Abstract
Introduction
The Nottingham Prognostic Index (NPI) is an established prognostication tool in the management of breast cancers (BCs). Latest ten-year survival data have demonstrated an improved outlook for each NPI category and the latest UK five- and ten-year survival from BC has been reported to be 85% and 77%, respectively. We compared survival of each NPI category for BCs diagnosed within the national breast screening service in Wales (Breast Test Wales (BTW)) to the latest data, and reviewed its validity in unselected cases within a screened population.
Methods
All women screened between 1998 and 2001 within BTW were included. The NPI score for each cancer was calculated using the size, nodal status, and grade of the primary tumour. Survival data (all-cause) were calculated after ten years of follow-up.
Results
In the three-year screening period, 199,082 women were screened. A total of 1,712 cancers were diagnosed, and 1,546 had data available for calculating the NPI. Overall five-year and ten-year survival was 94% and 82%, respectively.
Conclusions
Overall five-year and ten-year survival (all-cause) has improved even when compared with UK data for BC-specific survival. We found that the NPI remains valid for BC treatment, and that our data provide a reference for updating the all-cause survival of women diagnosed with BCs within a screened population.
Keywords: Breast cancer, Screening, Survival, Nottingham Prognostic Index
Prognostication of breast cancers (BCs) helps clinicians in decision-making by using information to stratify risk and tailor individual treatment plans. Multiple prognostication models are available within the UK. Commonly used prognostic indicators include the Nottingham Prognostic Index (NPI),1–3 and the Internet-based Adjuvant! Online (AO).4,5
The NPI takes into account the size of the tumour, number of lymph nodes involved, and tumour grade. The NPI was devised by Galea in 1982. The NPI was used to predict survival of 80%, 42% and 13% in three groups of patients according to the NPI score. Since then, the NPI has been refined and segregates patients into four groups, and is used to predict five-year survival (in accordance with the more commonly used time scales for survival of other types of cancers). It is, therefore, used as a surrogate marker of aggressiveness of BCs. The NPI has been applied to prospective validation in multiple centres with comparable results.1,6–9
AO, in contrast, is an Internet-based analytical tool used to project the outcomes of patients with or without adjuvant therapy based on epidemiology, tumour characteristics, estimated efficacy of endocrine therapy or chemotherapy and, finally, a Bayesian statistical method to predict the long-term prognosis. In comparison, calculation of the NPI is simpler, requires fewer data, and can more easily provide gross projection of survival.
Five- and ten-year survival from BC in the UK has been reported to be 85%10 and 77%, respectively.11 The most recently updated survival data a vailable with reference to the NPI are related to consecutive BCs and BC-specific survival in a single centre in Nottingham (Table 1).12 That study revalidated the value of the NPI in segregating BCs into groups with varying prognoses.
Table 1.
Predicted ten-year breast cancer-specific survival and categories of the Nottingham Prognostic Index
| NPI | Score | Cancer-specific ten-year survival | All-cause ten-year survival |
|---|---|---|---|
| I (Excellent) | ≤2.4 | 96% | 88% |
| II (Good) | >2.4 but ≤3.4 | 93% | 86% |
| III (Moderate) | >3.4 but ≤5.4 | 78% | 74% |
| IV (Poor) | >5.4 | 44% | 42% |
We reviewed all-cause survival data from the national population breast screening service in Wales (Breast Test Wales (BTW)) within one screening cycle with a follow-up of ≥10 years. These cases were unselected and treatment carried out across multiple centres within Wales. We wished to evaluate all cancers diagnosed within BTW in one screening cycle and present all-cause survival data for these patients, and then compare these data with the results from the single centre in Nottingham. Also, we wished to demonstrate that the NPI is still useful in unselected cases but may now need to be updated to make it a valuable prognostication tool within a screened population.
Methods
BTW holds a carefully maintained central database for all cancers diagnosed in women who have attended breast screening. All women aged 50–65 years who attended breast screening between April 1998 and April 2001 were followed up for ≥10 years. BTW received notifications of all BCs diagnosed as well as any deaths that occurred during follow-up. The study period of 1998 to 2001 was chosen because that particular screening round was the tenth year since the inception of BTW. At this juncture, BTW would be considered to be running without major problems.
BC patients underwent standardised treatment according to local protocols as agreed by discussion within the regional multidisciplinary team. Surgical treatment for BCs was conducted at respective treatment centres. Subsequent adjuvant therapy was carried out in a regional oncology centre.
