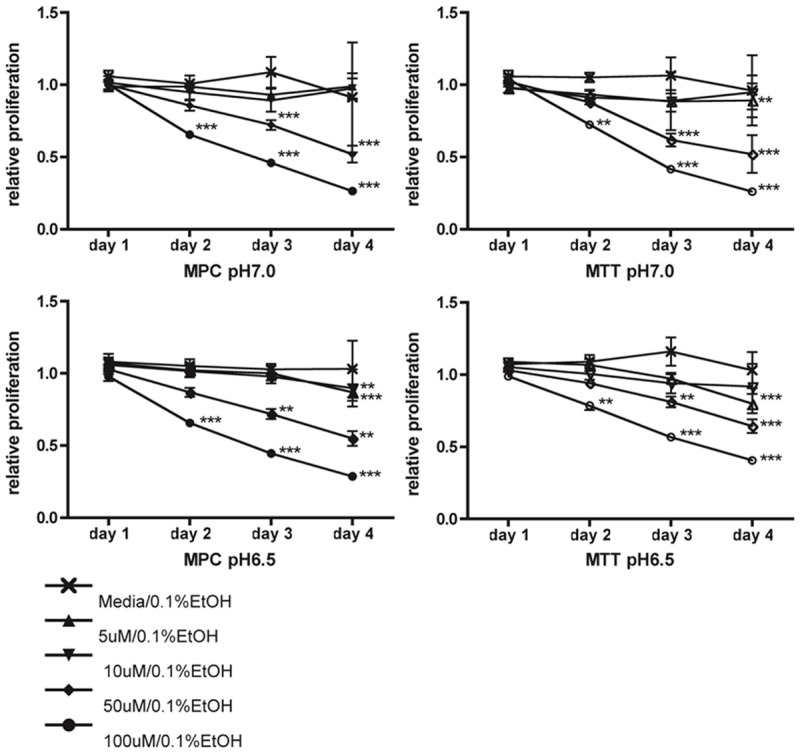
Targeted treatment with resveratrol. Proliferation of MPC (left) and MTT (right) cells treated with indicated concentrations of resveratrol in pH 7.0 (top) and pH 6.5 (bottom) for four consecutive days relative to vehicle, as assessed by MTT assay. Cell proliferation was inhibited by day two under all tested conditions with 100 μM resveratrol. Resveratrol at 50 μM significantly decreased proliferation by day three. **: p<0.01, ***: p<0.001.
Discussion
Within the past 15 years more and more evidence for an important role of ATP synthase dysregulation in cancer has appeared. In several cancers up- or down-regulation of specific ATP synthase subunits has been shown [1,35-38], but in addition, its localization on the cell surface in tumor supporting endothelial cells [7] as well as on tumor cells themselves [13-15,17-20] has been observed. However, some other normal cells/tissues have been reported to carry ATP synthase on their surface (e.g. neurons/brain, adipocytes/fat, hepatocytes/liver) [9]. Nevertheless, targeting cell surface ATP synthase on tumor supporting endothelial cells and/or directly on tumor cells with angiostatin, ATP synthase antibodies, resveratrol, aurovertin, sangivamycin, and other drugs has been shown to be efficient in killing tumor cells [5,9].
Here we show that ATP5B is present on the cell surface of PHEO/PGL cells: in vitro on MPC and MTT cells and on primary cells of two retroperitoneal human PGLs as well as tumor tissue of an SDHB-derived cardiac PGL, while being virtually absent from the surface of bovine primary chromaffin cells. Chromaffin cell origin was assured by positive labeling of tyrosine hydroxylase (Figure 2), showing strong positive staining in bovine chromaffin cells, while the signal was less pronounced in human primary PGL cells. This could be due to a species-related difference in antibody affinity or relatively lower catecholamine synthesis in the human paraganglioma. No differences in staining intensity for the ATP5B antibody have been observed, suggesting that its affinity is not influenced by the species of tested cells. Cell surface signal for ATP synthase was intense for MPC and MTT as well as for the primary cells of human SDHB PGL2, while appearing punctate in the primary cells of human SDHB PGL1 as has been observed in other human tumor cells [19]. Variability in the amount of ATP synthase on the surface of PGLs will have to be evaluated in additional tumors.