All cases in the present study were identified via the BTW and National Breast Screening System (NBSS) database. NBSS holds all the death records of women who attended screening regardless of their age, thereby providing all-cause survival data.
If possible, histological assessment was carried out to provide parameters for calculation of the NPI using the following formula:
NPI = maximum invasive cancer size (S) × 0.2 + lymph node stage (LN = 1, 2, or 3) + histological grade (H = 1, 2, or 3).
Patients were grouped into four categories according to the NPI score: I (excellent) ≤2.4; II (good) >2.4 but ≤3.4; III (moderate) >3.4 but ≤5.4; and IV (poor) >5.4. Kaplan–Meier survival curves were constructed using SPSS v19 (IBM, Armonk, NY, USA) using all-cause survival data.
Results
The total number of women who attended screening in the years 1998 to 2001 was 199,082. The overall uptake was 77.3%.
A total of 1,712 patients were diagnosed with invasive BCs having undergone screening in the three-year period. There were 1,020 screening-detected BCs, and the overall prevalence of cancer detection on screening for these three years was 0.51% (5.1 per 1000 women screened). However, 692 women with ‘normal’ screening mammograms subsequently developed interval BCs, which equates to a prevalence of interval BC of 0.38% (3.48 per 1,000 women screened).
Data were incomplete for 166 patients so the NPI could not be calculated. Therefore, 1,546 cases were included in this study. Table 2 shows the number of cancers in each NPI category as well as overall five- and ten-year survival.
Table 2.
Number of cancers in each category of the Nottingham Prognostic Index and survival
| NPI | Total number of cases | Five-year survival | Ten-year survival |
|---|---|---|---|
| I | 322 (20.8%) | 97% | 89% |
| II | 537 (34.7%) | 93% | 84% |
| III | 536 (34.7%) | 90% | 77% |
| IV | 151 (9.8%) | 88% | 73% |
| Total | 1,546 | 94% | 82% |
Figure 1 shows the Kaplan–Meier all-cause survival curve of BCs diagnosed in women who underwent screening within one screening cycle, and is broken down into each NPI category. It demonstrates clear segregation of the survival curve for each NPI category with no significant crossover.
Figure 1.
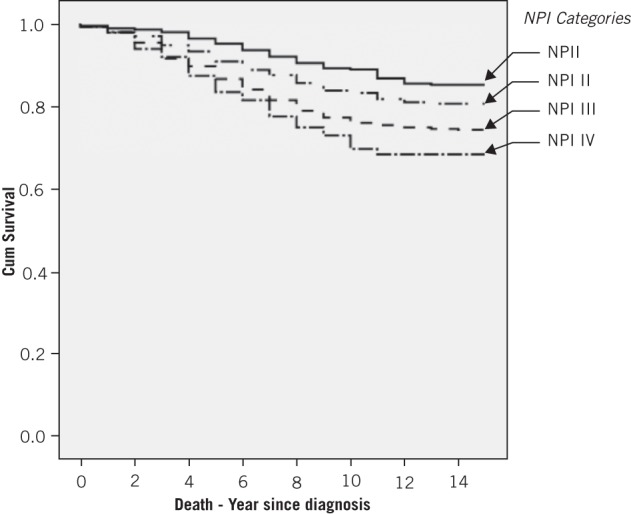
Kaplan–Meier all-cause survival curve
Discussion
Tabar et al. 13 described one of the longest follow-ups of BCs patients within a screening programme. After 29 years of follow-up, they demonstrated that the relative risk of BC mortality in screened women was 0.69–0.73 compared with unscreened women. This figure was comparable with their publication of a 30% reduction in BC mortality in 1985.14
Overall all-cause ten-year survival in the present study was 82%, which is better than that observed in a study in Sweden and that of recent UK data (77%).11 Five-year survival of 94% was a marked improvement from the five-year UK figure of 85%.10 These UK figures relate to cancer-specific survival, and our data are for all-cause survival. Purists would argue that such comparisons are not compatible but we stress: (i) that any improvement in overall survival is always an achievement worth noting; (ii) if available, BC-specific survival data for our cohort would be even better than that presented here. This trend was also observed in the study from a single centre in Nottingham. Our data may be used as a reference for updating all-cause survival data specifically within a screened population in which treatment was carried out in multiple centres.
1998 to 2001 was the third screening round for BTW. Each screening round is three years. With the first two screening rounds broadly achieving National Health Service Breast Screening Programme standards, we are confident in the quality of the data provided here. Importantly, two-view mammography was introduced for all rounds in BTW in 1998. Prevalent round screens used two view mammography since 1989; data from previous screening rounds were not included in the present study to prevent the introduction of bias. Data from more recent screening rounds were not included because the women who attended screening would not have completed ten-year follow-up to provide the required survival figure.
The validity of the NPI as a prognostic tool for BC has been reviewed.1,6–9 The most recent major review was carried out in Nottingham,12 with a detailed analysis and internal validation demonstrating the robustness of their data. Our data were comparable with their data with the exception of NPI VI. This difference could be because our patients were diagnosed more recently than those reviewed by the Nottingham team. Also, our patients were thought to have improvements in treatment (especially with introduction of analyses of the status of hormone receptors and hormonal manipulation), so NPI VI patients may have benefited greatly.
Most ‘developed’ countries are running BC screening, so our data highlight the validity of the NPI. The NPI is applicable in most screened populations in which one key centre oversees the screening programme, and where surgical/oncological treatments are carried out in multiple local centres. We analysed all cancers within one screening cycle in a population-based screening programme, and our patients were treated in multiple local centres. Each treatment centre would review each case in their respective multidisciplinary meeting and recommend appropriate treatment according to agreed protocols. This strategy represents how most screening programmes are run.
Other prognostication indices are available and are being used in conjunction with the NPI in the planning of treatment of BCs. For example, AO is highly valuable in helping to formulate adjuvant treatment and is used widely in multidisciplinary meetings. We propose that the NPI should remain one of many tools available to clinicians when formulating treatment plans. The NPI is easy to use and does not require cumbersome and significant clinical, epidemiological or histological assessment.
Conclusions
Overall five-year and ten-year all-cause survival has improved even when compared with BC-specific survival. We found that the NPI remains valid and valuable for BC treatment. However, our data suggest that the NPI should be updated for all-cause survival of women diagnosed with BCs within a screened population.
References
- 1. Balslev I, Axelsson CK, Zedeler K, Rasmussen BB, Carstensen B, Mouridsen HT. The Nottingham Prognostic Index applied to 9,149 patients from the studies of the Danish Breast Cancer Cooperative Group (DBCG). Breast Cancer Res Treat 1994; 32: 281–290. [DOI] [PubMed] [Google Scholar]
- 2. Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992; 22: 207–219. [DOI] [PubMed] [Google Scholar]
- 3. Haybittle JL, Blamey RW, Elston CW et al. A prognostic index in primary breast cancer. Br J Cancer 1982; 45: 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olivotto IA, Bajdik CD, Ravdin PM et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 2005; 23: 2,716–2,725. [DOI] [PubMed] [Google Scholar]
- 5. Ravdin PM, Siminoff LA, Davis GJ et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 2001; 19: 980–991. [DOI] [PubMed] [Google Scholar]
- 6. D’ Eredita G, Giardina C, Martellotta M, Natale T, Ferrarese F. Prognostic factors in breast cancer: the predictive value of the Nottingham Prognostic Index in patients with a long-term follow-up that were treated in a single institution. Eur J Cancer 2001; 37: 591–596. [DOI] [PubMed] [Google Scholar]
- 7. Sundquist M, Thorstenson S, Brudin L, Nordenskjold B. Applying the Nottingham Prognostic Index to a Swedish breast cancer population. South East Swedish Breast Cancer Study Group. Breast Cancer Res Treat 1999; 53: 1–8. [DOI] [PubMed] [Google Scholar]
- 8. Sundquist M, Thorstenson S, Brudin L, Wingren S, Nordenskjold B. Incidence and prognosis in early onset breast cancer. Breast 2002; 11: 30–35. [DOI] [PubMed] [Google Scholar]
- 9. Todd JH, Dowle C, Williams MR et al. Confirmation of a prognostic index in primary breast cancer. Br J Cancer 1987; 56: 489–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Office of National Statistics. Cancer survival in England – patients diagnosed 2005–2009 and followed up to 2010. National Archives, London; 2011. [Google Scholar]
- 11. Coleman MP, Forman D, Bryant H et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011; 377: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blamey RW, Ellis IO, Pinder SE et al. Survival of invasive breast cancer according to the Nottingham Prognostic Index in cases diagnosed in 1990–1999. Eur J Cancer 2007; 43: 1,548–1,555. [DOI] [PubMed] [Google Scholar]
- 13. Tabar L, Vitak B, Chen TH et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011; 260: 658–663. [DOI] [PubMed] [Google Scholar]
- 14. Tabar L, Fagerberg CJ, Gad A et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1985; 1: 829–832. [DOI] [PubMed] [Google Scholar]