Assuming that the presence of ATP5B on the surface of PHEO/PGL cells represents a functional ATP synthase complex as has been observed in other tumors and normal cells [29,39,40], we evaluated the effect of in vitro treatment of the mouse PHEO cells with an ATP5B antibody and resveratrol. ATP synthase antibody was effective in inhibiting proliferation at the highest applied concentration of 50 μg/ml by at least 10% within 24 h of treatment, which was comparable to oligomycin, a well described ATP synthase inhibitor and cytotoxic agent. However, lower antibody concentrations and non-immune IgG had no effect on proliferation. It is unlikely that the applied antibody penetrates the cell membrane, so the effect is likely due to inhibition of the cell surface ATP synthase. While effective in mouse cells, potential interspecies differences in antibody affinity may be of concern when applying the ATP5B antibody to human cells. Thus, special consideration should be given to high affinity to human ATP synthase when attempting the evaluation of treatment of human PGLs.
Resveratrol dosed at 100 μM inhibited proliferation relative to media up to 44% (MPC) and up to 27% (MTT) on the second day of treatment and up to 73% (MPC) and 74% (MTT) on treatment day 4 compared to vehicle. A dose of 50 μM inhibited proliferation by up to 28% (MPC) and 38 % (MTT) on day 3 and 48% (MPC and MTT) on day 4 (Figure 5).
Resveratrol has been shown to have a cytostatic effect on cells carrying cell surface ATP synthase [20]. In addition, resveratrol has been shown to target a wide variety of molecular pathways, exhibiting chemopreventive and chemotherapeutic effects in a variety of cell and animal models of cancer (for excellent recent reviews see [41-43]). Clinical trials showed that its cancer preventive effects are promising while toxicity seems to be negligible [44-46]. Doses at or below 1 g/day were reported as non-cytotoxic in short-term tests in humans. However, long-term side effects remain to be evaluated (reviewed in [47]). The bioavailability of resveratrol is limited due to its hydrophobic capabilities as well as a rapid and complex turnover (reviewed by Santos et al. [48]). After oral administration of up to 5 mg of resveratrol, peak plasma concentrations in the low micromolar range (~2.3 µM) can be reached (reviewed in [47]). Currently, development of more efficient ways for systemic and targeted administration of resveratrol is underway [48]. Johnson et al. have recently reported that co-administration of piperine with resveratrol has led to a 1544% increased maximum serum concentration compared to resveratrol alone in mice [49]. Thus, the effective concentrations for in vitro treatment of MPC and MTT cells described here may not be reached by systemic administration of resveratrol in humans, but the intratumoral administration or utilization of new delivery systems, including direct tumor perfusion, have excellent and promising potential to reach therapeutic concentrations of resveratrol in a tumor.
In models of neurovegetative diseases using PC12 or neuroblastoma cells, resveratrol was shown to be neuroprotective in attenuating damage by reactive oxygen species [50,51]. However, neuroblastoma cell proliferation was also effectively inhibited by resveratrol [52]. In addition, resveratrol has been shown to be cardioprotective [53] and to improve longevity [54]. Bauer and collaborators showed that at least 127 different pathways may be affected by resveratrol [55]. Most described resveratrol targets are located inside the treated cells. For example, resveratrol was shown to interact with estrogen [56] and androgen [57] receptors and affect their expression as well as that of their target genes, suggesting a beneficial effect in breast and prostate cancer. Amongst effects on additional transcription factors, resveratrol was shown to decrease hypoxia inducible factor α levels and metastatic potential in colon carcinoma cells [58].
Interaction of resveratrol with ATP synthase has been previously reported [59]. Gledhill and colleagues reported that resveratrol binds to α and β subunits of the complex, inhibiting ATPase as well as ATP-synthase activity [60]. Treatment of human umbilical vein cells that carry ATP synthase on the surface with resveratrol was shown to inhibit proliferation based on inhibited cell surface ATP synthesis [61]. The possibility of resveratrol penetrating into the cells was rendered unlikely by the authors, since intracellular ATP levels were barely affected. Thus, the authors conclude that the efficacy of the drug was related to its inhibition of cell surface ATP synthase. Thus the detected ATP5B subunit on the cell surface may represent a functional ATP synthase complex that promotes PHEO/PGL survival. However, additional cell surface or intracellular targets for ATP synthase antibody and resveratrol cannot be ruled out.
Previously, low extracellular pH, as expected for glycolytic tumor cells, has been shown to enhance toxicity of cell surface ATP synthase inhibition with angiostatin and ATP synthase antibodies [16]. Thus, we applied the treatments in low pH media (pH 6.5) as well as neutral media (pH 7.0). However, we did not observe any pH-related difference in effect of the treatment with neither ATP5B antibody nor resveratrol. Thus, survival of MPC and MTT in an acidic environment may not depend on cell surface ATP synthase. Future studies are necessary to determine the functionality and role of ATP synthase on the cell surface of PGLs.
In addition to resveratrol and ATP synthase antibodies other drugs targeting cell surface ATP synthase have been shown to effectively inhibit proliferation of tumor cells. For example aurovertin has been shown to inhibit proliferation of breast cancer cell lines while having little effect on normal cells [17]. An expert review summarizes reports on cell surface ATP synthase inhibition in various cancers [6].
Despite the promising potential of ATP synthase inhibitors for the treatment of cancers, similar to any chemotherapeutic or other drug, adverse effects cannot be excluded since ATP synthase is located on the surface of other cells including heart, liver, neurons, and T lymphocytes. Therefore, any future clinical studies must be conducted carefully and organ function must be well monitored for any adverse effects when ATP synthase inhibitors are used. If these inhibitors are used for intratumoral administration or perfusion, side effects on other organs can be minimal.
In the present study, chromaffin cells or PGLs of murine, bovine, and human origin have been evaluated. While interspecies differences cannot be excluded, a comparison of chromaffin cells and PGLs from the same species is currently not possible. To date no cell line of human PGLs or chromaffin cells exists. Collection of mouse primary chromaffin cells is difficult due to the small size of the murine adrenal gland and requires large numbers of animals to collect sufficient cells for experiments. While the amount of chromaffin cells that can be collected from a bovine adrenal gland is satisfactory, bovine PGLs have not been reported.
The data presented here suggests that ATP synthase is localized on the cell surface of mouse and human PHEO/PGL cells, while it is virtually absent from the surface of bovine primary chromaffin cells. Treatment of MPC and MTT cells with ATP synthase antibody and resveratrol, which have been reported to target cell surface ATP synthase, inhibited cell proliferation of MPC and MTT cells. Thus, resveratrol and ATP synthase inhibitors may represent a promising new option for targeted treatment of metastatic or inoperable human PGLs.
Acknowledgements
We are indebted to V. Schram, L. Holtzclaw, and L. Dye from the NICHD imaging core facility for their expert assistance in microscopy imaging. This study was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD and supported by the institute’s imaging core facility. AST was supported by a grant from the Pheo-Para Alliance www.pheo-para-alliance.org/.
Disclosure of conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Chang HJ, Lee MR, Hong SH, Yoo BC, Shin YK, Jeong JY, Lim SB, Choi HS, Jeong SY, Park JG. Identification of mitochondrial FoF1-ATP synthase involved in liver metastasis of colorectal cancer. Cancer Sci. 2007;98:1184–1191. doi: 10.1111/j.1349-7006.2007.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin YK, Yoo BC, Chang HJ, Jeon E, Hong SH, Jung MS, Lim SJ, Park JG. Down-regulation of Mitochondrial F1F0-ATP Synthase in Human Colon Cancer Cells with Induced 5-Fluorouracil Resistance. Cancer Res. 2005;65:3162–3170. doi: 10.1158/0008-5472.CAN-04-3300. [DOI] [PubMed] [Google Scholar]
- 3.Willers IM, Cuezva JM. Post-transcriptional regulation of the mitochondrial H(+)-ATP synthase: A key regulator of the metabolic phenotype in cancer. Biochim Biophys Acta. 2010;1807:543–51. doi: 10.1016/j.bbabio.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen PL. The cancer cell’s “power plants” as promising therapeutic targets: an overview. J Bioenerg Biomembr. 2007;39:1–12. doi: 10.1007/s10863-007-9070-5. [DOI] [PubMed] [Google Scholar]
- 5.Chi SL, Pizzo SV. Cell surface F1Fo ATP synthase: a new paradigm? Ann Med. 2006;38:429–438. doi: 10.1080/07853890600928698. [DOI] [PubMed] [Google Scholar]
- 6.Mowery YM, Pizzo SV. Targeting cell surface F1F0 ATP synthase in cancer therapy. Cancer Biol Ther. 2008;7:1836–1838. doi: 10.4161/cbt.7.11.7155. [DOI] [PubMed] [Google Scholar]
- 7.Moser TL, Stack MS, Asplin I, Enghild JJ, Hojrup P, Everitt L, Hubchak S, Schnaper HW, Pizzo SV. Angiostatin binds ATP synthase on the surface of human endothelial cells. Proc Natl Acad Sci U S A. 1999;96:2811–2816. doi: 10.1073/pnas.96.6.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurup A, Lin CW, Murry DJ, Dobrolecki L, Estes D, Yiannoutsos CT, Mariano L, Sidor C, Hickey R, Hanna N. Recombinant human angiostatin (rhAngiostatin) in combination with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer: a phase II study from Indiana University. Ann Oncol. 2006;17:97–103. doi: 10.1093/annonc/mdj055. [DOI] [PubMed] [Google Scholar]
- 9.Vantourout P, Radojkovic C, Lichtenstein L, Pons V, Champagne E, Martinez LO. Ecto-F-ATPase: a moonlighting protein complex and an unexpected apoA-I receptor. World J Gastroenterol. 2010;16:5925–5935. doi: 10.3748/wjg.v16.i47.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippe G, Bisetto E, Comelli M, Contessi S, Di Pancrazio F, Mavelli I. Mitochondrial and cell-surface F0F1ATPsynthase in innate and acquired cardioprotection. J Bioenerg Biomembr. 2009;41:151–157. doi: 10.1007/s10863-009-9208-8. [DOI] [PubMed] [Google Scholar]
- 11.Ohta T, Ikemoto Y, Usami A, Koide T, Wakabayashi S. High affinity interaction between histidine-rich glycoprotein and the cell surface type ATP synthase on T-cells. Biochim Biophys Acta. 2009;1788:1099–1107. doi: 10.1016/j.bbamem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Yavlovich A, Viard M, Zhou M, Veenstra TD, Wang JM, Gong W, Heldman E, Blumenthal R, Raviv Y. Ectopic ATP synthase facilitates transfer of HIV-1 from antigen-presenting cells to CD4(+) target cells. Blood. 2012;120:1246–1253. doi: 10.1182/blood-2011-12-399063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung KH, Song SH, Paik JY, Koh BH, Choe YS, Lee EJ, Kim BT, Lee KH. Direct targeting of tumor cell F(1)F(0) ATP-synthase by radioiodine angiostatin in vitro and in vivo. Cancer Biother Radiopharm. 2007;22:704–712. doi: 10.1089/cbr.2007.369. [DOI] [PubMed] [Google Scholar]
- 14.Shi XJ, Knowles AF. Prevalence of the mercurial-sensitive EctoATPase in human small cell lung carcinoma: characterization and partial purification. Arch Biochem Biophys. 1994;315:177–184. doi: 10.1006/abbi.1994.1487. [DOI] [PubMed] [Google Scholar]
- 15.Lu ZJ, Song QF, Jiang SS, Song Q, Wang W, Zhang GH, Kan B, Chen LJ, Yang JL, Luo F, Qian ZY, Wei YQ, Gou LT. Identification of ATP synthase beta subunit (ATPB) on the cell surface as a non-small cell lung cancer (NSCLC) associated antigen. BMC Cancer. 2009;9:16. doi: 10.1186/1471-2407-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi SL, Pizzo SV. Angiostatin is directly cytotoxic to tumor cells at low extracellular pH: a mechanism dependent on cell surface-associated ATP synthase. Cancer Res. 2006;66:875–882. doi: 10.1158/0008-5472.CAN-05-2806. [DOI] [PubMed] [Google Scholar]
- 17.Huang TC, Chang HY, Hsu CH, Kuo WH, Chang KJ, Juan HF. Targeting Therapy for Breast Carcinoma by ATP Synthase Inhibitor Aurovertin B. J Proteome Res. 2008;7:1433–1444. doi: 10.1021/pr700742h. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Han Y, Liang J, Cheng X, Yan L, Wang Y, Liu J, Luo G, Chen X, Zhao L, Zhou X, Wu K, Fan D. Effect of a novel inhibitory mAb against beta-subunit of F1F0 ATPase on HCC. Cancer Biol Ther. 2008;7:1829–1835. doi: 10.4161/cbt.7.11.6861. [DOI] [PubMed] [Google Scholar]
- 19.Yonally SK, Capaldi RA. The F(1)F(0) ATP synthase and mitochondrial respiratory chain complexes are present on the plasma membrane of an osteosarcoma cell line: An immunocytochemical study. Mitochondrion. 2006;6:305–314. doi: 10.1016/j.mito.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Ravera S, Aluigi MG, Calzia D, Ramoino P, Morelli A, Panfoli I. Evidence for Ectopic Aerobic ATP Production on C6 Glioma Cell Plasma Membrane. Cell Mol Neurobiol. 2010;31:313–21. doi: 10.1007/s10571-010-9624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harguindey S, Arranz JL, Wahl ML, Orive G, Reshkin SJ. Proton transport inhibitors as potentially selective anticancer drugs. Anticancer Res. 2009;29:2127–2136. [PubMed] [Google Scholar]
- 22.Vicha A, Taieb D, Pacak K. Current views on cell metabolism in SDHx-related pheochromocytoma and paraganglioma. Endocr Relat Cancer. 2014;21:R261–277. doi: 10.1530/ERC-13-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, Giscos-Douriez I, De Reynies A, Bertherat J, Badoual C, Tissier F, Amar L, Libe R, Plouin PF, Jeunemaitre X, Rustin P, Gimenez-Roqueplo AP. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4:e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Jimenez E, Gomez-Lopez G, Leandro-Garcia LJ, Munoz I, Schiavi F, Montero-Conde C, de Cubas AA, Ramires R, Landa I, Leskela S, Maliszewska A, Inglada-Perez L, de la Vega L, Rodriguez-Antona C, Leton R, Bernal C, de Campos JM, Diez-Tascon C, Fraga MF, Boullosa C, Pisano DG, Opocher G, Robledo M, Cascon A. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24:2382–2391. doi: 10.1210/me.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fliedner SM, Kaludercic N, Jiang XS, Hansikova H, Hajkova Z, Sladkova J, Limpuangthip A, Backlund PS, Wesley R, Martiniova L, Jochmanova I, Lendvai NK, Breza J, Yergey AL, Paolocci N, Tischler AS, Zeman J, Porter FD, Lehnert H, Pacak K. Warburg Effect’s Manifestation in Aggressive Pheochromocytomas and Paragangliomas: Insights from a Mouse Cell Model Applied to Human Tumor Tissue. PLoS One. 2012;7:e40949. doi: 10.1371/journal.pone.0040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae TJ, Kim MS, Kim JW, Kim BW, Choo HJ, Lee JW, Kim KB, Lee CS, Kim JH, Chang SY, Kang CY, Lee SW, Ko YG. Lipid raft proteome reveals ATP synthase complex in the cell surface. Proteomics. 2004;4:3536–3548. doi: 10.1002/pmic.200400952. [DOI] [PubMed] [Google Scholar]
- 27.Vantourout P, Martinez LO, Fabre A, Collet X, Champagne E. Ecto-F1-ATPase and MHC-class I close association on cell membranes. Mol Immunol. 2008;45:485–492. doi: 10.1016/j.molimm.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Zhang LH, Kamanna VS, Zhang MC, Kashyap ML. Niacin inhibits surface expression of ATP synthase beta chain in HepG2 cells: implications for raising HDL. J Lipid Res. 2008;49:1195–1201. doi: 10.1194/jlr.M700426-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Ma Z, Cao M, Liu Y, He Y, Wang Y, Yang C, Wang W, Du Y, Zhou M, Gao F. Mitochondrial F1Fo-ATP synthase translocates to cell surface in hepatocytes and has high activity in tumor-like acidic and hypoxic environment. Acta Biochim Biophys Sin (Shanghai) 2010;42:530–537. doi: 10.1093/abbs/gmq063. [DOI] [PubMed] [Google Scholar]
- 30.Erlic Z, Rybicki L, Peczkowska M, Golcher H, Kann PH, Brauckhoff M, Mussig K, Muresan M, Schaffler A, Reisch N, Schott M, Fassnacht M, Opocher G, Klose S, Fottner C, Forrer F, Plockinger U, Petersenn S, Zabolotny D, Kollukch O, Yaremchuk S, Januszewicz A, Walz MK, Eng C, Neumann HP. Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clin Cancer Res. 2009;15:6378–6385. doi: 10.1158/1078-0432.CCR-09-1237. [DOI] [PubMed] [Google Scholar]
- 31.Powers JF, Evinger MJ, Tsokas P, Bedri S, Alroy J, Shahsavari M, Tischler AS. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000;302:309–320. doi: 10.1007/s004410000290. [DOI] [PubMed] [Google Scholar]
- 32.Martiniova L, Lai EW, Elkahloun AG, Abu-Asab M, Wickremasinghe A, Solis DC, Perera SM, Huynh TT, Lubensky IA, Tischler AS, Kvetnansky R, Alesci S, Morris JC, Pacak K. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin Exp Metastasis. 2009;26:239–250. doi: 10.1007/s10585-009-9236-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hahm SH, Hsu CM, Eiden LE. PACAP activates calcium influx-dependent and -independent pathways to couple met-enkephalin secretion and biosynthesis in chromaffin cells. J Mol Neurosci. 1998;11:43–56. doi: 10.1385/JMN:11:1:43. [DOI] [PubMed] [Google Scholar]
- 34.Lodish MB, Powell AC, Abu-Asab M, Cochran C, Lenz P, Libutti SK, Pingpank JF, Tsokos M, Gorden P. Insulinoma and gastrinoma syndromes from a single intrapancreatic neuroendocrine tumor. J Clin Endocrinol Metab. 2008;93:1123–1128. doi: 10.1210/jc.2007-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuezva JM, Krajewska M, de Heredia ML, Krajewski S, Santamaria G, Kim H, Zapata JM, Marusawa H, Chamorro M, Reed JC. The Bioenergetic Signature of Cancer: A Marker of Tumor Progression. Cancer Res. 2002;62:6674–6681. [PubMed] [Google Scholar]
- 36.He QY, Chen J, Kung HF, Yuen AP, Chiu JF. Identification of tumor-associated proteins in oral tongue squamous cell carcinoma by proteomics. Proteomics. 2004;4:271–278. doi: 10.1002/pmic.200300550. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Rios F, Sanchez-Arago M, Garcia-Garcia E, Ortega AD, Berrendero JR, Pozo-Rodriguez F, Lopez-Encuentra A, Ballestin C, Cuezva JM. Loss of the Mitochondrial Bioenergetic Capacity Underlies the Glucose Avidity of Carcinomas. Cancer Res. 2007;67:9013–9017. doi: 10.1158/0008-5472.CAN-07-1678. [DOI] [PubMed] [Google Scholar]
- 38.Isidoro A, Martinez M, Fernandez PL, Ortega AD, Santamaria G, Chamorro M, Reed JC, Cuezva JM. Alteration of the bioenergetic phenotype of mitochondria is a hallmark of breast, gastric, lung and oesophageal cancer. Biochem J. 2004;378:17–20. doi: 10.1042/BJ20031541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moser TL, Kenan DJ, Ashley TA, Roy JA, Goodman MD, Misra UK, Cheek DJ, Pizzo SV. Endothelial cell surface F1-F0 ATP synthase is active in ATP synthesis and is inhibited by angiostatin. Proc Natl Acad Sci U S A. 2001;98:6656–6661. doi: 10.1073/pnas.131067798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Gao F, Yu LL, Peng Y, Liu HH, Liu JY, Yin M, Ni J. Dual functions of a monoclonal antibody against cell surface F1F0 ATP synthase on both HUVEC and tumor cells. Acta Pharmacol Sin. 2008;29:942–950. doi: 10.1111/j.1745-7254.2008.00830.x. [DOI] [PubMed] [Google Scholar]
- 41.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 42.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila) 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 43.Kundu JK, Surh YJ. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 2008;269:243–261. doi: 10.1016/j.canlet.2008.03.057. [DOI] [PubMed] [Google Scholar]
- 44.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli G, Brown K, Steward WP, Gescher AJ, Brenner DE. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 47.Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health - A comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–41. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- 48.Santos AC, Veiga F, Ribeiro AJ. New delivery systems to improve the bioavailability of resveratrol. Expert Opin Drug Deliv. 2011;8:973–990. doi: 10.1517/17425247.2011.581655. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, Mukhtar H, Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol Nutr Food Res. 2011;55:1169–76. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 51.Albani D, Polito L, Batelli S, De Mauro S, Fracasso C, Martelli G, Colombo L, Manzoni C, Salmona M, Caccia S, Negro A, Forloni G. The SIRT1 activator resveratrol protects SK-N-BE cells from oxidative stress and against toxicity caused by alpha-synuclein or amyloid-beta (1-42) peptide. J Neurochem. 2009;110:1445–1456. doi: 10.1111/j.1471-4159.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 52.Liontas A, Yeger H. Curcumin and resveratrol induce apoptosis and nuclear translocation and activation of p53 in human neuroblastoma. Anticancer Res. 2004;24:987–998. [PubMed] [Google Scholar]
- 53.Das DK, Sato M, Ray PS, Maulik G, Engelman RM, Bertelli AA, Bertelli A. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res. 1999;25:115–120. [PubMed] [Google Scholar]
- 54.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 55.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lappano R, Rosano C, Madeo A, Albanito L, Plastina P, Gabriele B, Forti L, Stivala LA, Iacopetta D, Dolce V, Ando S, Pezzi V, Maggiolini M. Structure-activity relationships of resveratrol and derivatives in breast cancer cells. Mol Nutr Food Res. 2009;53:845–858. doi: 10.1002/mnfr.200800331. [DOI] [PubMed] [Google Scholar]
- 57.Shi WF, Leong M, Cho E, Farrell J, Chen HC, Tian J, Zhang D. Repressive effects of resveratrol on androgen receptor transcriptional activity. PLoS One. 2009;4:e7398. doi: 10.1371/journal.pone.0007398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu H, Liang X, Fang Y, Qin X, Zhang Y, Liu J. Resveratrol inhibits hypoxia-induced metastasis potential enhancement by restricting hypoxia-induced factor-1 alpha expression in colon carcinoma cells. Biomed Pharmacother. 2008;62:613–621. doi: 10.1016/j.biopha.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 59.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130:1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci U S A. 2007;104:13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arakaki N, Nagao T, Niki R, Toyofuku A, Tanaka H, Kuramoto Y, Emoto Y, Shibata H, Magota K, Higuti T. Possible role of cell surface H+ -ATP synthase in the extracellular ATP synthesis and proliferation of human umbilical vein endothelial cells. Mol Cancer Res. 2003;1:931–939. [PubMed] [Google Scholar]


